Summary
Background
We report on the efficacy and side effects of granulocyte collection, which is comparatively infrequently performed in Germany.
Methods
Data from 378 healthy donors who underwent 914 granulocyte collections between 1999 and 2007 were retrospectively analyzed. Donors received G-CSF (lenograstim) at a median dose of 5.58 (3.25–7.36) μg/kg body weight with (n = 243) or without (n = 57) 4 mg dexamethasone. Side effects were recorded by donor monitoring and interview (questionnaire).
Results
The median granulocyte yield in apheresis products was 8.47 × 1010 (3.07–14.92 × 1010). Granulocyte yields correlated significantly with gender, baseline WBC, PMN and PLT counts, and nicotine consumption. Dexamethasone and lenograstim administration was more effective than lenograstim administration alone (p < 0.001). Side effects of granulocyte mobilization were generally mild: bone pain in 31.4%, headache in 19.6%, and fatigue in 15.7% of donors. During follow-up (4 weeks), pruritus and/or exanthema were reported in 17.6% of donors.
Conclusions
Granulocyte mobilization with lenograstim with or without dexamethasone was a safe and effective regimen for granulocyte mobilization. Side effects were tolerable and milder than those seen in peripheral blood stem cell donors. Long-term monitoring of granulocyte donors is important to establish optimal standards for the procedure.
KeyWords: Granulocyte collection, Allogeneic donors, G-CSF
Introduction
Severe neutropenia (<0.5 × 109/l) and neutrophil dysfunction are major risk factors for the development of serious mycotic and bacterial infections in patients undergoing intensive chemotherapy and hematopoietic stem cell transplantation [1, 2, 3, 4]. Granulocyte transfusion (GTX) therapy is a logical approach to restore normal polymorphonuclear neutrophils (PMNs) to sufficient numbers in these patients. GTX to neutropenic patients has been studied since the 1930s [5]. Studies performed in the 1960s showed that granulocytes harvested from donors with chronic myeloid leukemia (CML) had a dose-dependent therapeutic benefit when transfused to neutropenic patients [6]. In the 1970s, granulocyte yields obtained from healthy donors improved on the introduction of continuous flow leukapheresis, use of hydroxyethyl starch (HES) as a sedimenting agent, and stimulation of granulocytes using glucocorticoids [5, 6, 7, 8, 9, 10, 11]. Despite these improvements, granulocyte yields from CML patients were still considerably higher than those from glucocorticoid-stimulated healthy donors. Subsequently, interest in GTX declined because the achievable cell dose was only marginally adequate, and antibiotic and antimycotic therapy of neutropenic patients had substantially improved [5, 12, 13, 14]. The introduction of recombinant human granulocyte colony-stimulating factor (rhG-CSF) in the 1990s enabled increasing neutrophil numbers by three- to fivefold with a single subcutaneous administration [15, 16, 17, 18]. Furthermore, the combination of rhG-CSF and glucocorticoid resulted in even higher granulocyte yields than rhG-CSF alone [10, 19, 20, 21, 22, 23, 24]. In Germany, there are no reliable data available regarding the amount of granulocyte concentrates collected per year, because the annual survey of the Paul-Ehrlich-Institut included this information only since 2012. Compared with allogeneic stem cell collection, which is a well-established procedure in university hospitals and other medical facilities, granulocyte collection is only performed in a few centers.
At the University Hospital of Dresden, granulocyte concentrates have been harvested since 1997. In our retrospective study, we evaluated the granulocyte yields achieved with lenograstim, which is an rhG-CSF (5.0–7.0 μg/kg body weight; our standard mobilization regimen), and assessed the effect of donor characteristics. In addition, we studied the side effects of granulocyte mobilization and collection and compared them with the well-known side effects of peripheral blood stem cell (PBSC) mobilization and collection [25].
Material and Methods
Study Design and Granulocyte Mobilization
A database of clinical data of 378 granulocyte donors who donated granulocytes between April 1999 and December 2007 was compiled retrospectively; all donors were either relatives or friends of the recipients. Donor eligibility was established by medical history, complete physical examination, ECG, and laboratory tests. Complete blood counts (CBC) with differentials were performed at baseline (in median 4 days before the first donation) and before and after each leukapheresis. Granulocyte mobilization and collection was approved by the Ethics Committee of the University Hospital of Dresden.
Granulocyte mobilization was achieved using a single subcutaneous injection of lenograstim (rhG-CSF) at a median dose of 5.58 (3.25–7.36) μg/kg body weight 12–16 h before leukapheresis. Donors without contraindications to corticosteroids (e.g., diabetes, obesity, hypertension, gastric ulcer, or mental disorder) were orally administered 4 mg of dexamethasone 12–16 h before the first and second apheresis. All donors were thoroughly informed that both mobilizing agents are still not approved for granulocyte mobilization in Germany.
Granulocyte Collection
Granulocyte concentrates were collected using a continuous flow blood cell separator (Cobe Spectra; CaridianBCT, Lakewood, CO; Version 7.0, PMN program) via bilateral peripheral venipuncture in the forearm or cubital vein. During each granulocyte apheresis, donor blood was processed at a rate of 50–60 ml/min for approximately 3 h, a median of 2.0 (0.44–4.56) times the total blood volume (TBV). The target volume of the granulocyte concentrate was 500 ml, and products with 172–539 ml volume were obtained. Trisodium citrate (2.4% in HES; Plasmasteril 450/0.7; Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany) was used as an anticoagulant at a blood:anticoagulant ratio of 13:1. During apheresis, vital signs and potential adverse events were continuously monitored. Calcium gluconate was infused intravenously, as necessary. Each donor was allowed to donate a maximum of 4 granulocyte concentrates, and 914 granulocyte concentrates were harvested. In detail, the apheresis procedures were performed as follows: 378 1st aphereses, 300 2nd aphereses, 169 3rd aphereses and 67 4th aphereses.
Intervals between apheresis procedures varied according to donor availability and the number of donors on call for one patient. Minimum intervals between apheresis procedures were 2 days, median intervals 3 days. Administration of G-CSF with or without dexamethasone was performed on each evening preceding the next apheresis.
All apheresis products were examined for leukocyte and neutrophil counts by routine methods.
Follow-Up Investigations
Donors received a questionnaire to assess specific complaints that may have emerged because of mobilization and apheresis within 4 weeks of final granulocyte donation. At the same time CBC with differential count, lactate dehydrogenase examination, and alkaline phosphatase examination were performed either by the referring physician or the University Hospital of Dresden.
Data Analysis
All data were compiled using Microsoft Office Access 2002 database software (Microsoft® Corporation, Redmond, WA, USA). Statistical analysis was performed with SPSS (version 15.0) (IBM, Armonk, NY, USA). Data are provided as median and ranges unless otherwise noted. Univariate analyses for significance in metric variables were performed using Wilcoxon-Mann-Whitney test. Correlations of metric variables were assessed using Spearman's rank correlation coefficient. Linear models were fitted to model the simultaneous effect of two or more variables on a dependent variable. All analyses are considered explorative; no adjustment was performed for multiple testing.
Results
Donor Demographics
A total of 378 donors (157 female, 221 male) underwent 914 leukapheresis procedures; of these, 78, 131, 102 and 67 individuals donated on 1, 2, 3 and 4 occasions, respectively. The median interval between each apheresis was 3 (1–1,098) days, and the median donor age was 39 (18–70) years.
Effect of Donor Characteristics, Donor Blood Counts and Mobilization Regimen on Apheresis Yields
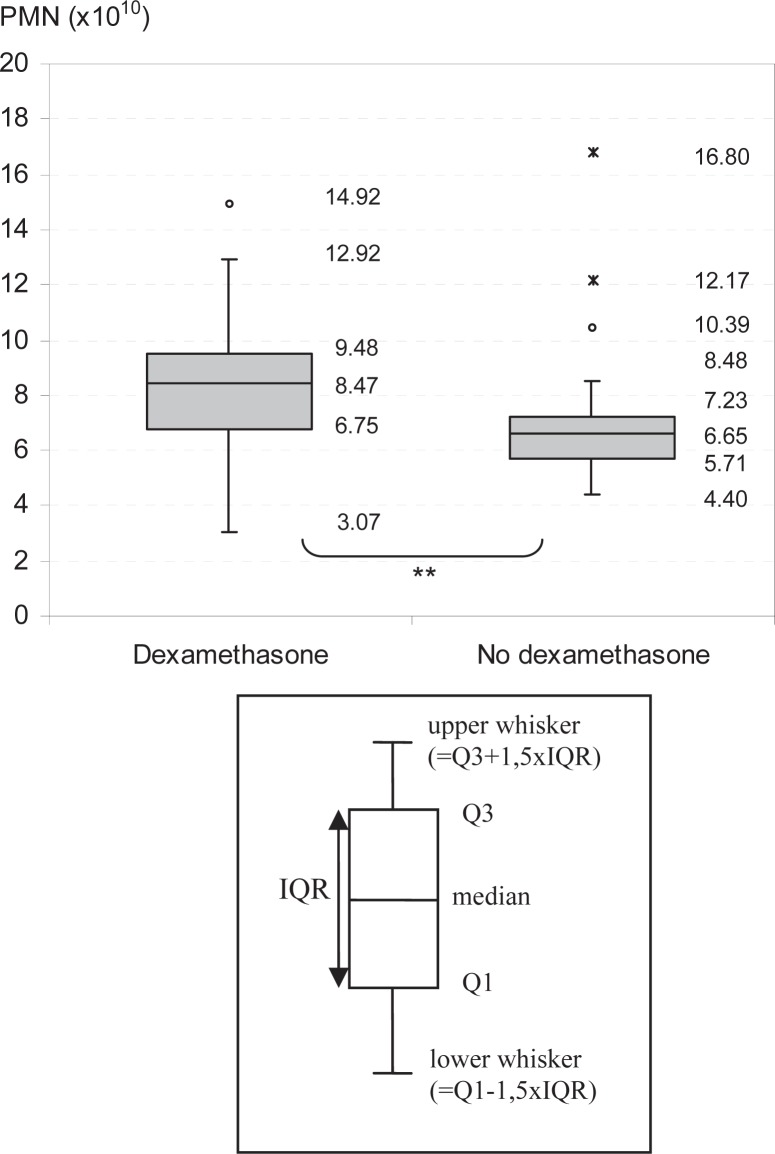
The characteristics of apheresis results are shown in table 1. To eliminate potential variability due to differences in the durations between multiple aphereses, only the yield of the first apheresis from each donor was considered for further analyses. Furthermore, only donors with a lenograstim dose of 5.0–7.0 μg/kg body weight and granulocyte concentrates with a volume of more than 450 ml were included. The median pre-apheresis PMN (prePMN) count of donors after stimulation with a combination of lenograstim and dexamethasone (n = 141) was 31.90 (17.40–53.02) Gpt/l, and the median granulocyte yield (n = 144) was 8.47 (3.07–14.92) × 1010. By comparison, the median prePMN count of donors receiving lenograstim only was 24.83 (16.78–59.51) Gpt/l, and the median granulocyte yield 6.65 (4.40–16.80) × 1010. This difference was statistically significant (p < 0.001; fig. 1).
Table 1.
Parameter of 1st aphereses and all aphereses performed
| 1st aphereses | All aphereses | |
|---|---|---|
| PMN yields × 1010 | 7.43 (1.10–14.91) n = 369 | 7.62 (1.1–18.97) n = 890 |
| WBC yields × 1010 | 8.75 (1.38–17.77) n = 371 | 9.43 (1.38–20.13) n = 895 |
| Platelet yields × 1011 | 2.13 (0.53–3.45) n = 366 | 1.89 (0.27–4.20) n = 880 |
| Volume of product, ml | 504 (172–623) n = 378 | 504 (29–623) n = 914 |
| Processed volume, l | 10.0 (3.1–16.0) n = 378 | 10.4 (1.9–16.0) n = 914 |
| Duration of apheresis, min | 176 (67–253) n = 378 | 176 (55–253) n = 914 |
Fig. 1.
Effect of dexamethasone administration on granulocyte yields. Granulocyte yields (PMN) of donors with (n = 144) and without dexamethasone (n = 42) administration before first apheresis. **Significant.
We analyzed the effect of lenograstim doses on granulocyte yields in donors administered 4 mg of dexamethasone during mobilization. Granulocyte yields from donors administered 5.0–7.0 μg/kg body weight of lenograstim (n = 144) were not significantly different than those from donors administered 3.69–4.98 μg /kg body weight of lenograstim (n = 63). There was no significant difference between granulocyte yields from donors with an age of 18–39 years (n = 97) and those of donors with an age of 39–70 years (n = 108) (p = 0.099).
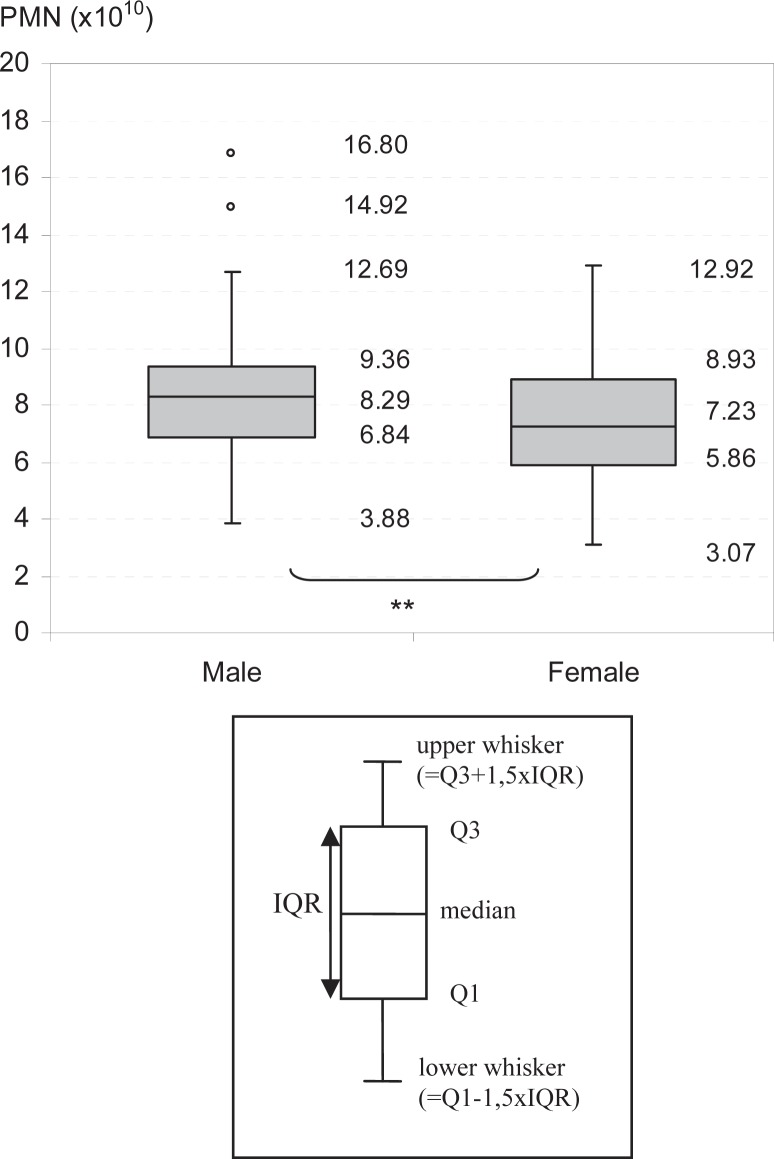
Gender was a significant variable affecting granulocyte yields (p = 0.016). Males (n = 120) had a median granulocyte yield of 8.29 × 1010 (3.88–16.80 × 1010) compared to 7.23 × 1010 (3.07–12.92 × 1010) in females (n = 85; p = 0.016; fig. 2). However, baseline granulocyte counts were similar in males and females. Although female donors (n = 84) mobilized more effectively than males (n = 121; p = 0.033), as indicated by significantly higher prePMN counts (p = 0.036), PMN counts following apheresis were similar. The processed blood volume in each apheresis procedure was adjusted to the target product volume and depended on the blood flow, but not directly on the donor's TBV. For that reason, the overall blood volume processed in male donors (1.93 times the TBV) was significantly lower than that in female donors (2.32 times the TBV; p < 0.001).
Fig. 2.
Effect of gender on granulocyte yields. Granulocyte yields of male and female donors. **Significant.
Donors with either higher baseline WBC (n = 121) and PMN (n = 120) counts had significantly higher granulocyte yields (r = 0.375 and r = 0.364, respectively; p < 0.001). A positive correlation was observed between the baseline platelet counts and granulocyte yields (r = 0.242, p = 0.001, n = 199).
Alcohol consumption had no effect on granulocyte yields (p = 0.419), whereas regular smokers (n = 74) had significantly higher granulocyte yields (median = 8.35 × 1010 (4.15–16.80 × 1010) than occasional or non-smokers (n = 130, median = 7.54 × 1010 (3.07–12.92 × 1010); p = 0.005). In addition to significantly higher baseline granulocyte counts (smokers: median = 7.09 (3.40–13.04) Gpt/l, non-smokers: median = 6.03 (2.76–11.34) Gpt/l; p = 0.001), regular smokers had higher pre-apheresis granulocyte counts (smokers: median = 37.0 (21.60–57.00) Gpt/l, non-smokers: median = 31.49 (19.28–63.69) Gpt/l; p < 0.001).
Multivariate Analysis of Factors Affecting Granulocyte Yields
We performed multivariate stepwise regression analysis of the relationships between granulocyte yields and baseline WBC, PMN and PLT counts, baseline hemoglobin (Hb) levels, gender, age, height, weight, blood volume, alcohol consumption, smoking, common cold 4 weeks before first examination, dexamethasone administration as well as lenograstim dosage. In order to exclude other confounding factors, we only included concentrates of the first apheresis with a volume of more than 450 ml and only those from donors receiving 5.0–7.0 μg/kg body weight lenograstim (n = 177). In this multivariate analysis donor weight, baseline PMN and PLT counts, dexamethasone administration, smoking status, and donor height were the key determinants, accounting for 41% of variance. Higher donor weight and height as well as baseline PMN and PLT counts were associated with better granulocyte yields. The stimulation with dexamethasone (and lenograstim) and the consumption of nicotine also resulted in higher granulocyte yields.
Development of Granulocyte Yields during the Course of Consecutive Leukaphereses
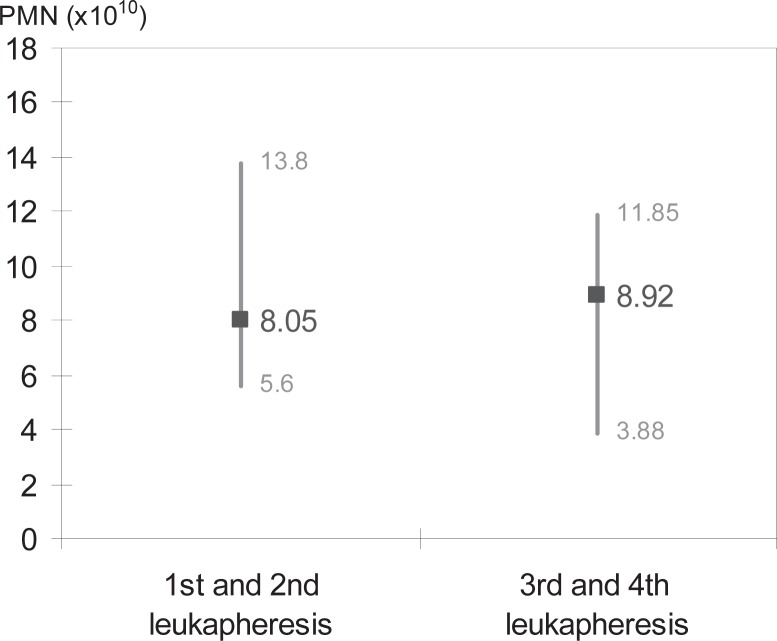
According to German guidelines, donors are allowed to donate granulocytes only 4 times a year. We compared the concentrates of the 1st and 2nd leukaphereses of a donor with the 3rd and 4th of the same donor. Among the concentrates with a volume of 450 ml or more from donors administered 5.0–7.0 μg/kg body weight of lenograstim and who donated multiple times, we found no significant changes in granulocyte yields between the 1st and 2nd aphereses and the 3rd and 4th aphereses (p = 0.429, n = 38, fig. 3).
Fig. 3.
Development of granulocyte yields over multiple apheresis. Granulocyte yields between 1st and2nd and 3rd and 4th leukapheresis were compared, shown as median values and range.
Side Effects of Lenograstim Administration and Leukapheresis
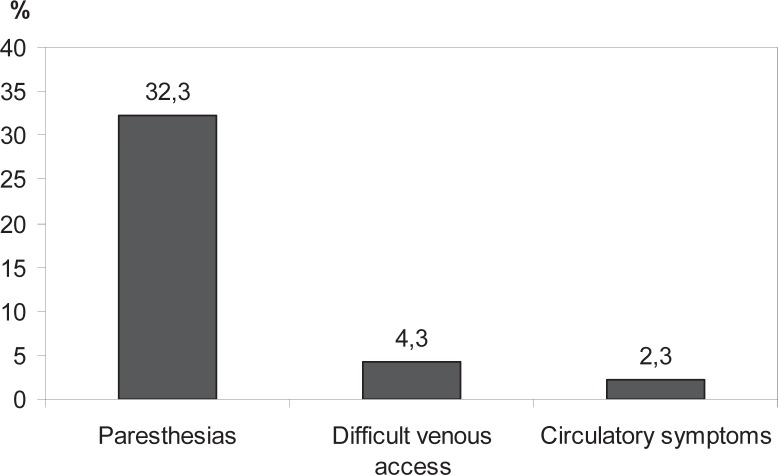
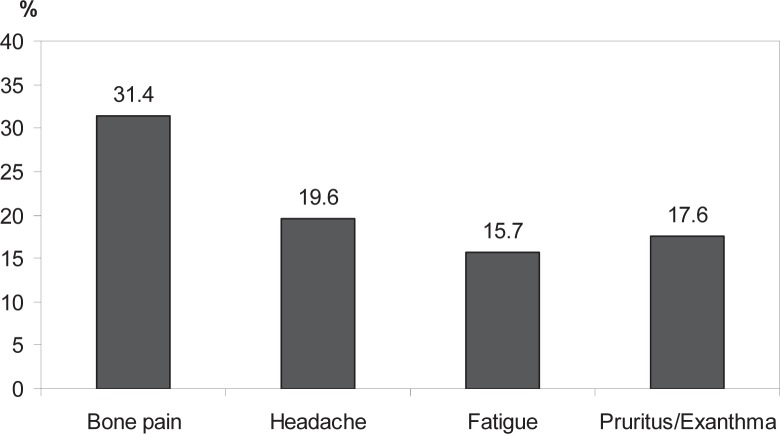
The most common complications occurring during leukapheresis are shown in figure 4 (criteria for inclusion: volume of concentrate > 450 ml; administration of 5.0–7.0 μg/kg body weight lenograstim, n = 514). The most frequent complaints reported within 4 weeks following final leukapheresis are shown in figure 5 (all donors have been included in this analysis). Other side effects reported by more than 2% of the donors were common cold or flu-like symptoms, abnormal hemorrhage, nausea, circulatory symptoms, paresthesia, and edema. These data were obtained from 102 of 378 questionnaires evaluated.
Fig. 4.
Most frequent side effects during leukapheresis. Percentage of donors reporting side effects during apheresis.
Fig. 5.
Most frequent side effects within 4 weeks after final leukapheresis. Percentage of donors reporting side effects within 4 weeks after final leukapheresis.
There was no serious adverse event among the donors investigated.
Blood Cell Counts and Chemistry
The kinetics of blood cell counts and blood chemistry at baseline, before first leukapheresis and 4 weeks after final apheresis are shown in table 2. Pre-apheresis Hb levels and PLT counts declined in donors that underwent multiple leukaphereses. The median Hb level after 4th leukapheresis was 7.2 mmol/l and the median PLT count was 168 × 109/l. One donor had PLT counts of 89 × 109/l and 24 × 109/l after 1st and 2nd leukapheresis, respectively. Although he had no spontaneous hemorrhages, he was unable to attend work for 2 weeks until his PLT count normalized. WBC, PMN and lymphocyte counts as well as Hb levels 4 weeks after apheresis were all significantly lower than their baseline values.
Table 2.
Blood cell counts and chemistry at baseline, before first apheresis, and 4 weeks after final apheresis
| Baseline | Before first apheresis | After 4 weeks | |
|---|---|---|---|
| WBC, × 109/l | 6.29 (n = 206) | 33.54* (n = 208) | 5.62** (n = 155) |
| PMN, × 109/l | 3.67 (n = 204) | 30.30* (n = 202) | 3.25** (n = 140) |
| Lymphocyte, × 109/l | 1.9 (n = 161) | 1.6** (n = 140) | |
| Hgb, mmol/l | 9.10 (n = 206) | 9.20 * (n = 208) | 8.70** (n = 155) |
| PLT, × 109/l | 236 (n = 206) | 249* (n = 208) | 238* (n = 154) |
| LDH, mmol/s × l | 3.29 (n = 207) | 4.38* (n = 202) | 2.66** (n = 138) |
| AP, mmol/s × l | 1.21 (n = 204) | 1.38* (n = 202) | 1.11** (n = 139) |
LDH = lactate dehydrogenase, AP = alkaline phosphatase.
Significantly higher compared with baseline.
Significantly lower compared with baseline.
Discussion
The efficacy and side effects of granulocyte mobilization and collection were analyzed in 378 donors in whom 914 leukaphereses had been performed. Our standard mobilization regimen of 5.0–7.0 μg/kg body weight of lenograstim and 4 mg of dexamethasone was very effective in obtaining high granulocyte yields and PMN counts for subsequent transfusion, which were consistent with those obtained in previous studies [19, 20, 21, 22, 23, 24]. Higher doses of lenograstim did not result in significantly higher granulocyte yields but increased the rate of side effects as shown by a study of Heuft et al. [26]. Granulocyte yields from donors mobilized with a combination of 3.69–4.98 μg/kg body weight (median 4.33 μg/kg body weight) of lenograstim and 4 mg of dexamethasone were similar to yields from donors administered higher lenograstim doses (5.0–7.0 μg/kg body weight). Lenograstim is delivered in packages of 105 μg and 263 μg. We found the combination of dexamethasone and a single vial of 263 μg of lenograstim to be a sufficient dose for donors with a body weight of 70 kg or less.
Next, we compared the granulocyte yields in donors administered a combination of dexamethasone and lenograstim with those administered lenograstim only. Donors administered the combination had significantly higher granulocyte yields (about 30% more in the median) than those administered lenograstim only. The efficacy of the combination of G-CSF and dexamethasone has been previously reported and could become a standard mobilization regimen for donors without contraindications to steroid treatment in the future [16, 17, 19, 23, 24]. Nevertheless it has to be emphasized that both mobilizing agents have still not been approved for granulocyte collection in Germany. Despite considerable evidence of a beneficial impact in severely neutropenic patients with refractory infections, the clinical efficacy of GTX also remains to be confirmed in prospective, randomized trials [27]. There are also no clear data regarding the clinical impact of the functionality of neutrophiles mobilized with G-CSF versus G-CSF and dexamethasone.
In our retrospective study, granulocyte yields from males were significantly higher than those from females, although female donors had higher peripheral blood PMN counts before harvesting. PMN counts after apheresis were similar in both genders. These findings are probably due to the significantly lower blood volumes of female donors compared to males (p < 0.001). Because of this, 1.93 times the TBV of male donors was processed during apheresis in contrast to 2.32 times the TBV of females (p < 0.001). Interestingly, Sachs et al. (Sachs, personal communication, February 2012) reported that female donors have higher granulocyte yields than males, although these authors used a significantly lower blood volume (approximately 7 l) during apheresis than that used in our study. To the best of our knowledge, there is no evidence regarding recruitment of granulocytes during the apheresis procedure in the literature, but this phenomenon would be worth to be investigated in future studies.
Donor age had no effect on granulocyte yields; however, younger donors mobilized more PMN into their peripheral blood than older donors. However, Chatta et al. [28] found no difference in prePMN counts due to age, although they compared significantly different age groups (20–30 years vs. 70–80 years) than that in our study [28].
We found a significant correlation between the donor baseline PMN, WBC and PLT counts and granulocyte yields; this re-confirmed the results of Quillen et al. [24]. The same study group found an association between baseline PLT and pre-apheresis CD34+ cell counts in PBSC donors [29].
Alcohol consumption had no effect while smoking had a significant effect on granulocyte yields. Regular smokers had significantly higher baseline PMN counts, prePMN counts, and granulocyte concentrate yields than occasional or non-smokers.
In our multivariate analysis, we found that donor baseline PMN and PLT counts, weight, height, male gender, and regular smoking significantly affected granulocyte yields. A combination of 4 mg dexamethasone and lenograstim administration resulted in significantly higher granulocyte yields in the apheresis products.
There was no decrease in prePMN counts or granulocyte yields during 4 sequential donations performed with a median interval of 3 days, possibly because of cumulating action of lenograstim. The most common side effects during leukapheresis were paresthesia, difficulties with venous access, and circulatory symptoms. Paresthesia may occur because of hypocalcemia emerging from anticoagulation with trisodium citrate. This side effect was observed significantly more frequently in females than in males, most likely because of the higher dose of trisodium citrate per body weight used in female donors. Circulatory symptoms were typically vasovagal syncopes that often occurred at the beginning or the end of leukapheresis. Within the 4 weeks following final apheresis nearly 18% of donors complained of pruritus and/or exanthema. In one donor, pruritus persisted for approximately 1 year. These are side effects of leukapheresis that are caused by the sedimenting agent HES [31]. One donor could not be admitted for further donations because of edema after first leukapheresis. To minimize HES-associated side effects, the maximum dose of HES administered to the donors should be limited in future protocols.
In our study, we found that lenograstim was well tolerated (fig. 5). No donors were incapable of working due to these side effects. Due to the considerably lower dose and shorter administration interval of lenograstim used in these donors, the adverse effects of mobilization were lower than in PBSC donors; bone pain was reported by 93.5% of PBSC donors [25], but by only 31.4% of granulocyte donors. Our data are in accordance with those of other studies using lenograstim as a mobilization agent; bone pain, headache and fatigue occurred in 23.3%, 20% and 6.7%, respectively, of donors in a study by Moog [16] using a mobilization regimen of lenograstim and dexamethasone; McCullough et al. [31] reported only mild side effects due to lenograstim stimulation; Stroncek et al. [22] found that lenograstim and dexamethasone administration together was not more toxic than lenograstim alone.
Four weeks after final apheresis, WBC, PMN and PLT counts and Hb levels were significantly diminished. The decrease in Hb levels is not found in PBSC donors and can be explained by the significant loss of erythrocytes during granulocyte donation. In contrast, a greater reduction in WBCs is observed in PBSC donors – median WBC of 5.1 × 109/l and median PMN 2.9 × 109/l [25] have been reported 4 weeks after donation [22, 26]. The reason for this remains unclear, but others have proposed that it may involve depletion of slowly self-renewing hematopoietic stem cells, down-regulation of the G-CSF receptor, or another alteration of cytokine signaling [25, 32]. The values 4 weeks after final leukapheresis were all within the standard range, and the rate of infections in donors appeared to be low. Lactate dehydrogenase and alkaline phosphatase are known to be decreased below baseline 4 weeks after final apheresis [16, 22].
In conclusion, effective PMN mobilization and collection is possible with lenograstim at a dose of 5.0–7.0 μg/kg of body weight. The administration of 4 mg dexamethasone further increased prePMN counts and granulocyte yields. We identified several demographic and laboratory variables that significantly correlated with the quantity of granulocyte concentrates. Besides the donor with a thrombocytopenia WHO grade 4, no other short-term serious side effects of the complete donation procedure were observed up to 4 weeks after final leukaphereses.
Although short-term toxicity of this mobilization regimen seems to be acceptable, some aspects have to be further evaluated before the general implementation into routine is possible. In contrast to stem cell donation, granulocyte collection conceivably could be performed repeatedly in healthy allogeneic blood donors over a number of years. Besides the unknown effects of long-term recurrent administration of G-CSF, also the possible side effects of repetitive application of corticosteroids become an issue of concern. The higher risk of the development of posterior subcapsular cataract (PSC) in granulocyte donors was initially reported by Ghodsi and Strauss [33]. Two other studies [34, 35] published more recently could not confirm a significant higher incidence of PSC in granulocyte donors compared with platelet donors. In spite of that, both studies revealed a clear association of PSC frequency with the number of granulocyte donations and the cumulative dose of steroids. In our donor cohort, periods of granulocyte donation of the individual donors were always dedicated to one corresponding recipient. Hereby chronic exposition to mobilizing agents was avoided.
Thorough documentation and accurate long-term monitoring of granulocyte donors remain absolutely essential to establish reliable guidelines for the frequency of rhG-CSF with or without steroid administration and leukapheresis.
Disclosure Statement
KH received speaking fees from Chugai Pharma and Genzyme. The other authors have no conflicts of interest to declare.
References
- 1.Price TH. Granulocyte transfusion therapy. J Clin Apher. 2006;21:65–71. doi: 10.1002/jca.20088. [DOI] [PubMed] [Google Scholar]
- 2.Hughes WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, Feld R, Pizzo P, Rolston KVI, Shenep JL, Young LS. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Clin Infect Dis. 1997;25:551–573. doi: 10.1086/513764. [DOI] [PubMed] [Google Scholar]
- 3.Dale DC, Liles WC, Price TH. Renewed interest in granulocyte transfusion therapy. Br J Haematol. 1997;98:497–501. doi: 10.1046/j.1365-2141.1997.3023119.x. [DOI] [PubMed] [Google Scholar]
- 4.Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328:1323–1332. doi: 10.1056/NEJM199305063281808. [DOI] [PubMed] [Google Scholar]
- 5.Robinson SP, Marks DJ. Mini review granulocyte transfusion in the G-CSF era. Where do we stand? Bone Marrow Transplant. 2004;34:839–846. doi: 10.1038/sj.bmt.1704630. [DOI] [PubMed] [Google Scholar]
- 6.Freireich EJ, Judson G, Levin RH. Separation and collection of leukocytes. Cancer Res. 1965;25:1516–1520. [PubMed] [Google Scholar]
- 7.Winton EF, Vogler WR. Development of a practical oral dexamethasone premedication schedule leading to improved granulocyte yields with the continuous-flow centrifugal blood cell separator. Blood. 1978;52:249–253. [PubMed] [Google Scholar]
- 8.Lee JH, Leitmann SF, Klein HG. A controlled comparison of the efficacy of hetastarch and pentastarch in granulocyte collections by centrifugal leukapheresis. Blood. 1995;86:4662–4666. [PubMed] [Google Scholar]
- 9.Mishler JM. Hydroxyethyl starch as an experimental adjunct to leukocyte separation by centrifugal means: review of safety and efficacy. Transfusion. 1975;15:449–460. doi: 10.1046/j.1537-2995.1975.15576082219.x. [DOI] [PubMed] [Google Scholar]
- 10.Sussman LN, Colli W, Pichetshote C. Harvesting of granulocytes using a hydroxyethyl starch solution. Transfusion. 1975;15:461–465. doi: 10.1046/j.1537-2995.1975.15576082220.x. [DOI] [PubMed] [Google Scholar]
- 11.Shoji M, Vogler WR. Effects of hydrocortisone on the yield and bactericidal function of granulocytes collected by continuous-flow centrifugation. Blood. 1974;44:435–443. [PubMed] [Google Scholar]
- 12.Strauss RG. Therapeutic granulocyte transfusions in 1993 (editorial) Blood. 1993;81:1675–1678. [PubMed] [Google Scholar]
- 13.Wright DC, Kauffmann JC, Chusid MJ, Herzig Gallin JI. Functional abnormalities of human neutrophils collected by continuous flow filtration leukopheresis. Blood. 1975;46:901–911. [PubMed] [Google Scholar]
- 14.Han P, Vincent PC. Letter: Problems with filtration-collected neutrophils. N Engl J Med. 1976;294:729. [PubMed] [Google Scholar]
- 15.Caspar CB, Seger RA, Burger J, Gmür J. Effective stimulation of donors for granulocyte transfusions with recombinant methionyl granulocyte colony-stimulating factor. Blood. 1993;11:2866–2871. [PubMed] [Google Scholar]
- 16.Moog R. Donor tolerance and results of stimulation with G-CSF alone or in combination with dexamethasone for the collection of granulocytes. J Clin Apher. 2004;19:115–118. doi: 10.1002/jca.20013. [DOI] [PubMed] [Google Scholar]
- 17.Liles WC, Rodger E, Dale DC. Combined administration of G-CSF and dexamethasone for the mobilization of granulocytes in normal donors: optimization of dosing. Transfusion. 2000;40:642–644. doi: 10.1046/j.1537-2995.2000.40060642.x. [DOI] [PubMed] [Google Scholar]
- 18.Liles WC, Huang JE, Llewellyn C, SenGupta D, Price TH, Dale DC. A comparative trial of granulocyte-colony-stimulating factor and dexamethasone, separately and in combination, for the mobilization of neutrophils in the peripheral blood of normal volunteers. Transfusion. 1997;37:182–187. doi: 10.1046/j.1537-2995.1997.37297203521.x. [DOI] [PubMed] [Google Scholar]
- 19.Leitman SF, Yu M, Lekstrom J. Pair-controlled study of granulocyte colony stimulating factor (G-CSF) plus dexamethasone (Dexa) for granulocytapheresis donors. Transfusion. 1995;35(suppl):53S. [Google Scholar]
- 20.Dale DC, Liles WC, Llewellyn C, Rodger E, Price TH. Neutrophil transfusion: kinetics and functions of neutrophils mobilized with granulocyte-colony-stimulating factor and dexamethasone. Transfusion. 1998;38:713–721. doi: 10.1046/j.1537-2995.1998.38898375509.x. [DOI] [PubMed] [Google Scholar]
- 21.Price TH, Bowden RA, Boeckh M, Bux J, Nelson K, Liles WC, Dale DC. Phase I/II trial of neutrophil transfusion from donors stimulated with G-CSF and dexamethasone for treatment of patient with infections in hematopoietic stem cell transplantation. Blood. 2000;95:3302–3309. [PubMed] [Google Scholar]
- 22.Stroncek DF, Yau AA, Oblitas J, Leitman SF. Administration of G-CSF plus dexamethasone produces greater granulocyte concentrate yields while causing no more donor toxicity than G-CSF alone. Transfusion. 2001;41:1037–1044. doi: 10.1046/j.1537-2995.2001.41081037.x. [DOI] [PubMed] [Google Scholar]
- 23.Heuft HG, Goudeva L, Sel S, Blasczyk R. Equivalent mobilization and collection of granulocytes for transfusion after administration of glycosylated G-CSF (3μg/kg) plus dexamethasone versus glycosylated G-CSF (12μg/kg) alone. Transfusion. 2002;42:928–934. doi: 10.1046/j.1537-2995.2002.00133.x. [DOI] [PubMed] [Google Scholar]
- 24.Quillen K, Yau YY, Leitman SF. The determinants of granulocyte yield in 1198 granulocyte concentrates collected form unrelated volunteer donors mobilized with dexamethasone and granulocyte-colony-stimulating factor: a 13-year experience. Transfusion. 2009;49:421–426. doi: 10.1111/j.1537-2995.2008.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hölig K, Kramer M, Kroschinsky F, Bornhäuser M, Mengling T, Schmidt AH, Rutt C, Ehninger G. Safety and efficacy of hematopoietic stem cell collection form mobilized peripheral blood in unrelated volunteers: 12 years of single-center experience in 3928 donors. Blood. 2009;114:3757–3763. doi: 10.1182/blood-2009-04-218651. [DOI] [PubMed] [Google Scholar]
- 26.Heuft HG, Goudeva L, Pulver N, Grigull L, Schwella N, Blasczyk R. A dose-response analysis of lenograstim plus dexamethasone for neutrophil mobilization and collection. Transfusion. 2005;45:604–612. doi: 10.1111/j.0041-1132.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- 27.Peters C. Granulocyte transfusions in neutropenic patients: beneficial effects proven? Vox Sang. 2009;96:275–283. doi: 10.1111/j.1423-0410.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 28.Chatta GS, Price TH, Allen RC, Dale DC. Effects of in vivo recombinant methionyl human granulocyte colony-stimulating factor on the neutrophil response and peripheral blood colony-forming cells in healthy young and elderly adult volunteers. Blood. 1994;84:2923–2929. [PubMed] [Google Scholar]
- 29.Vasu S, Leitman SF, Tisdale JF, Hsieh MM, Childs RW, Barrett J, Fowler DH, Bishop MR, Kang EM, Malech HL, Dunbar CE, Khuu HM, Wesley R, Yau YY, Bolan CD. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112:2092–2100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bork K. Pruritus precipitated by hydroxyethyl starch: a review. Br J Dermatol. 2005;152:3–12. doi: 10.1111/j.1365-2133.2004.06272.x. [DOI] [PubMed] [Google Scholar]
- 31.McCullough J, Clay M, Herr G, Smith J, Stroncek D. Effects of granulocyte-colony-stimulating factor an potential normal granulocyte donors. Transfusion. 1999;39:1136–1140. doi: 10.1046/j.1537-2995.1999.39101136.x. [DOI] [PubMed] [Google Scholar]
- 32.de Haas M, Kerst JM, van der Schoot CE, Calafat J, Hack CE, Nuijens JH, Roos D, Oers RH. Granulocyte colony-stimulating factor administration to healthy volunteers: analysis of the immediate activating effects on circulating neutrophils. Blood. 1994;84:3885–3894. [PubMed] [Google Scholar]
- 33.Ghodsi Z, Strauss R. Cataracts in neutrophil donors stimulated with adrenal corticosteroids. Transfusion. 2001;41:1464–1468. doi: 10.1046/j.1537-2995.2001.41121464.x. [DOI] [PubMed] [Google Scholar]
- 34.Burch JW, Mair DC, Meny GM, Moroff G, Ching SS, Naidoff MA, Steuer ER, Loftus SA, Armstrong J, Clemons TE, Klein BE. The risk of posterior subcapsular cataracts in granulocyte donors. Transfusion. 2005;45:1701–1708. doi: 10.1111/j.1537-2995.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- 35.Clayton JA, Vitale S, Kim J, Conry-Cantilena C, Byrne P, Reed GF, Ferris FL, Leitman SF. Prevalence of posterior subcapsular cataracts in volunteer cytapheresis donors. Transfusion. 2011;51:921–928. doi: 10.1111/j.1537-2995.2010.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]