Abstract
Toxicity of polycations has been recognized since their first use in gene delivery. Bioreducible polycations attract attention because of their improved safety due to selective intracellular degradation by glutathione (GSH). Here we present a systematic study of the toxicity of bioreducible poly(amido amine)s (PAA). PAA with increasing content of disulfide bonds were synthesized by Michael addition. Toxicity of PAA was evaluated in two cell lines with different innate levels of intracellular GSH. Increasing the content of disulfide bonds decreased the toxicity of PAA, with more significant decrease observed in cells with high GSH. Depleting intracellular GSH by diethyl maleate resulted in increased toxicity of bioreducible PAA. In contrast, increasing the GSH concentrations by growing cells in hypoxic conditions resulted in further decreased toxicity compared with cells grown in normoxic conditions. The presence of exofacial plasma membrane thiols selectively increased toxicity of bioreducible PAA while having no effect on non-degradable controls. These results improve our understanding of the cellular mechanisms of polycation toxicity. They also shed light on the opposing effects of different cellular thiol pools on the toxicity of bioreducible polycations.
Keywords: non-viral gene delivery, polycations, bioreducible polycations, glutathione, toxicity
1. INTRODUCTION
Successful implementation of novel gene therapy protocols requires that we develop better strategies to effectively deliver nucleic acids to disease targets. Synthetic delivery vectors based on self-assembly of nucleic acids and polycations (polyplexes) continue to gain strength as viable alternatives to viral vectors. Clinical success of polyplexes continues to be hampered by adverse toxic effects associated with the use of polycations and by poor therapeutic response due to low transfection. Significant effort has been devoted to the design of safer polyplexes and growing evidence suggests that their toxicity can be decreased to a significant extent by using biodegradable polycations [1–4].
The toxicity of polycations such as poly(ethylenimine) (PEI) has been recognized since their first use in gene delivery [5, 6]. Structural factors known to be involved in the toxicity of polycations include molecular weight, molecular architecture, and charge density. In general, flexible polycations with high molecular weight and high charge density exhibit high cytotoxicity. However, the cellular mechanisms behind polycation toxicity are still not fully understood and remain a subject of intensive research [7–9]. The most common approaches to decreasing toxicity rely on decreasing the total number of charges and charge density of the polycations. Biodegradability in particular, has been widely explored and many biodegradable polycations indeed show lower toxicity than their non-degradable counterparts.
Bioreducible polycations are among the most investigated biodegradable polycations. Numerous groups reported synthesis of various bioreducible polycations, including polycations based on cysteine-containing peptides, polymethacrylates, poly(β-amino ester)s (PBAE), and poly(amido amine)s (PAA) [10–17]. Regardless of the chemical nature, all bioreducible polycations exhibit decreased toxicity when compared with non-degradable controls. Apart from decreased toxicity, bioreducible polycations offer additional benefits, such as better spatial control of DNA release, especially when compared with hydrolytically degradable polycations. Among the reported polycations, PAA and PBAE emerged as an important class of nonviral delivery vectors because of their chemical versatility that allows use of a broad range of amines in the synthesis. Indeed, multiple PBAE polycation libraries have been synthesized by this approach [18].
The overall goal of this study was to elucidate the mechanism of decreased toxicity of bioreducible PAA. We set to understand the effect of disulfide content in PAA and the role of intracellular and membrane thiol pools in controlling PAA toxicity. We have hypothesized that toxicity of PAA will be directly related to the intracellular glutathione (GSH) content and that altering concentrations of intracellular GSH will affect the toxicity of reducible polymers. We further postulated that toxicity caused by direct plasma membrane perturbation by polycations will not be affected by the biodegradability of PAA because plasma membrane interaction precedes PAA degradation inside of the cells.
1. MATERIALS AND METHODS
2.1. Materials
Branched polyethylenimine 25 kDa (PEI), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), and N,N′-dimethyldipropylenetriamine (DMDPTA) were purchased from Sigma (St. Louis, MO). Diethyl maleate (DEM) was purchased from Alfa Aesar (Ward Hill, MA). N,N′-hexamethylenebisacrylamide (HMBA) and N,N′-cystaminebisacrylamide (CBA) were obtained from Polysciences, Inc. (Warrington, PA). Dulbecco’s Modified Eagle Medium (DMEM), Dulbecco’s Phosphate Buffered Saline (PBS), and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, CA). All other reagents and chemicals were obtained from Fisher Scientific or VWR International unless otherwise noted.
2.2. Synthesis of PAA
A series of five PAAs containing different disulfide content was synthesized by Michael addition reaction of a triamine monomer DMDPTA and different molar ratio of reducible CBA and non-reducible HMBA as described in a previously published method [10]. The polymers are designated as PAA-m, where m is the molar percent of the acrylamide units containing disulfide bond (m = 100*[CBA]/([CBA]+[HMBA]). Average molecular weights of the polymers were determined by size exclusion chromatography using Shimadzu LC-10ADVP liquid chromatography equipped with a multiangle light scattering detector and a refractive index detector (Wyatt Technology, Santa Barbara, CA). Sodium acetate (300 mM, pH 4.5) was used as an eluent at a flow rate of 1.0 mL/min at 35 °C.
2.3. Cell culture
Two human pancreatic cancer cell lines were used in all the studies. Both cell lines, human ductal pancreatic adenocarcinoma cell line Panc-1 and human pancreatic adenocarcinoma cell line Panc-28, were kind gifts from Dr. Sarkar (Karmanos Cancer Institute, Detroit, MI) and were maintained at 37 °C in 5% CO2 atmosphere in DMEM supplemented with 10% FBS. For experiments under hypoxic conditions, cells were maintained at 37 °C in 5% CO2 and 5% O2 atmosphere using incubator equipped with an oxygen controller (ProOx 110, BioSpherix, Lacona, NY).
2.4. Determination of total intracellular GSH content
The total intracellular GSH content (i.e., both oxidized and reduced forms) was determined using a previously published enzymatic recycling method [19]. Briefly, 3×105 Panc-1 and Panc-28 cells were seeded in a 6-well plate. The cells were harvested after 24 h, pelleted, and deproteinated using 5% solution of 5-sulfosalicylic acid. GSH content was then determined colorimetrically using DTNB and the following assay buffer: potassium phosphate buffer (95 mM, pH 7.0), EDTA (0.95 mM), NADPH (0.038 mg/mL), DTNB (0.031 mg/mL), and GSH reductase (0.115 units/mL). Absorbance was measured at 410 nm using kinetic mode on a Synergy2 Multi-Mode Microplate Reader (Biotek Instruments, Inc., Winooski, VT). A calibration curve was constructed with known concentrations of reduced GSH. Protein content in the cell pellet was measured using the BCA™ Protein Assay Reagent kit (Thermo Fisher Scientific Inc., Waltham, MA). The results were normalized to protein content and expressed as mmol GSH/mg protein ± SEM (n=4).
2.5. Determination of total intracellular protein thiol (PSH) content
Panc-28 and Panc-1 cells (3 × 105 cells/well) were seeded in a 6-well plate. After 24 h, the cells were incubated with 2.5 mM DEM in culture medium for 60 min to deplete intracellular GSH. The cells were detached using non-enzymatic cell dissociation solution (Cellstripper, Mediatech, Herndon, VA) and pelleted. Cellular proteins were precipitated by addition of 100 μL of methanol to the cell pellet. The mixture was further centrifuged down and the precipitate was thoroughly mixed with 1 mM DTNB in PBS. The total cellular protein thiol (PSH) content was determined by measuring absorbance at 410 nm using Synergy2 Multi-Mode Microplate Reader. The results were normalized to protein content and expressed as mmol PSH/mg protein ± SEM (n=4).
2.6. Determination of cellular surface protein thiol content
Panc-28 and Panc-1 cells were seeded in a 6-well plate (3×105 cells/well). The culture medium was replaced after 24 h with 1 mM DTNB in PBS and the plates were shaken gently on a plate shaker for 30 min at room temperature. Absorbance was measured at 410 nm and the content of cell surface protein thiols was determined using a calibration curve constructed with known concentrations of reduced GSH. The results were normalized to cell number and expressed as mmol/106 cells ± SEM (n=4).
2.7. Determination of PAA toxicity by MTS assay
Toxicity of PAA in Panc-28 and Panc-1 cells was determined by MTS assay using CellTiter 96® Aqueous Cell Proliferation Assay (Promega, Madison, WI). The cells were plated in 96-well microplates at 10,000 cells/well. After 24 h, culture medium was replaced with 200 μL of serial dilutions of polycations prepared in complete culture medium and the cells were incubated for another 24 h. The medium containing PAA was aspirated and replaced by a mixture of 100 μL serum-free media and 20 μL of MTS reagent. After 1 h incubation, the absorbance was measured at 505 nm. The relative cell viability (%) was calculated as [A]sample/[A]untreated × 100%. IC50 values were determined using Boltzmann Sigmoidal nonlinear regression and the results are expressed as IC50 ± S.D. (n = 4 – 8).
2.8. Determination of acute membrane toxicity of PAA by lactate dehydrogenase (LDH) release assay
LDH release from polycation-treated cells was measured using CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega). Panc-28 and Panc-1 cells were seeded in 96-well plates at 10,000 cells/well. After 24 h, the cells were incubated with increasing concentrations of PAA-0 or PAA-100 for 30 min. The plates were then centrifuged at 250×g for 4 min and 50 μL of the supernatant in each well was transferred to another assay plate. LDH assay substrate mix (50 μL) was added to each well and incubated at room temperature for 30 min in dark. Then, 50 μL of stop solution was added to each well and absorbance was measured at 490 nm. LDH release (%) was calculated as [A]sample/[A]max × 100%, where [A]max is the absorbance value of a positive control (Triton X-100) in which complete cell lysis occurred.
2.9. Analysis of apoptosis induction by PAA using caspase 3/7 activity apoptosis assay
Induction of apoptosis by the polycations was determined by measuring caspase 3/7 activities using Caspase-Glo® 3/7 Assay (Promega). Panc-28 and Panc-1 cells were seeded in white 96-well plates with optical bottom at 10,000 cells/well. After 24 h, the cells were incubated with different concentrations of PAA-0 or PAA-100 in 100 μL culture medium for different periods of time. The caspase 3/7 reagent (100 μL) was then added and the mixture was incubated at room temperature in dark for another 30 min before measuring luminescence on Synergy2 Multi-Mode Microplate Reader. Results were normalized to luminescence of untreated cells and expressed as a mean fold increase ± SEM (n = 4).
3. RESULTS AND DISCUSSION
3.1. Synthesis of PAA
There is a great interest in developing biodegradable polycations as a way of overcoming their well-documented toxicity. Although decreased toxicity is often explained by degradability of such polycations, systematic studies quantifying specific contribution of degradation to their toxicity are rare. Existing studies are too often compromised by multiple confounding factors including differences in chemical structure, differences in charge density, or rate of degradation too slow to affect toxicity typically measured within 24 h of exposure. The main goal of this study was to conduct systematic study of the effects of reductive biodegradability on the toxicity of polycations. We have selected poly(amido amine)s (PAA) for this study because of the versatility of their synthesis which allows easy control of chemical composition and properties.
Using our previously published protocol, we have synthesized a series of PAA (Figure 1) with increasing content of disulfide bonds by increasing the relative content of the disulfide-containing monomer CBA in the reaction mixture (Table 1). Detailed evaluation of transfection activity of these polymers was reported previously [10]. Chemical similarity of the non-degradable monomer (HMBA) and the bioreducible monomer CBA in which two methylene groups are replaced with a disulfide minimizes potential influence of structural differences and charge density on the observed results. Bioreducible polycations offer additional advantages when compared with hydrolytically degradable polycations. Rapid cleavage of disulfide bonds inside the cells and the possibility to alter polycation degradation rates by biochemically modifying the redox state of the cells allow us to directly correlate the observed effects with degradation of the polycations. The synthesized bioreducible polycations (PAA-25, 50, 75, and 100) all had similar molecular weight and molecular weight polydispersity. The non-degradable control (PAA-0) exhibited about a 2-fold higher molecular weight than the bioreducible PAA. While higher molecular weight is associated with higher toxicity of polycations, the molecular weight difference between PAA-0 and the rest of PAA was deemed acceptable for our study, which was primarily concerned with the relative effects of changes in reducing environment on PAA toxicity.
Figure 1. Chemical structure of bioreducible poly(amido amine)s (PAA).
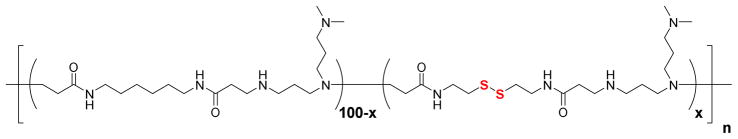
PAA with increasing disulfide content (x) were synthesized: x = 0, 25, 50, 75, and 100%.
Table 1.
Characterization of PAA
| PAA | Disulfide content (%) | Mn (kDa) | Mw/Mn |
|---|---|---|---|
| PAA-100 | 100 | 12.6 | 1.34 |
| PAA-75 | 75 | 13.7 | 1.53 |
| PAA-50 | 50 | 11.7 | 1.20 |
| PAA-25 | 25 | 12.2 | 1.22 |
| PAA-0 | 0 | 22.4 | 2.67 |
3.2. Characterization of thiol pools in Panc-1 and Panc-28 cells
A redox potential gradient exists between extracellular environment and various subcellular organelles. Disulfide bonds present in the structure of PAA are readily reduced in the reducing intracellular environment, while they are generally preserved in the oxidizing extracellular space. The intracellular degradation of PAA is mediated by thiol/disulfide exchange reactions with small redox molecules like GSH and thioredoxin; either alone or with the help of redox enzymes. GSH (L-γ-glutamyl-L-cysteinylglycine) is the most abundant intracellular thiol present in mM concentrations inside the cell but only in μM concentrations in the blood plasma [20].
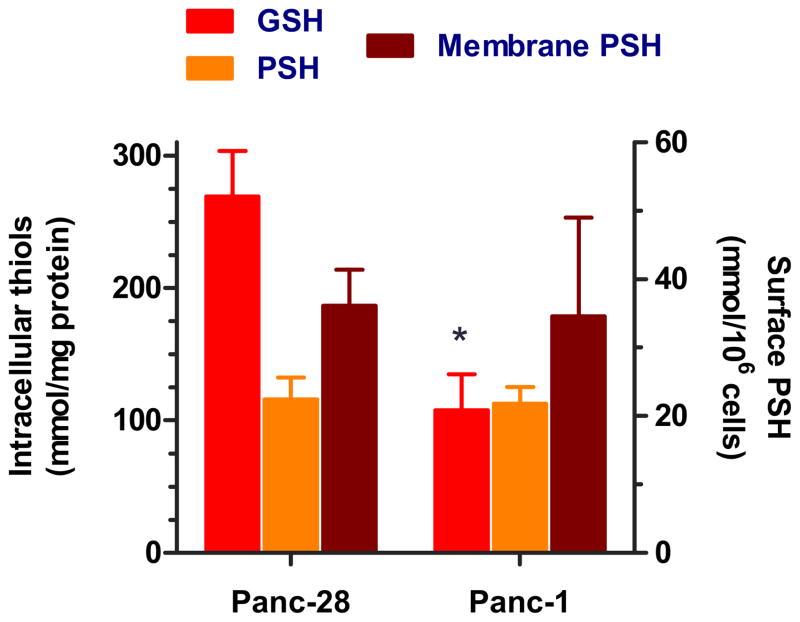
Because of the importance of GSH for degradation of PAA, we have selected two pancreatic cancer cell lines that in our previous studies demonstrated large innate differences in intracellular GSH content [13]. The intracellular GSH concentration is an additive function of both its oxidized (GSSG) and reduced form (GSH). The glutathione redox ratio ([GSH]:[GSSG]) is maintained and determined by the activity of glutathione reductase, NADPH concentrations, and transaldolase activity. The redox state of the GSH/GSSG couple is used as an indicator of the overall redox environment of the cell. In most cancer cells, the ratio [GSH]:[GSSG] typically exceeds the ratio found in normal cells and it is not unusual to observe ratios >100:1. We have therefore determined only the total intracellular GSH (i.e., GSH + GSSG) using enzymatic recycling method. In this method, any GSSG present in deproteinated cell lysate is converted into GSH by the action of GSH reductase. We observed that Panc-28 had 2.5-times higher intracellular GSH concentration than Panc-1 (Figure 2). In addition to GSH, the total cellular pools of thiols also consist of protein thiols (PSH) that may affect or participate in the degradation of PAA. Thus, we determined total concentration of PSH using a recently reported method of Hansen et al. [21]. Unlike GSH, Panc-1 and Panc-28 showed no significant differences in intracellular PSH levels (Figure 2). The last cellular pool of thiols we quantified were redox-active PSH present on the surface of plasma membrane. Similar to intracellular PSH, no significant differences in plasma membrane PSH were observed between the two cell lines.
Figure 2. Characterization of different thiol pools in Panc-28 and Panc-1 cells.
The following pools of cellular thiols were determined: (i) total intracellular GSH, (ii) total intracellular protein thiols (PSH), and (iii) exofacial plasma membrane PSH. All results expressed as normalized mean thiol content ± SEM (n = 4–6). *P < 0.005 vs. Panc-28.
3.3. Effect of disulfide content on PAA toxicity
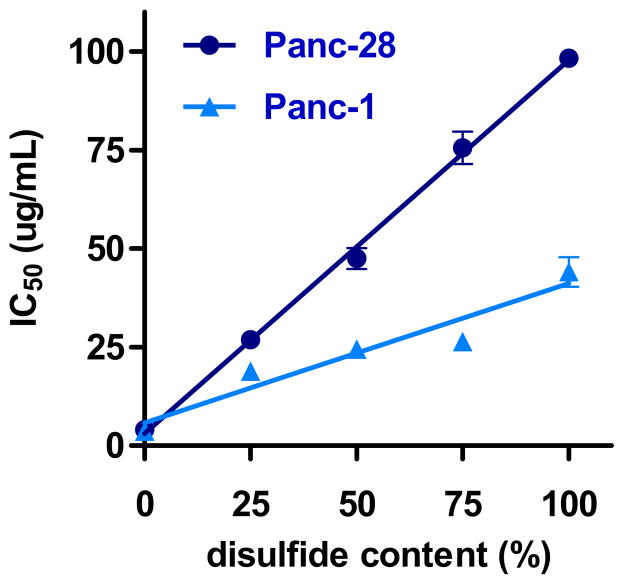
The effect of increasing disulfide content on PAA toxicity was investigated by incubating the cells for 24 h with increasing concentrations of PAA and measuring cell viability using mitochondrial activity-determining MTS assay. The concentration range of PAA used in the studies was from 2 to 500 μg/mL. At least 10 different concentrations were used and the observed cell viability values were fit to a Boltzmann sigmoidal equation and the concentrations of PAA necessary to decrease cell growth by 50% (IC50) were calculated (Figure 3). As expected, increasing the disulfide content resulted in decreased toxicity of PAA in both cell lines, as indicated by increasing IC50 values. Toxicity of non-degradable PAA-0 was similar in both cell lines as was the toxicity of control PEI (IC50 = 8.59 ± 0.35 μg/mL (Panc-28) and 6.30 ± 0.64 μg/mL (Panc-1)). The toxicity of PAA with the highest disulfide content (PAA-100) decreased 25-fold in high GSH Panc-28 cells and 12-fold in low GSH Panc-1 when compared with PAA-0. Furthermore, PAA-100 was 2.2-fold less toxic in Panc-28 than in Panc-1 cells. The toxicity dependence on disulfide content (x) followed linear relationship that could be described by the following equations: IC50(PAA-x) = IC50(PAA-0) + 0.95x for Panc-28 cells and IC50(PAA-0) = IC50(PAA-0) + 0.35x for Panc-1 cells. The fact that biodegradability proved more beneficial for decreasing toxicity in cells with high GSH (Panc-28) than in cells with low GSH (Panc-1) is likely a result of faster intracellular degradation of PAA, and thus shorter exposure of the intracellular environment to high molecular weight polycations. This conclusion is supported by the fact that no significant differences in cytotoxicity were observed for non-reducible polymers. These findings point to a possible direct correlation between the rate of intracellular polycation degradation and toxicity.
Figure 3. Effect of increasing disulfide content on cytotoxicity of PAA in Panc-28 and Panc-1 cells.
Cell viability was measured by MTS assay after 24 h incubation with Panc-28 (●) and Panc-1 (▲) cells with increasing concentrations of PAA with increasing disulfide content. Results are expressed as IC50 values ± SEM (n = 5).
3.4. Effect of bioreducibility on acute membrane toxicity of PAA
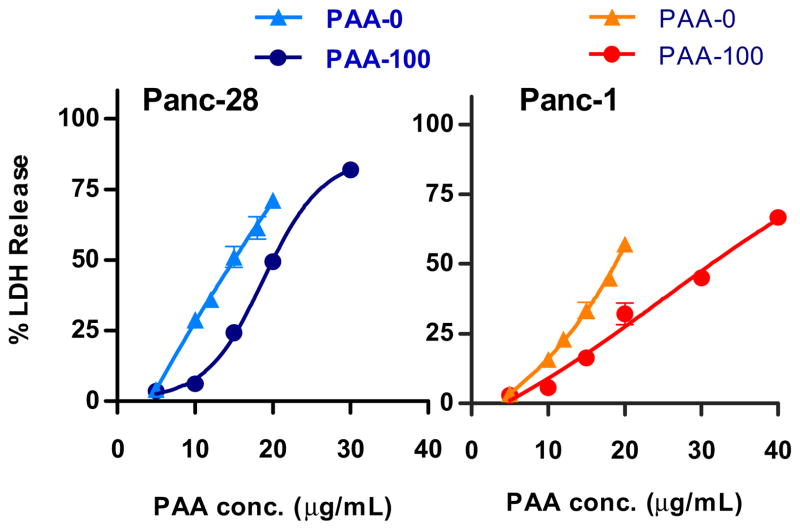
The ability of polycations to disrupt cellular membranes and induce membrane leakage due to pore formation has been well-documented [6, 22, 23]. As proposed by Moghimi, the membrane perturbation is part of the initial, phase 1, toxicity of polycations [7, 24]. One of the hallmarks of this phase is the release of lactate dehydrogenase (LDH) within the first hour of incubation and rapid redistribution of phosphotidylserine from the inner plasma membrane to the outer cell surface. Degradation of bioreducible PAA takes place predominantly in the cytoplasm of the cells. We therefore hypothesized that any potential adverse effects of PAA during their initial interaction with cells will be largely independent of their biodegradability. To test the hypothesis, we have investigated acute membrane toxicity of PAA-100 and PAA-0 by measuring LDH release after 30 min incubation of the cells with polycations (Figure 4). We have observed only small differences between PAA-0 and PAA-100 in their ability to disrupt membranes. While the non-degradable PAA-0 caused higher LDH release at all tested concentrations, the differences were relatively small when compared with the 25-fold difference in IC50 values determined after 24 h incubation. The results in Figure 4 suggest that degradation is a prerequisite for decreased ability of biodegradable PAA to disrupt cellular membranes. The results also suggest that no significant degradation of PAA-100 takes place in the extracellular space (culture medium). We believe that the increased LDH release by PAA-0 is due to its higher molecular weight compared with PAA-100.
Figure 4. Effect of reducibility on acute membrane toxicity of PAA in Panc-28 and Panc-1 cells.
Acute membrane toxicity was measured by LDH assay after 30 min incubation with bioreducible (PAA-100) and non-degradable (PAA-0) polycation. Results are expressed as mean % LDH release ± SEM (n = 5).
3.5. Effect of changes in intracellular GSH concentration on toxicity of bioreducible PAA
The above results suggested that there is a direct correlation between intracellular GSH levels and toxicity of bioreducible PAA. To obtain more supporting evidence, we have hypothesized that artificially decreasing the intracellular GSH content will decrease the rate and/or extent of PAA degradation and that this will be demonstrated by increased cytotoxicity of bioreducible PAA-100 but not control non-degradable PAA-0. To test the hypothesis, we treated the cells for 1 h with a GSH-conjugating agent DEM to deplete GSH levels before incubation with PAA and determining changes in IC50. Exposure to DEM resulted in a rapid (usually in 30–60 min) and significant decrease in GSH levels in both Panc-1 and Panc-28 cells (Figure 5). In our setup, DEM alone caused no decrease in cell viability. Prior evidence shows that both the cytosolic and the mitochondrial pools are affected by DEM treatment [25]. Importantly, GSH depletion persists for many hours even after DEM removal from the medium (50% recovery of GSH was reported 8 h after DEM treatment [26]). In our experiment, we observed differences in the rate of GSH recovery between the two cell lines, with Panc-28 recovering faster than Panc-1. We also observed slower rate of GSH recovery in cells treated with PAA.
Figure 5. Effect of altered intracellular GSH on toxicity of polycations.
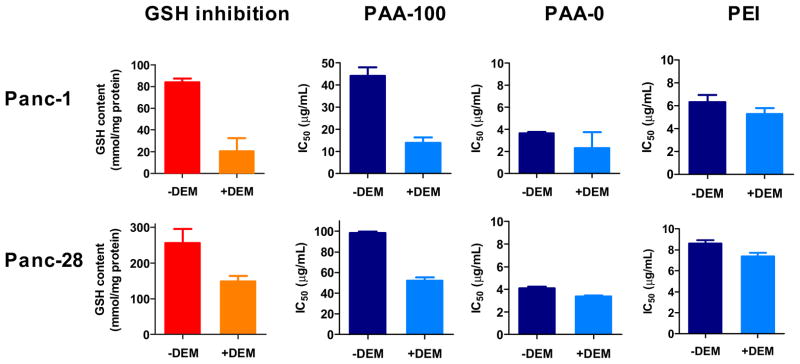
Intracellular GSH was depleted in Panc-28 and Panc-1 cells by 1 h preincubation with 2.5 mM DEM. Toxicity of PAA-100, PAA-0, and PEI was evaluated by incubating DEM-treated and control untreated cells with increasing concentrations of the polycations and determining IC50 values using MTS assay after 24 h incubation. (mean ± SEM, n = 4)
GSH depletion exerted significant effect on the toxicity of PAA-100 while having only a small effect on the toxicity of PAA-0 and control PEI (Figure 5). The IC50 of PAA-100 decreased substantially in both cell lines, indicating importance of intracellular GSH for decreased toxicity of bioreducible PAA. The IC50 values of PAA-100 decreased 1.9-times and 3.2-times in Panc-28 and Panc-1 cells, respectively. The observed changes in toxicity are consistent with changes in the rate of intracellular degradation of PAA-100 which proceeds via thiol-disulfide exchange between GSH and the polycation [27]. The observed increase in toxicity of non-degradable PAA-0 and PEI controls is most likely a result of sensitization of cells with depleted GSH to chemical injury.
Exposure of cancer cells to hypoxic conditions results in marked increase of GSH levels [28–30]. We have utilized this phenomenon to further confirm relationship between intracellular GSH concentrations and toxicity of bioreducible PAA. The cells were incubated under hypoxia (5% O2) and IC50 values were determined as above. As shown in Figure 6, toxicity of bioreducible PAA-100 decreased 2.4-times in Panc-28 and 2.0-times in Panc-1 cells. In contrast, hypoxia had no significant effect on the toxicity of non-degradable PAA-0. This finding suggests that hypoxia-induced increase of GSH levels promotes faster degradation of bioreducible polycations, which in turn results in further decreased toxicity.
Figure 6. Effect of hypoxia on toxicity of PAA-100 and PAA-0.
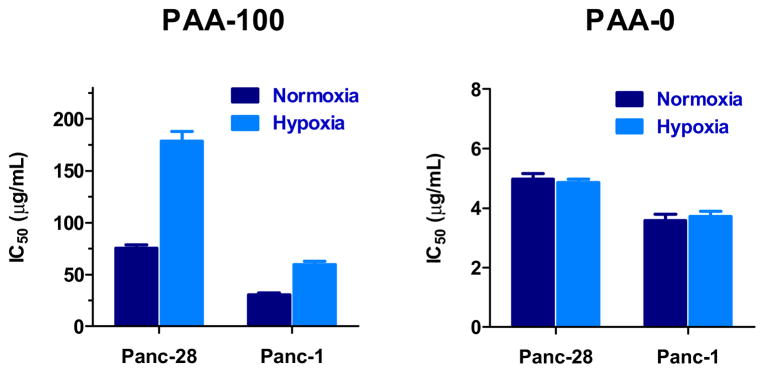
Panc-28 and Panc-1 cells were incubated either under standard 5% CO2 conditions (normoxia) or in 5% O2 + 5% CO2 (hypoxia). Toxicity of PAA-100 and PAA-0 was evaluated by incubating the cells with increasing concentrations of the polycations and determining IC50 values using MTS assay after 24 h incubation. (mean ± SEM, n = 5)
3.6. Effect of cell surface PSH on toxicity of bioreducible PAA
After elucidating the role of intracellular thiol pools in the toxicity of bioreducible PAA, we focused our attention on a pool of redox-active protein thiols present on the surface of plasma membrane. Although the membrane thiol pool is relatively small, mounting evidence suggests that it may play a crucial role in cell uptake of bioreducible polycation/DNA polyplexes [31–33]. Similar thiol-disulfide reactions on the cell surface are required for cell entry of viruses like HIV and play a role in cell entry of disulfide-containing peptides [34–37]. An early study also suggested the reducing cell surface thiols are involved in cell uptake of bioreducible lipoplexes [38]. Therefore, it is reasonable to assume that interactions between membrane PSH could also affect toxicity of bioreducible PAA, either through increased cell uptake and more effective membrane attachment or through early degradation of the polycations. The goal of this experiment was to investigate the contribution of membrane PSH to the toxicity of bioreducible PAA.
As shown above in Figure 2, there were no differences in the membrane PSH content between Panc-1 and Panc-28 cells. Both cell lines contained around 35 nmol PSH/106 cells. To determine the effect of membrane PSH on the cytotoxicity of PAA, the cells were treated for 30 min with cell impermeable nonspecific thiol blocker DTNB to mask all the membrane PSH [39]. The cells were then treated with increasing concentrations of the polycations and IC50 values were determined. As shown in Figure 7, blocking of the membrane PSH resulted in a remarkable 2-fold and a 2.6-fold decrease in the toxicity of PAA-100 in Panc-28 and Panc-1 cells, respectively. In contrast, blocking of the membrane PSH increased slightly the toxicity of non-degradable PAA-0 and PEI controls.
Figure 7. Effect of membrane PSH thiols on toxicity of polycations.
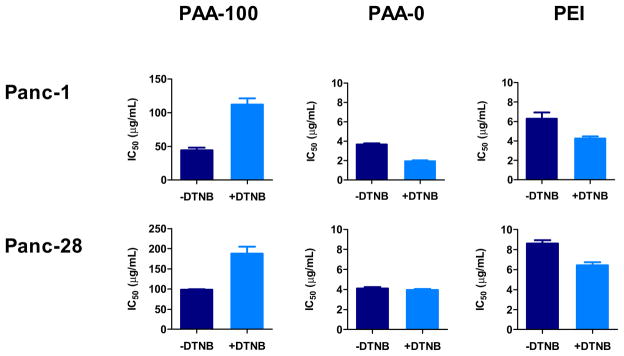
Membrane thiols were blocked by preincubating Panc-28 and Panc-1 cells with 1 mM of membrane impermeable DTNB for 30 min. Toxicity of PAA-100, PAA-0, and PEI was evaluated by incubating DTNB-treated and control untreated cells with increasing concentrations of the polycations and determining IC50 values using MTS assay after 24 h incubation. (mean ± SEM, n = 5)
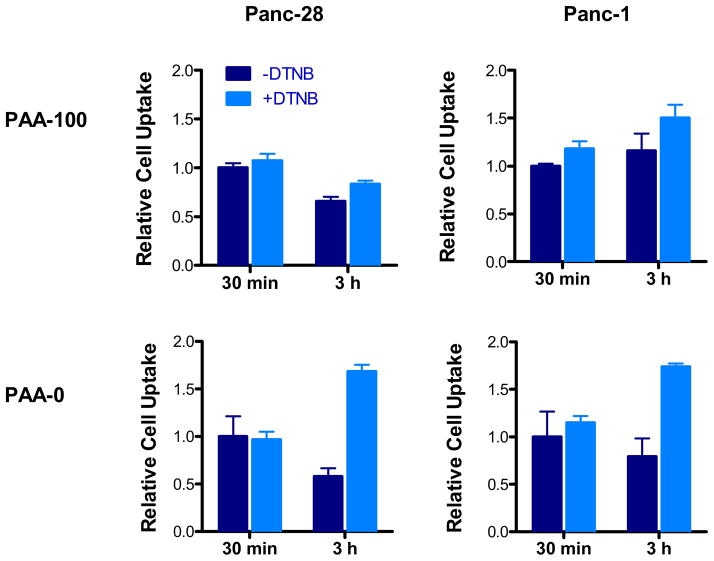
Thiol-disulfide exchange with membrane PSH can lead to endocytosis and this mechanism of uptake can be inhibited by DTNB [38, 40, 41]. To determine the effect of membrane PSH on cell uptake of PAA, Panc-28 and Panc-1 cells were treated with DTNB prior to incubating with FITC-labeled PAA-100 and PAA-0 for 30 min and 3 h. No differences between DTNB-treated and untreated cells were observed at 30 min. At the later time point, however, the uptake of both PAA-0 and PAA-100 increased in cells treated with DTNB (Figure 8). Interestingly, DTNB treatment increased uptake of PAA-0 more than PAA-100 in both cell lines. This effect is most likely due to inhibition of exocytosis by DTNB reported previously [42]. The fact that there was no decrease in the total amount of PAA-100 after inhibiting membrane PSH suggests that other factors were responsible for the decreased toxicity in DTNB-treated cells.
Figure 8. Effect of membrane PSH thiols on cell uptake of PAA-100 and PAA-0.
Membrane thiols were blocked by preincubating Panc-28 and Panc-1 cells with 1 mM DTNB for 30 min before incubation with FITC-labeled PAA-100 and PAA-0. Cell uptake was determined after 30 min and 3 h incubation by measuring cell-associated fluorescence intensity. Cell uptake in untreated cells after 30 min incubation was set as = 1 and the results are expressed relative to this value. (mean ± SEM, n = 3)
Our results establish, for the first time, that thiol-disulfide reactions at the cell membrane play a key role in enhancing toxicity of bioreducible PAA. This effect is most likely due to increased ability of polycations covalently attached to membrane PSH to disrupt membranes. Our findings also emphasize contrasting effect of intracellular thiols and membrane thiols on the toxicity of bioreducible PAA. Limiting interactions of bioreducible polycations with membrane PSH by PEGylation is likely to eliminate their contribution to the toxicity of bioreducible polycations.
3.7. Effect of bioreducible PAA on apoptosis
Phase 2 toxicity of polycations arises from their intracellular effects responsible for induction of apoptosis demonstrated by significant activation of the effector caspase 3 [6, 43]. Polycation-induced apoptosis is mainly the result of their effect on mitochondrial membrane [9]. However, leakage of lysosomal proteases into cytoplasm as a result of endo/lysosomal escape of the polycations could be another contributing factor to the apoptosis [7, 44].
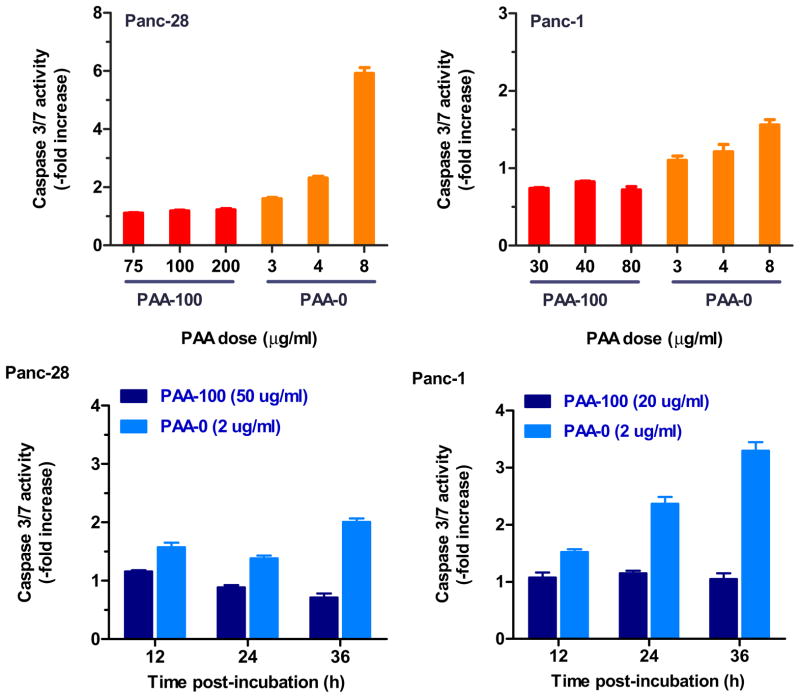
Mitochondria participate actively in key apoptosis events, including the loss of mitochondrial transmembrane potential and the release of proapoptotic cytochrome c which activates effector caspases (e.g., caspase 3) [45]. Mitochondria are important intracellular organelles that store about 10% of intracellular GSH. Because of a possible effect of bioreducible PAA on mitochondrial redox state and membrane stability, it was important to evaluate whether the observed differences in toxicity are reflected also in differential ability of PAA-100 and PAA-0 to induce apoptosis. We have determined the effect of PAA-100 and PAA-0 on activation of caspases 3 and 7 using a commercial luminescence-based assay. As shown in Figure 9, bioreducible PAA-100 exhibited no measurable activation of the caspases at 6 h after incubation even when tested up to 200 μg/mL PAA-100 in Panc-28 and 80 μg/mL in Panc-1. In contrast, non-degradable PAA-0 displayed significant enhancement of the caspase 3/7 activity already at a concentration of 8 μg/mL. To determine whether PAA-100 may induce apoptosis at later time points, we have incubated the cells with sub-lethal (approx. ½ of the respective IC50) concentrations of PAA-100 and PAA-0. The results show that PAA-100 caused no significant increase in caspase 3/7 activity at least until 36 h. PAA-0, on the other hand, caused a markedly enhanced activity of caspase 3/7 even though PAA-0 was used at 25-times (Panc-28) and 10-times (Panc-1) lower concentrations than PAA-100.
Figure 9. Effect of bioreducibility on induction of apoptosis measured by activation of caspase 3/7.
(top panels) Panc-28 and Panc-1 cells were incubated with increasing concentrations of PAA-100 and PAA-0 and activity of caspase 3/7 was measured after 6 h and normalized to untreated cells. (bottom panels) Panc-28 and Panc-1 cells were incubated with the indicated concentrations of PAA-100 and PAA-0 for 12, 24, and 36 h and caspase 3/7 activity was measured and normalized to untreated cells. (mean ± SEM, n = 4)
CONCLUSIONS
This study presented systematic evaluation of the mechanism of toxicity of bioreducible polycations. We have found that toxicity decreases in a linear fashion with increasing disulfide content in PAA. The observed toxicity is cell-dependent, with lower toxicity found in cells containing higher concentrations of GSH. Decrease of the toxicity of bioreducible PAA is directly related to the intracellular GSH concentrations as demonstrated by increased toxicity upon GSH depletion and decreased toxicity upon elevation of GSH in hypoxic conditions. Unlike intracellular thiols that are responsible for the decrease of toxicity, plasma membrane protein thiols exert an opposite effect and are responsible for increased toxicity of bioreducible PAA. Intracellular degradation of bioreducible PAA results also in greatly decreased induction of apoptosis. In conclusion, this work increases our understanding of the mechanisms of polycation toxicity as it directly demonstrates a link between intracellular degradation of bioreducible polycations and decreased toxicity and the distinct roles of different cellular thiol pools in the toxicity of bioreducible polycations.
Acknowledgments
This study was supported by the National Institutes of Health (CA 109711, EB 014570).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akinc A, Anderson DG, Lynn DM, Langer R. Synthesis of poly(beta-amino ester)s optimized for highly effective gene delivery. Bioconjug Chem. 2003;14:979–88. doi: 10.1021/bc034067y. [DOI] [PubMed] [Google Scholar]
- 2.Lee J-S, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG. Gold, Poly(β-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009;9:2402–6. doi: 10.1021/nl9009793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russ V, Elfberg H, Thoma C, Kloeckner J, Ogris M, Wagner E. Novel degradable oligoethylenimine acrylate ester-based pseudodendrimers for in vitro and in vivo gene transfer. Gene Ther. 2008;15:18–29. doi: 10.1038/sj.gt.3303046. [DOI] [PubMed] [Google Scholar]
- 4.Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong ZY, Lin C, Engbersen JFJ, Feijen J. Reducible poly(amido ethylenimine)s designed for triggered intracellular gene delivery. Bioconjug Chem. 2006;17:1233–40. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- 5.Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–80. doi: 10.1016/s0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- 6.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–5. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Parhamifar L, Larsen AK, Hunter AC, Andresen TL, Moghimi SM. Polycation cytotoxicity: a delicate matter for nucleic acid therapy-focus on polyethylenimine. Soft Matter. 2010;6:4001–9. [Google Scholar]
- 8.Matz RL, Erickson B, Vaidyanathan S, Kukowska-Latallo JF, Baker JR, Orr BG, Banaszak Holl MM. Polyplex exposure inhibits cell cycle, increases inflammatory response, and can cause protein expression without cell division. Mol Pharm. 2013;10:1306–17. doi: 10.1021/mp300470d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grandinetti G, Smith AE, Reineke TM. Membrane and nuclear permeabilization by polymeric pDNA vehicles: Efficient method for gene delivery or mechanism of cytotoxicity? Mol Pharm. 2012;9:523–38. doi: 10.1021/mp200368p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Wu C, Oupicky D. Bioreducible hyperbranched poly(amido amine)s for gene delivery. Biomacromolecules. 2009;10:2921–7. doi: 10.1021/bm900724c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You YZ, Zhou QH, Manickam DS, Wan L, Mao GZ, Oupicky D. Dually responsive multiblock copolymers via reversible addition-fragmentation chain transfer polymerization: Synthesis of temperature- and redox-responsive copolymers of poly(N-isopropylacrylamide) and poly(2-(dimethylamino)ethyl methacrylate) Macromolecules. 2007;40:8617–24. doi: 10.1021/ma071176p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.You YZ, Manickam DS, Zhou QH, Oupicky D. Reducible poly(2-dimethylaminoethyl methacrylate): synthesis, cytotoxicity, and gene delivery activity. J Control Release. 2007;122:217–25. doi: 10.1016/j.jconrel.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manickam DS, Li J, Putt DA, Zhou QH, Wu C, Lash LH, Oupicky D. Effect of innate glutathione levels on activity of redox-responsive gene delivery vectors. J Control Release. 2010;141:77–84. doi: 10.1016/j.jconrel.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CP, Kim JS, Steenblock E, Liu D, Rice KG. Gene transfer with poly-melittin peptides. Bioconjug Chem. 2006;17:1057–62. doi: 10.1021/bc060028l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zugates GT, Anderson DG, Little SR, Lawhorn IEB, Langer R. Synthesis of poly(beta-amino ester)s with thiol-reactive side chains for DNA delivery. J Am Chem Soc. 2006;128:12726–34. doi: 10.1021/ja061570n. [DOI] [PubMed] [Google Scholar]
- 16.Namgung R, Brumbach JH, Jeong JH, Yockman JW, Kim SW, Lin C, Zhong ZY, Feijen J, Engbersen JFJ, Kim WJ. Dual bio-responsive gene delivery via reducible poly(amido amine) and survivin-inducible plasmid DNA. Biotechnol Lett. 2010;32:755–64. doi: 10.1007/s10529-010-0219-7. [DOI] [PubMed] [Google Scholar]
- 17.Jeong JH, Kim SH, Christensen LV, Feijen J, Kim SW. Reducible poly(amido ethylenimine)-based gene delivery system for improved nucleus trafficking of plasmid DNA. Bioconjug Chem. 2010;21:296–301. doi: 10.1021/bc9003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008;41:749–59. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manickam DS, Hirata A, Putt DA, Lash LH, Hirata F, Oupicky D. Overexpression of Bcl-2 as a proxy redox stimulus to enhance activity of non-viral redox-responsive delivery vectors. Biomaterials. 2008;29:2680–8. doi: 10.1016/j.biomaterials.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 21.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A. 2009;106:422–7. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Hessler JA, Putchakayala K, Panama BK, Khan DP, Hong S, Mullen DG, DiMaggio SC, Som A, Tew GN, Lopatin AN, Baker JR, Holl MMB, Orr BG. Cationic nanoparticles induce nanoscale disruption in living cell plasma membranes. J Phys Chem B. 2009;113:11179–85. doi: 10.1021/jp9033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong S, Leroueil PR, Janus EK, Peters JL, Kober M-M, Islam MT, Orr BG, Baker JR, Banaszak Holl MM. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem. 2006;17:728–34. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 24.Hunter AC, Moghimi SM. Cationic carriers of genetic material and cell death: A mitochondrial tale. Biochim Biophys Acta. 2010;1797:1203–9. doi: 10.1016/j.bbabio.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: Studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825–34. [PubMed] [Google Scholar]
- 26.Esteban-Pretel G, Pilar Lopez-Garcia M. An experimental design for the controlled modulation of intracellular GSH levels in cultured hepatocytes. Free Radic Biol Med. 2006;41:610–9. doi: 10.1016/j.freeradbiomed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Kalinina EV, Chernov NN, Saprin AN. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry (Mosc) 2008;73:1493–510. doi: 10.1134/s0006297908130099. [DOI] [PubMed] [Google Scholar]
- 28.O’Dwyer PJ, Yao K-S, Ford P, Godwin AK, Clayton M. Effects of hypoxia on detoxicating enzyme activity and expression in HT29 colon adenocarcinoma cells. Cancer Res. 1994;54:3082–7. [PubMed] [Google Scholar]
- 29.Moreno-Merlo F, Nicklee T, Hedley D. Association between tissue hypoxia and elevated non-protein sulphydryl concentrations in human cervical carcinoma xenografts. Br J Cancer. 1999;81:989. doi: 10.1038/sj.bjc.6690797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vukovic V, Nicklee T, Hedley DW. Differential effects of buthionine sulphoximine in hypoxic and non-hypoxic regions of human cervical carcinoma xenografts. Radiother Oncol. 2001;60:69–73. doi: 10.1016/s0167-8140(01)00331-0. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Manickam DS, Chen J, Oupicky D. Effect of cell membrane thiols and reduction-triggered disassembly on transfection activity of bioreducible polyplexes. Eur J Pharm Sci. 2012;46:173–80. doi: 10.1016/j.ejps.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezzoli D, Zanda M, Chiesa R, Candiani G. The yin of exofacial protein sulfhydryls and the yang of intracellular glutathione in in vitro transfection with SS14 bioreducible lipoplexes. J Control Release. 2013;165:44–53. doi: 10.1016/j.jconrel.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Yan Y, Wang Y, Heath JK, Nice EC, Caruso F. Cellular association and cargo release of redox-responsive polymer capsules mediated by exofacial thiols. Adv Mater. 2011;23:3916–21. doi: 10.1002/adma.201101609. [DOI] [PubMed] [Google Scholar]
- 34.Markovic I, Stantchev TS, Fields KH, Tiffany LJ, Tomiç M, Weiss CD, Broder CC, Strebel K, Clouse KA. Thiol/disulfide exchange is a prerequisite for CXCR4-tropic HIV-1 envelope-mediated T-cell fusion during viral entry. Blood. 2004;103:1586–94. doi: 10.1182/blood-2003-05-1390. [DOI] [PubMed] [Google Scholar]
- 35.Jain S, McGinnes LW, Morrison TG. Thiol/disulfide exchange is required for membrane fusion directed by the Newcastle disease virus fusion protein. J Virol. 2007;81:2328–39. doi: 10.1128/JVI.01940-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryser HJ, Levy EM, Mandel R, DiSciullo GJ. Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc Natl Acad Sci U S A. 1994;91:4559–63. doi: 10.1073/pnas.91.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aubry S, Burlina F, Dupont E, Delaroche D, Joliot A, Lavielle S, Chassaing G, Sagan S. Cell-surface thiols affect cell entry of disulfide-conjugated peptides. FASEB J. 2009;23:2956–67. doi: 10.1096/fj.08-127563. [DOI] [PubMed] [Google Scholar]
- 38.Kichler A, Remy JS, Boussif O, Frisch B, Boeckler C, Behr JP, Schuber F. Efficient gene delivery with neutral complexes of lipospermine and thiol-reactive phospholipids. Biochem Biophys Res Commun. 1995;209:444–50. doi: 10.1006/bbrc.1995.1522. [DOI] [PubMed] [Google Scholar]
- 39.Gowthaman U, Jayakanthan M, Sundar D. Molecular docking studies of dithionitrobenzoic acid and its related compounds to protein disulfide isomerase: computational screening of inhibitors to HIV-1 entry. BMC Bioinformatics. 2008;9:S14. doi: 10.1186/1471-2105-9-S12-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai F, Wang F, Lin FK, Liu C, Ma LQ, Liu J, Wu WN, Wang W, Wang JH, Chen JG. Redox modulation of long-term potentiation in the hippocampus via regulation of the glycogen synthase kinase-3beta pathway. Free Radic Biol Med. 2008;45:964–70. doi: 10.1016/j.freeradbiomed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Ryser HJ, Mandel R, Ghani F. Cell surface sulfhydryls are required for the cytotoxicity of diphtheria toxin but not of ricin in Chinese hamster ovary cells. J Biol Chem. 1991;266:18439–42. [PubMed] [Google Scholar]
- 42.Zoccarato F, Cavallini L, Valente M, Alexandre A. Modulation of glutamate exocytosis by redox changes of superficial thiol groups in rat cerebrocortical synaptosomes. Neurosci Lett. 1999;274:107–10. doi: 10.1016/s0304-3940(99)00680-1. [DOI] [PubMed] [Google Scholar]
- 43.Merkel OM, Beyerle A, Beckmann BM, Zheng M, Hartmann RK, Stöger T, Kissel TH. Polymer-related off-target effects in non-viral siRNA delivery. Biomaterials. 2011;32:2388–98. doi: 10.1016/j.biomaterials.2010.11.081. [DOI] [PubMed] [Google Scholar]
- 44.Klemm AR, Young D, Lloyd JB. Effects of Polyethyleneimine on Endocytosis and Lysosome Stability. Biochem Pharmacol. 1998;56:41–6. doi: 10.1016/s0006-2952(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 45.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]