Abstract
Objectives:
To provide a detailed semiology to aid the clinical recognition of psychogenic pseudosyncope (PPS), which concerns episodes of apparent transient loss of consciousness (TLOC) that mimic syncope.
Methods:
We analyzed all consecutive tilt-table tests from 2006 to 2012 showing proven PPS, i.e., apparent TLOC had occurred without EEG changes or a decrease in heart rate (HR) or blood pressure (BP). We analyzed baseline characteristics, video data, EEG, ECG, and continuous BP measurements on a 1-second time scale. Data were compared with those of 69 cases of tilt-induced vasovagal syncope (VVS).
Results:
Of 800 tilt-table tests, 43 (5.4%) resulted in PPS. The majority (74%) were women. The median duration of apparent TLOC was longer in PPS (44 seconds) than in VVS (20 seconds, p < 0.05). During the event, the eyes were closed in 97% in PPS but in only 7% in VVS (p < 0.0001). A sudden head drop or moving down the tilt table was more common in PPS than in VVS (p < 0.01), but jerking movements occurred more frequently in VVS (p < 0.0001). In PPS, both HR and BP increased before and during apparent TLOC (p < 0.0001).
Conclusions:
PPS is clinically distinct from VVS and can be diagnosed accurately with tilt-table testing and simultaneous EEG monitoring. Compared with VVS, eye closure during the event, long periods of apparent TLOC, and high HR and BP are highly specific for PPS. Improved understanding of the semiology of PPS as a clinical entity is vital to ensure accurate diagnosis.
Transient loss of consciousness (TLOC) is the reason for approximately 3% of all emergency department visits.1 The most common major causes of apparent TLOC are syncope, epileptic seizures, and psychogenic events.2–4 The pathophysiology of psychogenic and somatic apparent TLOC is different, but they can be difficult to distinguish clinically.
Psychogenic apparent TLOC bears various labels. When episodes involve pronounced movements, they resemble epilepsy and are frequently labeled psychogenic nonepileptic seizures (PNES).5 Episodes without pronounced movements resemble syncope and are generally labeled psychogenic pseudosyncope (PPS).3
PNES and PPS are probably manifestations of the same underlying psychiatric disorder, but their different presentation has important consequences for diagnosis. PNES is relatively well known, with a reported prevalence of up to 30% in patients in epilepsy clinics.5 In contrast, PPS is rarely mentioned in the literature on syncope, and the reported prevalence of PPS in those analyzed for presumed syncope is lower, ranging from 0% to 8%.6–8 Some authors suspect that it is insufficiently recognized.9,10
At present, the gold standard for PPS is a demonstration of the absence of circulatory abnormalities causing cerebral hypoperfusion during an event. Psychogenic events are amenable to suggestion and can be induced by tilt-table testing.9,11–13 A clinical suspicion of PPS is a recognized indication for tilt-table testing by the European Society of Cardiology.3
Herein, we present the semiology of PPS based on the analysis of consecutive episodes of tilt-evoked proven psychogenic apparent TLOC documented with video, EEG, ECG, and blood pressure (BP) measurements.
METHODS
Patients.
Tilt-table tests performed at the Department of Neurology of Leiden University Medical Centre were gathered from April 1, 2006, when a video camera was attached to the tilt table, to April 1, 2012. Patients were referred largely from the outpatient clinic of the last author (J.G.v.D.) with a special interest in syncope. Patients had all been referred because of recurrent episodes of apparent TLOC. A suspicion of PNES was not an exclusion criterion. Inclusion required an episode of apparent TLOC during tilt-table testing without EEG changes and without decreases in heart rate (HR) or BP. The event had to be recognized by the patient or a relative (present during the test) as typical of the patient's episodes. Note that the occurrence of both syncope and PPS during tilt-table testing was not a reason for exclusion; in such cases, there was a period with changes in HR, BP, or EEG indicative of syncope as well as an episode of apparent TLOC without these changes.
Age, sex, history of psychological problems, prior treatment by a psychiatrist or psychologist, the use of psychotropic medication, and whether the patient had received an implantable loop recorder were noted. Psychiatric data were obtained by history taking and reviewing available correspondence.
For comparison, data from a consecutive series of 69 patients with tilt-induced vasovagal syncope (VVS) were analyzed. Baseline characteristics recorded in the VVS series were age and sex of the patient.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the hospital medical ethics board. Dutch law does not require individual informed consent for the publication of anonymous patient data gathered for purposes of patient care.
Clinical tilt protocol and data retrieval.
The “Italian protocol” was used to test for reflex syncope and for PPS14: 10 minutes of supine rest, followed by 20 minutes of head-up tilt at 60°. When syncope was not evoked during this period, 400 μg nitroglycerin was administered sublingually followed by another 20 minutes of observation. The aim of tilt-table testing is the provocation of an episode of apparent TLOC.3,14 When patients stated specific triggers for their events, other provocative maneuvers were occasionally used.10
A technician and a neurology resident were always present. For syncope, the decision to tilt back to supine was based on a profound decrease of HR or BP, usually together with recognition of presyncopal complaints by the patient, or slowing of the EEG. Because low BP or EEG slowing do not occur in PPS, patients were generally tilted back after recognition of the nature of the event by the resident.
BP was recorded continuously with either a Finometer (Finapres Medical Systems, Amsterdam, the Netherlands) or a Nexfin (BMEye, Amsterdam, the Netherlands) device. A 16-channel EEG, at least one channel of ECG, and video were routinely recorded in all tilt-table tests. An EEG machine (Nicolet 2100; Nihon Kohden, Tokyo, Japan) was used to record all signals using a sampling rate of 200 Hz, ensuring synchrony between all signals.
Data analysis—clinical events.
The start and end time of apparent TLOC were recorded from video data with an accuracy of 1 second. Apparent TLOC was defined as a state of unconsciousness as it would appear from the perspective of a lay person. The times of tilting up and back to supine were recorded.
The following signs were recorded before and after the event: yawning, sweating, pallor, and crying. Signs recorded during events included eye closure at the start of apparent TLOC, motor changes indicative of a loss of muscle tone (dropping the head, sliding down the tilt table, and falling against the restraints), and jerking movements of the limbs or body. These signs were also recorded in the series of VVS patients.
Data analysis—BP and HR.
ECG and BP records from 15 minutes before to 15 minutes after the start of the event were analyzed. When events occurred within 15 minutes of upward tilting, the period from tilting up to the event was used to avoid effects of posture. Periods after the event could last less than 15 minutes if tests had been terminated earlier. The RR intervals of the ECG were used to calculate a continuous series of HR data. The continuous BP data were used to calculate systolic BP (SBP) and diastolic BP (DBP) time series, and the smoothed mean arterial pressure (sMAP) was calculated from the recorded BP data by smoothing the continuous BP data over 9 seconds, i.e., a “rolling window.” All continuous signals were then resampled at 1 second. The baseline period was defined as a 120-second period in the upright position, either directly after tilting up if the event had happened within 15 minutes of tilting, or starting 15 minutes before the event. The mean values of HR, SBP, DBP, and sMAP were calculated for this baseline period and noted for the second at which the event began.
Statistics.
Data were analyzed with MATLAB (version 7.14.0; The MathWorks Inc., Natick, MA) and SPSS (version 17; SPSS Inc., Chicago, IL) software. Differences between mean values were tested for significance with the paired 2-sided Student t test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Categorical data were analyzed with Fisher exact test. The significance threshold was set at p < 0.05.
RESULTS
Baseline characteristics.
From April 1, 2006 to April 1, 2012, 800 tilt-table tests were performed, resulting in tilt-induced PPS in 43 patients (5.4%). Patients with PPS were divided into those with pure PPS (n = 31) and those with a mixed pattern (n = 12), in whom changes in BP or HR occurred indicating VVS or presyncope (n = 11) or orthostatic hypotension (n = 1). These changes were usually followed immediately by a period of nonresponsiveness during which HR, BP, and EEG were normal, but could occur after an interval or before PPS. Group characteristics are summarized in table 1. The majority of patients were female. A considerable proportion had a history of psychiatric disease and used psychotropic drugs. Four patients had received an implantable loop recorder.
Table 1.
Baseline characteristics of patients with pure PPS, mixed PPS, and VVSa
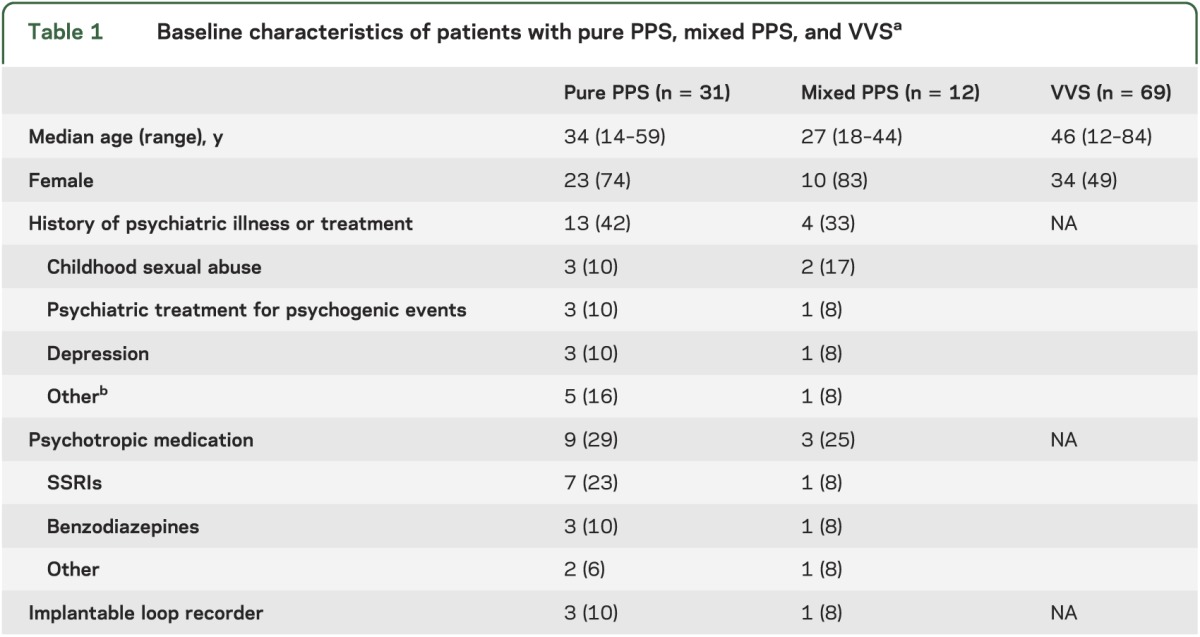
The mean age of patients with VVS was 46 years (range: 12–84 years), comparable to that of the pure and mixed PPS groups. In the VVS series, 34 patients (49%) were female, fewer than in the pure PPS group (p < 0.05, Fisher exact test).
Seven patients were excluded from subsequent analysis because video data were insufficiently clear to define the start and end of apparent TLOC. The clinical and physiologic features of the remaining patients with pure PPS (n = 27) and mixed PPS (n = 9) were analyzed separately.
Clinical features of PPS.
Figure e-1 (on the Neurology® Web site at www.neurology.org) shows a comparison of a typical tilt-table test recording in PPS with an example of VVS. PPS occurred almost always in the upward-tilted position: in 93% for the pure group and 100% in the mixed group. In the other 2 cases, apparent TLOC was provoked by repetitive squatting or repeated Mini-Mental State Examinations while standing because such triggers habitually provoked events in these patients. The median duration of apparent TLOC was 44 seconds (range: 2 seconds to 13 minutes 31 seconds) in the pure PPS group and 1 minute 13 seconds (range: 35 seconds to 2 minutes 23 seconds) in the mixed group (figure e-2). Apparent TLOC in the pure PPS group lasted longer than in VVS (median 20 seconds; range 4–55 seconds; p < 0.05, Mann-Whitney U test).
Table 2 shows recorded clinical signs. Both before and after apparent TLOC, sweating and pallor occurred less frequently in the pure PPS group than in the VVS group (p < 0.001, Fisher exact test, pure PPS vs VVS). The eyes were almost always closed at the start of apparent TLOC in pure and mixed PPS groups, whereas they were almost always open in the VVS group (p < 0.0001, Fisher exact test, pure PPS vs VVS). The only exception in the pure group was a patient who kept the eyes half closed in a position in which there was no eye contact. In the mixed group, the eyes were open in 2 patients while BP and HR were low, indicative of syncope. These patients remained unresponsive after HR, BP, and EEG had restored to normal values, and during that period their eyes were closed.
Table 2.
Clinical signs in PPS, mixed PPS (PPS followed or preceded by [pre]syncope), and VVSa
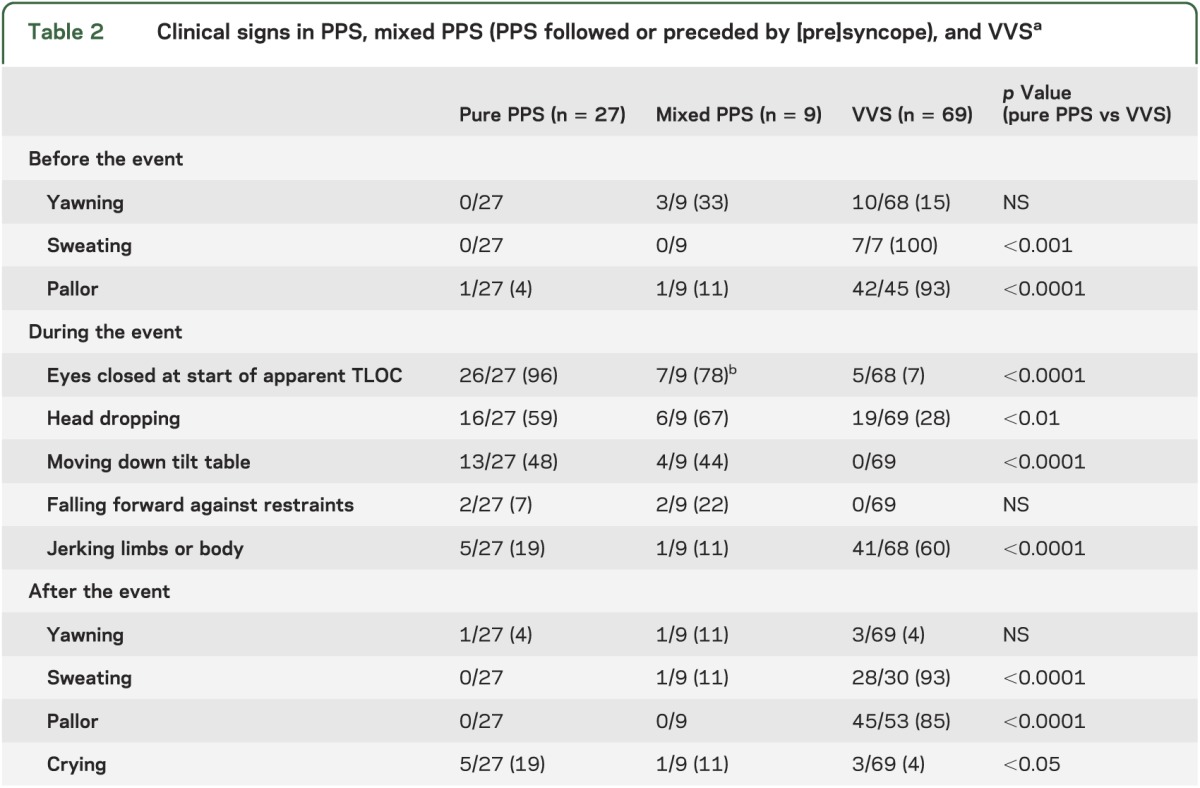
In virtually all patients with pure PPS, the start of the event was marked by a sudden loss of muscle tone. Patients either dropped the head sideways or forward at the start of the event, moved partly down the tilt table, or fell forcefully forward against the restraints. Dropping the head and moving down the table against restraints were less common in VVS than in the pure PPS group (p < 0.01 and p < 0.0001, Fisher exact test). Conversely, jerking movements of the limbs were uncommon in the pure PPS group, but occurred frequently in VVS (p < 0.0001, Fisher exact test).
Physiology of PPS: Changes in HR and BP.
At the start of apparent TLOC, mean HR, SBP, sMAP, and DBP were all higher than at baseline in pure PPS (table 3). In the mixed group, HR was lower at the start of the event compared with baseline (p = 0.04), whereas SBP, sMAP, and DBP did not differ (p = 0.05 for all 3). To examine the changes of HR and BP in more detail, their changes over time relative to the start of apparent TLOC were plotted (figures 1 and 2). In the pure group, mean HR started to increase approximately 6 minutes before the start of the event (figure 1A), reaching a peak at the start of apparent TLOC, followed by a return to baseline after the event. SBP, sMAP, and DBP showed a similar pattern (figure 1B), but their increase started approximately 1 minute before the start of the event. Individual HR and BP data varied considerably: there were 4 patients in whom there was no increase in HR and 5 in whom BP did not change.
Table 3.
SBP, sMAP, DBP, and HR at baseline and at the start of PPSa
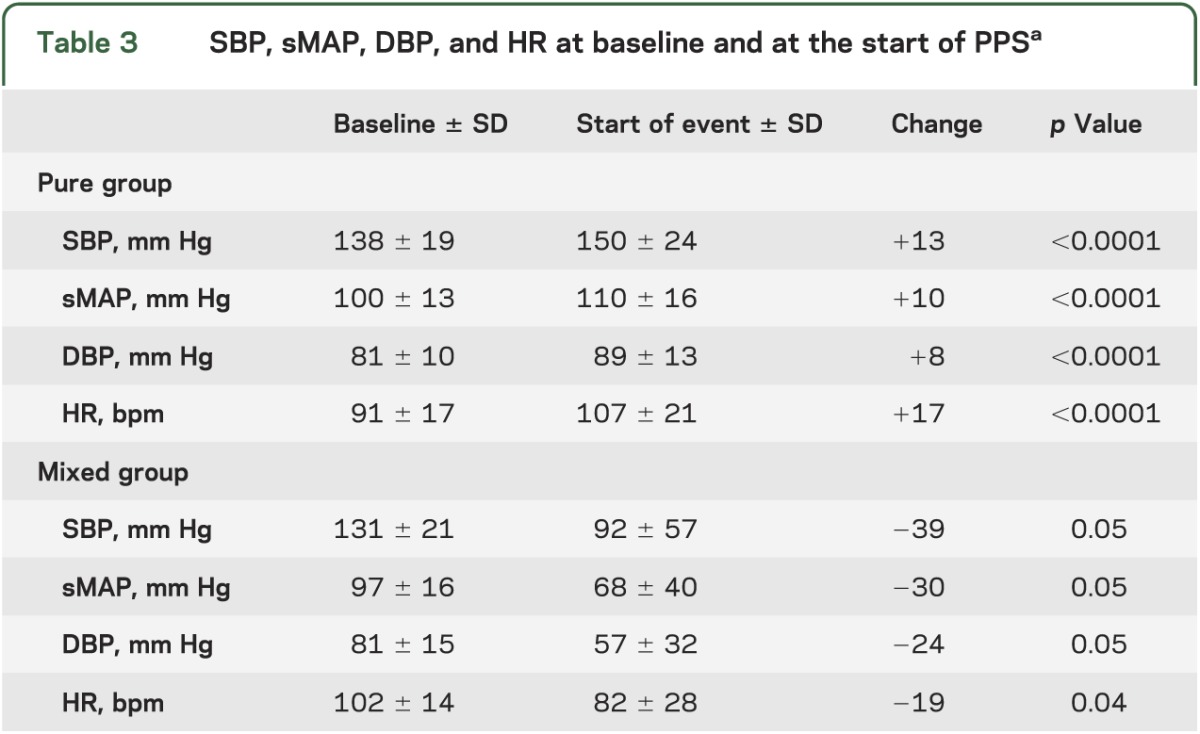
Figure 1. Mean heart rate and blood pressure of the pure psychogenic group (n = 27).
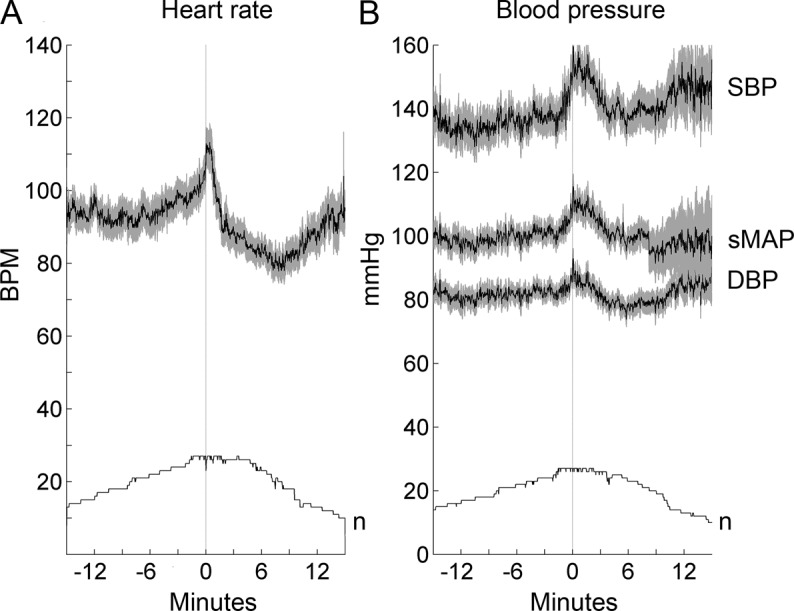
(A) Mean heart rate in beats per minute (bpm). (B) Mean values for systolic blood pressure (SBP) and diastolic blood pressure (DBP) as well as the smoothed mean arterial pressure (sMAP) in mm Hg. The number of valid measurements per time point (n) is shown in both graphs. The time of apparent transient loss of consciousness is indicated with a line at time zero. The horizontal scale shows time in minutes. Gray zones indicate 1 standard error above and below mean values. Note the increase in heart rate and blood pressure before the onset of the event.
Figure 2. Mean heart rate and blood pressure of the mixed psychogenic group (n = 9).

Both heart rate and blood pressure decreased sharply before apparent transient loss of consciousness, in the manner typical of reflex syncope. bpm = beats per minute; DBP = diastolic blood pressure; SBP = systolic blood pressure; sMAP = smoothed mean arterial pressure.
In the mixed group, average HR and BP decreased sharply before the onset of apparent TLOC, in the manner typical of reflex syncope (figure 2).
DISCUSSION
In this study, we describe the semiology of tilt-evoked PPS, concerning 5.4% of 800 consecutive tilt-table tests. The median duration of apparent TLOC was longer in PPS than in VVS. During the event, the eyes were closed in 97% in PPS but in only 7% in VVS. A sudden head drop or moving down the tilt table was more common during PPS, but jerking movements occurred more frequently in VVS. In PPS, both HR and BP increased before and during apparent TLOC.
In this study, we did not aim to examine the sensitivity of tilt-table testing for PPS, but a sensitivity of 81% has been reported for PNES.12 PPS can also be provoked with placebo maneuvers, leading to apparent TLOC in 90% of patients with PPS.10 The diagnosis of PPS does not exclude additional diagnoses, particularly of a somatic nature.15 In fact, 28% of our PPS patients had both PPS and syncope during the test, reminiscent of the well-known co-occurrence of epileptic seizures and PNES.5
The majority of patients with PPS in our series were female (74%). This percentage was higher than in the parallel series of patients with VVS, although it should be noted that the VVS series consisted of a selection of patients in a tertiary referral center. Reported prevalence rates in PNES are also higher in women.5 The percentage of patients in the pure PPS group with a history of psychiatric illness or treatment by a psychiatrist (42%) was fairly high, but in the absence of a formal control group, we cannot judge whether this was higher than expected. In addition, information regarding psychiatric disease may well be incomplete: traumatic experiences are not always asked about, or answers may have been withheld.
PPS episodes resemble syncope more than epileptic seizures, in that they involve apparent loss of consciousness without appreciable jerking movements. Somewhat ironically, a sudden onset of loss of muscle tone occurred more often and jerking movements occurred less often in PPS than in VVS. It should be noted that the video-based nature of the study allows more jerks to be noted than can be expected to be reported by an eyewitness after the fact. A typical feature of PPS is that the eyes are virtually always closed during the event, in contrast to VVS and epileptic seizures.16 In PNES, the eyes are also usually closed during apparent TLOC.16–18 Besides eye closure, the distinction from VVS can also be made by measuring the duration of the apparent TLOC, which is longer in PPS. In our series, patients with syncope never remained unresponsive for more than 1 minute, whereas PPS could last up to 14 minutes. While a long duration of PPS is well known, our study suggests that the majority of PPS episodes last as short a time as VVS.
Novel findings of this study were that both HR and BP were increased before and during apparent TLOC, and that the increases started well before the event, with HR starting to increase 6 minutes before. Ictal HR in PNES was recently shown to be higher than pre- and postictal values.19 These increases are presumably the result of psychological stress, which activates the hypothalamic-pituitary-adrenal axis causing the release of adrenaline.20 The increase in HR before the increase in BP points toward the involvement of central autonomic networks.21 These autonomic changes may be explained by altered subconscious processing, as described previously in patients experiencing PNES.22
Our study has several limitations. The patient population described here was largely derived from an outpatient clinic specialized in syncope. A clinical suspicion of PNES was not a formal exclusion criterion for tilt-table testing, but referral selection will have excluded the majority of these patients nonetheless. This may have affected the prevalence of jerking movements. In 2 patients, physical and mental exertion was used to provoke apparent TLOC because this was how their episodes were usually provoked. Ethically, the use of provocations is justified as the diagnostic benefit of documenting an event is generally accepted to outweigh any potential drawback of such maneuvers.3 The failure to elicit apparent TLOC in these 2 patients with tilt-table testing can be regarded as an intention-to-diagnose failure, but the occurrence of PPS during tilting probably relies more on suggestibility than on a gravitational circulatory challenge. In some cases, more creative maneuvers than tilting may be needed.
PPS is part of the spectrum of conversion disorder. The scarcity of reports on PPS suggests that it is either rarely diagnosed or is dealt with by physicians with a limited affinity for conversion disorder. We contend that PPS is a clinical entity that can be clearly distinguished from VVS using both positive (eye closure, increase in HR and BP) and negative (absence of EEG changes, no decrease in HR or BP) criteria. We have not compared PPS with PNES directly, but suspect that there may be an overlap in symptoms between the 2 conditions. It is in fact likely that PNES and PPS represent the same psychiatric disorder, and that the presence or absence of pronounced movements is their only difference. Nonetheless, this difference has important practical consequences: patients with PNES will be referred to neurologists, and, if diagnosed incorrectly, are likely to receive treatment with antiepileptic drugs.5 Patients with PPS are most likely to be referred to cardiologists in a search for a cause of the presumed syncope. When cardiac abnormalities are not found, they are likely either to receive “no diagnosis” or to be labeled as “unclassified syncope,”6,7 resulting in prolonged periods of undiagnosed and untreated episodes of apparent TLOC. In PNES, such a diagnostic delay negatively affects the outcome of treatment5,23 and can have serious adverse effects, even including death.24
An insightful interview study revealed that neurologists appeared to view conversion disorder as feigning of symptoms.25 If this attitude, which is probably shared by cardiologists, results in an inability to discuss the PPS diagnosis with sincerity, patients are likely to reject it. Therefore, the authors believe that diagnosing PPS with a tilt test obliges those ordering the test to explain the diagnosis to patients in a way that allows them to understand and accept it.23 In our experience, the psychological nature of the events is usually accepted by patients and relatives, provided that enough time is taken for explanation.2
PPS can be diagnosed with certainty if tilt-table testing results in apparent TLOC without EEG changes or a decrease in HR or BP. Improved recognition of the semiologic features described herein will provide valuable tools to aid its clinical diagnosis.
Supplementary Material
GLOSSARY
- BP
blood pressure
- DBP
diastolic blood pressure
- HR
heart rate
- PNES
psychogenic nonepileptic seizure
- PPS
psychogenic pseudosyncope
- SBP
systolic blood pressure
- sMAP
smoothed mean arterial pressure
- TLOC
transient loss of consciousness
- VVS
vasovagal syncope
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
The study was designed by M.R.T. and J.G.v.D. The data were analyzed by M.R.T., J.v.N., R.H.R., and J.G.v.D. All authors contributed in writing the manuscript. All authors viewed and approved the final version of the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Day SC, Cook EF, Funkenstein H, Goldman L. Evaluation and outcome of emergency room patients with transient loss of consciousness. Am J Med 1982;73:15–23 [DOI] [PubMed] [Google Scholar]
- 2.Sutton R, Brignole M, Benditt DG. Key challenges in the current management of syncope. Nat Rev Cardiol 2012;9:590–598 [DOI] [PubMed] [Google Scholar]
- 3.Moya A, Sutton R, Ammirati F, et al. ; The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC) Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J 2009;30:2631–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dijk JG, Thijs RD, Benditt DG, Wieling W. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol 2009;5:438–448 [DOI] [PubMed] [Google Scholar]
- 5.Bodde NMG, Brooks JL, Baker GA, et al. Psychogenic non-epileptic seizures—definition, etiology, treatment and prognostic issues: a critical review. Seizure 2009;18:543–553 [DOI] [PubMed] [Google Scholar]
- 6.Sule S, Palaniswamy C, Aronow WS, et al. Etiology of syncope in patients hospitalized with syncope and predictors of mortality and rehospitalization for syncope at 27-month follow-up. Clin Cardiol 2011;34:35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brignole M, Ungar A, Casagranda I, et al. Prospective multicentre systematic guideline-based management of patients referred to the Syncope Units of general hospitals. Europace 2010;12:109–118 [DOI] [PubMed] [Google Scholar]
- 8.Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet 2001;357:348–353 [DOI] [PubMed] [Google Scholar]
- 9.Luzza F, Di Rosa S, Pugliatti P, Andò G, Carerj S, Rizzo F. Syncope of psychiatric origin. Clin Auton Res 2004;14:26–29 [DOI] [PubMed] [Google Scholar]
- 10.Benbadis SR, Chichkova R. Psychogenic pseudosyncope: an underestimated and provable diagnosis. Epilepsy Behav 2006;9:106–110 [DOI] [PubMed] [Google Scholar]
- 11.Petersen ME, Williams TR, Sutton R. Psychogenic syncope diagnosed by prolonged head-up tilt testing. QJM 1995;88:209–213 [PubMed] [Google Scholar]
- 12.Zaidi A, Crampton S, Clough P, Fitzpatrick A, Scheepers B. Head-up tilting is a useful provocative test for psychogenic non-epileptic seizures. Seizure 1999;8:353–355 [DOI] [PubMed] [Google Scholar]
- 13.Linzer M, Varia I, Pontinen M, Divine GW, Grubb BP, Estes NA., III Medically unexplained syncope: relationship to psychiatric illness. Am J Med 1992;92:18S–25S [DOI] [PubMed] [Google Scholar]
- 14.Bartoletti A, Alboni P, Ammirati F, et al. “The Italian Protocol”: a simplified head-up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace 2000;2:339–342 [DOI] [PubMed] [Google Scholar]
- 15.Kanjwal K, Kanjwal Y, Karabin B, Grubb BP. Psychogenic syncope? A cautionary note. Pacing Clin Electrophysiol 2009;32:862–865 [DOI] [PubMed] [Google Scholar]
- 16.Chung SS, Gerber P, Kirlin KA. Ictal eye closure is a reliable indicator for psychogenic nonepileptic seizures. Neurology 2006;66:1730–1731 [DOI] [PubMed] [Google Scholar]
- 17.Syed TU, Arozullah AM, Suciu GP, et al. Do observer and self-reports of ictal eye closure predict psychogenic nonepileptic seizures? Epilepsia 2008;49:898–904 [DOI] [PubMed] [Google Scholar]
- 18.Syed TU, LaFrance WC, Jr, Kahriman ES, et al. Can semiology predict psychogenic nonepileptic seizures? A prospective study. Ann Neurol 2011;69:997–1004 [DOI] [PubMed] [Google Scholar]
- 19.Reinsberger C, Perez DL, Murphy MM, Dworetzky BA. Pre- and postictal, not ictal, heart rate distinguishes complex partial and psychogenic nonepileptic seizures. Epilepsy Behav 2012;23:68–70 [DOI] [PubMed] [Google Scholar]
- 20.Gregg ME, James JE, Matyas TA, Thorsteinsson EB. Hemodynamic profile of stress-induced anticipation and recovery. Int J Psychophysiol 1999;34:147–162 [DOI] [PubMed] [Google Scholar]
- 21.Silvani A, Magosso E, Bastianini S, Lenzi P, Ursino M. Mathematical modeling of cardiovascular coupling: central autonomic commands and baroreflex control. Auton Neurosci 2011;162:66–71 [DOI] [PubMed] [Google Scholar]
- 22.Bakvis P, Roelofs K, Kuyk J, Edelbroek PM, Swinkels WA, Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia 2009;50:1001–1011 [DOI] [PubMed] [Google Scholar]
- 23.Thompson R, Isaac CL, Rowse G, Tooth CL, Reuber M. What is it like to receive a diagnosis of nonepileptic seizures? Epilepsy Behav 2009;14:508–515 [DOI] [PubMed] [Google Scholar]
- 24.Reuber M, Baker GA, Gill R, Smith DF, Chadwick DW. Failure to recognize psychogenic nonepileptic seizures may cause death. Neurology 2004;62:834–835 [DOI] [PubMed] [Google Scholar]
- 25.Kanaan R, Armstrong D, Barnes P, Wessely S. In the psychiatrist’s chair: how neurologists understand conversion disorder. Brain 2009;132:2889–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


