Abstract
Objective:
To demonstrate that U-2 pilot occupational exposure to hypobaria leads to increased incidence of white matter hyperintensities (WMH) with a more uniform distribution throughout the brain irrespective of clinical neurologic decompression sickness history.
Methods:
We evaluated imaging findings in 102 U-2 pilots and 91 controls matched for age, health, and education levels. Three-dimensional, T2-weighted, high-resolution (1-mm isotropic) imaging data were collected using fluid-attenuated inversion recovery sequence on a 3-tesla MRI scanner. Whole-brain and regional WMH volume and number were compared between groups using a 2-tailed Wilcoxon rank sum test.
Results:
U-2 pilots demonstrated an increase in volume (394%; p = 0.004) and number (295%; p < 0.001) of WMH. Analysis of regional distribution demonstrated WMH more uniformly distributed throughout the brain in U-2 pilots compared with mainly frontal distribution in controls.
Conclusion:
Pilots with occupational exposure to hypobaria showed a significant increase in WMH lesion volume and number. Unlike the healthy controls with predominantly WMH in the frontal white matter, WMH in pilots were more uniformly distributed throughout the brain. This is consistent with our hypothesized pattern of damage produced by interaction between microemboli and cerebral tissue, leading to thrombosis, coagulation, inflammation, and/or activation of innate immune response, although further studies will be necessary to clarify the pathologic mechanisms responsible.
The United States Air Force (USAF) operates the U-2 high-altitude reconnaissance aircraft, which maintains a cabin altitude of approximately 9,000 m (28,000–30,000 ft) while operating above 21,000 m.1 Decompression sickness (DCS), including CNS neurologic DCS (NDCS), is a known occupational risk from exposure to low ambient pressure (hypobaria) in high-altitude aviators.2 The risk of DCS per flight increased from 0.076% pre-2006 to 0.23% during the 2006–2010 operation years, believed to be related to more frequent and longer periods of exposure for the pilots.3,4 Importantly, 44% of episodes were diagnosed as NDCS, with symptoms ranging from mild, such as complaints of slowed thought processes, to severe, including anomia, confusion, unresponsiveness,5 and permanent cognitive decline.6
We previously reported that clinical NDCS was associated with an increase in white matter hyperintensities (WMH).7 Herein, we examine the volume, number, and regional distribution of subcortical WMH in a healthy, young population of age-, health-, and education-matched pilots and controls who lack the common risk factors for recognized WMH etiologies, leaving occupational exposure to hypobaria as the main intergroup contrast. We hypothesized that this increase in WMH was directly or indirectly related to microbubbles of predominantly nitrogen gas formed during hypobaria. Specifically, we hypothesized the entire U-2 pilot population would exhibit significantly greater subcortical WMH volume and number with a more uniform regional distribution throughout the brain than a normative control group.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was reviewed and approved by the Air Force Research Laboratory Institutional Review Board. All study subjects were active duty members of the US Armed Forces. All participants were recruited with strict adherence to Department of Defense requirements regarding protection of human subjects' research. Participation in this study was voluntary without commander involvement or knowledge. All participants acknowledged this was not an anonymous study and results would become a permanent part of their electronic military medical record and provided informed consent.
All high-altitude U-2 pilots currently on active duty in the USAF were invited to participate, with a participation rate exceeding 90%. All active-duty military members with a doctorate degree assigned to duty within the continental United States were eligible to participate as normal controls, although recruitment was predominantly from the 2 San Antonio graduate medical education military facilities through presentations at professional staff meetings. Additionally, presentations were made seeking volunteers at international aerospace meetings and through electronic messaging such as facility daily bulletins. All pilots were healthy at the time of testing, meeting USAF Flying Class II standards8 and on active USAF flying status. All normative subjects also met USAF Flying Class II neurologic standards. Military medical records were reviewed to confirm self-reported medical information. Briefly, exclusionary criteria included a history of any of the following: head trauma with any loss of consciousness or amnesia; migraine headache; psychiatric or psychological disease requiring any medication or hospitalization; hypertension requiring more than a single angiotensin-converting enzyme inhibitor for control; hyperlipidemia requiring more than a single statin for control; diabetes or glucose intolerance; any neurologic disease including infection, seizure, or stroke; familial degenerative neurologic disease; substance or drug abuse or dependence; or any systemic disease with the potential for neurologic involvement. All subjects were between the ages of 26 and 50 years (37.0 ± 6.0 years, range 28–50 years, and age 36.0 ± 6.2 years, range 26–50 years, for pilots and controls, respectively, p = 0.063). Both sexes were included. All subjects were evaluated between May 2011 and December 2012. Subjects did not receive compensation for participation in this study, but subjects' travel costs were reimbursed as permitted under federal government travel regulations.
Imaging.
Structural MRI data for the pilots were collected at the Research Imaging Institute, University of Texas Health Science Center, San Antonio, using a Siemens 3T Tim Trio scanner (Siemens AG, Erlangen, Germany) equipped with a 12-channel phase array coil. Structural MRI data for the normative doctorate-degree subjects were collected at Wilford Hall Ambulatory Surgical Clinic, 59th Medical Wing, Lackland AFB, TX, using a Siemens 3T Verio scanner equipped with a 32-channel phase array coil. Both scanners were operated under quality control and assurance guidelines in accordance with recommendations by the American College of Radiology and utilized the same imaging sequence. T2-weighted, 3-dimensional (3D), high-resolution (isotropic 1-mm), fluid-attenuated inversion recovery (FLAIR) data were collected using a turbo-spin sequence with the following parameters: repetition time/echo time/inversion time/flip angle/echo train length = 5 seconds/353 milliseconds/1.8 seconds/180°/221. This 3D FLAIR protocol was specifically designed to overcome the limitations of a 2-dimensional thick-slice (5- to 10-mm) clinical imaging protocol and to permit increased detection of smaller lesions with accurate tracing of lesion boundaries.9,10 This 3D FLAIR sequence uses a nonselective inversion radiofrequency (RF) pulse to suppress CSF pulsation artifacts to reduce false-negative hyperintense artifacts seen near CSF-containing structures in the 2-dimensional FLAIR sequences.11
In addition to FLAIR data, high-resolution (isotropic 0.8 mm, voxel size = 0.5 mm3) T1-weighted data were collected with the following sequence parameters: echo time/repetition time/inversion time = 3.04/2,100/785 milliseconds, flip angle = 11°. A retrospective motion-correction technique12 was used to reduce subject motion-related artifacts. Magnetic resonance spectroscopy, arterial spin labeling, and diffusion tensor imaging data were collected but not included in this report.
A cross-calibration protocol was performed to calculate linear scaling coefficients for the volume and number of subcortical WMH. Under this protocol, 11 subjects with variable count and volume of WMH were imaged at both sites (see complete calibration analysis, figures e-1 to e-3 on the Neurology® Web site at www.neurology.org). Linear regression demonstrated excellent reproducibility between the 2 MRI scanners (r = 0.96 and 0.99 for volume and number of WMH, respectively). Results and analysis are provided for both adjusted and raw FLAIR data.
Analysis of FLAIR images.
WMH number and volume quantification was performed as previously described.7,13 Briefly, FLAIR images were preprocessed by removal of nonbrain tissue using FSL BET (brain extraction tool), freely available from the Oxford Centre for Functional MRI of the Brain (FMRIB).14 Next, FLAIR images for individual subjects were registered to their corresponding T1-weighted images using FSL FLIRT (FMRIB's linear image registration tool) and then registered to a common Talairach atlas–based stereotactic frame using FSL FLIRT15 and 9-parameter (3 each for rotation, translation, and scaling) global normalization transformation. The purpose of this step is to reduce interindividual anatomical variance in the global brain size, shape, and orientation and to permit the use of automated labeling approaches by using a digital brain atlas.16 Next, all images were corrected for RF inhomogeneity artifact using the FSL BET method with default parameters. RF inhomogeneity artifact manifests itself as a low-frequency variation of MRI intensity that impedes intensity-based image analysis unless corrected. WMH regions were then manually delineated in 3D space using in-house software (http://ric.uthscsa.edu/mango) by a single experienced neuroanatomist, blinded for scanner and group information, with high intrarater (r = 0.95) test-retest reproducibility and high interrater correlation (r = 0.92). During the labeling, WMH regions were coded as ependymal regions, contiguous with CSF structures, and as subcortical regions as previously described.17 Finally, the volume and location of WMH were analyzed using the boundaries for the frontal, insula, limbic, occipital, parietal, sublobar, and temporal regions extracted from the digital Talairach atlas (http://www.talairach.org).
Statistical analysis.
Two sets of analyses were performed to compare the prevalence and regional distribution of WMH in pilots to the control group. Group-wise analyses of the difference in the volume and number of WMH were performed using a nonparametric Wilcoxon rank sum 2-tailed statistical model. A nonparametric test was used because WMH data are not normally distributed. We considered p < 0.05 as the threshold for significance. The volume and number of WMH for U-2 pilots were adjusted using the linear regression coefficients obtained from the calibration study to accommodate for a higher signal-to-noise ratio of the Wilford Hall Ambulatory Surgical Clinic imaging center (see figures e-1 to e-3). Group-wise comparison was performed for both original and adjusted data.
Analyses of regional distribution of WMH were performed using the methods described elsewhere.7 In short, we performed intergroup comparisons of the volume and number of WMH for the major cerebral partitions based on the 3D Talairach atlas.18 Further, we tested the regional nonuniformity of lesion distribution by adjusting the fractional volume of lesions per brain region by the fractional volume of region obtained from the atlas. A uniform distribution of lesions would lead to this ratio not being significantly different from the unity. We tested this hypothesis by calculating the significance of the z value obtained by subtracting 1.0 from the normalized fraction of lesion volume and dividing the residual by normalized SD. All regional analyses were performed on raw volume and numbers.
RESULTS
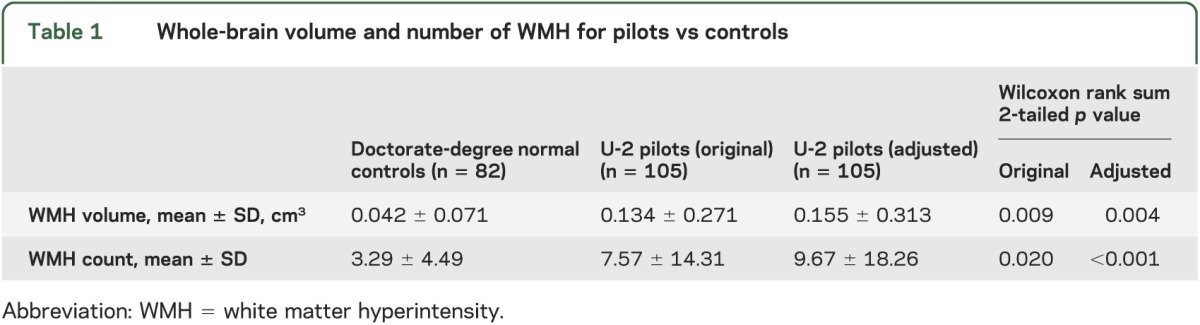
An example of punctate subcortical WMH findings in a 39-year-old U-2 pilot who denied ever experiencing symptoms of DCS is shown in figure 1. Population-wide overlap of individual subcortical WMH demonstrated both a much larger number of lesions in pilots and a more uniform regional distribution of WMH than in normal controls (figure 2). Pilots demonstrated a nearly 4-fold increase in volume (375%) and a 3-fold increase in the number (294%) of WMH; this difference was significant for both the raw data and the site-specific adjusted data (table 1). Spearman correlation coefficient between lesion volume and age approached significance for the normal controls but not for the pilots (p = 0.07 and 0.16; r = 0.19 and 0.14 for controls and pilots, respectively) (see figure e-4, top). The correlation coefficients between the number of lesions and age were not different (p = 0.14 and 0.2; r = 0.15 and 0.13 for controls and pilots, respectively) (see figure e-4, bottom). Likewise, the correlation coefficients between the volume and number of WMH and the number of U-2 flight hours were not different for the pilots (p > 0.5; r = 0.05 and 0.03 for volume and number, respectively) (see figure e-5).
Figure 1. T2-weighted FLAIR image of a 39-year-old pilot who never experienced NDCS shows multiple punctate WMH.
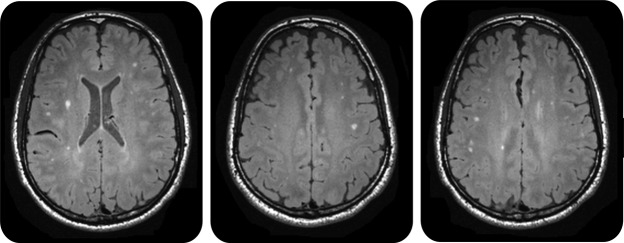
FLAIR = fluid-attenuated inversion recovery; NDCS = neurologic decompression sickness; WMH = white matter hyperintensities.
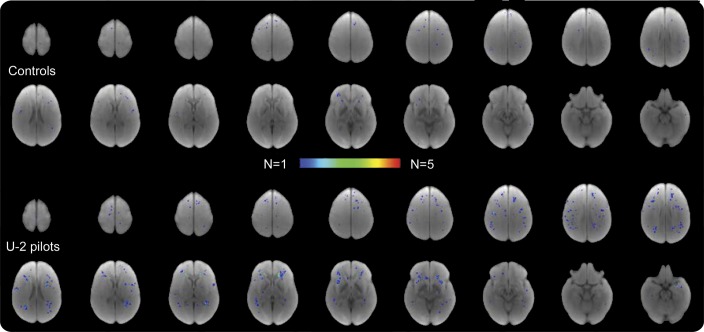
Figure 2. Summary of WMH lesions for each group.
U-2 pilots showed a higher number and a more regionally uniform distribution of lesions, including some overlapping lesions in the frontal area compared with more frontal distribution in controls. WMH = white matter hyperintensity.
Table 1.
Whole-brain volume and number of WMH for pilots vs controls
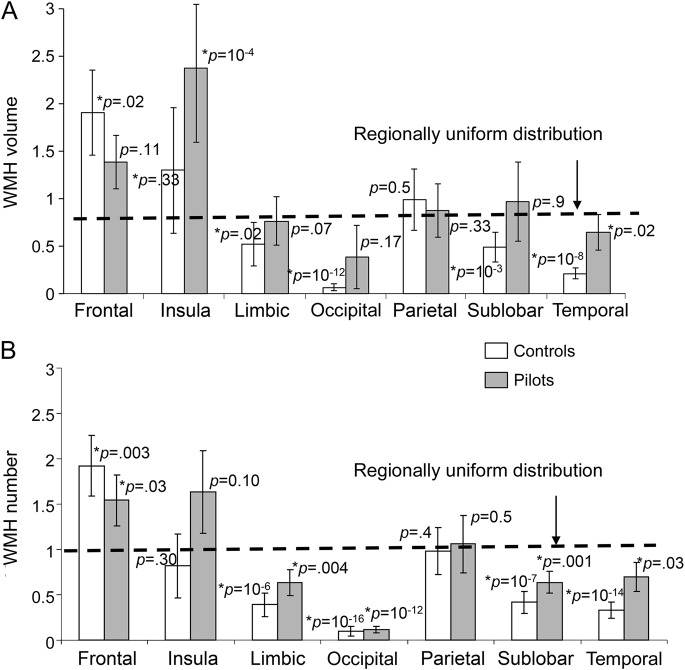
Regional analysis revealed that frontal lobe lesions constituted the largest fraction of both volume and number of WMH loci in both U-2 pilots (50% and 56% for volume and number, respectively) and normative controls (69% and 70% for volume and number, respectively) (see tables e-1 and e-2). Pilots had a higher volume (p < 0.03) of WMH in the frontal, insula, limbic, sublobar, and temporal regions and a higher number (p < 0.01) of WMH in the insula, limbic, temporal, and sublobar regions. No difference was noted in the occipital and parietal regions. Analysis of regional heterogeneity demonstrated that the fractional WMH volume deviated from the homogeneous distribution for 2 regions in pilots (insula and temporal lobe) vs 5 regions in normal controls (figure 3A). The same analysis was performed on the number of lesions, demonstrating that the regional distribution for pilots relative to the regional volume was more uniform than in the controls (figure 3B).
Figure 3. Regional uniformity of WMH lesion distribution was tested by calculating the fraction of WMH volume (A) and numbers (B) over the fractional volume of each brain region.
Regional uniform distribution for WMH lesions, where the lesion volume and number are explained by volume of the region, corresponds to ratios that are not significantly different from the unity (1.0). Pilots demonstrate a more uniform distribution than controls. WMH = white matter hyperintensity.
DISCUSSION
The volume and count of WMH are important markers of cerebral integrity.9 In addition, increasing volume and number of WMH are linked to age-related cognitive decline, particularly in executive functioning,19 processing speed, and general cognitive status.20 Histopathologic findings of MRI-localized punctate WMH reveal areas of demyelination and atrophy of the neuropil around fibrohyalinotic arterioles, halo-like rarefaction of myelinated fibers surrounding the atrophic neuropil, and a suggestion of focally decreased permeability of the vessel walls.21 There is a significant regional heterogeneity in the distribution of subcortical WMH in normal aging.10 The majority (60%–80%) of WMH are found in the frontal area, presumably because its high metabolic demand makes it more vulnerable to age-related cerebrovascular disorders,22,23 while a more uniform distribution of WMH is a hallmark finding in many neuroinflammatory disorders and traumatic brain injury and may be used to gauge disease severity and progression.24,25
This study demonstrated that pilots exposed to hypobaria had increased volume and number of subcortical WMH compared with a healthy, age- and education-matched normative population. WMH in pilots were more uniformly distributed throughout the brain than in normal controls and did not increase with age in pilots, suggesting that hypobaric exposure produces white matter damage different from that occurring in normal aging. Both findings suggest injury produced by microemboli entering cerebral circulation at random.
Three potential sources of microemboli should be considered: microbubbles of gas, presumably nitrogen; platelet-based thrombi; and microparticles. The symptoms of DCS classically are believed secondary to nitrogen gas bubbles exerting direct pressure on tissues, blocking small arteriolar vessels, and interacting with blood proteins.5 All of the WMH observed in pilots were located in deep white matter rather than in the cerebral cortex, suggesting that simple compression of white matter or arteriolar occlusion by gas bubbles cannot be the complete explanation. Additionally, arterial gas emboli are relatively uncommon, reported in only 6 of more than 1,500 altitude chamber exposure cases.26 However, the WMH may still be caused by occlusion or injury to small (5- to 30-μm) deep cerebral vessels from microembolic gas bubbles that are smaller than the 30-μm detection limit of clinical ultrasound scanners used for bubble detection.27 A second potential mechanism is occlusion of small cerebral vessels by platelet thrombi produced by accelerated coagulation of blood in the presence of venous nitrogen gas bubbles; in rabbits, microthrombi were noted in medium-sized and large lung arteries after DCS.28 This mechanism is also supported by the decreased platelet count with increased concentration of venous nitrogen gas bubbles in humans due to increases in platelet adhesion and aggregation.29 A third potential mechanism is microparticle release, 0.1- to 1.0-μm vesicles with proinflammatory potential, with induction of neutrophil activation and vascular damage in response to intravascular bubbles as demonstrated in mice.30 In scuba divers, microparticles are increased 3.4-fold, neutrophil activation occurs, and increased neutrophil interactions with platelet membranes are noted.31 Such an inflammatory mechanism might explain the clinical relapse we observed in 3 NDCS pilots after successful hyperbaric treatment (US Navy Treatment Table 6; 100% fraction of inspired oxygen; 2.8 atm absolute) when subsequently exposed to commercial airline cabin altitudes within the first 2 weeks after the incident. Further studies in laboratory animals are necessary to clarify the precise pathologic mechanisms responsible for formation of hypobaric-induced WMH.
Increased WMH burden was previously reported in high-altitude mountain climbers, attributed to a combination of hypoxia and hypobaria.32,33 Oxygenation status was maintained in our pilots; therefore, we can exclude hypoxia as a potential cause of our increased WMH.34 Another argument for ruling out hypoxia is increased WMH in divers who experience decompression going from depths to sea level. Increased WMH were detected in 23% (26/113) of Turkish military divers without a history of DCS compared with 11% (7/65) of controls.35 Similarly, an increase in WMH was observed in French military divers lacking a history of DCS when compared with normal controls.36 This suggests that the prevalence of WMH is increased in populations whose occupation subjects them to deviations in atmospheric pressure.
Our study suggests that occupational exposure to hypobaria induces WMH, presumably secondary to white matter injury, even in the absence of clinical symptoms of NDCS. The etiology of this is unknown but is believed to be secondary to microembolic gas bubbles, “thrombo-inflammatory” mechanisms, or microparticle-induced neutrophil activation and vascular damage rather than simple gas bubble occlusion of cerebral arterioles. Our study provides radiologic evidence supporting the premise of microemboli showering cerebral tissues and provides evidence of cerebral injury as a consequence of this activation. Cerebrovascular-induced WMH is typically permanent, but the long-term ramifications of hypobaric-induced WMH are unknown. More complete understanding of this pathologic mechanism will require development of a laboratory animal model of hypobaria-related white matter damage to detect the biological/neuropathologic mechanism and to develop neuroprotection/neurotreatment therapies designed to mitigate this damage.
Supplementary Material
ACKNOWLEDGMENT
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the United States Air Force. The authors thank Ms. LeeAnn Zarzabal, 59th Medical Wing, Lackland AFB, TX, for statistical assistance.
GLOSSARY
- BET
brain extraction tool
- DCS
decompression sickness
- FLAIR
fluid-attenuated inversion recovery
- FMRIB
Functional MRI of the Brain
- NDCS
neurologic decompression sickness
- RF
radiofrequency
- 3D
3-dimensional
- USAF
United States Air Force
- WMH
white matter hyperintensities
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. McGuire: study concept, design, performance analysis, interpretation, and principal author of the manuscript. Dr. Sherman: study concept, design, performance, analysis and interpretation. Dr. Profenna, Dr. Grogan, Dr. Sladky, Dr. Brown, and Dr. Robinson: acquisition of data, analysis and interpretation. Dr. Rowland, Dr. Hong, and Ms. Patel: analysis and interpretation. Dr. Tate: analysis, interpretation, and critical revision of the manuscript for important intellectual content. Ms. Kawano: critical scientific editorial assistance. Dr. Fox: study concept and design. Dr. Kochunov: study concept, design, performance, data analyses, and critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Supported by United States Air Force Surgeon General grants (Log I-11-10 and I-11-44) to S.M.
DISCLOSURE
S. McGuire is supported by USAF Surgeon General grants Log I-11-10 and I-11-44. P. Sherman and L. Profenna report no disclosures P. Grogan has received speaker honoraria from USWorldMeds. J. Sladky, A. Brown, and A. Robinson report no disclosures. L. Rowland serves as an editorial board member of Schizophrenia Bulletin, and is funded by NIH grants R01MH094520 and R01MH096263. E. Hong and B. Patel report no disclosures. D. Tate has been a consultant to the Brigham and Women's Hospital, Boston, MA, for an NIH funded grant of brain injury. E.S. Kawano reports no disclosures. P. Fox reports that his UTHSCSA salary is derived, in part, by various grants from the NIH, the Department of Defense, and the Department of Veterans Affairs. Dr. Fox receives royalty income from Wiley-Blackwell. P. Kochunov reports no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.U-2 high-altitude reconnaissance aircraft, United States of America. In: airforce-technology.com [online]. Available at: http://www.airforce-technology.com/projects/u2. Accessed December 1, 2012
- 2.Vann RD, Butler FK, Mitchell SJ, Moon RE. Decompression illness. Lancet 2011;377:153–164 [DOI] [PubMed] [Google Scholar]
- 3.Jersey SL, Hundemer GL, Stuart RP, West KN, Michaelson RS, Pilmanis AA. Neurological altitude decompression sickness among U-2 pilots: 2002–2009. Aviat Space Environ Med 2011;82:673–682 [DOI] [PubMed] [Google Scholar]
- 4.Hundemer GL, Jersey SL, Stuart RP, Butler WP, Pilmanis AA. Altitude decompression sickness incidence among U-2 pilots: 1994–2010. Aviat Space Environ Med 2012;83:968–974 [DOI] [PubMed] [Google Scholar]
- 5.Balldin UI, Pilmanis AA, Webb JT. Central nervous system decompression sickness and venous gas emboli in hypobaric conditions. Aviat Space Environ Med 2004;75:969–972 [PubMed] [Google Scholar]
- 6.Jersey SL, Baril RT, McCarty RD, Millhouse CM. Severe neurological decompression sickness in a U-2 pilot. Aviat Space Environ Med 2010;81:64–68 [DOI] [PubMed] [Google Scholar]
- 7.McGuire SA, Sherman PM, Brown AC, et al. Hyperintense white matter lesions in 50 high-altitude pilots with neurologic decompression sickness. Aviat Space Environ Med 2012;83:1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Air Force Medical Examinations and Standards. Washington, DC: Department of the Air Force; 2009. Air Force Instruction 48-123 [Google Scholar]
- 9.Kochunov P, Thompson PM, Coyle TR, et al. Relationship among neuroimaging indices of cerebral health during normal aging. Hum Brain Mapp 2008;29:36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochunov P, Glahn D, Winkler A, et al. Analysis of genetic variability and whole genome linkage of whole-brain, subcortical, and ependymal hyperintense white matter volume. Stroke 2009;40:3685–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakshi R, Caruthers SD, Janardhan V, Wasay M. Intraventricular CSF pulsation artifact on fast fluid-attenuated inversion-recovery MR images: analysis of 100 consecutive normal studies. AJNR Am J Neuroradiol 2000;21:503–508 [PMC free article] [PubMed] [Google Scholar]
- 12.Kochunov P, Lancaster JL, Glahn DC, et al. Retrospective motion correction protocol for high-resolution anatomical MRI. Hum Brain Mapp 2006;27:957–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochunov P, Thompson PM, Lancaster JL, et al. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage 2007;35:478–487 [DOI] [PubMed] [Google Scholar]
- 14.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23:S208–S219 [DOI] [PubMed] [Google Scholar]
- 16.Mazziotta JC, Toga AW, Evans AC, Fox PT, Lancaster JL. Digital brain atlases. Trends Neurosci 1995;18:210–211 [DOI] [PubMed] [Google Scholar]
- 17.Henry Feugeas MC, De Marco G, Peretti II, Godon-Hardy S, Fredy D, Schouman Claeys ES. Age-related cerebral white matter changes and pulse-wave encephalopathy: observations with three-dimensional MRI. Magn Reson Imaging 2005;23:929–937 [DOI] [PubMed] [Google Scholar]
- 18.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000;10:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kochunov P, Robin DA, Royall DR, et al. Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp 2009;30:2581–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galluzzi S, Lanni C, Pantoni L, Filippi M, Frisoni GB. White matter lesions in the elderly: pathophysiological hypothesis on the effect on brain plasticity and reserve. J Neurol Sci 2008;273:3–9 [DOI] [PubMed] [Google Scholar]
- 21.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurolology 1993;43:1683–1689 [DOI] [PubMed] [Google Scholar]
- 22.Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res 2009;1297:41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochunov P, Coyle T, Lancaster J, et al. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage 2010;49:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maillard P, Carmichael O, Harvey D, et al. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol 2013;34:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moen KG, Skandsen T, Folvik M, et al. A longitudinal MRI study of traumatic axonal injury in patients with moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry 2012;83:1193–1200 [DOI] [PubMed] [Google Scholar]
- 26.Pilamnis AA, Meissner FW, Olson RM. Left ventricular gas emboli in six cases of altitude-induced decompression sickness. Aviat Space Environ Med 1996;67:1092–1096 [PubMed] [Google Scholar]
- 27.Olson RM. Echo Imaging Techniques Determine the Size of Intravascular Bubbles in Decompression Sickness. Brooks Air Force Base, TX: Armstrong Laboratory, Crew Systems Directorate. AL/CF-TR-1994-0033; 1994
- 28.Tanoue K, Mano Y, Kuroiwa K, Suzuki H, Shibayama M, Yamazaki H. Consumption of platelets in decompression sickness of rabbits. J Appl Physiol 1987;62:1772–1779 [DOI] [PubMed] [Google Scholar]
- 29.Pontier JM, Jimenez C, Blatteau JE. Blood platelet count and bubble formation after a dive to 30 msw for 30 min. Aviat Space Environ Med 2008;79:1096–1099 [DOI] [PubMed] [Google Scholar]
- 30.Thom S, Yang M, Bhopale V, Huang S, Milovanova T. Microparticles initiate decompression-induced neutrophil activation and subsequent vascular injuries. J Appl Physiol 2011;110:340–351 [DOI] [PubMed] [Google Scholar]
- 31.Thom S, Milovanova T, Bogush M, Bhopale V, et al. Microparticle production, neutrophil activation, and intravascular bubbles following open-water SCUBA diving. J Appl Physiol 2012;112:1268–1278 [DOI] [PubMed] [Google Scholar]
- 32.Fayed N, Modrego PJ, Morales H. Evidence of brain damage after high-altitude climbing by means of magnetic resonance imaging. Am J Med 2006;119:168.e1–168.e6 [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Lin J, Sun Y, et al. Compromised white matter microstructural integrity after mountain climbing: evidence from diffusion tensor imaging. High Alt Med Biol 2012;13:118–125 [DOI] [PubMed] [Google Scholar]
- 34.Harding RM. Pressure changes and hypoxia in aviation. In: Pandolf KB, Burr RE, editors. Medical Aspects of Harsh Environments, Vol 2. Washington, DC: Office of the Surgeon General; 2002:984–1012 [Google Scholar]
- 35.Erdem I, Yildiz S, Uzun G, et al. Cerebral white-matter lesions in asymptomatic military divers. Aviat Space Environ Med 2009;80:2–4 [DOI] [PubMed] [Google Scholar]
- 36.Gempp E, Sbardella F, Stephant E, et al. Brain MRI signal abnormalities and right-to-left shunting in asymptomatic military divers. Aviat Space Environ Med 2010;81:1008–1012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.