Abstract
Objective:
To determine the effect of a lower dose of rituximab in depleting B lymphocytes, maintaining low B-cell counts, and relapse in patients with neuromyelitis optica (NMO) and NMO spectrum disorders.
Methods:
We treated 5 Chinese patients with deteriorating NMO and NMO spectrum disorders with a 100-mg IV infusion of rituximab once a week for 3 consecutive weeks, followed by additional infusion of the same dosage depending on circulating B-cell repopulation.
Results:
This reduced dosage of rituximab was sufficient to deplete B cells and maintain low B-cell counts. None of the treated patients experienced relapse, and all patients exhibited stabilized or improved neurologic function during the 1-year follow-up period. MRI revealed the absence of new lesions, no enhancement in spinal cord and brain, a significant shrinkage of spinal cord segments, and a reduction/disappearance of previous brain lesions.
Conclusion:
A lower dosage of rituximab may be sufficient in depleting B cells, maintaining low B-cell counts, and preventing disease progression in Chinese patients with NMO.
Neuromyelitis optica (NMO) is an autoimmune disease predominantly affecting the optic nerves and spinal cord.1 NMO is less common than multiple sclerosis (MS) in Caucasians and the proportion of NMO to MS is greater in East Asia. Currently, it remains difficult to control the relapses of NMO, in part because these patients respond poorly to traditional MS therapies. Among the very few disease-modifying medications that have been tested in patients with NMO, rituximab therapy has achieved a 70% or greater response rate by depleting B cells.2–6 However, most studies were performed in Caucasians, the dose of rituximab was adopted from those targeting B-cell malignancies, and the potential long-term side effects of rituximab are not known.1
The dosage of rituximab given to patients with NMO is expected to be different than that given to B-cell lymphoma patients considering the different targeted treatments of these 2 diseases. In addition, rituximab therapy is considerably more expensive in China, where it is not covered by insurance for patients with NMO. Herein, we report that repeated dosages of 100 mg of rituximab are very effective in depleting circulating B cells and preventing relapses in Chinese patients with NMO and NMO spectrum disorders.
METHODS
Subjects.
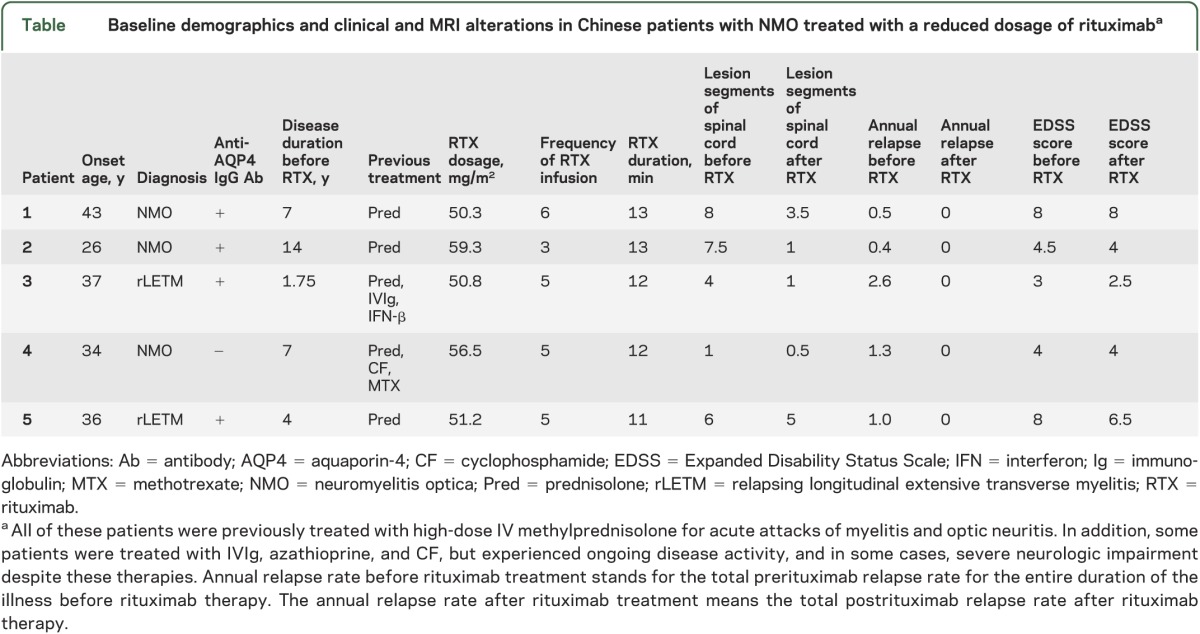
The 5 patients who were cared for in our clinic met the revised diagnostic criteria as proposed by Wingerchuk7 in 2007. General clinical features, as well as presence of anti–aquaporin-4 immunoglobulin G antibody, are presented in the table.
Table.
Baseline demographics and clinical and MRI alterations in Chinese patients with NMO treated with a reduced dosage of rituximaba
Standard protocol approvals, registrations, and patient consents.
All subjects signed fully informed written consent before they were enrolled in the study. The use of human subjects for this study was approved by the Tianjin Medical University Ethical Review Board.
Rituximab dosing and monitoring of circulating B cells.
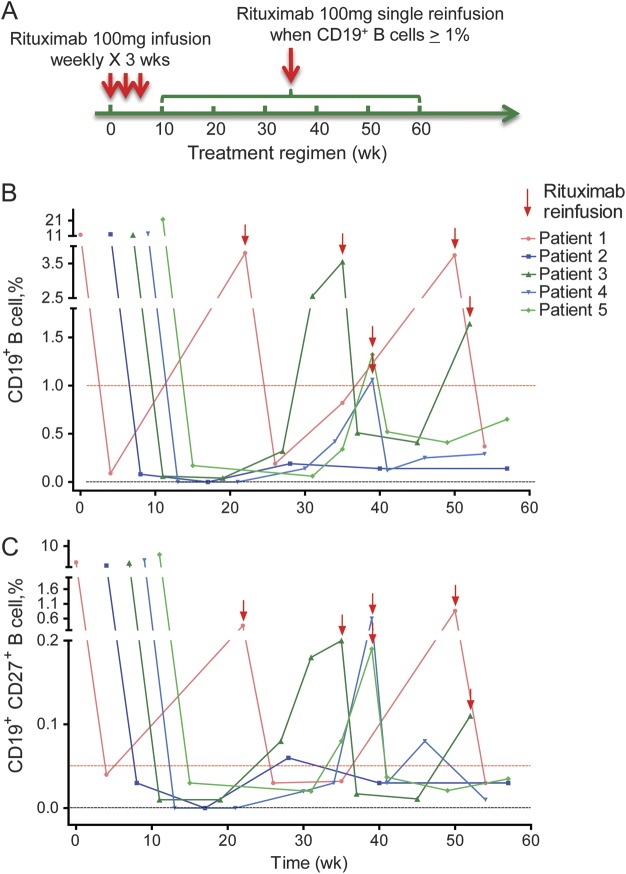
All 5 patients were treated with rituximab (Biogen-Idec, Cambridge, MA, and Genentech, San Francisco, CA) 100 mg (equivalent of 50–59 mg/m2) IV, one infusion per week for 3 consecutive weeks (figure 1A). Detailed rituximab administration and methodology of monitoring circulating B cells are explicitly described in the legend of figure 1.
Figure 1. Kinetics of the B-cell population in patients treated with repeated dosage of 100 mg rituximab.
(A) Reduced-dosage rituximab treatment regimen. We administered rituximab 100 mg IV, once per week for 3 consecutive weeks. Continued dosage was dependent on the percentage of circulating CD19+ B-cell counts from patients with neuromyelitis optica. Whenever it reached 1% of total lymphocyte population, rituximab 100 mg was reinfused except in patient 3 because a delay was caused by patient scheduling issues. (B) The repopulation of CD19+ B cells in patients with neuromyelitis optica during rituximab therapy. (C) The repopulation of CD19+CD27+ B cells parallels that of CD19+ B cells after rituximab treatment. In all 5 patients, whenever the percentage of CD19+ B cells reached 1%, CD19+CD27+ B cells were higher than 0.05%. We used simple whole-blood staining to monitor B-cell kinetics directly from the circulation. We immunostained whole-blood samples within 60 minutes of blood draw using antibodies directed against CD27/CD19 with isotype controls, followed by red blood cell lysis and immediate acquisition and analysis by flow cytometry. Before infusion, a percentage of circulating CD19+ cells among the total lymphocytes was determined as the baseline level. The percentage of CD19+ cells was determined again after 3 rituximab infusions at 4- to 8-week intervals. Infusion was stopped when these cells reached <1%, and reinfusion was continued when CD19+ cells exceeded 1% (A and B). All patients gave consent before receiving treatment.
Data analysis.
An individual dosage of rituximab was analyzed separately in the context of CD19+ B-cell percentage and patients' clinical data and MRI scan. Day 0 in figure 1 refers to the initial infusion. The signed-rank test was used to assess pre- and postrituximab median Expanded Disability Status Scale (EDSS) score; the 2-sided sign test was used to assess the median relapse rates.
RESULTS
Kinetics of the B-cell population in patients with NMO and NMO spectrum disorders treated with repeated dosage of 100 mg rituximab.
A 100-mg infusion for 3 consecutive weeks was sufficient to reduce total CD19+ B cells (≤1%), as well as the memory component of CD19+CD27+ B cells (≤0.05%), in all 5 patients with NMO (figure 1, B and C). These cells started to increase in 4 of the 5 patients 20 weeks after initial infusion. Whenever CD19+ B-cell counts reached 1%, an additional infusion was effective to maintain them <1% (figure 1B). We also observed kinetics of CD19+CD27+ B cells (figure 1C). At week 53 after initial infusion, CD19+ B-cell counts were still <1% in one patient, whose CD19+CD27+ B-cell counts were <0.05% (figure 1, B and C). We observed no serious side effects, such as complications of renal and hepatic functions, and no infections in these patients during the 1.5-year follow-up period. One patient complained of fatigue after infusion, which spontaneously disappeared within a week. Another patient felt transient tight throat and chest distress during the first and third infusion of rituximab, which was controlled by IV administration of 40 mg of methylprednisolone.
Efficacy of repeated dosage of 100 mg rituximab in progression of NMO.
Before rituximab therapy, the median relapse rate in the 5 patients with NMO was 1.0 (table). None of the patients experienced relapse after rituximab therapy during the 1-year follow-up period (relapse rate 0). The median EDSS score for the 5 patients before therapy was 4.5. Reassessment of EDSS scores 1 year after rituximab therapy revealed that the median score was 4; 3 patients presented with a tendency of EDSS score reduction, whereas 2 experienced no change (table). Although the sum of EDSS score reduction did not achieve statistical significance, overall the patients experienced significant improvements in pyramidal, sensory, and bowel functions after therapy (p < 0.05 for 3 comparisons).
Alterations of neuroimaging in patients treated with repeated dosages of 100 mg rituximab.
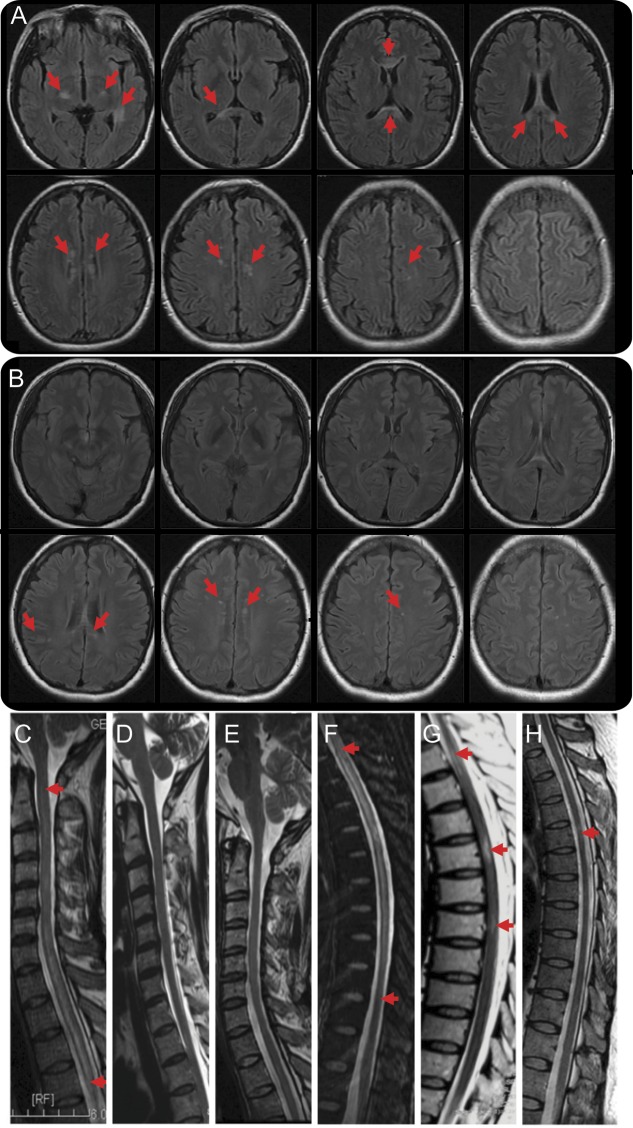
MRI assessment revealed no new T2 lesions and no enhancement in the CNS in all 5 patients treated with rituximab during the 1-year follow-up period. The median of total T2 lesions in brain and spinal cord before and after rituximab therapy was 3 and 2.5, respectively (figure 2). The median length of spinal cord lesions before and after therapy was 5 and 2.5 vertebral segments, respectively (figure 2), which was significantly reduced over the 1-year period (p < 0.05).
Figure 2. Brain and spinal cord MRIs of a patient treated with repeated dosages of 100 mg rituximab.
(A) Axial brain MRI scan of patient 3 illustrated multiple lesions bilaterally at bilateral basal ganglia, the thalamus, corpus callosum, and centrum ovale (arrows) on T2 fluid-attenuated inversion recovery before rituximab infusion. (B) Dramatic reduction and/or disappearance of lesions (arrows) were noted 1 year after rituximab therapy at the corresponding segments to panel A. (C–E) Sagittal T2W1 MRI of C1-T4 segments of spinal cord exhibits long segments of diffused signal enhancement before rituximab infusion (C); a gradual reduction in the extent of these lesions was observed between the sixth (D) and tenth (E) months during rituximab therapy. (F–H) No new lesions were found. Similar alterations in lesions were observed in T1-11 segments (arrows, F–H).
DISCUSSION
A decrease of autoantigen-specific humoral immune response and inhibitory inflammatory responses orchestrated by pathogenic B cells rationalize B-cell–targeted therapy in patients with NMO. The current results, together with previous ones, demonstrate rituximab as a promising medication for NMO.2–6 To further confirm the efficacy of rituximab in patients with NMO, multicenter, double-blind, randomized, placebo-controlled studies must be performed. In planning such a clinical trial, several issues, including the dosage of rituximab and challenges to recruit a sufficient number of patients, must be considered.
In nearly all previous reports, the treatment protocol was similar to that used in B-cell lymphoma, i.e., 4 weekly doses of 375 mg/m2. Our study suggested that the therapeutic objectives in patients with NMO may be accomplished with lower dosages of rituximab (50–59 mg/m2), whereas in B-cell lymphoma, complete removal of the B-cell lineage may be required. The primary motivation for B-cell depletion is different for these 2 conditions. In support of this notion, there are a few reports documenting reduced rituximab dosage in myasthenia gravis and autoimmune cytopenias.8,9 A study comparing 100- and 1,000-mg rituximab treatment suggests earlier repopulation of B cells (99 vs 184 days) and relapse in 2 patients with NMO.4 However, these 2 patients were considered rituximab “nonresponders,” which may not be associated with 100-mg rituximab treatment.4
One recent study documented the effectiveness of the treatment, in which Korean patients with NMO received an identical dosage to that of Caucasian patients with NMO.6 Our study also suggests that Asian NMO responds to rituximab. Furthermore, responsiveness can be achieved with a lower dosage of rituximab. Despite the patient tolerability in the current and standard dosages, data on the long-term safety considerations of rituximab in autoimmune diseases are to be established, and there is concern about the risk of opportunistic infections after rituximab therapy, including, but not limited to, progressive multifocal leukoencephalopathy.10 Because of the small sample size and lack of controls, we cannot provide a definite evaluation of the benefit of using a low dosage of rituximab in achieving B-cell depletion to stop the progression of NMO. Nevertheless, the study suggests that low-dosage rituximab treatment is a promising therapy for patients with NMO, given a cost and accessibility advantage. The average cost of rituximab in this study was only about 20% of the usual regimen (375 mg/m2/wk for 4 weeks). It may also promote the need for an organized controlled clinical trial using rituximab at different dosages in a larger population of patients with NMO in China and other Asian countries where NMO is more prevalent.
ACKNOWLEDGMENT
The authors thank the patients for participating in this study and Miss A. Kousari for substantive editing.
GLOSSARY
- EDSS
Expanded Disability Status Scale
- MS
multiple sclerosis
- NMO
neuromyelitis optica
AUTHOR CONTRIBUTIONS
C.-S.Y., L.Y., and F.-D.S. recruited the patients, executed the treatment regimen, assessed the patients, and analyzed data. D.-Q.Z. and T.L. assessed patients during the therapy. W.-N.J., M.-S.L., and Q.L. processed the blood samples and performed flow cytometry and analyzed laboratory results. C.-S.Y., L.Y., C.Y., Q.L., and N.S. participated in manuscript preparation. Z.-H.S. advised on rituximab dosage and shared the flow cytometry facility. N.Z. and C.Y. acquired and analyzed neuroimaging data. F.-D.S. formulated the study concept, supervised the execution of the study, provided funding, and wrote the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
C.-S. Yang reports no disclosures. L. Yang has received research support from the National Science Foundation of China (81171183). T. Li, D.-Q. Zhang, W.-N. Jin, M.-S. Li, N. Su, N. Zhangning, Q. Liu, Z.-H. Shao, and C. Yu report no disclosures. F.-D. Shi has received research support from the National Basic Science Program of China (81230028), National Science Foundation of China (2013CB966900), US Muscular Dystrophy Association (159281), and US NIH (RO1AI083294). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012;11:535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedi GS, Brown AD, Delgado SR, Usmani N, Lam BL, Sheremata WA. Impact of rituximab on relapse rate and disability in neuromyelitis optica. Mult Scler 2011;17:1225–1230 [DOI] [PubMed] [Google Scholar]
- 3.Cree BA, Lamb S, Morgan K, Chen A, Waubant E, Genain C. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 2005;64:1270–1272 [DOI] [PubMed] [Google Scholar]
- 4.Greenberg BM, Graves D, Remington G, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler 2012;18:1022–1026 [DOI] [PubMed] [Google Scholar]
- 5.Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol 2008;65:1443–1448 [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011;68:1412–1420 [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM. Diagnosis and treatment of neuromyelitis optica. Neurologist 2007;13:2–11 [DOI] [PubMed] [Google Scholar]
- 8.Blum S, Gillis D, Brown H, et al. Use and monitoring of low dose rituximab in myasthenia gravis. J Neurol Neurosurg Psychiatry 2011;82:659–663 [DOI] [PubMed] [Google Scholar]
- 9.Provan D, Butler T, Evangelista ML, Amadori S, Newland AC, Stasi R. Activity and safety profile of low-dose rituximab for the treatment of autoimmune cytopenias in adults. Haematologica 2007;92:1695–1698 [DOI] [PubMed] [Google Scholar]
- 10.Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 2010;9:425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]