Abstract
Objective:
To evaluate the association between cardiovascular disease (CVD) and spinal cord injury (SCI) in a large representative sample.
Methods:
Data were compiled from more than 60,000 individuals from the 2010 cycle of the cross-sectional Canadian Community Health Survey (CCHS). Multivariable logistic regression analysis was conducted to examine this relationship, adjusting for confounders and using probability weighting to account for the CCHS sampling method.
Results:
After adjusting for age and sex, SCI was associated with a significant increased odds of heart disease (adjusted odds ratio [OR] = 2.72, 95% confidence interval [CI] 1.94–3.82) and stroke (adjusted OR = 3.72, 95% CI 2.22–6.23).
Conclusions:
These remarkably heightened odds highlight the exigent need for targeted interventions and prevention strategies addressing modifiable risk factors for CVD in individuals with SCI.
Over the last decade, there have been marked changes in the trends of morbidity and mortality among individuals with spinal cord injury (SCI). With advances in acute care and in the management of septicemia, renal failure, and pneumonia, cardiovascular complications are now a leading cause of death in those with SCI.1 Moreover, several risk factors for cardiovascular disease (CVD) are amplified in individuals with SCI compared with able-bodied individuals, including physical inactivity, dyslipidemia, blood pressure irregularities, chronic inflammation, and abnormal glycemic control.2–22
While most of the literature with respect to CVD and SCI has shown a higher prevalence of risk factors for CVD,2–22 relatively few studies have examined the prevalence of CVD itself and corresponding risk estimates.23–26 None of these studies has provided direct comparisons of risk estimates for multiple CVD outcomes in the SCI population compared to a non-SCI population, with appropriate adjustment for confounding, in a large representative sample.
It thus remains unknown whether there is excess risk of both heart disease and stroke (after adjustment for potential confounders) in individuals with SCI. The current study addresses this knowledge gap by utilizing the national Canadian Community Health Survey (CCHS), which is comprised of comprehensive, up-to-date, cross-sectional data. Our aim was to estimate the prevalence of heart disease and stroke outcomes in the SCI population, to compare their risk with a non-SCI population, and to investigate this relationship after controlling for confounders.
METHODS
Data source.
This study utilized data from the CCHS 2010 Annual Component. The CCHS is a comprehensive national cross-sectional survey conducted by Statistics Canada. It provides data obtained by trained interviewers on individuals aged 12 and over residing in households in all the provinces and territories. Those living on reserves or Crown lands, full-time members of the Canadian armed forces, and those living in institutions (prisons, hospitals, universities) are excluded from the survey. The CCHS includes data on a range of topics, including access to health care services, health care utilization, lifestyle behaviors, sociodemographic information, and health status. Statistics Canada utilizes a multistage, stratified cluster sampling design, more details of which are provided elsewhere.27
Standard protocol approvals, registrations, and patient consents.
Ethical approval for the use of the data was obtained via the publicly available data clause from the University of British Columbia, in accordance with the Tri-Council Policy Statement.
Exposure and outcomes.
The primary outcome variables in this analysis were self-reported heart disease and stroke. Stroke status was obtained with the following question: “Do you suffer from the effects of a stroke?” Heart disease status was obtained with the following question: “Do you have heart disease?” An individual could respond “Yes” to both questions asking about heart disease and stroke. The primary explanatory variable in this analysis was self-reported SCI. SCI status was obtained with the following question: “Do you have a neurological condition caused by a spinal cord injury?” During the survey, individuals were given the following reminder: “Remember, we're interested in conditions diagnosed by a health professional.” Only those with valid responses for the primary explanatory variable and outcome variables were included in the analysis. Nonrespondents (those in the categories of “don't know,” “refusal,” and “not stated”) were excluded.
Confounding: Definitions and variable selection.
Confounding was assessed both from a theoretical (i.e., causal) perspective based on previous studies as well as a statistical perspective (i.e., in examining changes in effect sizes in the presence/absence of possible confounders). We used the commonly accepted definition of confounding: a factor “x” is a confounder if factor x is a known risk factor for the outcome/disease (in this case, heart disease or stroke), and factor x is associated with the primary explanatory variable (in this case, SCI), but is not a result of the primary explanatory variable.28 Thus, factors that are associated only with CVD but not SCI, factors that are associated only with SCI but not CVD, or factors that are the result of SCI that might lead to CVD did not meet the criteria for confounding, and are not adjusted for in the final analysis.
Risk factors for CVD have been well-documented in the Framingham study, which identified the following risk factors and used these to calculate a 10-year absolute risk of sex-specific general CVD: age, diabetes, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status.29 The recent Canadian global INTERHEART study identified similar risk factors for myocardial infarction, including dyslipidemia, smoking status, diabetes, hypertension, abdominal obesity, stress, lack of daily consumption of fruits and vegetables, and lack of daily exercise, in more than 37,000 individuals from 55 different countries and cultural environments.30,31 Risk factors for traumatic SCI include age and sex.32 Risk factors for nontraumatic SCI are more difficult to examine, as nontraumatic SCI includes tumor-related, congenital/developmental (e.g., spina bifida), infectious (viral, bacterial, fungal, parasitic), inflammatory (e.g., multiple sclerosis), and ischemic causes, as well as several others.33 However, sex and age are well-known risk factors for the majority of nontraumatic SCIs.32 Sex and age were thus selected a priori as possible confounders.23–25 Sensitivity analyses were however performed to examine the effect of additional self-report covariates (daily energy expenditure, obesity [using body mass index], hypertension, smoking status, daily alcohol consumption, daily consumption of fruits and vegetables, and diabetes) on the reported effect sizes.
Statistical analyses.
Logistic regression models were obtained separately for the binary outcome stroke and the binary outcome heart disease with SCI (binary) as the main explanatory variable. Both bivariable and multivariable logistic regression models were developed. Multivariable logistic models also included age (treated as a continuous variable) and sex. Using the results from the logistic models, both unadjusted and adjusted odds ratios (ORs), with corresponding 95% confidence intervals (CIs), are presented. The CCHS sampling design (clustering and stratification) was accounted for in the analyses using probability weighting. Probability weights were obtained by dividing the frequency weights provided by Statistics Canada (these correspond to the number of persons represented by the individual) by the average frequency weight for the given sample. Reported percentages and ORs are weighted. SAS statistical software (SAS Institute, Cary, NC, version 9.3) was used for all analyses.
RESULTS
Study sample.
After excluding those with invalid responses for the primary explanatory and outcome variables, the final study sample included 60,959 individuals for the SCI/heart disease analysis and 61,031 individuals for the SCI/stroke analysis (table 1). There was a similar proportion of male and female subjects (table 1) in both analytic samples. The median age category for both analytic samples is also shown in table 1.
Table 1.
Characteristics of the 2 analytic samples: Sample sizes and percentagesa
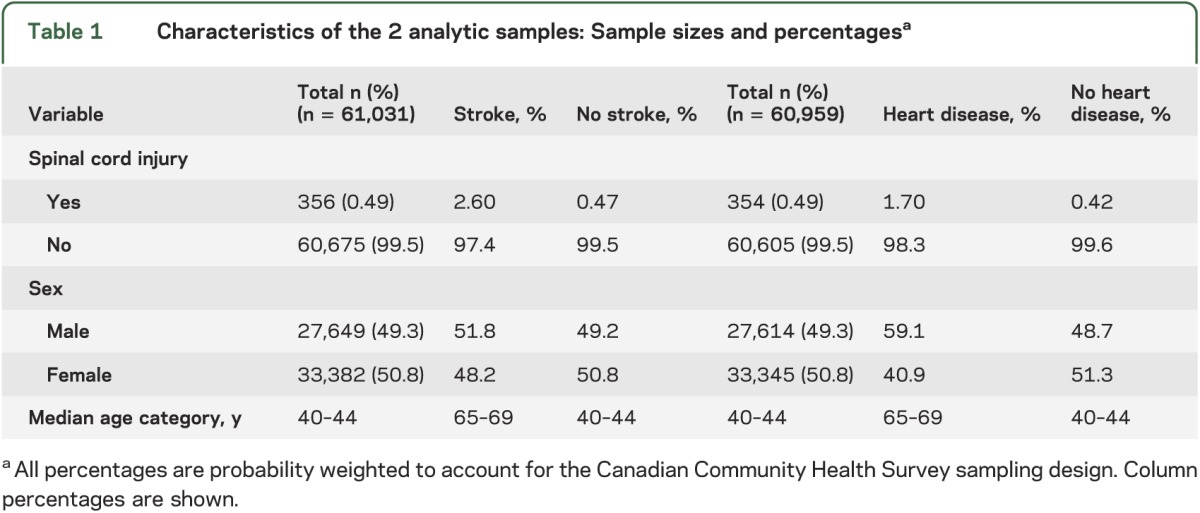
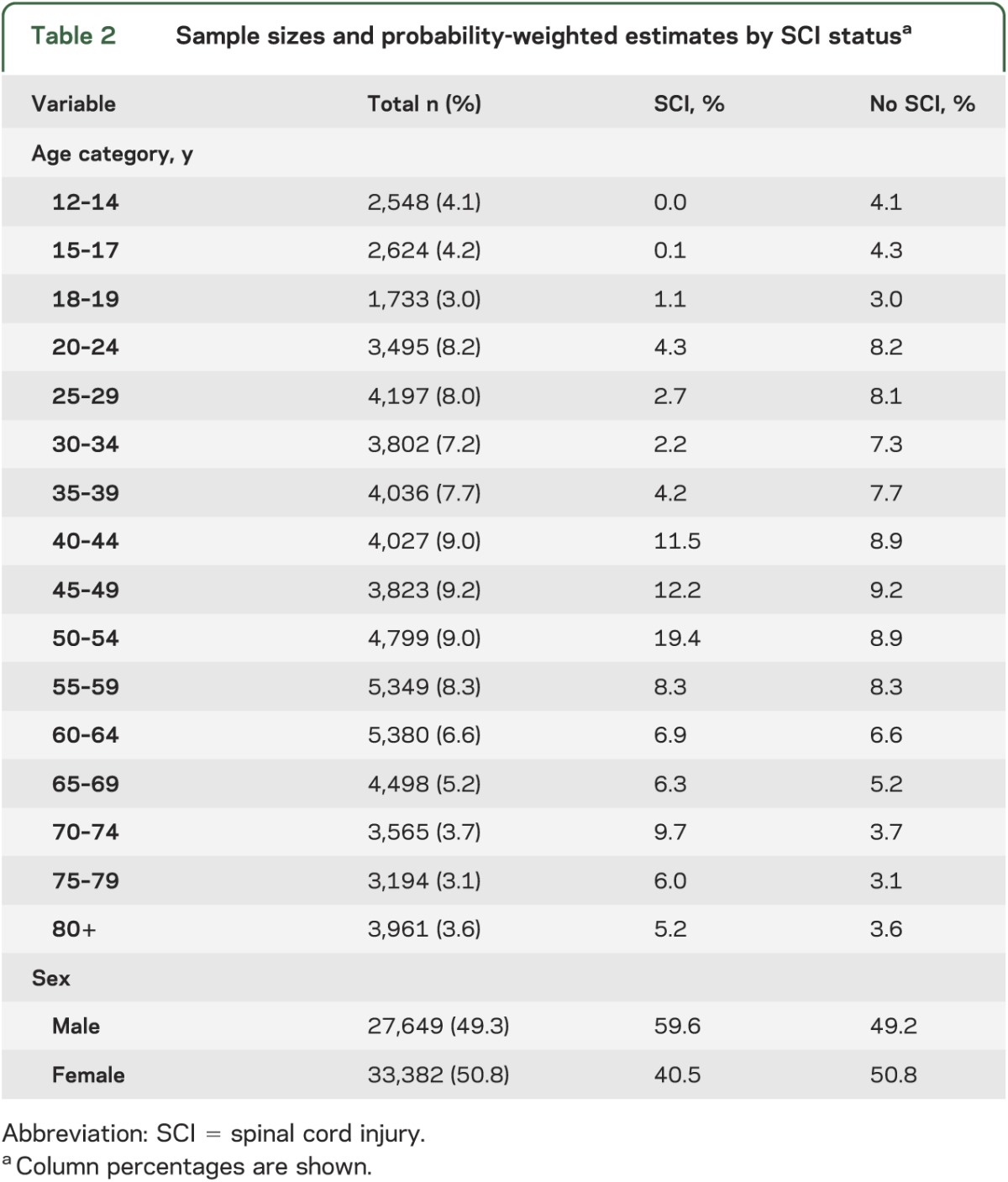
There were a total of 354 unique individuals with SCI who had a valid response to the heart disease question and 356 unique individuals with SCI who had a valid response to the stroke question (table 1). This yielded a prevalence of 0.49% for SCI in both analytic samples (table 1). Among the individuals with SCI, there was a higher proportion of males vs females (table 2). The age distribution for those with SCI is also shown in table 2, with the highest prevalence among the 50–54 years age group.
Table 2.
Sample sizes and probability-weighted estimates by SCI statusa
Among the entire sample, the prevalence of stroke and heart disease was 1.1% and 5.0%, respectively. The prevalence of individuals with both stroke and heart disease was 0.38%. The proportion of male subjects with stroke was higher than that of female subjects with stroke (table 1). Similarly, the proportion of male subjects with heart disease was higher than that of female subjects with heart disease (table 1). The median age category for individuals with stroke and heart disease was higher than for those without (table 1). The proportion of individuals with stroke and heart disease steadily increased with age, with 75% of strokes and 72% of heart disease cases accounted for by individuals greater than 60 years of age.
SCI and CVD.
Among individuals with SCI, the prevalence of stroke was 5.7% compared to 1.1% in individuals without SCI. Similarly, among those with SCI, the prevalence of heart disease was 17.1% compared to 4.9% in individuals without SCI. Thus, the prevalence of stroke and heart disease was higher among individuals with SCI compared to those without.
ORs: SCI and heart disease.
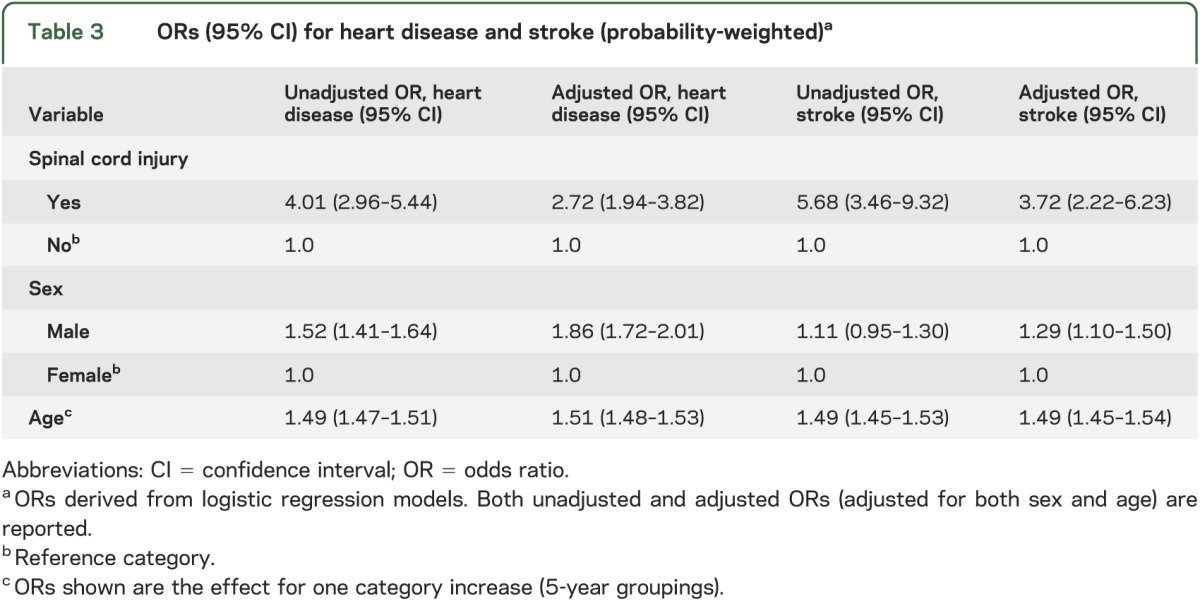
Table 3 provides unadjusted and adjusted ORs for heart disease. The odds of heart disease was 4.01 times greater in individuals with SCI vs individuals without SCI (95% CI 2.96–5.44). After adjusting for sex and age, the heightened odds persisted but was reduced; the fully adjusted OR for heart disease was 2.72 (95% CI 1.94–3.82), suggesting confounding by sex and age. In examining the adjusted ORs from the model adjusted for sex only and the model adjusted for age only, it appears that both age and sex were important confounders (results not shown).
Table 3.
ORs (95% CI) for heart disease and stroke (probability-weighted)a
ORs: SCI and stroke.
Table 3 provides unadjusted and adjusted ORs for stroke. The odds of stroke was 5.68 times greater in individuals with SCI vs individuals without SCI (95% CI 3.46–9.32). After adjusting for sex and age, the OR for stroke was 3.72 (95% CI 2.22–6.23), suggesting confounding by sex and age. In comparing the adjusted ORs from the model adjusted for sex only vs the model adjusted for age only, it appears that age was a more important confounder (results not shown).
Nonrespondents.
For the SCI/heart disease analysis, 1,950 individuals were excluded based on nonresponse; for the SCI/stroke analysis, 1,878 individuals were excluded based on nonresponse. Since sex and age were collected on all participants regardless of their response to the SCI/CVD questions, both sex and age of the nonresponders were examined. The sex distribution of nonresponders (52.0% male; 48.0% female) showed a slightly higher proportion of male subjects than in the responders. The median age category for nonresponders was the same for responders (40–44 years).
Sensitivity analyses.
Variables such as smoking, obesity, hypertension, physical inactivity, lack of consumption of fruits and vegetables, alcohol consumption, and diabetes status were examined in the multivariable models. Although it is debatable whether these variables meet the criteria for confounding, due to the nature of these composite variables, we wanted to examine the robustness of our results to their inclusion in the models. The inclusion of each of these variables did not significantly change the estimated ORs adjusted for sex and age only (for either stroke or heart disease). With stepwise inclusion of these additional variables into the regression models, the adjusted ORs for heart disease ranged from 2.63 to 3.10, all remaining statistically significant. Likewise, the ORs for stroke ranged from 3.35 to 3.82, all remaining statistically significant. Thus, these covariates were likely not confounding factors.
DISCUSSION
The present study utilized a comprehensive national survey with data collected from more than 60,000 individuals to investigate the relationship between CVD and SCI. Here, we demonstrate for the first time in a large representative population that SCI is independently associated with a significant increased odds of both heart disease and stroke—a more than 2- and 3-fold increase, respectively. To put these values into context, the heightened ORs reported here are similar in magnitude to the estimated ORs in the general population for the relationship between smoking and myocardial infarction (MI) and diabetes and MI, and are in fact higher than those for the relationship between hypertension and MI and abdominal obesity and MI.30
That individuals with SCI are at an increased risk of CVD is in line with previous evidence. Namely, risk factors for CVD are amplified in individuals with SCI, relative to able-bodied individuals.2–22 In addition to the immobility caused by SCI, individuals with SCI have unique disadvantages that may further contribute to these risks relating to the disconnection between autonomic circuits and supraspinal control. As an example, significant lability in blood pressure, from extreme hypotension during episodes of orthostatic hypotension to extreme hypertension during episodes of autonomic dysreflexia, are typical post SCI and unique features of SCI.8,9 Researchers have speculated that this blood pressure instability could result in vascular injury, and consequently results in a greater risk for arterial disease in individuals with SCI.34
In addition to these studies examining risk factors, others have reported a hazard ratio of 2.85 for stroke in individuals with SCI (using age-, sex-, and propensity score– matched controls) from a longitudinal sample of more than 20,000 Taiwanese individuals, but this study did not examine other cardiovascular outcomes such as heart disease.25 Similarly, a longitudinal study among diabetic American veterans with SCI reported an increased risk of “macrovascular complications” (including stroke and heart disease) compared to nondiabetic veterans with SCI, but this study did not include separate risk estimates for stroke and heart disease, and did not include a control group.23 In addition, another group demonstrated an increased risk of ischemic heart disease in a small convenience sample of male subjects injured before 1974.24 Taken together, these biological and epidemiologic results support the hypothesis that SCI is associated with an increased risk of CVD.
There are possible limitations to the current study. First, the data are derived from a cross-sectional study design; therefore, it is impossible to determine whether SCI preceded the stroke or heart disease. However, we did perform a sensitivity analysis, restricting the study sample to a group of younger individuals, to reduce the likelihood that stroke or heart disease preceded SCI. The increased odds of both stroke and heart disease persisted, but had very wide CIs due to the reduced power (results not shown).
Another limitation of this study is the lack of detailed neurologic and cardiovascular examination records. The CCHS provides no information on neurologic level, completeness of injury, etiology of SCI, or class of heart disease and stroke. The variable “heart disease” may, for example, include nonatherosclerotic heart disease (such as rheumatic or congenital heart disease). However, the prevalence of these conditions compared to atherosclerotic-related heart disease is relatively small. In addition, although the data are from self-report, self-reported heart disease and stroke have been validated against clinical records in other studies, demonstrating a relatively high accuracy of self-report, generally with higher specificity than sensitivity.35,36 Any misclassification of these outcomes would likely be nondifferential by SCI status, which tends to bias effect sizes toward the null. With respect to SCI status, the prevalence rate reported here was surprisingly high. However, if misclassification of SCI status occurred, it would likely occur for less severe “injuries.” Given that completeness and severity (neurologic level) are correlated with cardiovascular risk,37 the inclusion of these individuals with less severe injuries would likely dilute the reported effect size, and would also likely be nondifferential by outcome status.
These limitations notwithstanding, the drastic increased odds of CVD among individuals with SCI is an impetus for future investigations. Although there is physiologic plausibility for a causal relationship between SCI and CVD, future research is needed to better understand this and whether interventions can modify CVD risk. Additional cohort and case-control studies, or links with clinical records, with the use of SCI-specific registries, such as the Rick Hansen Spinal Cord Injury Registry,38 are needed to build on this evidence. Results from this study may inform the data collected prospectively in such registries (e.g., the type and severity of stroke/heart disease, age at onset, etiology of SCI, neurologic level, and completeness). Finally, since heart disease is also a risk factor for stroke, this further highlights the need for epidemiologic studies to examine temporality, causality, and possible interactions between heart disease and stroke in the context of SCI.
Moreover, current clinical practice guidelines for the management of CVD following SCI are by and large based on short-term “efficacy” outcomes; thus these results may better inform clinical practice guidelines for individuals with SCI.39 In addition, findings from this research will be important from a health care planning perspective since there is a change in the epidemiology of SCI—an increase in the average age for traumatic SCI and a rise in nontraumatic SCI.32 In sum, research of this kind will ultimately lead to interventions and targeted prevention strategies addressing modifiable risk factors for CVD in individuals with SCI.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Mieke Koehoorn (School of Population and Public Health, University of British Columbia) for review of the manuscript.
GLOSSARY
- CCHS
Canadian Community Health Survey
- CI
confidence interval
- CVD
cardiovascular disease
- MI
myocardial infarction
- OR
odds ratio
- SCI
spinal cord injury
Footnotes
Editorial, page 700
AUTHOR CONTRIBUTIONS
J.J. Cragg was responsible for the study concept/design, analysis/interpretation of the data, and drafting the manuscript. Dr. Noonan was responsible for the study concept/design, interpretation of the data, and revising the manuscript for intellectual content. Dr. Krassioukov was responsible for the study concept/design, interpretation of the data, and revising the manuscript for intellectual content. Dr. Borisoff was responsible for the study concept/design, interpretation of the data, and revising the manuscript for intellectual content.
STUDY FUNDING
J.J. Cragg is supported by a University of British Columbia Killam Doctoral Scholarship. Dr. Noonan is an employee of the Rick Hansen Institute. Dr. Krassioukov is supported by grants from the Canadian Heart and Stroke Foundation, Canadian Institute of Health Research, Canadian Foundation for Innovation, Christopher and Dana Reeve Foundation, and Craig Neilsen Foundation. Dr. Borisoff is the Canada Research Chair in Rehabilitation Engineering Design.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Garshick E, Kelley A, Cohen SA, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord 2005;43:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollis B, Lafavor J, Mauder V, Mokshagundam S, Olive J. Inflammation and insulin sensitivity in spinal cord injured subjects. Med Sci Sports Exerc 2009;41:73 [Google Scholar]
- 3.Gibson AE, Buchholz AC, Martin Ginis KA; SHAPE-SCI Research Group C-reactive protein in adults with chronic spinal cord injury: increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord 2008;46:616–621 [DOI] [PubMed] [Google Scholar]
- 4.Liang H, Mojtahedi MC, Chen D, Braunschweig CL. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil 2008;89:36–41 [DOI] [PubMed] [Google Scholar]
- 5.Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc 2007;106:919–928 [DOI] [PubMed] [Google Scholar]
- 6.Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil 2007;88:1384–1393 [DOI] [PubMed] [Google Scholar]
- 7.Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86:312–317 [DOI] [PubMed] [Google Scholar]
- 8.Krassioukov A, Eng JJ, Warburton DE, Teasell R; Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of the management of orthostatic hypotension after spinal cord injury. Arch Phys Med Rehabil 2009;90:876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krassioukov A, Warburton DE, Teasell R, Eng JJ; Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 2009;90:682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahoney ET, Bickel CS, Elder C, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil 2005;86:1502–1504 [DOI] [PubMed] [Google Scholar]
- 11.Lee MY, Myers J, Hayes A, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med 2005;28:20–25 [DOI] [PubMed] [Google Scholar]
- 12.Karlsson AK, Attvall S, Jansson PA, Sullivan L, Lonnroth P. Influence of the sympathetic nervous system on insulin sensitivity and adipose tissue metabolism: a study in spinal cord-injured subjects. Metabolism 1995;44:52–58 [DOI] [PubMed] [Google Scholar]
- 13.Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism 1994;43:749–756 [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46:466–476 [DOI] [PubMed] [Google Scholar]
- 15.Lieberman JA, Hammond FM, Barringer TA, et al. Comparison of coronary artery calcification scores and national cholesterol education program guidelines for coronary heart disease risk assessment and treatment paradigms in individuals with chronic traumatic spinal cord injury. J Spinal Cord Med 2011;34:233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldenberg D, Rubinstein A, Levtov O, Werbin B, Tamir I. Serum lipids and lipoprotein concentrations in young quadriplegic patients. Atherosclerosis 1981;39:163–167 [DOI] [PubMed] [Google Scholar]
- 17.Brenes G, Dearwater S, Shapera R, LaPorte RE, Collins E. High density lipoprotein cholesterol concentrations in physically active and sedentary spinal cord injured patients. Arch Phys Med Rehabil 1986;67:445–450 [PubMed] [Google Scholar]
- 18.Bauman WA, Spungen AM, Zhong YG, Rothstein JL, Petry C, Gordon SK. Depressed serum high density lipoprotein cholesterol levels in veterans with spinal cord injury. Paraplegia 1992;30:697–703 [DOI] [PubMed] [Google Scholar]
- 19.Krum H, Howes LG, Brown DJ, et al. Risk factors for cardiovascular disease in chronic spinal cord injury patients. Paraplegia 1992;30:381–388 [DOI] [PubMed] [Google Scholar]
- 20.Zlotolow SP, Levy E, Bauman WA. The serum lipoprotein profile in veterans with paraplegia: the relationship to nutritional factors and body mass index. J Am Paraplegia Soc 1992;15:158–162 [DOI] [PubMed] [Google Scholar]
- 21.Schmid A, Halle M, Stutzle C, et al. Lipoproteins and free plasma catecholamines in spinal cord injured men with different injury levels. Clin Physiol 2000;20:304–310 [DOI] [PubMed] [Google Scholar]
- 22.Ozgurtas T, Alaca R, Gulec M, Kutluay T. Do spinal cord injuries adversely affect serum lipoprotein profiles? Mil Med 2003;168:545–547 [PubMed] [Google Scholar]
- 23.Banerjea R, Sambamoorthi U, Weaver F, Maney M, Pogach LM, Findley T. Risk of stroke, heart attack, and diabetes complications among veterans with spinal cord injury. Arch Phys Med Rehabil 2008;89:1448–1453 [DOI] [PubMed] [Google Scholar]
- 24.Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R. The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia 1989;27:58–62 [DOI] [PubMed] [Google Scholar]
- 25.Wu JC, Chen YC, Liu L, et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology 2012;78:1051–1057 [DOI] [PubMed] [Google Scholar]
- 26.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001;39:310–317 [DOI] [PubMed] [Google Scholar]
- 27.Beland Y. Canadian community health survey: methodological overview. Health Rep 2002;13:9–14 [PubMed] [Google Scholar]
- 28.Gordis L. Epidemiology. Philadelphia: Saunders; 2009 [Google Scholar]
- 29.D'Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753 [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–952 [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya OK, Estey EA, Cheng AY; Canadian Diabetes Association 2008 Update on the Canadian Diabetes Association 2008 clinical practice guidelines. Can Fam Physician 2009;55:39–43 [PMC free article] [PubMed] [Google Scholar]
- 32.Noonan VK, Fingas M, Farry A, et al. Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology 2012;38:219–226 [DOI] [PubMed] [Google Scholar]
- 33.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet 2002;359:417–425 [DOI] [PubMed] [Google Scholar]
- 34.Steins SA, Johnson MC, Lyman PJ. Cardiac rehabilitation in patients with spinal cord injuries. Phys Med Rehabil Clin N Am 1995;6:236–296 [Google Scholar]
- 35.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol 1998;147:969–977 [DOI] [PubMed] [Google Scholar]
- 36.Engstad T, Bonaa KH, Viitanen M. Validity of self-reported stroke: the Tromsø Study. Stroke 2000;31:1602–1607 [DOI] [PubMed] [Google Scholar]
- 37.Bauman WA, Adkins RH, Spungen AM, Waters RL. The effect of residual neurological deficit on oral glucose tolerance in persons with chronic spinal cord injury. Spinal Cord 1999;37:765–771 [DOI] [PubMed] [Google Scholar]
- 38.Noonan VK, Kwon BK, Soril L, et al. The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord 2012;50:22–27 [DOI] [PubMed] [Google Scholar]
- 39.Cragg JJ, Stone JA, Krassioukov AV. Management of cardiovascular disease risk factors in individuals with chronic spinal cord injury: an evidence-based review. J Neurotrauma 2012;29:1999–2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


