Abstract
Otocephaly-dysgnathia complex is characterized by mandibular hypo- or aplasia, ear abnormalities, microstomia, and microglossia. Mutations in the orthodenticle homeobox 2 (OTX2) and paired related homeobox 1 (PRRX1) genes have recently been identified in some cases. We screened 4 otocephalic cases for these 2 genes and identified OTX2 mutations in 2 of them, thus confirming OTX2 is implicated in otocephaly. No PRRX1 mutation was identified. Interestingly, ocular involvement is not a constant feature in otocephalic cases with an OTX2 mutation. In one case, the mutation was inherited from a microphthalmic mother. The mechanism underlying this intrafamilial phenotypic variability remains unclear, but other genetic factors are likely to be necessary for the manifestation of the otocephalic phenotype.
Key Words: Agnathia, Microphthalmia, Otocephaly, OTX2, PRRX1
Otocephaly-dysgnathia complex (ODC, OMIM 202650) is a rare malformation characterized by the association of agnathia or mandibular hypoplasia, anteromedial malposition of the ears (melotia) which can be merged into one median ear (synotia), microstomia, and aglossia or lingual hypoplasia [Gekas et al., 2010]. Association with holoprosencephaly, microphthalmia or anophthalmia, pituitary hypoplasia, and malformation of the extremities have been reported [Gekas et al., 2010]. The incidence of ODC is estimated to be 1 per 70,000 births. Paired related homeobox 1 (PRRX1) was the first gene to be associated with ODC, and to date, PRRX1 mutations have been identified in 4 cases [Sergi and Kamnasaran, 2011; Celik et al., 2012; Donnelly et al., 2012; Dasouki et al., 2013]. Mutations of the orthodenticle homeobox 2 (OTX2) gene were more recently implicated in ODC in 2 families [Chassaing et al., 2012]. Apart from a personal or family history of ocular involvement described in some ODC patients with OTX2 mutations, the phenotypes appear similar between ODC patients mutated either in OTX2 or PRRX1. The molecular basis of this severe developmental disorder remains unclear in the majority of cases [Celik et al., 2012; Chassaing et al., 2012]. Here, we report 4 cases with ODC, of which one was described previously [Kauvar et al., 2010]. We searched for mutations in the OTX2 and PRRX1 genes and identified OTX2 mutations in 2 cases. The clinical and molecular results of our 4 cases are described and compared to other reports.
Patients and Methods
Cases
Case 1
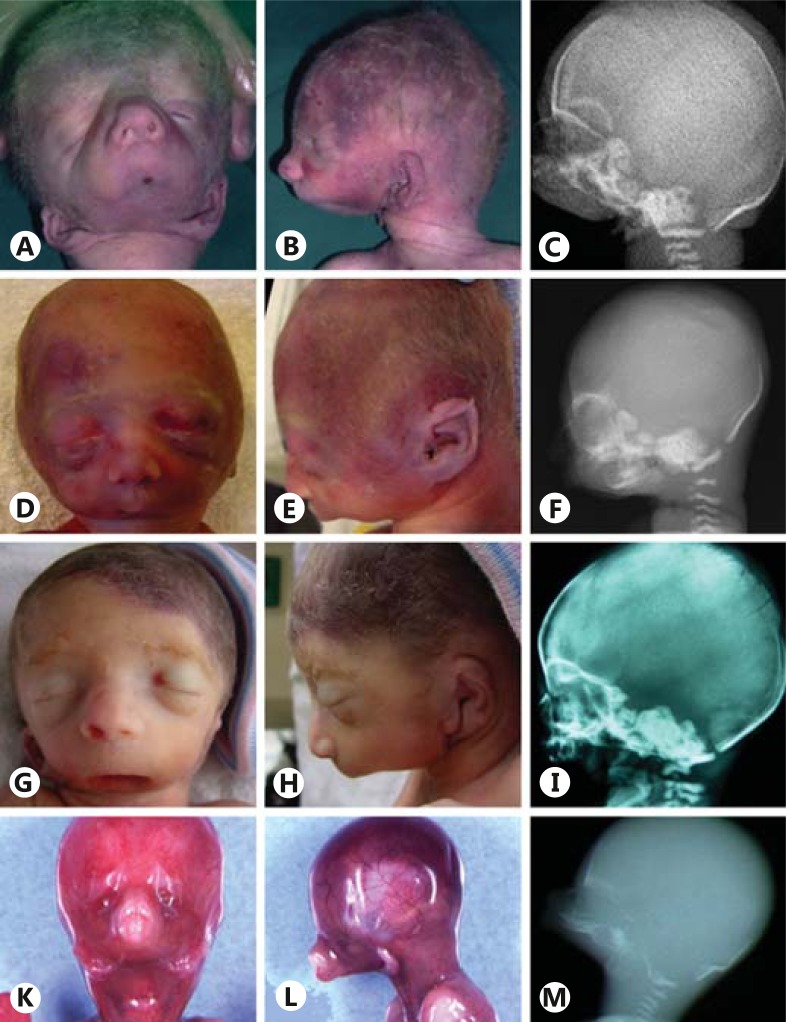
This boy was the second child of healthy, nonconsanguineous parents (father 39, mother 36 years old). The pregnancy was uneventful until 28 weeks of gestation, when an amniocentesis was performed because of an unexplained polyhydramnios. The karyotype was normal: 46,XY. The boy was born at 30 weeks of gestation. At birth, the absence of the mandible resulted in an airway obstruction causing an immediate respiratory distress. Intubation was impossible because of a severe microstomia. He died within the first hour of life. The boy weighed 1,505 g (40th percentile) with a length of 41.5 cm (50th percentile) and head circumference of 29.5 cm (60th percentile). He had a severe malformation of the face, including agenesis of the mandible, microstomia and persistent buccopharyngeal membrane (fig. 1A-C). The ears were dysmorphic and anterocaudally positioned. On external inspection, the eyes were normal, and besides mild club feet, no other malformations were noted. No aberrations of the central nervous system were found on macroscopic or microscopic neuropathological examination. Heart, urogenital and intestinal organs were normal. There was no situs abnormality.
Fig. 1.
Photographs and X-rays of otocephalic case 1 (A-C), case 2 (D-F), case 3 (G-I), and case 4 (K-M). Cardinal features of ODC include: severe micrognathia (cases 2 and 3) or agnathia (cases 1 and 4), microstomia (present in all 4 cases) and low set ears (present in all 4 cases) with sometimes anteromedial position (melotia, cases 1 and 4). All 4 patients have downslanting palpebral fissures.
Case 2
This female has been reported previously [Kauvar et al., 2010]. She weighed 525 g (10th percentile) with a length of 31 cm (10-50th percentile) and a head circumference of 19.5 cm (3rd-10th percentile) at almost 24 weeks of gestation after an, until then, uncomplicated pregnancy (mother was 17 years old). She died during delivery and had extreme micrognathia with microstomia but a normal position of the ears with overfolded helices, downslanting palpebral fissures and camptodactyly of the fourth and fifth digits. Postmortem X-rays showed extreme micrognathia (fig. 1D-F) and 13 ribs bilaterally. Head and brain MRI showed absence of the mandible, probable choanal atresia and signs of semilobar holoprosencephaly (no central sulcus, dorsally fused ventricles, absent falx cerebri). A full-body MRI showed small kidneys and a hypoplastic spleen, but no situs abnormality. No abnormalities of the eyes were noted. The karyotype was normal: 46,XX. The parents were examined physically for signs of the holoprosencephaly spectrum, but showed no hypotelorism, midface hypoplasia, single central incisor, anosmia, or micrognathia.
Case 3
She was the first child of healthy, nonconsanguineous parents. The family history was unremarkable. A routine ultrasound at a gestational age of 25 weeks revealed a polyhydramnios. An amniocentesis was performed showing a normal 46,XX karyotype. An additional ultrasound showed severe microretrognathia and ear abnormalities. She was born at 26 weeks and 2 days of gestation and died within 90 min of birth due to severe respiratory failure. She weighed 800 g (50th percentile) and had a length of 31.5 cm (20th percentile) and a head circumference of 24.5 cm (60th percentile). Severe micrognathia due to a hypoplastic mandibulum, low set dysmorphic ears, a skin tag at the left ear, and microstomia were observed (fig. 1G-I). Eyes, thorax, abdomen, genitalia, and extremities were normal. Internal organs were normal apart from a relatively high weight of heart and adrenals. No situs inversus was noticed. An X-ray revealed fusion of some ribs and a hemivertebra. Neuropathology revealed a macroscopically and microscopically normal cerebrum and cerebellum.
Case 4
The pregnancy of this male fetus was terminated at 16 weeks gestation because of agnathia associated with bilateral microphthalmia. The mother had isolated severe unilateral microphthalmia and was known to have a nonsense mutation (p.Arg97*) in the OTX2 gene. She had already undergone a pregnancy interruption at 22 weeks gestation for unilateral microphthalmia without signs of mandibular malformation. Her father and her paternal grandmother were also affected by isolated unilateral microphthalmia. The male fetus presented with agnathia, astomia and aglossia, and had low, posteriorly rotated, paramedian and convergent ears. The pharyngeal floor was absent. He had bilateral microphthalmia with downslanted palpebral fissures (fig. 1 K-M). The optic chiasma and pituitary gland could not be found. The abdominal and thoracic organs were normal, and there was no situs abnormality. X-rays confirmed the absence of the mandibular bone. The petrosal bone appeared smooth, and the semicircular canals were not well-shaped, but the cochlea was normal. Abnormalities of the extremities including brachymesophalangy of the fifth finger and bilateral talus valgus were present.
Molecular Screening
Informed consent for molecular analysis was obtained for each case. Genetic investigations included array-CGH (Agilent 105K) and direct sequencing of exons and flanking regions of the PRRX1 and OTX2 genes using previously published primer pairs [Chassaing et al., 2012]. Sequence variations were numbered considering the adenine of the ATG initiation codon as the first nucleotide (OTX2 GenBank accession NM_021728.2).
Results
Array-CGH Analysis
Case 1 had an approximately 400-kb deletion in 14q23.1 including the whole OTX2 gene. The proximal breakpoint was located between 56,184,175 and 56,268,038 and the distal breakpoint between 56,661,990 and 56,699,192 (oligo positions given in NCBI build 36.1/hg18 assembly). No other genes were included in the deletion. Parental analysis showed that this deletion had appeared de novo. Array-CGH revealed no nonpolymorphic rearrangements for cases 2, 3 and 4.
Direct Sequencing of the PRRX1 Gene
No mutations were found in the PRRX1 gene in our 4 cases.
Direct Sequencing of the OTX2 Gene
No additional mutations were identified in the OTX2 gene in case 1 and no mutations were detected in cases 2 and 3. Case 4 had inherited the nonsense mutation c.289C>T (p.Arg97*) from his mother, who suffered from unilateral severe microphthalmia. He also carried a heterozygous synonymous variation c.525C>G (p.(=)) inherited from his asymptomatic father.
Discussion
The genetic basis of otocephaly is still largely unclear. To date, mutations in only 2 genes, OTX2 and PRRX1, have been identified in a small number of ODC cases [Celik et al., 2012; Chassaing et al., 2012]. The most likely explanation for this is that only a small proportion of the causative genes have been identified as yet. In our small case cohort, we confirmed the role of OTX2 in ODC and the broad phenotypic variability seen in familial OTX2 mutations.
The first otocephalic gene described in humans was PRRX1, which has been involved in 3 cases reported to date. A candidate gene approach identified a heterozygous missense mutation (p.Phe113Ser) in a 30-week fetus with ODC [Sergi and Kamnasaran, 2011]. The PRRX1 gene also appeared to be responsible for an autosomal recessive form of otocephaly. An infant born to consanguineous parents carried a nonpolymorphic PRRX1 missense mutation (p.Ala231Pro) inherited from both parents [Celik et al., 2012]. Functional studies indicated that both missense mutations decrease the ability of the mutant protein to regulate the tenascin-C gene promoter, and as a consequence, they were considered deleterious. The finding of PRRX1 de novo frameshift mutations (c.267delA, c.266_269dupAAAA) in 2 unrelated families confirmed its role in autosomal dominant otocephaly [Donnelly et al., 2012; Dasouki et al., 2013]. However, mutations in this gene may be involved in fewer than 15% of ODC cases [Celik et al., 2012]; in the 4 cases reported here, no PRRX1 mutations were identified.
The second otocephalic gene identified in humans is OTX2. OTX2 mutations were firstly described in cases affected by microphthalmia or anophtalmia [Ragge et al., 2005], associated with a variable ocular phenotype and incomplete penetrance. More recently, we extended the phenotype associated with an OTX2 mutation to otocephaly [Chassaing et al., 2012]. OTX2 is the vertebrate homologue of the gene orthodenticle (otd) identified in drosophila as one of the major genes controlling the development of the head [Simeone et al., 1993]. In mice, the Otx2 gene is expressed in the mesencephalic neural crest cells which are distributed to the developing mandibular region and rostral brain region [Kimura et al., 1997]. Homozygous Otx2 mutant mice displayed early developmental failure and total absence of the structures corresponding to the anterior (rostral) part of the head [Matsuo et al., 1995]. Heterozygous Otx2 mutants were found to display ODC phenotypes in variable proportions depending on the genetic background of the mice, suggesting a role of a modifier gene [Matsuo et al., 1995]. These mutants also inconstantly displayed anophthalmia and holoprosencephaly.
Here, we report 2 additional OTX2-mutation ODC cases. The phenotype of OTX2-mutated cases seems to be indistinguishable from that of nonmutated ODC cases. Particularly eye involvement, which was the first phenotype associated with OTX2 mutations, is absent in 2 out of the 4 ODC cases so far reported to be due to an OTX2 mutation. We show that such mutations in ODC cases not only occur de novo (case 1 and Chassaing et al. [2012]) but that they can also be inherited from a microphthalmic parent (case 4 and Chassaing et al. [2012]). This has serious implications for the counseling of carriers of OTX2 mutations. The extreme intrafamilial phenotypic variability has already been reported [Chassaing et al., 2012] and argues against any genotype/phenotype correlation. All the OTX2 mutations reported so far in otocephalic cases are assumed to lead to complete loss-of-function of the protein or absence of protein production (whole gene deletion, premature nonsense or frame shift mutations), but mutations with a similar effect have also been described in microphthalmic cases [reviewed in Chassaing et al., 2012]. The hypothesis of modifier genes was proposed to explain this extreme variability in expression, even within families. In vivo modeling experiments in zebrafish demonstrated that otx2 can interact genetically with 3 genes (pgap1, prrx1 and msx1) which when suppressed in concert with otx2 lead to exacerbated craniofacial defects far exceeding the defects that result from otx2 suppression alone. This suggests that the combinatorial effect of additional molecular lesions in the genome may explain the phenotypic variability associated with OTX2 mutations [Chassaing et al., 2012]. However, which modifier genes are implicated in humans is unknown. Case 4, who was unique for his otocephaly in a 4-generation pedigree of autosomal dominant microphthalmia, inherited a synonymous OTX2 variation from his healthy father, in addition to the nonsense OTX2 mutation from his affected mother. Since the father had no abnormalities on ocular examination, it is unlikely that the silent OTX2 variation explains the intrafamilial phenotypic variability between the fetus and his affected relatives.
Finally, the genetic cause remains unidentified in most cases of ODC. This argues for its broad genetic heterogeneity. Candidate gene approaches previously failed to identify any other genes. Further screening of cases without mutations in the OTX2 and PRRX1 genes and using next generation sequencing should help to decipher the molecular basis of this severe developmental defect.
Acknowledgements
The authors would like to thank the families for their participation and the following physicians for their assistance: Tania Attie-Bitach, Ton van Essen and Bert Visser (pathologist).
This work was supported by grants from the Clinical Research Hospital Program from the French Ministry of Health (PHRC 09 109 01) and from Retina France.
References
- 1.Celik T, Simsek PO, Sozen T, Ozyuncu O, Utine GE, et al. PRRX1 is mutated in an otocephalic newborn infant conceived by consanguineous parents. Clin Genet. 2012;81:294–297. doi: 10.1111/j.1399-0004.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- 2.Chassaing N, Sorrentino S, Davis EE, Martin-Coignard D, Iacovelli A, et al. OTX2 mutations contribute to the otocephaly-dysgnathia complex. J Med Genet. 2012;49:373–379. doi: 10.1136/jmedgenet-2012-100892. [DOI] [PubMed] [Google Scholar]
- 3.Dasouki M, Andrews B, Parimi P, Kamnasaran D. Recurrent agnathia-otocephaly caused by DNA replication slippage in PRRX1. Am J Med Genet A. 2013;161:803–808. doi: 10.1002/ajmg.a.35879. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly M, Todd E, Wheeler M, Winn VD, Kamnasaran D. Prenatal diagnosis and identification of heterozygous frameshift mutation in PRRX1 in an infant with agnathia-otocephaly. Prenat Diagn. 2012;32:903–905. doi: 10.1002/pd.3910. [DOI] [PubMed] [Google Scholar]
- 5.Gekas J, Li B, Kamnasaran D. Current perspectives on the etiology of agnathia-otocephaly. Eur J Med Genet. 2010;53:358–366. doi: 10.1016/j.ejmg.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Kauvar EF, Solomon BD, Curry CJ, van Essen AJ, Janssen N, et al. Holoprosencephaly and agnathia spectrum: presentation of two new patients and review of the literature. Am J Med Genet C Semin Med Genet. 2010;154C:158–169. doi: 10.1002/ajmg.c.30235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura C, Takeda N, Suzuki M, Oshimura M, Aizawa S, Matsuo I. Cis-acting elements conserved between mouse and pufferfish Otx2 genes govern the expression in mesencephalic neural crest cells. Development. 1997;124:3929–3941. doi: 10.1242/dev.124.20.3929. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S. Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 1995;9:2646–2658. doi: 10.1101/gad.9.21.2646. [DOI] [PubMed] [Google Scholar]
- 9.Ragge NK, Brown AG, Poloschek CM, Lorenz B, Henderson RA, et al. Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet. 2005;76:1008–1022. doi: 10.1086/430721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sergi C, Kamnasaran D. PRRX1 is mutated in a fetus with agnathia-otocephaly. Clin Genet. 2011;79:293–295. doi: 10.1111/j.1399-0004.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- 11.Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D'Apice MR, et al. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]