Summary
Background
In patients failing successful conventional mobilization of hematopoietic progenitor cells (HPC) plerixafor (Mozobil®) seems to be an alternative. We report a series of 14 patients with multiple myeloma or NHL successfully mobilized and harvested by plerixafor together with large-volume leukaphereses (LVL).
Methods
In a first series (GI), 5 patients were mobilized with G-CSF and plerixafor. In the second series (GII), 9 patients were mobilized by chemotherapy, G-CSF, and plerixafor.
Results
In GI and GII, addition of plerixafor led to a significant (p < 0.01) increase of leukocytes and CD34+ cells in peripheral blood (PB). In GII, the median number of CD34+ cells in PB before and after addition of plerixafor was significantly (p = 0.019) higher compared to GI (9 vs. 5 and 50 vs. 24 cells/μl, respectively). In GI and GII, a median number of three or one aphereses was performed. In GII, the median yield (6.7 × 106 CD34+ cells/kg) of the first apheresis and the median intra-apheresis recruitment of CD34+ cells were significantly (p < 0.05) higher compared to GI (2.94 × 106 CD34+ cells/kg). All patients transplanted, 5 in GI and 8 in GII, exhibited successful engraftment.
Conclusions
Plerixafor and G-CSF mobilization or the addition of plerixafor during non-optimal chemotherapy and G-CSF mobilization together with LVL enabled, independent of leukocyte count and even without detectable CD34+ cells before addition of plerixafor, sufficient harvest of HPC numbers for transplantation. Addition of plerixafor during chemotherapy and G-CSF mobilization led to an increased intra-apheresis recruitment and a significantly higher yield of CD34+ cells compared to plerixafor and G-CSF steady-state mobilized patients.
KeyWords: Plerixafor, HPC products, Large-volume leukapheresis, Poor or non-optimal mobiliser
Introduction
Transplantation of hematopoietic progenitor cells (HPC) has become a widely accepted therapeutic option, particularly for patients with chemotherapy-sensitive hematological malignancies. Transplantation of HPC offers several advantages compared to bone marrow. Collection of HPC can be performed without general anesthesia, engraftment is faster, and supportive care and costs are reduced. HPC are harvested by leukapheresis after mobilization with chemotherapy and/or G-CSF [1, 2].
A decisive factor for patients being transplanted in an autologous setting is the dose of transplanted HPC usually determined by measurement of CD34+ cells. Some data suggest that transplantation with less than 2 million of CD34+ cells/kg body weight (bw) is associated with a prolonged hematologic engraftment and worse outcome, whereas a dose of more than 5 million CD34+ cells/kg bw was of benefit [3]. In addition several data suggest that a minimum of 1.5 million [4], 2.5 million, or more than 5 million CD34+ cells might result in better outcome because of more rapid hematological engraftment and a decrease in infectious episodes [5]. Some data even suggest that patients might benefit of a dose higher than 15 million CD34+ cells/kg bw with regard to engraftment and hospitalization time after transplantation [6]. Therefore, 2–4 × 106 CD34+ cells/kg bw were recently defined as minimum and 8–10 × 106 CD34+ cells/kg bw as optimum dose for autologous (tandem) transplantation in patients with multiple myeloma (MM) [7].
To achieve a sufficient number of CD34+ cells for transplantation, it is necessary to optimally mobilize and harvest HPC. Several factors have been identified being associated with poor mobilization, e.g., number of previous chemotherapy cycles, chemotherapy with stem cell-toxic substances like fludarabine, melphalan or lenalidomide, previous radiotherapy, and disease status [3, 8, 9, 10, 11, 12]. Depending on diagnosis, different mobilization failure rates up to about 30% are reported in the literature [13]. Therefore, strategies to identify poor mobilizing patients upfront or alternatives to improve mobilization regimens in non-optimally mobilizing patients are needed. Recently published data [14] suggest that patients exhibiting a peak count of less than 20 CD34+ cells/μl could be considered poor mobilizer.
Plerixafor (Mozobil®; Genzyme GmbH, Neu-Isenburg, Germany) is such a new alternative. It is an inhibitor of the CXCR4 chemokine receptor and blocks binding of its cognate ligand stromal cell-derived factor-1 alpha (SDF-1α) [15, 16]. In two prospective, randomized, placebo-controlled phase III trials in patients with non-Hodgkin's lymphoma (NHL) [17] or MM [18], it was shown that administration of plerixafor led to a significantly higher number of CD34+ cells/kg bw with less aphereses compared to those obtained in the G-CSF and placebo groups.
Additionally, not only the mobilization strategy but also the apheresis should be tailored to the patient's needs. It was already shown that large-volume leukaphereses (LVL) may result in an intra-apheresis recruitment of CD34+ cells in the range of up to 3.5 × 106 CD34+ cells/kg bw, therefore representing an additional tool for improving the yield in poor or non-optimally mobilizing patients [19, 20, 21, 22].
We compared mobilization and apheresis data of two groups of patients undergoing mobilization with plerixafor. In the first group (GI), plerixafor was added during a steady-state mobilization with G-CSF after previous failure of HPC mobilization, whereas in the second group (GII) plerixafor was added during an ongoing chemotherapy and G-CSF mobilization on the basis of poor or non-optimal CD34+ cell counts in peripheral blood (PB) not allowing for harvest of sufficient numbers of CD34+ cells for transplantation in a single apheresis.
Material and Methods
Patients
According to institutional policies, all patients were eligible for high-dose therapy and subsequent support with autologous HPC. In GI, 5 patients were treated during the compassionate use program after failure of a previous mobilization cycle. Patients’ diagnoses were MM (n = 4) or NHL (n = 1). The median age of the patients was 52 years (range 44–65 years) and three were male and two were female. In GII, 9 patients (4 female and 5 male) with MM (n = 5) or NHL (n = 4) and a median age of 61 years (range 54–69 years) were treated with plerixafor on the basis of CD34+ cell counts in PB not sufficient for harvest of the minimum or optimum number of CD34+ cells/kg bw in a single apheresis based on a formerly published algorithm [23]. Patients’ data are summarized in table 1. A median of 4 previous cycles of chemotherapy was given in both GI and GII.
Table 1.
Summary of patients characteristics
| Patient number | Sex | Age, years | Diagnosis | Previous radiation | Previous cycles of chemotherapy | Number of aphereses | Times the processed total peripheral blood volume, a / b |
|---|---|---|---|---|---|---|---|
| Steady state mobilization, GI | |||||||
| 1 | m | 52 | MM | yes | 3× VelCD, 1× Cy 2 g/m2 | 3 | 4.1 / 13.1 |
| 2 | m | 44 | MM | none | 1× VelCD, 2× VelCD, | 2 | 3.4 / 7.1 |
| 1× Cy 3g/m2, 1× Cy 2 g/m2 | |||||||
| 3 | f | 45 | NHL | none | 5× R-CHOP, 6× R-FC, | 2 | 3.9 / 9.6 |
| 2× R-DHAP | |||||||
| 4 | f | 65 | MM | none | 3× VM, 1× Cy 3 g/m2 | 3 | 5.6 / 18.2 |
| 5 | m | 58 | MM | yes | 3× VelCD, 1× Cy 3 g/m2 | 3 | 4.4 / 14.7 |
| Mobilization with chemotherapy + G-CSF, GII | |||||||
| 6 | f | 69 | MM | yes | 1× VMP, 3× VelCD | 1 | 5.1 / – |
| 7 | m | 61 | NHL | yes | 6× R-CHOP | 1 | 3.6 / – |
| 8 | f | 68 | MM | none | none | 1 | 5.8 / – |
| 9 | m | 56 | MM | none | 3× VelCD | 1 | 4.9 / – |
| 10 | f | 54 | MM | none | 1× VelCD | 1 | 4.0 / – |
| 11 | m | 56 | MM | none | 4× VelCD | 1 | 4.3 / – |
| 12 | m | 61 | NHL | none | 6× R-CHOP | 1 | 4.2 / – |
| 13 | f | 60 | NHL, CNS | none | 4× MTX | 1 | 4.8 / – |
| 14 | m | 67 | NHL, CNS | none | 4× MTX | 1 | 3.5 / – |
m = male; f = female; MM = multiple myeloma; VelCD = Velcade, cyclophosphamide, dexamethasone; Cy = cyclophosphamide; R-CHOP = rituximab, doxorubicine, vincristine, prednisone; R-FC = rituximab, fludarabine, cyclophosphamide; R-DHAP = rituximab, dexamethasone, cytosine arabinoside, cisplatin; VM =Velcade, melphalan; VMP = Velcade, melphalan, prednisone; MTX = methotrexate; a = first apheresis after plerixafor application; b = summary of all aphereses with plerixafor application.
Mobilization and Apheresis
In GI, patients were mobilized with G-CSF 5 μg/kg bw subcutaneously twice daily and addition of plerixafor, 240 μg/kg bw subcutaneously 10 h before apheresis starting in the evening of the 4th day of G-CSF administration. G-CSF and plerixafor administrations were continued for a maximum of 3 apheresis days or until the minimum transplantation dose of 2 × 106 CD34+ cells/kg bw in patients with NHL or if feasible 4×106 CD34+ cells/kg bw in patients with MM was reached. In GII, mobilization regimens consisted of cyclophosphamide and G-CSF in MM patients, of ICE (ifosfamide, etoposide, and carboplatin) or of DexaBEAM (dexamethasone, BCNU, etoposide, cytosine arabinoside, and melphalan) in patients with NHL or of high-dose cytosine arabinoside and thiotepa in patients with primary CNS NHL. Aphereses were performed via a central venous line using a COBE Spectra® (CARIDIAN BCT, Heimstetten, Germany) cell separator with program version 6.1. Apheresis size, i.e., normal-volume apheresis or LVL according to earlier publications [19], was tailored according to patients’ needs. The targeted minimum transplantation dose for autologous transplantation in patients with NHL was 2 × 106 CD34+ cells/ kg bw with an additional ‘back-up’ autograft and in patients with MM 4 × 106 CD34+ cells/ kg bw split in two bags, with an additional ‘back-up’ graft in both cases.
Laboratory Methods Flow Cytometric Analysis
Immunophenotyping of CD34+ cells was performed using a commercially available kit (Stem Kit®; Beckman Coulter, Krefeld, Germany) as a single platform method according to the ISHAGE guidelines [24]. This kit consists of an anti-CD45-FITC monoclonal antibody (moAb), an anti-CD34-PE moAb, a respective isoclone control moAb, and the viability dye 7-AAD (7-aminoactinomycin A). These conjugated moAbs are already provided in defined combinations ready to use. Furthermore, stem count fluorospheres and ammonium chloride are provided with the kit.
All flow cytometric analyses were performed in duplicate, and the mean was calculated from the results of two analyses. Flow cytometric analyses were performed on an EPICS XL or a FC500 flow cytometer (Beckman Coulter).
CD34+ cells were analyzed in all patients of GI in PB starting on day 4 of G-CSF administration and on each day before apheresis. In GII patients, CD34+ cell monitoring in PB started on the day the leukocyte count reached 1/nl for the first time after chemotherapy-induced aplasia. Monitoring was continued each day until the last apheresis. In 6 of the 9 patients, CD34+ cell numbers were also evaluated in PB after the apheresis after addition of plerixafor.
Analysis of Clonogenic Growth
Clonogenic growth was analyzed using a commercially available kit (Stemcell Technologies Inc., Vancouver, BC, Canada). In brief, a satellite tube of the product was thawed at +37 °C and quickly diluted with cold phosphate-buffered saline (PBS); based on a previous flow cytometric analysis [25] of another tube of the same graft, 200 viable CD34+ cells were plated without washing steps in semisolid medium (Cellgrowth; Stemcell Technologies). The medium is supplemented with GM-CSF (granulocyte macrophage-colony stimulating factor), IL-3, and SCF (stem cell factor). Culture dishes were incubated at +37 °C, 5% CO2 in a humidified atmosphere for 14 days. Cultures were analyzed for CFU-GM (colony forming units-granulocyte macrophage) growth with an inverted microscope (Invertoskop, Zeiss, Jena, Germany); based on the recommendations of the manufacturer, colonies containing at least 20 cells were counted as colonies.
Transplantation
Patients were transplanted after successful harvest of at least 2 × 106 CD34+ cells/kg bw. For patients with MM, conditioning regimens consisted of melphalan in concentrations of 100, 120, 140 or 200 mg/m2, depending on the patient's age and condition. Patients with NHL were conditioned with Z-BEAM or R-BEAM (Y-90-Zevalin® 1,200 MBq, Rituximab® 375 mg/m2, BCNU (carmustine) 300 mg/m2, etoposide 4 × 200 mg/m2, cytosine arabinoside 4 × 200 mg/m2, and melphalan 100 or 140 mg/m2), and patients with primary NHL of the CNS were conditioned with Rituximab® 375 mg/m2, BCNU 400 mg/m2, and thiotepa 2 × 5 mg/kg bw.
Statistics
If not stated otherwise, data are given as median and range. The data of the different examinations were compared by the distribution-free Mann-Whitney if not stated otherwise using the Graph Pad PRISM program (San Diego, CA, USA), version 3.03. Results were considered statistically significant if the p value was < 0.05.
Results
Peak Counts of Leukocytes or CD34+ Cells before and after Apheresis
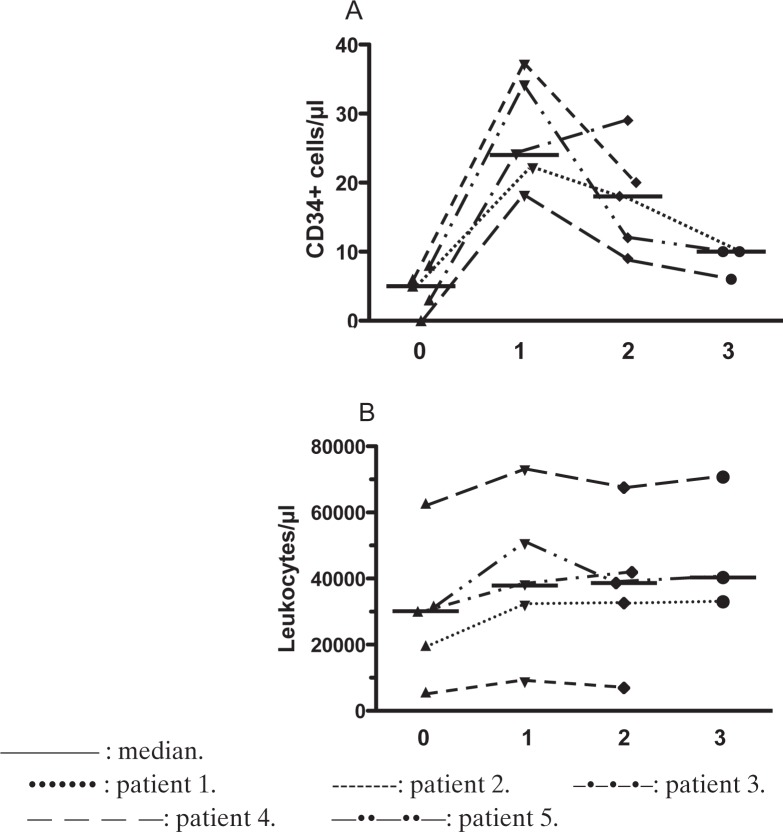
In GI, the median peak number of CD34+ cells in PB in a previous mobilization cycle consisting of chemotherapy and G-CSF was 6 cells/μl (range 2.5–14 cells/μl). On day 4 of the consecutive steady-state mobilization cycle with application of G-CSF in a daily subcutaneous dose of 10μg/kg bw, the median number of CD34+ cells was 5/μl (range 0–8/μl) in PB. These numbers increased significantly (p < 0.01) to a median number of 24 CD34+ cells/μl (range 18–37 CD34+ cells/μl). Interestingly, in patient #4, CD34+ cells were initially not detectable but increased after plerixafor administration to 18 cells/μl Regarding leukocyte counts, an increase after plerixafor addition was detectable, but this was not significant. Patient #2 exhibited an extraordinary behavior of the leukocytes during G-CSF as well as plerixafor mobilization. On day 4 of G-CSF administration, he presented with an unexpectedly normal leukocyte count of 5,600/μl but detectable CD34+ cells of 6/μl. After addition of plerixafor, the leukocyte count increased to 8,500/μl but still remained in the normal range. At the same time, the CD34 cell number increased to 37/μl. Kinetics of leukocytes and of CD34+ cells are summarized in figure 1.
Fig. 1.
Summary of kinetics of leukocytes and CD34+ cells in GI A before and B after administration of plerixafor. 0: cell numbers in the morning before the first application of plerixafor. 1: cell numbers in the morning after the first application of plerixafor. 2: cell numbers in the morning after the second application of plerixafor. 3: cell numbers in the morning after the third application of plerixafor.
In GII, the median number of leukocytes increased significantly (p < 0.01) from 11,200/μl (range 2,900–19,800/μl) on the morning of the day of plerixafor addition to 28,300/μl (range 7,600–48,600/μl) in the morning after adding plerixafor. Additionally, by adding plerixafor the median number of CD34+ cells in PB increased significantly (p < 0.01) from a median number of 9 cells/μl (range 4–20/μl) to 50 cells/μl (range 22–112 cells/μl). In 6 of the patients, the enumeration of CD34+ cells/μl after the apheresis revealed a median of 26 cells/μl (range 17–45 cells/μl). Results are summarized in detail in figure 2. In addition, 4 of the patients in GII underwent a harvest in the morning of the day of plerixafor addition and had a median of 11 CD34+ cells/μl (range 9–11 CD34+ cells/μl).
Fig. 2.
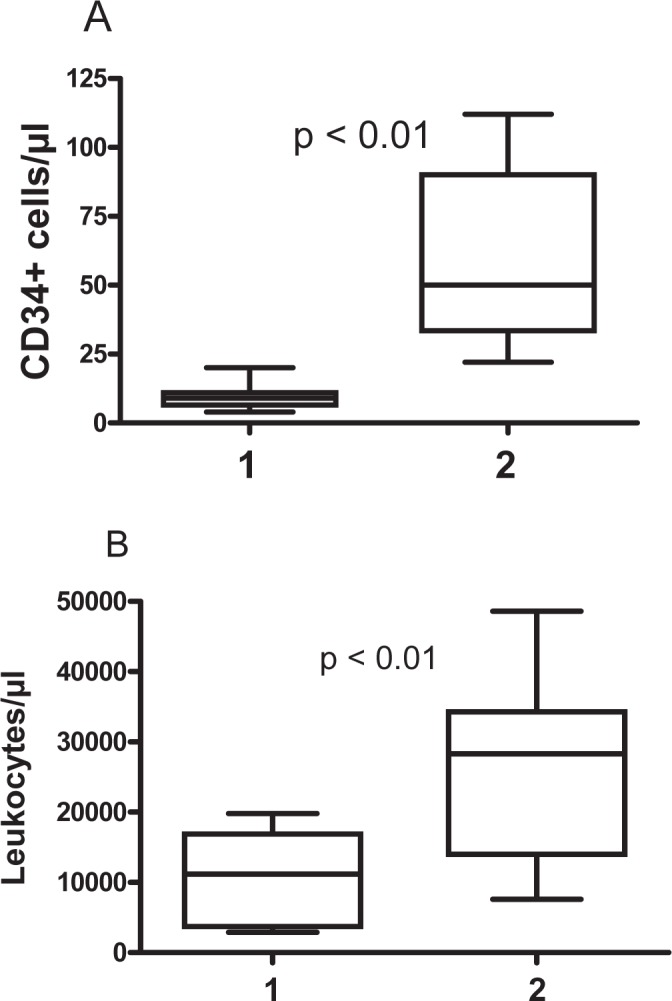
Summary of kinetics of leukocytes and CD34+ cells in GII before A and after B administration of plerixafor. 1: cell numbers in the morning before the first application of plerixafor. 2: cell numbers in the morning after the first application of plerixafor.
Comparing the CD34+ cell numbers in PB between GI and GII, the median numbers of CD34+ cells were significantly (p = 0.019) higher in the chemo-mobilization group GII at both analyzed time points, i.e., in the morning before adding plerixafor in the evening as well in the morning after its addition (table 2).
Table 2.
Summary of cell kinetics before and after plerixafor administration with respect to leukocytes and CD34+ cells
| Patient number | A CD34+ cells/μl | B CD34+ cells/μl | C / D / E CD34+ cells/μl | B WBC/μl | C / D / E WBC/μl | Yield of CD34+ cells, F / G /H / I, × 106/kg bw | Intra-apheresis recruitment of CD34+ cells/kg bw in the first apheresis with plerixafor* | Intra-apheresis recruitment of CD34+ cells/kg bw in the first apheresis with plerixafor per one processed TPBV# |
|---|---|---|---|---|---|---|---|---|
| Steady state mobilization, GI | ||||||||
| 1 | 5 | 5 | 22 / 18 / 10 | 19,700 | 31,700 / 32,600 / 33,000 | – / 2.94 / 2.11 / 1.35 | 1.48 × 106 | 3.61 × 105 |
| 2 | 14 | 6 | 37 / 20 / – | 5,600 | 8,500 / 6,900 / – | – / 3.94 / 3.62 | 1.7 × 106 | 4.97 × 105 |
| 3 | 2.5 | 3 | 24 / 29 / – | 30,100 | 37,900 / 41,900 / – | – / 2.62 / 3.44 | 1.1 × 106 | 2.96 × 105 |
| 4 | 6.5 | 0 | 18 / 9 / 6 | 62,800 | 72,600 / 67,500 / 70,700 | – / 0.96 / 1.17 / 0.74 | –1.01 × 105 | –1.80 × 104 |
| 5 | 6 | 8 | 34 / 12 / 10 | 31,700 | 50,300 / 38,600 / 40,300 | – / 3.27 / .1.92 / 1.35 | 1.01 × 106 | 2.29 × 105 |
| Mobilisation with chemotherapy + G-CSF, GII | ||||||||
| 6 | – | 9 | 42 | 11,200 | 31,400 / – / – | 1.06 / 4.66 / – | 1.60 × 106 | 3.12 × 105 |
| 7 | – | 11 | 58 | 19,800 | 48,600 / – / – | 1.34 / 5.07 / – | 3.64 × 104 | 1.02 × 104 |
| 8 | – | 11 | 44 | 15,700 | 28,300 / – / – | 1.22 / 6.7 / – | 4.00 × 106 | 6.85 × 105 |
| 9 | – | 4 | 50 | 2,900 | 11,700 / – / – | – / 8.24 / – | 4.71 × 106 | 9.55 × 105 |
| 10 | – | 20 | 112 | 11,400 | 37,100 / – / – | – / 13.56 / – | 6.84 × 106 | 1.72 × 106 |
| 11 | – | 9 | 22 | 4,200 | 7,600 / – / – | – / 3.84 / – | 2.65 × 106 | 6.14 × 105 |
| 12 | – | 11 | 25 | 18,100 | 20,400 / – / – | 1.24 / 3.94 / – | 2.39 × 106 | 5.67 × 105 |
| 13 | – | 7 | 68 | 3,290 | 16,300 / – / – | – / 10.59 / – | 6.39 × 106 | 1.34 × 106 |
| 14 | – | 6 | 112 | 4,220 | 30,200 / – / – | – / 10.78 / – | 2.34 × 106 | 6.70 × 105 |
TPBV = Total blood volume.
A–I = Different time points of measurements or harvest: A: in PB during the previous mobilization cycle consisting of chemotherapy and G-CSF; B: in PB in the morning of the first day of plerixafor administration; C: in PB in the morning after the first administration of plerixafor; D: in PB in the morning after the second administration of plerixafor; E: in PB in the morning after the third administration of plerixafor; F: in the harvest in the first apheresis on the day before the evening administration of plerixafor; G: in the harvest in the apheresis on the day after the first evening administration of plerixafor; H: in the harvest in the apheresis on the day after the second evening administration of plerixafor; I: in the harvest in the apheresis on the day after the third evening administration of plerixafor.
Intra-apheresis recruitment/kg is defined as ((number of CD34+ cells in the graft) – (total number of CD34+ cells in PB before apheresis)) / kg bw.
Intra-apheresis recruitment as defined above per one TPBV (normalized to one processed TPBV).
Aphereses’ Characteristics
Comparing the processed number of total peripheral blood volumes (TPBV) during the apheresis on the first day after addition of plerixafor, in GI a median of 4.1 (range 2.9–5.6) and in GII a median of 4.3 (range 3.5–5.8) times the TPBV was processed. There was no difference between both groups. In GII, 4 patients underwent an apheresis before addition of plerixafor, and a median of 3.8 (range 3.3–4.1) times the TPBV was processed. After addition of plerixafor, all patients in GII were only harvested once. In GI, a median number of 3 (range 2–3) aphereses was performed. A Wilcoxon signed rank test comparing against a theoretical median of 1 displayed a significant (p = 0.03) difference.
Yield of HPC and Their Intra-Apheresis Recruitment
The median yield of the first and only apheresis after addition of plerixafor in the chemotherapy group GII (6.7 × 106 CD34+ cells/kg bw (range 3.84–13.56 × 106 CD34+ cells/kg bw)) was not different from the total median yield (6.4 × 106 CD34+ cells/kg bw (range 2.87–7.56 × 106 CD34+ cells/kg bw)) of all aphereses in GI. Comparing only the yield of the first apheresis after addition of plerixafor between both groups, the median yield was significantly (p = 0.001) lower in GI (2.94 × 106 CD34+ cells/kg bw (range 0.96–3.94 × 106 CD34+ cells/kg bw)) than in the chemotherapy GII. In 3 of the 4 patients with MM in GI and in 4 of the 5 patients with MM in GII, two transplantation dosages of at least 2 × 106 CD34+ cells/kg bw for potential tandem transplantation could be achieved.
In addition, the intra-apheresis recruitment of HPCs was estimated by simply calculating the difference of the yield of CD34+ cells in the apheresis and the total amount of CD34+ cells in PB before start of apheresis. The median recruitment per kg bw and per 1 processed TPBV was significantly (p = 0.029) higher in tGII (0.67 × 106 CD34+ cells/kg bw (range 0.010–1.72 × 106 CD34+ cells/kg bw and processed TPBV)) compared with GI (0.3 × 106 CD34+ cells/kg bw (range −0.018 to 0.5 × 106 CD34+ cells/kg bw and processed TPBV)). By multiplication with the processed TPBV in the chemotherapy group GII the total median intra-apheresis recruitment was higher than 1 transplantation dose (2.65 × 106 CD34+ cells/kg bw (range 0.036–6.84 × 106 CD34+ cells/kg bw)), whereas in GI the median recruitment was below the transplantation threshold (1.16 × 106 CD34+ cells/kg bw (range −0.10 to 1.70 × 106 CD34+ cells/kg bw)) and significantly (p < 0.019) different from 1 in GI. These results are summarized in figure 3. The median intra-apheresis recruitment in the 4 aphereses performed in GII before addition of plerixafor was 0.47 × 106 CD34+ cells/kg bw (range 0.39–0.56 × 106 CD34+ cells/kg bw).
Fig. 3.
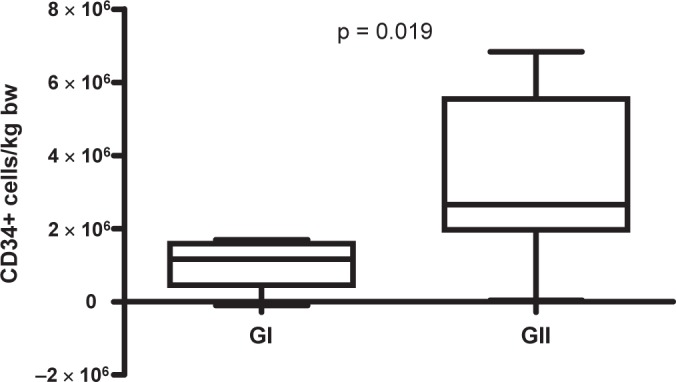
Summary of intraapheresis recruitment of CD34+ cells/kg bw in GI and in GII.
In 6 of the 9 patients of GII, CD34+ cells were measured after the apheresis as well, enabling for a more accurate estimation of the intra-apheresis recruitment per kg bw by the following calculation: (yield of CD34+ cells in the harvest) – ((total amount of CD34+ cells in PB before apheresis) – (total amount of CD34+ cells in PB after apheresis)) / kg bw. Comparing these values with the values deduced from the up-mentioned simple calculation, the median recruited amount increased for the 6 analyzable patients from 3.33 × 106 CD34+ cells/kg bw (range 2.34–6.39 × 106 CD34+ cells/kg bw) to 5.54 × 106 CD34+ cells/kg bw (range 3.63–8.61 × 106 CD34+ cells/kg bw).
Clonogenic Growth
In all 14 patients, except for one of GII, clonogenic growth was analyzed in at least one product derived from aphereses after addition of plerixafor. CFU-GM assays were performed from 25 different products produced from 20 different aphereses of the 14 patients. In each assay colonies were detectable. A median number of 35 CFU-GM (range 12–54 CFU-GM) per 200 viable CD34+ cells were detected.
Transplantation
So far, all patients in GI and 8 of the 9 patients in GII were transplanted. Patients in GI received a median dose of 3.03 × 106 CD34+ cells/kg bw (range 2.52–3.32 × 106 CD34+ cells/kg bw) and those in GII a median dose 3.69 × 106 CD34+ cells/kg bw (range 2.33–7.06 × 106 CD34+ cells/kg bw). All patients exhibited leukocyte and platelet engraftment after transplantation. In GI and GII, a leukocyte count above 1/nl was reached after a median time of 10 days (range 8–11 days) and 11 days (range 8–12 days) after transplantation, respectively.
Discussion
In a substantial proportion of patients, transplantation of autologous HPC represents an accepted part of a multimodal treatment. A decisive prerequisite for planning, success, and assessing the risk profile of an autologous transplantation is the availability of a sufficient number of HPC, namely of CD34+ cells/kg bw. Therefore, mobilization and harvest of CD34+ cells need to be optimized with respect to the amount of collected cells as well as to the number of aphereses necessary to achieve high numbers of CD34+ cells. Several factors affecting the mobilization success have been identified, e.g., patient's age, previous chemotherapy, and previous irradiation. Recently published randomized phase III studies [17, 18] showed that in NHL and MM patients the addition of a novel chemokine receptor antagonist, plerixafor (Mozobil) to a steady-state mobilization with G-CSF led to a significantly higher proportion of patients achieving the targeted transplantation dose of more than 5 and 6 × 106 CD34+ cells/kg bw, respectively, in significantly less aphereses. Our findings in GI showed that patients mobilized subsequently with G-CSF alone after a previous unsuccessful chemo-mobilizing cycle present with less than 10 CD34+ cells/μl on day 4 of the steady-state mobilization. Three of the 5 patients even presented with CD34+ cell counts equal to or below 5 CD34+ cells/μl. In GII, all but one patient, who had 20 CD34+ cells/μl had less than 20 cells/μl, and in 5 patients the CD34+ cell count was below 10/μl. According to recently published literature [14], patients with a peak count of less than 20 CD34+ cells/μl after chemotherapy and G-CSF mobilization were considered poor mobilizers. In addition, those with counts between 6 and 10/μl were regarded as relative and those with counts below 6/μl as absolute poor mobilizers. In our GI patients, the addition of plerixafor shifted all but 1 of the 5 patients to counts above 20 CD34+ cells/μl threshold. The one patient (#4) reaching 18 CD34+ cells/μl in PB the morning after addition of plerixafor had no detectable CD34+ cells before its addition. These data underline that low counts of CD34+ cells or even non-detectable CD34+ cells in PB before potential addition of plerixafor are not predictive for its effect. Additionally, an unexpectedly low or no increase of the leukocyte count after 4 days of G-CSF mobilization seems to be negligible. In GII, all 9 patients exhibited a CD34+ cell count equal or below 20 cells/μl, and 5 patients even had counts below 10 CD34+ cells/μl After administration of plerixafor, the number of CD34+ cells/μl increased above 20 CD34+ cells/μl for all 9 patients.
In GI, all patients achieved the goal of one transplantation dose of at least 2 × 106 CD34+ cells/kg bw. In this group more than one transplantation dose of 2 × 106 CD34+ cells/kg bw could be harvested in 4 patients after repeated administrations of plerixafor and 2–3 LVL. In GII, all patients achieved the minimum transplantation dose of east 2 × 106 CD34+ cells/kg bw already after the first dose of plerixafor and one LVL, and 6 of the 9 patients (66%) achieved the optimal dose of more than 4–5 × 106 CD34+ cells/kg bw by this approach.
Our data show as already published in a different study some years ago [19] that the yield in the aphereses was in both groups higher than the number of CD34+ cells circulating in the PB before apheresis. Interestingly, the intra-apheresis recruitment was highest in the patients of GII after addition of plerixafor compared with the patients from GI when normalized by one processed TPBV. Furthermore, the recruitment was smallest in the 4 aphereses performed in patients of GII before application of plerixafor. In addition, the recruitment calculated for 6 of the GII patients with considering postapheresis CD34+ cell counts even revealed a substantially higher recruitment compared to the formerly published data [26]. Possible explanations for the different extent of recruitment between GI and GII might be the different pre-apheresis CD34+ cell counts in PB or the difference in mobilization scheme, i.e., mobilization with chemotherapy and G-CSF or mobilization with G-CSF alone. The difference in recruitment in GII patients before and after addition of plerixafor might be due to the very low numbers of CD34+ cells before. Additionally a possible alternative explanation might be the addition of plerixafor itself which might be as well the explanation for the difference to earlier published data.
Finally, the addition of plerixafor increased the numbers of CD34+ cells in all but 1 patient so that the patients were no longer considered poor mobilizers. Nevertheless, especially in GI patients only the combination with LVL enabled the harvest of sufficient numbers of HPC for one or two transplantations. Apart from this, the scheduling of aphereses in poor or non-optimally mobilizing patients could become more easy and calculable by the addition of plerixafor. Regarding the intra-apheresis recruitment, further studies should be performed to elucidate the extent of potential influence of plerixafor and the apheresis’ size on it.
Disclosure Statement
A.H. received remunerations from the company GENZYME / SANOFI-AVENTIS for talks and for participation in advisory boards.
References
- 1.Korbling M, Dorken B, Ho AD, Pezzutto A, Hunstein W, Fliedner TM. Autologous transplantation of blood-derived hemopoietic stem cells after myeloablative therapy in a patient with Burkitt's lymphoma. Blood. 1986;67:529–532. [PubMed] [Google Scholar]
- 2.Gillespie TW, Hillyer CD. Peripheral blood progenitor cells for marrow reconstitution: mobilization and collection strategies. Transfusion. 1996;36:611–624. doi: 10.1046/j.1537-2995.1996.36796323060.x. [DOI] [PubMed] [Google Scholar]
- 3.Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, Gooley T, Demirer T, Schiff-man K, Weaver C, et al. Factors that influence collection and engraftment of autologous peripheral-blood stem cells. J Clin Oncol. 1995;13:2547–2555. doi: 10.1200/JCO.1995.13.10.2547. [DOI] [PubMed] [Google Scholar]
- 4.Desikan KR, Tricot G, Munshi NC, Anaissie E, Spoon D, Fassas A, Toor A, Zangari M, Badros A, Morris C, Vesole DH, Siegel D, Jagannath S, Barlogie B. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112:242–247. doi: 10.1046/j.1365-2141.2001.02498.x. [DOI] [PubMed] [Google Scholar]
- 5.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, West W. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–3969. [PubMed] [Google Scholar]
- 6.Ketterer N, Salles G, Raba M, Espinouse D, Sonet A, Tremisi P, Dumontet C, Moullet I, Eljaafari-Corbin A, Neidhardt-Berard EM, Bouafia F, Coiffier B. High CD34(+) cell counts decrease hematologic toxicity of autologous peripheral blood progenitor cell transplantation. Blood. 1998;91:3148–3155. [PubMed] [Google Scholar]
- 7.Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, Comenzo RL, Kumar S, Munshi NC, Dispenzieri A, Kyle R, Merlini G, San Miguel J, Ludwig H, Hajek R, Jagannath S, Blade J, Lonial S, Dimopoulos MA, Einsele H, Barlogie B, Anderson KC, Gertz M, Attal M, Tosi P, Sonneveld P, Boccadoro M, Morgan G, Sezer O, Mateos MV, Cavo M, Joshua D, Turesson I, Chen W, Shimizu K, Powles R, Richardson PG, Niesvizky R, Rajkumar SV, Durie BG. International Myeloma Working Group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;23:1904–1912. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 8.Dreger P, Kloss M, Petersen B, Haferlach T, Loffler H, Loeffler M, Schmitz N. Autologous progenitor cell transplantation: prior exposure to stem cell-toxic drugs determines yield and engraftment of peripheral blood progenitor cell but not of bone marrow grafts. Blood. 1995;86:3970–3978. [PubMed] [Google Scholar]
- 9.Gordan LN, Sugrue MW, Lynch JW, Williams KD, Khan SA, Wingard JR, Moreb JS. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44:815–820. doi: 10.1080/1042819031000067585. [DOI] [PubMed] [Google Scholar]
- 10.Olivieri A, Brunori M, Capelli D, Montanari M, Massidda D, Gini G, Lucesole M, Poloni A, Offidani M, Candela M, Centurioni R, Leoni P. Salvage therapy with an outpatient dhap schedule followed by pbsc transplantation in 79 lymphoma patients: an intention to mobilize and transplant analysis. Eur J Haematol. 2004;72:10–17. doi: 10.1046/j.0902-4441.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 11.Akhtar S, Weshi AE, Rahal M, Khafaga Y, Tbakhi A, Humaidan H, Maghfoor I. Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. Leuk Lymphoma. 2008;49:769–778. doi: 10.1080/10428190701843213. [DOI] [PubMed] [Google Scholar]
- 12.Hosing C, Saliba RM, Ahlawat S, Korbling M, Kebriaei P, Alousi A, De Lima M, Okoroji JG, Mc-Mannis J, Qazilbash M, Anderlini P, Giralt S, Champlin RE, Khouri I, Popat U. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. Am J Hematol. 2009;84:335–337. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pusic I, Jiang SY, Landua S, Uy GL, Rettig MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein KE, Sempek DS, Smith AL, DiPersio JF. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14:1045–1056. doi: 10.1016/j.bbmt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Wuchter P, Ran D, Bruckner T, Schmitt T, Witzens-Harig M, Neben K, Goldschmidt H, Ho AD. Poor mobilization of hematopoietic stem cells – definitions, incidence, risk factors and impact on outcome of autologous transplantation. Biol Blood Marrow Transplant. 2010;16:490–499. doi: 10.1016/j.bbmt.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Gerlach LO, Skerlj RT, Bridger GJ, Schwartz TW. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J Biol Chem. 2001;276:14153–14160. doi: 10.1074/jbc.M010429200. [DOI] [PubMed] [Google Scholar]
- 16.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–262. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 17.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 18.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, Cooper D, Bridger G, Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 19.Humpe A, Riggert J, Munzel U, Kohler M. A prospective, randomized, sequential crossover trial of large-volume versus normal-volume leukapheresis procedures: effects on serum electrolytes, platelet counts, and other coagulation measures. Transfusion. 2000;40:368–374. doi: 10.1046/j.1537-2995.2000.40030368.x. [DOI] [PubMed] [Google Scholar]
- 20.Cassens U, Barth IM, Baumann C, Fischer RJ, Kienast J, Vormoor J, Sibrowski W. Factors affecting the efficacy of peripheral blood progenitor cells collections by large-volume leukaphereses with standardized processing volumes. Transfusion. 2004;44:1593–1602. doi: 10.1111/j.1537-2995.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamsen JF, Stamnesfet S, Liseth K, Hervig T, Bruserud O. Large-volume leukapheresis yields more viable CD34+ cells and colony-forming units than normal-volume leukapheresis, especially in patients who mobilize low numbers of CD34+ cells. Transfusion. 2005;45:248–253. doi: 10.1111/j.1537-2995.2004.04210.x. [DOI] [PubMed] [Google Scholar]
- 22.Majado MJ, Minguela A, Gonzalez-Garcia C, Salido E, Blanquer M, Funes C, Insausti CL, Garcia-Hernandez AM, Moraleda JM, Morales A. Large-volume-apheresis facilitates autologous transplantation of hematopoietic progenitors in poor mobilizer patients. J Clin Apher. 2009;24:12–17. doi: 10.1002/jca.20191. [DOI] [PubMed] [Google Scholar]
- 23.Humpe A, Riggert J, Meineke I, Kurz M, Eil A, Storkebaum B, Binder C, Munzel U, Funke I, Hocker P, Wiesneth M, Kohler M. A cell-kinetic model of CD34+ cell mobilization and harvest: development of a predictive algorithm for CD34+ cell yield in pbpc collections. Transfusion. 2000;40:1363–1370. doi: 10.1046/j.1537-2995.2000.40111363.x. [DOI] [PubMed] [Google Scholar]
- 24.Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;34:61–70. [PubMed] [Google Scholar]
- 25.Humpe A, Beck C, Schoch R, Kneba M, Horst HA. Establishment and optimization of a flow cytometric method for evaluation of viability of CD34+ cells after cryopreservation and comparison with trypan blue exclusion staining. Transfusion. 2005;45:1208–1213. doi: 10.1111/j.1537-2995.2005.00174.x. [DOI] [PubMed] [Google Scholar]
- 26.Humpe A, Riggert J, Munzel U, Repas-Humpe LM, Vehmeyer K, Brunner E, Wormann B, Kohler M. A prospective, randomized, sequential, crossover trial of large-volume versus normal-volume leukapheresis procedures: effect on progenitor cells and engraftment. Transfusion. 1999;39:1120–1127. doi: 10.1046/j.1537-2995.1999.39101120.x. [DOI] [PubMed] [Google Scholar]