Abstract
The endocrine pancreas is richly innervated with sympathetic and parasympathetic projections from the brain. In the mid-20th century, it was established that α-adrenergic activation inhibits, whereas cholinergic stimulation promotes, insulin secretion; this demonstrated the importance of the sympathetic and parasympathetic systems in pancreatic endocrine function. It was later established that insulin injected peripherally could act within the brain, leading to the discovery of insulin and insulin receptors within the brain and the receptor-mediated transport of insulin into the central nervous system from endothelial cells. The insulin receptor within the central nervous system is widely distributed, reflecting insulin's diverse range of actions, including acting as an adiposity signal to reduce food intake and increase energy expenditure, regulation of systemic glucose responses, altering sympathetic activity, and involvement in cognitive function. As observed with central insulin administration, the pancreatic hormones glucagon, somatostatin, pancreatic polypeptide, and amylin can each also reduce food intake. Pancreatic and also gut hormones are released cephalically, in what is an important mechanism to prepare the body for a meal and prevent excessive postprandial hyperglycemia.
Keywords: cephalic response, conditioning, insulin, islets, pancreatic innervation
this article is a summary of a presentation given at the meeting of the American Physiological Society in San Diego, CA, in 2012. It was part of a symposium/refresher course on complications of diabetes mellitus, and the specific topic was interactions between the brain and endocrine pancreas.
The pancreatic islets of Langerhans were first identified in the late 19th century (75). As described by Langerhans, the islets are richly innervated (75), and it is now recognized that this innervation includes sympathetic, parasympathetic, and sensory nerves (1). Despite accounting for only 1–2% of the pancreatic mass, islets are highly vascularized, receiving 10–20% of pancreatic blood flow (14, 67, 79). Although most cells found in islets are insulin- and amylin-secreting β-cells (61), there are also significant numbers of α-cells (glucagon) and δ-cells (somatostatin) and small numbers of F cells (pancreatic polypeptide) and ε-cells (ghrelin) (43, 61, 71, 74, 136). In this review, after a brief description of endocrine secretions of the islets, we describe the interactions between the central nervous system (CNS) and pancreatic islets, focusing on cephalic responses, conditioned hypoglycemia, insulin secretion, and the roles of insulin in the CNS. While all pancreatic hormones are discussed, the primary focus throughout this review is upon insulin.
The Endocrine Pancreas and Insulin
Of the many hormones produced in and excreted by cells in the islets, the first identified and best known is insulin. Two decades after the discovery of the islets, Minkowski and von Mering (133) reported that removal of the pancreas produced a diabetic phenotype, and it was subsequently reported that aqueous pancreatic extracts produced moderate reductions in glycosuria (92). The isolation of insulin from pancreatic islets was first performed by Banting and Best (13) in 1922, and exogenous insulin soon became the only effective treatment for insulin-deficient (type 1) diabetes mellitus (123).
Insulin is a 51-amino acid peptide hormone cleaved by proteases from preproinsulin and subsequently from proinsulin in the secretory vesicles of β-cells (104, 105). Insulin's best known action is its ability to reduce circulating glucose by activating glucose transporters on cell membranes, enabling the uptake of glucose into most peripheral tissues, where the glucose is used as a fuel or stored as glycogen (72). Insulin is secreted from β-cells in response to increases of local glucose levels, and both its basal and stimulated levels are directly proportional to body fat, with leaner individuals having reduced insulin secretion relative to individuals with greater adiposity (9, 10, 115, 142).
Shortly after the discovery of insulin, Kimball and Murlin (70) determined that extracts of pancreatic tissue also contain a substance that produces a hyperglycemic response, and this hormone was named glucagon. In the late 1960s and early 1970s, pancreatic polypeptide and somatostatin were described and identified in the pancreas (25, 71). Pancreatic polypeptide is made in and secreted from F cells, and somatostatin is made in and secreted from D cells. Amylin was identified as a pancreatic hormone cosecreted with insulin in the late 1980s (43, 136), and, more recently, ghrelin-secreting epsilon cells have been described in pancreatic islets (100).
Insulin and the CNS
Despite Langerhans' early work describing the rich innervation of the islets, there was little early interest in a possible influence of the CNS over the secretion of insulin. Indeed, the Endocrine Pancreas volume of the series on endocrinology published by the American Physiological Society in 1972 contained no mention of a possible CNS influence (114). The presumption that there was unlikely to be a significant brain-islet interaction was primarily based on two premises. First, the brain, unlike most other tissues, does not require insulin to take up glucose (63), i.e., the brain was considered to be insulin independent. Second, insulin was considered too large a molecule to cross the blood-brain barrier (BBB) (91), deeming it unlikely that the peptide could even enter the brain. The logic was that since insulin was not believed to act on CNS cells and that, in any case, it could not reach CNS cells, there could be no meaningful brain-islet-insulin axis. As a consequence, the prevailing theory leading up to the publication of the volume on the pancreatic islets in 1972 was that insulin was a key negative feedback molecule to prevent hyperglycemia; as glucose levels reaching the islet increased, β-cells responded by secreting insulin and consequently preventing further glucose increases and ultimately returning glucose to basal values (114). However, this model had no explanation as to why there is such a rich innervation of the islets with no known functional significance (151).
At the same time, evidence consistent with a functional role of the nervous system in the pancreatic islets was accumulating. Porte demonstrated that local α-adrenergic stimulation inhibits secretion (96), whereas β-adrenergic agonists stimulate insulin secretion (94, 95), strongly suggesting that insulin secretion is under sympathetic control. Campfield and colleagues (29, 31) subsequently observed that insulin secretion is stimulated by acetylcholine, indicating parasympathetic involvement. Cholinergic stimulation of insulin release was, in turn, decreased in the presence of epinephrine (30, 32), implicating a complex neural control involving both parasympathetic and sympathetic control over β-cells. These early studies paved the way to the currently supported view that the autonomic nervous system can have a powerful influence over the secretion of insulin and indeed all pancreatic hormones.
A Neural Reflex Eliciting Insulin Release
In the late 1960s and early 1970s, using a Pavlovian behavioral paradigm whereby an unconditioned stimulus is paired with a neutral stimulus over the course of several trials resulting in the neutral stimulus becoming a conditioned stimulus, one of us (S. C. Woods) found that rats could be conditioned to secrete insulin and become hypoglycemic (137, 139, 141, 143, 146, 147). In training sessions, experimental rats received a subcutaneous injection of insulin (the unconditioned stimulus) in the presence of a novel stimulus (usually an odor, the conditioned stimulus). Blood glucose decreased in response to insulin (the unconditioned response), whereas it increased slightly in control rats administered a placebo (saline) injection subcutaneously in association with the odor. After several such conditioning trials, a test day occurred in which all rats received only saline injections plus the odor, and those that been previously received insulin became hypoglycemic (Fig. 1). Subsequent experiments revealed that the conditioned hypoglycemia required an intact vagus nerve (139), could be blocked with the anti-cholinergic drug atropine (155), and was secondary to conditioned secretion of pancreatic insulin (141).
Fig. 1.
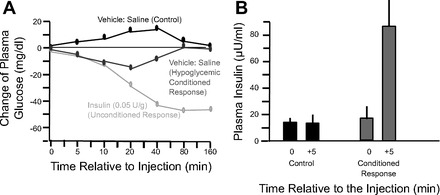
A: the conditioned hypoglycemic response. Saline injection in animals previously conditioned with a series of insulin injections produced a transient hypoglycemia relative to control animals injected with saline on the conditioning trials. B: conditioned insulin secretion. Animals conditioned with multiple injections increasing plasma and brain insulin have a fourfold increase of plasma insulin on a saline injection test trial. Graphs were redrawn from Woods et al. (147) (A) and from Woods et al. (141) (B).
There are several reviews of these early experiments on conditioned hypoglycemia (81, 138, 144), and it is important to note that the findings generated more questions than they answered. For example, what is the neural circuit that normally leads from the CNS to parasympathetic secretion of pancreatic insulin? Does subcutaneously administered insulin somehow (counterintuitively) trigger a reflex leading to endogenous insulin secretion? Does some of the administered insulin actually get into the brain on conditioning trials? If so, does this imply that brain cells can actually detect and respond to changes of local insulin?
While the phenomenon of conditioned hypoglycemia has been observed in numerous laboratories (e.g., Refs. 5, 45, 52, 53, 84, 120, and 146) and species (for reviews, see Refs. 4, 77, and 144), including humans (48, 118), it should be noted that these early conditioning experiments were not without controversy. Using a purportedly identical protocol, Siegel (110, 111) observed that rats developed a conditioned hyperglycemic response, as opposed to a hypoglycemic response. This makes teleological sense in that when a signal (the odor) occurs that has always previously predicted that the animal was about to receive an injection of exogenous insulin; if the rat learns anything, it should learn to increase its blood glucose to mitigate the inevitable hypoglycemia, i.e., the rat should learn to counter the upcoming reduction of blood glucose. In fact, when smaller, closer-to-physiological doses of insulin are administered on the training days, rats do just that. They develop conditioned hyperglycemia (54, 154).
On the other hand, there was evidence supporting the counterintuitive position. Peripheral administration of glucose had been previously used as the unconditioned stimulus in some classical conditioning experiments, and a slight conditioned hypoglycemia was developed relative to controls (90, 102). The glucose administration on conditioning trials presumably elicited endogenous insulin secretion, and subsequent experiments later determined that a change of blood glucose on conditioning trials is not a crucial component of the conditioning process; rather, during conditioning trials, the important factor for the development of a conditioned hypoglycemic response is the increase of insulin (137). Overall, the data suggest that an increase of insulin (either exogenous or endogenously produced) rather than a decrease of blood glucose is sufficient to produce conditioned hypoglycemia (and concomitant conditioned insulin secretion).
The implication, as well as the important point, from all of these studies reporting conditioned insulin secretion and hypoglycemia was that a sudden increase of insulin triggered a reflex reaction in which neurally elicited pancreatic insulin secretion occurred. Such a reflex had in fact been reported in dogs, but it only occurred when the administered insulin actually gained direct access to the brain (38, 124, 125). Only later was it recognized that when large doses of insulin are administered systemically, some insulin actually penetrates the BBB, i.e., after an intravenous administration of exogenous insulin, the level of insulin in the blood rises rapidly and glucose in both plasma and the cerebrospinal fluid (CSF) decreases (152). A sudden increase of insulin in the CSF, in and of itself, elicits a transient, neutrally elicited increase of pancreatic insulin secretion (36, 148). In summary, when sufficient exogenous insulin is administered that some of it enters the CNS, the insulin acts on brain circuits to trigger a vagally mediated parasympathetic increase of insulin secretion from the islets, and this reflexive response can be conditioned.
Subsequent experiments found insulin receptors on neurons and other brain cells in many areas of the CNS. In fact, by the mid 1980s, insulin receptors had been found to be abundant and widely distributed throughout both the developing and adult CNS (44, 62). The distributions of insulin and its receptor in the brain have been extensively characterized by immunohistochemistry (16), autoradiography (130, 131), and in situ hybridization of insulin receptor mRNA (83). High levels of insulin receptors are found in the choroid plexus, olfactory bulbs, and arcuate nucleus of the hypothalamus. The insulin receptor is also abundant in many other regions, including the cerebellum, cerebral cortex, hippocampus, and several hypothalamic nuclei (15, 16, 82, 83, 130, 131).
Insulin and the BBB
Once insulin had been identified within the brain and CSF, it was hypothesized that insulin enters the CSF from plasma via the choroid plexus, subsequently passing through the ependymal lining and acting at insulin receptors on nearby neurons (149). This seemed a reasonable explanation given the dense insulin binding sites in the choroid plexus and the observation that numerous nuclei in the ventral hypothalamus are close to the wall of the CSF-containing third ventricle and have a high density of insulin receptors (15, 59, 60, 130, 132, 157). However, more mechanistic experiments assessing the dynamics of insulin uptake into the CSF and the brain later determined that rather than entering the CNS via the choroid plexus and CSF, insulin is transported into the brain via an insulin receptor-mediated, saturable pathway in brain capillary endothelial cells (see Fig. 2) (17). Thus, the normal movement of insulin to the CNS fits a three-compartment model (plasma to brain interstitial fluid to CSF) (11, 12, 80, 106).
Fig. 2.
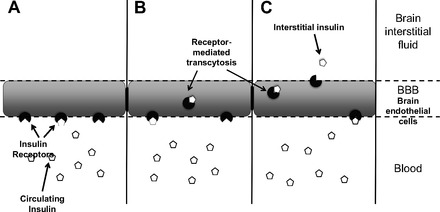
Transport of circulating insulin from the blood into the central nervous system. A: circulating insulin, which has been released into the blood from β-cells in the pancreas, binds to receptors on endothelial cells of the blood-brain barrier (BBB). B: insulin is transported through the endothelial cells via receptor-mediated transcytosis. C: insulin is released into the brain interstitial fluid, where it can then act on neuronal insulin receptors.
Cephalic Responses
Neurally elicited insulin secretion normally occurs at mealtime, and this natural reflex is easily conditionable to cues that reliably predict meal onset (155). These cues include food-predicting odors or the time of day that meals normally occur. These meal-related responses are called “cephalic” because insulin is not secreted in response to a local change of glucose or other nutrient in the pancreas but rather to a neural signal emanating from the brain. The importance of cephalic insulin to normal physiology is demonstrated by the observation that if cephalic insulin is blocked or prevented, animals appear diabetic when they eat, experiencing abnormally high elevations of blood glucose. The secretion of a small amount of insulin as the meal begins thus enables individuals to consume large caloric loads without becoming hyperglycemic, and in its absence, only small meals are generally consumed (140). There are many reviews of cephalic insulin and its importance (1, 23, 69, 99, 118, 119, 127, 128).
During meals, and especially during large meals, there are large fluxes of nutrients into and then out of the gastrointestinal system, into and out of the blood, and ultimately into tissues for immediate use or storage. Cephalic responses enable the body to prepare for these processes in advance and consequently to allow them to proceed smoothly and with the least metabolic perturbation (153). Importantly, many processes other than insulin secretion are involved. Before anticipated meals, there is also evidence for the cephalic secretion of other islet hormones, including pancreatic polypeptide (126), amylin (76), and glucagon (107). Gastrointestinal hormones are also secreted cephalically before the actual onset of eating, including ghrelin (46, 122), cholecystokinin (50), and glucagon-like peptide 1 (129). The important point is that the CNS initiates and coordinates a complex mix of premeal events that help the individual adequately prepare for and deal with the caloric load (see Fig. 3).
Fig. 3.
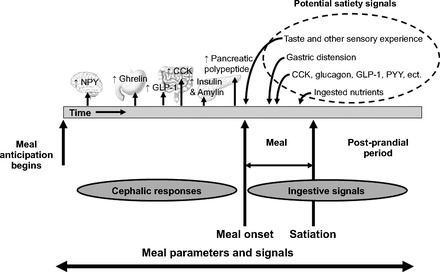
Meal parameters and signals in anticipation of, during, and after a meal. Cephalically induced neuropeptide and hormone release occurs to prepare the body for the incoming nutrients and prevent potentially damaging hyperglycemia. During a meal, numerous factors may act as satiation signals, and when the meal is completed, leading to the postprandial period, many of the same signals help complete the digestion process. NPY, neuropeptide Y; GLP, glucagon-like peptide; CCK, cholecystokinin; PYY, peptide YY.
In summary, contrary to earlier beliefs, insulin is now recognized to cross from the periphery to the brain via an insulin receptor-mediated transport process through brain capillary endothelial cells. Within the brain, insulin stimulates insulin receptors, which are abundantly located in many areas of the CNS. The widespread distribution of insulin and its receptors within the CNS suggests that there are diverse actions of insulin in the brain, likely influencing many behaviors in addition to those directly related to energy homeostasis.
The Functions of Central Insulin
Once within the CNS, insulin has a diverse range of actions. Central insulin alters food intake and energy expenditure (19, 106, 108) and systemic glucose responses to meals and fluctuations of plasma glucose (51, 78, 113). Insulin in the brain is also involved in reproductive function/development (28), hedonic responses (49), and sympathetic activity (101, 134). A particularly exciting topic at the present time concerns the ability of insulin, acting on receptors in the hippocampus and elsewhere in the CNS, to improve cognitive function (37, 116, 117). Most of these aspects of CNS insulin action are beyond the scope of this review.
Insulin and the Regulation of Body Adiposity
Soon after a specific insulin assay became available, it was determined that both basal insulin and insulin secreted in response to a glucose load are directly correlated with body weight, with insulin being elevated in individuals with greater adiposity (9, 10). Because insulin levels in the blood increase when humans (112) or animals (22) overeat and become fat, it was proposed that because insulin is able to enter the CNS, it may act as a negative feedback controller of adiposity (150). The concept was simple, i.e., if an individual overeats and gains weight, the elevated blood and consequently brain insulin would provide a signal triggering corrective responses to eat less and return weight to its former level. Conversely, if an individual fasts or diets, the reduced brain insulin signaling would trigger a reflex to eat more and regain weight. To test this hypothesis, Woods et al. (145) infused insulin into the CSF of baboons and observed a dose-dependent decrease of food intake and body weight over a several-week period, and these effects were reversed after the cessation of the infusions. Many studies have confirmed the effects of central insulin administration to reduce food intake and body weight, most commonly in rodents (2, 3, 64, 85). Importantly, the anorexia and weight loss are not secondary to aversion (35) or to decreased mobility of animals after insulin infusion (33). Consistent with these data, when the insulin signal in the CNS is reduced, by local administration of antibodies to insulin, by genetically knocking out insulin receptors on all CNS neurons, or by interfering with the insulin receptor mRNA message locally in the hypothalamus, animals have increased food intake and body weight (28, 56, 85, 121).
Central insulin-induced hypophagia has been observed in many species, including rats (2, 3, 21, 26, 42, 64, 93), baboons (145), mice (27, 66), chicks (109), and sheep (55). Importantly, humans also have reduced food intake after central insulin administration. With the use of an intranasal insulin administration technique to increase CSF insulin levels, a dose-dependent reduction of food intake was observed in human volunteers (58), and daily treatment resulted in significant weight loss after 6 wk (58). Followup experiments found that men were more sensitive to the anorectic effects of insulin than women (20). Similarly, in the rat, males are relatively more sensitive to the anorectic effects of central insulin, whereas females are relatively more sensitive to the anorectic effect of central leptin (40, 42). Consistent with this, females have higher circulating leptin and lower circulating insulin than comparably obese males (40). This appears to be related to fat distribution, as females generally have more subcutaneous fat, whereas males tend to have a higher proportion of visceral fat (24, 88), and leptin is secreted disproportionately more from subcutaneous fat (47, 86), whereas insulin secretion is more proportional to visceral fat (98). Sex differences observed in hypophagia after the central administration of insulin are due to the actions of estrogen in the hypothalamus, i.e., estrogen inhibits insulin effects on food intake while potentiating the effects of leptin (87). It should be noted that central insulin administration does not necessarily lead to a negative energy balance; rather, the magnitude of the brain insulin signal reduces the amount of body adiposity that is homeostatically maintained (i.e., central insulin infusion will not obligatorily reduce food intake) (33). These examples point to some of the complexities involved in the “regulation” of food intake and energy balance.
Central insulin administration is ineffective at reducing food intake in obese Zucker rats lacking functional leptin receptors (64), and the response to central insulin infusion is also reduced in animals made obese using diet-induced obesity models (7, 34, 39, 41) as well as in obese humans (57). This indicates that obesity results in insulin resistance in the CNS as well as in peripheral tissues. The transport of insulin through the BBB is also compromised in diet-induced obesity (18, 65), and it is possible that this effect is secondary to the leptin resistance that occurs with weight gain (73), given that there is some evidence indicating that the hypophagic effects of insulin require leptin functioning (64, 156). When insulin and leptin are both administered centrally, most doses elicit additive leptin-insulin hypophagic effects. However, at some doses, the reduction of food intake is significantly less than the sum of the individual effects (3). The interactions between insulin and leptin resistance in obesity remain an important area for future research, and a key question that remains is whether endogenous insulin has the same hypophagic action as exogenous insulin.
Other Pancreatic Hormones and the CNS
The hormones produced in the pancreas are all capable of being neurally stimulated, with insulin, amylin, glucagon, and pancreatic polypeptide all released during cholinergic stimulation (29, 68, 89), whereas somatostatin secretion is controlled adrenergically (103). Although they have different and sometimes opposite effects on blood glucose and other parameters, each of the islet hormones reduces food intake. Insulin, pancreatic polypeptide, and somatostatin all act at the hypothalamus to decrease food intake (6, 8, 145), whereas amylin acts at the area postrema in the hindbrain (97) and glucagon acts on the vagal afferent neurons (135). As stated above, pancreatic hormones are released cephalically, in what is an important mechanism for the body to prepare for a meal and prevent diabetes-like symptoms.
Summary
It is now well established that there are complex interactions between the brain and pancreatic islets. Cells within the islets are stimulated by CNS parasympathetic or sympathetic outputs to alter the secretion of peptide hormones, including insulin. Insulin acts within the brain to reduce the amount of body adiposity that is homeostatically maintained, generally reducing food intake and increasing energy expenditure. All other major islet hormones, including pancreatic polypeptide, somatostatin, amylin, and glucagon, reduce food intake, indicating mechanisms of feedback to the CNS from the endocrine pancreas.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-17844. D. P. Begg was supported by National Health and Medical Research Council Early Career Fellowship 1013264.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.B. prepared figures; D.P.B. drafted manuscript; D.P.B. and S.C.W. edited and revised manuscript; D.P.B. and S.C.W. approved final version of manuscript.
REFERENCES
- 1.Ahren B. Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia 43: 393–410, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav 72: 423–429, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology 143: 2449–2452, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Buylla R, Carrasco-Zanini J. A conditioned reflex which reproduces the hypoglycemic effect of insulin. Acta Physiol Latin Am 10: 153–158, 1960 [PubMed] [Google Scholar]
- 5.Alvarez Buylla R, Roces De Alvarez Buylla E. Hypoglycemic conditioned reflex in rats: preliminary study of its mechanism. J Comp Physiol Psychol 88: 155–160, 1975 [DOI] [PubMed] [Google Scholar]
- 6.Aponte G, Leung P, Gross D, Yamada T. Effects of somatostatin on food intake in rats. Life Sci 35: 741–746, 1984 [DOI] [PubMed] [Google Scholar]
- 7.Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol Regul Integr Comp Physiol 255: R974–R981, 1988 [DOI] [PubMed] [Google Scholar]
- 8.Asakawa A, Inui A, Ueno N, Fujimiya M, Fujino MA, Kasuga M. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides 20: 1445–1448, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Bagdade JD. Basal insulin and obesity. Lancet 2: 630–631, 1968 [DOI] [PubMed] [Google Scholar]
- 10.Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest 46: 1549–1557, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks WA. The source of cerebral insulin. Eur J Pharmacol 490: 5–12, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides 18: 1423–1429, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J 12: 141–146, 1922 [PMC free article] [PubMed] [Google Scholar]
- 14.Barbu A, Johansson A, Bodin B, Kallskog O, Carlsson PO, Sandberg M, Borjesson JL, Jansson L. Blood flow in endogenous and transplanted pancreatic islets in anesthetized rats: effects of lactate and pyruvate. Pancreas 41: 1263–1271, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Baskin DG, Porte D, Jr, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain. Endocrinology 112: 898–903, 1983 [DOI] [PubMed] [Google Scholar]
- 16.Baskin DG, Woods SC, West DB, van HM, Posner BI, Dorsa DM, Porte D., Jr Immunocytochemical detection of insulin in rat hypothalamus and its possible uptake from cerebrospinal fluid. Endocrinology 113: 1818–1825, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Baura G, Foster D, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. J Clin Invest 92: 1824–1830, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg DP, Mul JD, Liu M, Reedy BM, D'Alessio DA, Seeley RJ, Woods SC. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology doi: 10.1210/en.2012-1929. [DOI] [PMC free article] [PubMed]
- 19.Begg DP, Woods SC. The central insulin system and energy balance. Handb Exp Pharmacol 111–129, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 93: 1339–1344, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 22: 9048–9052, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein IL, Lotter EC, Kulkosky PJ, Porte D, Jr, Woods SC. Effect of force-feeding upon basal insulin levels of rats. Proc Soc Exp Biol Med 150: 546–548, 1975 [DOI] [PubMed] [Google Scholar]
- 23.Berthoud HR, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B. Cephalic phase, reflex insulin secretion. Neuroanatomical and physiological characterization. Diabetologia Suppl 20: 393–401, 1981 [PubMed] [Google Scholar]
- 24.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord 20: 291–302, 1996 [PubMed] [Google Scholar]
- 25.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179: 77–79, 1973 [DOI] [PubMed] [Google Scholar]
- 26.Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull 12: 571–575, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav 30: 687–691, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Campfield LA, Blocker DC. Simulation of the autonomic neural control of insulin secretion. Comput Biol Med 9: 191–203, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Campfield LA, Smith FJ. Neural control of insulin secretion: interaction of norepinephrine and acetylcholine. Am J Physiol Regul Integr Comp Physiol 244: R629–R634, 1983 [DOI] [PubMed] [Google Scholar]
- 31.Campfield LA, Smith FJ, Eskinazi RE. Glucose responsiveness and acetylcholine sensitivity of pancreatic β-cells after vagotomy. Am J Physiol Regul Integr Comp Physiol 246: R985–R993, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Campfield LA, Smith FJ, Settle JE, Sohaey R. Effect of the order of application of neural inputs on insulin secretion. Adv Exp Med Biol 211: 343–349, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci 109: 528–531, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Chavez M, Riedy CA, van Dijk G, Woods SC. Central insulin and macronutrient intake in the rat. Am J Physiol Regul Integr Comp Physiol 271: R727–R731, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Chavez M, Seeley RJ, Woods SC. A comparison between effects of intraventricular insulin and intraperitoneal lithium chloride on three measures sensitive to emetic agents. Behav Neurosci 109: 547–550, 1995 [PubMed] [Google Scholar]
- 36.Chen M, Woods SC, Porte D., Jr Effect of cerebral intraventricular insulin on pancreatic insulin secretion in the dog. Diabetes 24: 910–914, 1975 [DOI] [PubMed] [Google Scholar]
- 37.Cholerton B, Baker LD, Trittschuh EH, Crane PK, Larson EB, Arbuckle M, Hernandez H, McCurry SM, Bowen JD, McCormick WC, Craft S. Insulin and sex interactions in older adults with mild cognitive impairment. J Alzheimers Dis 31: 401–410, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chowers I, Lavy S, Halpern L. Effect of insulin administered intracisternally on the glucose level of the blood and the cerebrospinal fluid in vagotomized dogs. Exp Neurol 14: 383–389, 1966 [DOI] [PubMed] [Google Scholar]
- 39.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55: 978–987, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav 103: 10–16, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes 52: 682–687, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA 84: 8628–8632, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corp ES, Woods SC, Porte D, Jr, Dorsa DM, Figlewicz DP, Baskin DG. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett 70: 17–22, 1986 [DOI] [PubMed] [Google Scholar]
- 45.Deutsch R. Conditioned hypoglycemia: a mechanism for saccharin induced sensitivity to insulin in the rat. J Comp Physiol Psychol 86: 350–358, 1974 [DOI] [PubMed] [Google Scholar]
- 46.Drazen DL, Vahl TP, D'Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147: 23–30, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta 1500: 88–96, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Fehm-Wolfsdorf G, Gnadler M, Kern W, Klosterhalfen W, Kerner W. Classically conditioned changes of blood glucose level in humans. Physiol Behav 54: 155–160, 1993 [DOI] [PubMed] [Google Scholar]
- 49.Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. Am J Physiol Regul Integr Comp Physiol 295: R388–R394, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Figlewicz DP, Nadzan AM, Sipols AJ, Green PK, Liddle RA, Porte D, Jr, Woods SC. Intraventricular CCK-8 reduces single meal size in the baboon by interaction with type-A CCK receptors. Am J Physiol Regul Integr Comp Physiol 263: R863–R867, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Fisher SJ, Bruning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes 54: 1447–1451, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Flaherty CF, Becker HC. Influence of conditioned stimulus context on hyperglycemic conditioned responses. Physiol Behav 33: 587–593, 1984 [DOI] [PubMed] [Google Scholar]
- 53.Flaherty CF, Grigson PS, Brady A. Relative novelty of conditioning context influences directionality of glycemic conditioning. J Exp Psychol Anim Behav Process 13: 144–149, 1987 [PubMed] [Google Scholar]
- 54.Flaherty CF, Uzwiak AJ, Levine J. Apparent hyperglycemic and hypoglycemic conditioned responses with exogenous insulin as the unconditioned stimulus. Anim Learn Behav 8: 382–386, 1980 [Google Scholar]
- 55.Foster LA, Ames NK, Emery RS. Food intake and serum insulin responses to intraventricular infusions of insulin and IGF-I. Physiol Behav 50: 745–749, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Grillo CA, Tamashiro KL, Piroli GG, Melhorn S, Gass JT, Newsom RJ, Reznikov LR, Smith A, Wilson SP, Sakai RR, Reagan LP. Lentivirus-mediated downregulation of hypothalamic insulin receptor expression. Physiol Behav 92: 691–701, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallschmid M, Benedict C, Schultes B, Born J, Kern W. Obese men respond to cognitive but not to catabolic brain insulin signaling. Int J Obes (Lond) 32: 275–282, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes 53: 3024–3029, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Havrankova J, Brownstein M, Roth J. Insulin and insulin receptors in the rodent brain. Diabetologia 20: 268–273, 1981 [PubMed] [Google Scholar]
- 60.Havrankova J, Roth J, Browstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272: 827–829, 1978 [DOI] [PubMed] [Google Scholar]
- 61.Helmstaedter V, Feurle GE, Forssmann WG. Insulin-, glucagon-, and somatostatin-immunoreactive endocrine cells in the equine pancreas. Cell Tissue Res 172: 447–454, 1976 [DOI] [PubMed] [Google Scholar]
- 62.Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience 17: 1127–1138, 1986 [DOI] [PubMed] [Google Scholar]
- 63.Hoyer S. Brain glucose and energy metabolism during normal aging. Aging (Milano) 2: 245–258, 1990 [DOI] [PubMed] [Google Scholar]
- 64.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite 7: 381–386, 1986 [DOI] [PubMed] [Google Scholar]
- 65.Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, Porte D, Jr, Figewicz DP. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull 30: 571–575, 1993 [DOI] [PubMed] [Google Scholar]
- 66.Jaillard T, Roger M, Galinier A, Guillou P, Benani A, Leloup C, Casteilla L, Penicaud L, Lorsignol A. Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes 58: 1544–1549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jansson L, Hellerstrom C. Stimulation by glucose of the blood flow to the pancreatic islets of the rat. Diabetologia 25: 45–50, 1983 [DOI] [PubMed] [Google Scholar]
- 68.Kaneto A, Kosaka K. Stimulation of glucagon and insulin secretion by acetylcholine infused intrapancreatically. Endocrinology 95: 676–681, 1974 [DOI] [PubMed] [Google Scholar]
- 69.Katschinski M. Nutritional implications of cephalic phase gastrointestinal responses. Appetite 34: 189–196, 2000 [DOI] [PubMed] [Google Scholar]
- 70.Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem 58: 337–346, 1923 [Google Scholar]
- 71.Kimmel JR, Hayden LJ, Pollock HG. Isolation and characterization of a new pancreatic polypeptide hormone. J Biol Chem 250: 9369–9376, 1975 [PubMed] [Google Scholar]
- 72.Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu Rev Physiol 65: 313–332, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C, Lowe C, Schwartz MW, Shepherd PR, Anderson GM, Grattan DR, Tups A. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci 30: 16180–16187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ku SK, Lee HS. Distribution and frequency of endocrine cells in the pancreas of the ddY mouse: an immunohistochemical study. Eur J Histochem 49: 125–130, 2005 [PubMed] [Google Scholar]
- 75.Langerhans P. Beitrage zur Mikroscopischen Anatomie der Bauchspeichel Druse. Berlin: Gustav Lange, 1869 [Google Scholar]
- 76.Leahy JL, Fineman MS. Impaired phasic insulin and amylin secretion in diabetic rats. Am J Physiol Endocrinol Metab 275: E457–E462, 1998 [DOI] [PubMed] [Google Scholar]
- 77.Leites SM, Pavlov GT. Conditioned reaction to hypoglycemic effect of insulin in experimental diabetes. Zh Vyssh Nerv Deiat Im I P Pavlova 4: 234–244, 1954 [PubMed] [Google Scholar]
- 78.Levin BE, Magnan C, Dunn-Meynell A, Le Foll C. Metabolic sensing and the brain: who, what, where, and how? Endocrinology 152: 2552–2557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology 79: 466–473, 1980 [PubMed] [Google Scholar]
- 80.Liu M, Shen L, Begg DP, D'Alessio DA, Woods SC. Insulin increases central apolipoprotein E levels as revealed by an improved technique for collection of cerebrospinal fluid from rats. J Neurosci Methods 209: 106–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Louis-Sylvestre J. The cephalic phase of insulin secretion. Diabete Metab 13: 63–73, 1987 [PubMed] [Google Scholar]
- 82.Marks JL, Eastman CJ. Ontogeny of insulin binding in different regions of the rat brain. Dev Neurosci 12: 349–358, 1990 [DOI] [PubMed] [Google Scholar]
- 83.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinology 127: 3236, 1990 [DOI] [PubMed] [Google Scholar]
- 84.Matysiak J, Green L. On the directionality of classically-conditioned glycemic responses. Physiol Behav 32: 5–9, 1984 [DOI] [PubMed] [Google Scholar]
- 85.McGowan MK, Andrews KM, Grossman SP. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav 51: 753–766, 1992 [DOI] [PubMed] [Google Scholar]
- 86.Montague CT, Prins JB, Sanders L, Digby JE, O'Rahilly S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 46: 342–347, 1997 [DOI] [PubMed] [Google Scholar]
- 87.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104: 2501–2506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res 2: 321–327, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Okita M, Inui A, Baba S, Kasuga M. Central cholinergic regulation of pancreatic polypeptide secretion in conscious dogs. J Endocrinol 154: 311–317, 1997 [DOI] [PubMed] [Google Scholar]
- 90.Overduin J, Dworkin BR, Jansen A. Introduction and commentary to: M. I. Mityushov (1954) “Conditioned reflex secretion of insulin”. Integr Physiol Behav Sci 32: 228–246, 1997 [DOI] [PubMed] [Google Scholar]
- 91.Park CR, Johnson LH. Effect of insulin on transport of glucose and galactose into cells of rat muscle and brain. Am J Physiol 182: 17–23, 1955 [DOI] [PubMed] [Google Scholar]
- 92.Piper HA, Allen RS, Murlin JR. Aqueous extracts of pancreas. II. Physical and chemical behavior of insulin. J Biol Chem 58: 321, 1923 [Google Scholar]
- 93.Plata-Salaman CR, Oomura Y. Effect of intra-third ventricular administration of insulin on food intake after food deprivation. Physiol Behav 37: 735–739, 1986 [DOI] [PubMed] [Google Scholar]
- 94.Porte D., Jr Beta adrenergic stimulation of insulin release in man. Diabetes 16: 150–155, 1967 [DOI] [PubMed] [Google Scholar]
- 95.Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest 46: 86–94, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porte D, Jr, Williams RH. Inhibition of insulin release by norepinephrine in man. Science 152: 1248–1250, 1966 [DOI] [PubMed] [Google Scholar]
- 97.Potes CS, Turek VF, Cole RL, Vu C, Roland BL, Roth JD, Riediger T, Lutz TA. Noradrenergic neurons of the area postrema mediate amylin's hypophagic action. Am J Physiol Regul Integr Comp Physiol 299: R623–R631, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Pouliot MC, Despres JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, Tremblay A, Bouchard C. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 41: 826–834, 1992 [DOI] [PubMed] [Google Scholar]
- 99.Power ML, Schulkin J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite 50: 194–206, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing β cells in two mouse models of pancreas development. Proc Natl Acad Sci USA 101: 2924–2929, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114: 652–658, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Russek M, Pina S. Conditioning of adrenalin anorexia. Nature 193: 1296–1297, 1962 [DOI] [PubMed] [Google Scholar]
- 103.Samols E, Weir GC. Adrenergic modulation of pancreatic A, B, and D cells: α-adrenergic suppression and β-adrenergic stimulation of somatostatin secretion, α-adrenergic stimulation of glucagon secretion in the perfused dog pancreas. J Clin Invest 63: 230–238, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanger F, Tuppy H. The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochem J 49: 481–490, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanger F, Tuppy H. The amino-acid sequence in the phenylalanyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J 49: 463–481, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev 13: 387–414, 1992 [DOI] [PubMed] [Google Scholar]
- 107.Secchi A, Caldara R, Caumo A, Monti LD, Bonfatti D, Di Carlo V, Pozza G. Cephalic-phase insulin and glucagon release in normal subjects and in patients receiving pancreas transplantation. Metabolism 44: 1153–1158, 1995 [DOI] [PubMed] [Google Scholar]
- 108.Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci 4: 901–909, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Shiraishi J, Yanagita K, Fukumori R, Sugino T, Fujita M, Kawakami S, McMurtry JP, Bungo T. Comparisons of insulin related parameters in commercial-type chicks: evidence for insulin resistance in broiler chicks. Physiol Behav 103: 233–239, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Siegel S. Conditioning insulin effects. J Comp Physiol Psychol 89: 189–199, 1975 [PubMed] [Google Scholar]
- 111.Siegel S. Conditioning of insulin-induced glycemia. J Comp Physiol Psychol 78: 233–241, 1972 [DOI] [PubMed] [Google Scholar]
- 112.Sims EA, Danforth E, Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 29: 457–496, 1973 [DOI] [PubMed] [Google Scholar]
- 113.Sisley S, Sandoval D. Hypothalamic control of energy and glucose metabolism. Rev Endocr Metab Disord 12: 219–233, 2011 [DOI] [PubMed] [Google Scholar]
- 114.Steiner DF, Freinkel N. (editors). Handbook of Physiology. Endocrine Pancreas. Bethesda, MD: Am. Physiol. Soc., 1972, sect. 7, vol. I [Google Scholar]
- 115.Stephan F, Reville P, Thierry R, Schlienger JL. Correlations between plasma insulin and body weight in obesity, anorexia nervosa and diabetes mellitus. Diabetologia 8: 196–201, 1972 [DOI] [PubMed] [Google Scholar]
- 116.Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav 83: 47–54, 2004 [DOI] [PubMed] [Google Scholar]
- 117.Stockhorst U, Huenig A, Ziegler D, Scherbaum W. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol Behav 103: 31–38, 2011 [DOI] [PubMed] [Google Scholar]
- 118.Stockhorst U, Steingruber HJ, Scherbaum WA. Classically conditioned responses following repeated insulin and glucose administration in humans. Behav Brain Res 110: 143–159, 2000 [DOI] [PubMed] [Google Scholar]
- 119.Storlien LH, Bruce DG. Mind over metabolism: the cephalic phase in relation to non-insulin-dependent diabetes and obesity. Biol Psychol 28: 3–23, 1989 [DOI] [PubMed] [Google Scholar]
- 120.Storlien LH, Smith DJ, Atrens DM, Lovibond PF. Development of hypoglycemia and hyperglycemia as a function of number of trials in insulin conditioning. Physiol Behav 35: 603–606, 1985 [DOI] [PubMed] [Google Scholar]
- 121.Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav 19: 309–313, 1977 [DOI] [PubMed] [Google Scholar]
- 122.Sugino T, Yamaura J, Yamagishi M, Ogura A, Hayashi R, Kurose Y, Kojima M, Kangawa K, Hasegawa Y, Terashima Y. A transient surge of ghrelin secretion before feeding is modified by different feeding regimens in sheep. Biochem Biophys Res Commun 298: 785–788, 2002 [DOI] [PubMed] [Google Scholar]
- 123.Swann JP. Insulin: a case study in the emergence of collaborative pharmacomedical research. Pharm Hist 28: 65–74, 1986 [PubMed] [Google Scholar]
- 124.Szabo AJ, Szabo O. Influence of the insulin sensitive central nervous system glucoregulator receptor on hepatic glucose metabolism. J Physiol 253: 121–133, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Szabo O, Szabo AJ. Neuropharmacological characterization of insulin-sensitive CNS glucoregulator. Am J Physiol 229: 663–668, 1975 [DOI] [PubMed] [Google Scholar]
- 126.Taylor IL, Feldman M, Richardson CT, Walsh JH. Gastric and cephalic stimulation of human pancreatic polypeptide release. Gastroenterology 75: 432–437, 1978 [PubMed] [Google Scholar]
- 127.Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite 34: 206–213, 2000 [DOI] [PubMed] [Google Scholar]
- 128.Teff KL. How neural mediation of anticipatory and compensatory insulin release helps us tolerate food. Physiol Behav 103: 44–50, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vahl TP, Drazen DL, Seeley RJ, D'Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology 151: 569–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Houten M, Posner BI. Insulin binds to brain blood vessels in vivo. Nature 282: 623–625, 1979 [DOI] [PubMed] [Google Scholar]
- 131.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin-binding sites in the rat brain: in vivo localization to the circumventricular organs by quantitative radioautography. Endocrinology 105: 666–673, 1979 [DOI] [PubMed] [Google Scholar]
- 132.van Houten M, Posner BI, Kopriwa BM, Brawer JR. Insulin binding sites localized to nerve terminals in rat median eminence and arcuate nucleus. Science 207: 1081–1083, 1980 [DOI] [PubMed] [Google Scholar]
- 133.von Mering JV, Minkowski O. Diabetes mellitus nach pankreasextirpation. Zbl Kiln Med 10: 393–394, 1889 [Google Scholar]
- 134.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weatherford SC, Ritter S. Lesion of vagal afferent terminals impairs glucagon-induced suppression of food intake. Physiol Behav 43: 645–650, 1988 [DOI] [PubMed] [Google Scholar]
- 136.Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA 84: 3881–3885, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woods SC. Conditioned hypoglycemia. J Comp Physiol Psychol 90: 1164–1168, 1976 [DOI] [PubMed] [Google Scholar]
- 138.Woods SC. Conditioned hypoglycemia and conditioned insulin secretion. Adv Metab Disord 10: 485–495, 1983 [DOI] [PubMed] [Google Scholar]
- 139.Woods SC. Conditioned hypoglycemia: effect of vagotomy and pharmacological blockade. Am J Physiol 223: 1424–1427, 1972 [DOI] [PubMed] [Google Scholar]
- 140.Woods SC. The eating paradox: how we tolerate food. Psychol Rev 98: 488–505, 1991 [DOI] [PubMed] [Google Scholar]
- 141.Woods SC, Alexander KR, Porte D., Jr Conditioned insulin secretion and hypoglycemia following repeated injections of tolbutamide in rats. Endocrinology 90: 227–231, 1972 [DOI] [PubMed] [Google Scholar]
- 142.Woods SC, Decke E, Vasselli JR. Metabolic hormones and regulation of body weight. Psychol Rev 81: 26–43, 1974 [DOI] [PubMed] [Google Scholar]
- 143.Woods SC, Hutton RA, Makous W. Conditioned insulin secretion in the albino rat. Proc Soc Exp Biol Med 133: 964–968, 1970 [DOI] [PubMed] [Google Scholar]
- 144.Woods SC, Kulkosky PJ. Classically conditioned changes of blood glucose level. Psychosom Med 38: 201–219, 1976 [DOI] [PubMed] [Google Scholar]
- 145.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979 [DOI] [PubMed] [Google Scholar]
- 146.Woods SC, Makous W, Hutton RA. A new technique for conditioned hypoglycemia. Psychon Sci 10: 389–390, 1968 [Google Scholar]
- 147.Woods SC, Makous W, Hutton RA. Temporal parameters of conditioned hypoglycemia. J Comp Physiol Psychol 69: 301–307, 1969 [DOI] [PubMed] [Google Scholar]
- 148.Woods SC, Porte D., Jr Effect of intracisternal insulin on plasma glucose and insulin in the dog. Diabetes 24: 905–909, 1975 [DOI] [PubMed] [Google Scholar]
- 149.Woods SC, Porte D., Jr Insulin and the set-point regulation of body weight. In: Hunger: Basic Mechanisms and Clinical Implications, edited by Novin D, Bray GA, Wyrwichka W. New York: Raven, 1976, p. 273–280 [Google Scholar]
- 150.Woods SC, Porte D., Jr The central nervous system, pancreatic hormones, feeding, and obesity. Adv Metab Disord 9: 283–312, 1978 [DOI] [PubMed] [Google Scholar]
- 151.Woods SC, Porte D., Jr Neural control of the endocrine pancreas. Physiol Rev 54: 596–619, 1974 [DOI] [PubMed] [Google Scholar]
- 152.Woods SC, Porte D., Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol Endocrinol Metab Gastrointest Physiol 233: E331–E334, 1977 [DOI] [PubMed] [Google Scholar]
- 153.Woods SC, Ramsay DS. Food intake, metabolism and homeostasis. Physiol Behav 104: 4–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Woods SC, Shogren RE., Jr Glycemic responses following conditioning with different doses of insulin in rats. J Comp Physiol Psychol 81: 220–225, 1972 [DOI] [PubMed] [Google Scholar]
- 155.Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV. Conditioned insulin secretion and meal feeding in rats. J Comp Physiol Psychol 91: 128–133, 1977 [DOI] [PubMed] [Google Scholar]
- 156.Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115: 951–958, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Young WSI, Kuhar MJ, Roth J, Brownstein MJ. Radiohistochemical localization of insulin receptors in the adult and developing rat brain. Neuropeptides 1: 15–22, 1980 [Google Scholar]


