Abstract
This communication describes the development of a thiamin sensor based on the bacterial thiamin binding protein. A triple mutant (C48S, C50S, S62C) of TbpA was labeled on C62 with N-[2-(l-maleimidyl)ethyl]-7-(diethylamino)coumarin-3-carboxamide (MDCC). Thiamin binding to this protein reduced the coumarin fluorescence giving a thiamin sensor with low nanomolar sensitivity.
Vitamin B1, also known as thiamin, is an essential nutrient for all animals and its pyrophosphate derivative acts as a cofactor that plays a key role in the stabilization of acyl carbanion intermediates involved in carbohydrate and amino acid metabolism. Beriberi, a disease caused by thiamin deficiency occurs primarily in developing countries, is usually caused by malnutrition. In developed countries thiamin deficiency is generally thought to be rare but does occur in Wernicke-Korsakoff syndrome, a condition caused by alcoholism. However, more recently, low levels of thiamin were discovered to be prevalent in cases of type 1 and type 2 diabetes.1 Interestingly, in the same study, it was noted that, historically, these low levels have been masked by the use of a conventional assay methodology based upon the magnitude of erythrocyte transketolase activation upon the addition of exogenous thiamin pyrophosphate. In 2003, an outbreak in cases of encephalopathy in infants were reported and found to be due to formula being deficient in thiamin.2 Other animals such as large predatory fish (e.g., salmon, trout, and walleye) of the Laurentian Great Lakes and the Baltic Sea suffer from thiamin deficiency caused by consuming prey fish that contain high levels of bacterially derived thiaminase I in their gut.3 Thiaminase I is an enzyme that efficiently degrades thiamin, and despite knowledge of its activity for over 6 decades, there has been no clear explanation of a physiological function. This type of enzymatic poisoning has been observed to occur in humans too, but the source of thiaminase I in that case was likely the nardoo fern.4
The most common clinical methods for measuring thiamin concentrations include the indirect assay mentioned above based upon transketolase activation and a more direct, but laborious, HPLC-based assay which takes advantage of the oxidation of thiamin into a fluorescent compound, thiochrome, using alkaline potassium ferricyanide.5 Here we present the engineering of the E. coli periplasmic thiamin binding protein (TbpA) and show that this fluorescently labeled mutant is capable of detecting nanomolar levels of thiamin in a real-time, single reagent, format. The selected site-directed mutant (S62C) TbpA was thioconjugated to an environmentally sensitive fluorophore that responds to changes in protein structure induced by the binding of thiamin in the active site.
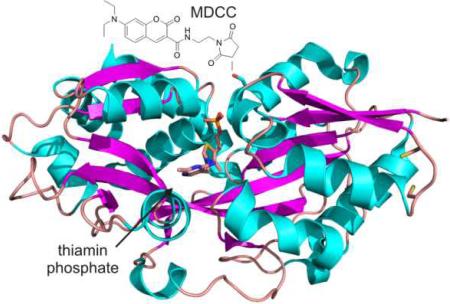
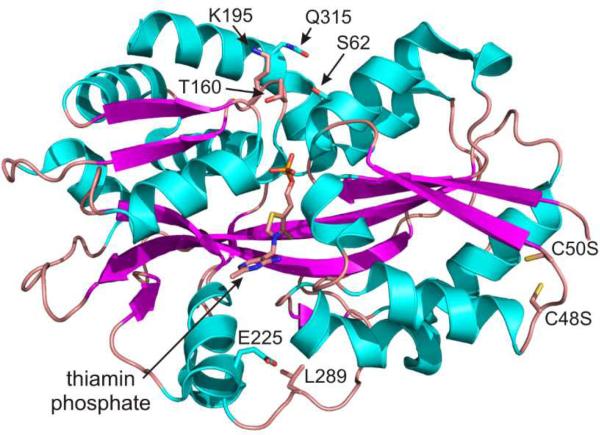
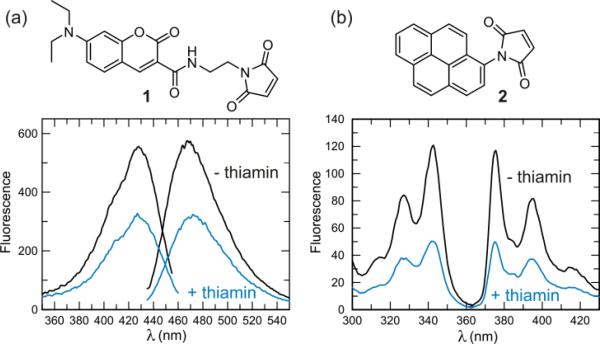
Selection of serine 62 as a site for thio-conjugation was made possible due to the fact that TbpA was recently structurally characterized (Figure 1.).6 Similar methodologies have been successfully undertaken in the engineering of other periplasmic solute binding proteins7,8, but to our knowledge, this is the first example of creating a vitamin-binding sensor in this way. The wild-type (WT) protein had two cysteine residues (C48 and C50) that were mutated to serine to prevent labeling at those positions. Several positions were attempted (S62C, T160C, K195C, E225C, L289C, and Q315C), but none gave the magnitude of fluorescence change as was seen with the S62C mutation. Conjugating the purified mutant protein with the thio-reactive maleimide probes, N- [2-( l-maleimidyl)ethyl]-7-(diethylamino)coumarin-3- carboxamide (MDCC; 1) or N-(1-pyrene)maleimide (pyrene; 2) resulted in a sensor that responded by a reduction in fluorescence of ~50% upon addition of a saturating concentration of thiamin or thiamin phosphate (thiamin addition shown in Figure 2.).
Figure 1.
Structure of E. coli TbpA with thiamin phosphate bound. The mutations made in engineering the protein are shown near each of the amino acid side chains.
Figure 2.
Measurement of the fluorescence change with MDCC (1) and pyrene (2) labeled TbpA. a) Fluorescence excitation and emission scans of 200 nM MDCC-TbpA. Upon addition of a saturating concentration of thiamin (2 μM), the fluorescence is reduced 47% at 467 nm. b) Fluorescence excitation and emission scans of 200 nM pyrene-TbpA. Upon addition of thiamin, the fluorescence is reduced 57% at 375 nm.
Important to the capability of detecting low concentrations of thiamin is the dissociation constant (Kd). Therefore, a series of titrations were performed using a computer-driven titration module that slowly adds ligand to a solution (magnetically stirred) while recording fluorescence changes real-time. As shown in Figure 3a, the apparent dissociation constant for thiamin was 9.5 ± 1 nM when the titration was performed at 50 nM MDCC-TbpA. Due to the relatively tight Kd, the data were fitted using a quadratic equation. Therefore, it should be stated that while this measurement provides a good appoximation of the true Kd, it is inherently difficult to measure under conditions where the protein concentration is comparable to the Kd.
Figure 3.
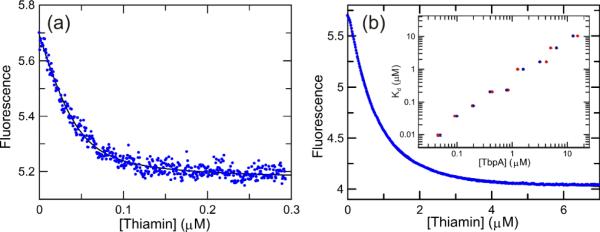
Titration of MDCC-TbpA with thiamin. A continuous titration of thiamin into a solution of 50 nM MDCC-TpbA performed over the course of 5 minutes is shown. An apparent Kd = 9.5 ± 1 nM and a binding-site concentration of 46 ± 3 nM was obtained (See supporting information). Excitation was at 425 nm and emission was measured using a 450 nm long-wave pass filter. The Y-axis is in arbitrary fluorescence units. b) Titration performed at a protein concentration of 800 nM. The data were fitted to extract an apparent Kd = 230 ± 3 nM and a binding site concentration of 850 ± 10 nM. Inset) A log-log plot of the apparent Kd as a function of either the protein concentration added (•) or the fitted binding site concentration (•).
Interestingly, the dissociation constant for thiamin increased in an approximately linear fashion as a function of protein concentration suggesting that possibly dimer formation could be negatively affecting the binding of thiamin (Figure 3b.). Indeed, this was suggested in the original report of the crystal structure where it appeared that dimer formation could obstruct the binding site.6 The physiological significance of this finding is presently unknown, but what is clear is that at low concentrations of protein, where it will most likely prove to be useful, nanomolar detection of thiamin is possible. The affinity of thiamin binding to pyrene-TbpA was in good agreement with these values (results not shown).
The binding of thiamin phosphate and thiamin pyrophosphate were also inspected. The binding of thiamin phosphate was essentially identical (Kd = 70 ± 8 nM at 200 nM MDCC-TbpA) to that of thiamin, as was seen with the wild-type protein, but thiamin pyrophosphate registered no discernable fluorescence change under the same conditions, which was in contrast to the wild-type protein (intrinsic protein fluorescence).6 A possible explanation for this observation can be made from inspection of the structure. The S62C mutation is in close proximity (less than ~5 Å) to where the β-phosphate would be positioned and upon conjugation with the MDCC, the binding of thiamin pyrophosphate may not be favorable. This gives the sensor a level of discrimination against the pyrophosphate that is not present in the wild-type protein offering a selective sensor for thiamin and thiamin phosphate. The removal of the pyrophosphate would presumably be facile using a non-specific phosphatase or other method if the concentration of the pyrophosphate form is of interest.
The kinetics of thiamin binding and dissociation were inspected to gain an understanding of the extent to which this probe might be useful in a “real-time” format. Shown in figure 4a are the results of a stopped-flow fluorescence experiment where 800 nM thiamin was rapidly mixed with 200 nM MDCC-TbpA. The binding data were biphasic with observed rates of 0.127 ± 0.001 s−1 and 0.048 ± 0.001 s−1, with the fast phase representing 68% of the total change. By and large, the overall change occurred in less than 1 min. Overall, the kinetics for thiamin binding to pyrene-TbpA were similar with observed rates of 0.206 ± 0.001 s−1 and 0.041 ± 0.001 s−1 (results not shown). Under all conditions examined, the observed rates appeared to be saturated, suggesting that these fluorescence changes represent some uni-molecular processes occurring following the binding reaction, probably conformational changes of the protein. Importantly, similar binding kinetics were observed using the WT protein, except that an additional, nearly diffusion limited binding reaction preceded two phases similar in rates to those measured here (results not shown). Increasing the initial protein concentration in a range from 200 nM to 1.8 μM (final after mixing) had no clear impact on the observed rates or relative amplitudes of the two phases. The dissociation reaction was inspected next using thiaminase I and DTT to rapidly degrade thiamin as it dissociates from the active site (Figure 4b). Thiaminase I degrades thiamin in the presence of free thiols such as DTT and other nucleophilic compounds.
Figure 4.
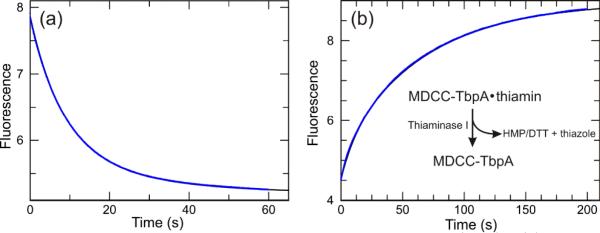
Kinetics of the fluorescence change associated with thiamin binding and dissociation from MDCC-TbpA. a) Time dependence of fluorescence change measured using a stopped-flow apparatus where 800 nM thiamin was rapidly mixed with 200 nM MDCC-TbpA. b) Time dependence of thiamin dissociation from MDCC-TbpA. A solution of 200 nM MDCC-TbpA was preincubated with 10 μM thiamin then rapidly mixed with 15 μM thiaminase I in buffer containing 5 mM DTT. Thiaminase I rapidly degrades free thiamin in the presence of a variety of nucleophilic co-substrates, including DTT. The concentration of thiaminase I was titrated to be sure that a sufficient amount was used. The Y-axes are in arbitrary fluorescence units.
The dissociation of thiamin also occurred in a biphasic fashion with rates of 0.092 ± 0.001 s−1 and 0.015 ± 0.001 s−1, with the slow phase representing 83% of the total change. Again the observed rates and relative amplitudes showed no clear trend as a function of the protein concentration in the range of 50-200 nM.
In conclusion, thiamin is an important vitamin and thiamin deficiency disease is still a significant medical problem. The current assays for thiamin are inadequate. Based on the structure of the thiamin binding protein, a new sensor capable of real-time detection of thiamin and thiamin phosphate in the nanomolar range was developed. This sensor is likely to be of use to clinicians, enzymologists and biosynthetic chemists with a need to run thiamin assays.
Supplementary Material
Acknowledgment
This research was funded by NIH grant DK67081 (to SEE) DK44083 (to TPB) and by the Robert A. Welch Foundation (A-0034)
Footnotes
Supporting Information Available: A detailed description of the purification and fluorophore conjugation of the mutant TbpA as well as a description of the fitting procedures can be found in the supporting information. This material is available free of charge via the Internet at
Notes and References
- 1.Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. Diabetologia. 2007;50(10):2164. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fattal-Valevski A, Kesler A, Sela BA, Nitzan-Kaluski D, Rotstein M, Mesterman R, Toledano-Alhadef H, Stolovitch C, Hoffmann C, Globus O, Eshel G. Pediatrics. 2005;115(2):233. doi: 10.1542/peds.2004-1255. [DOI] [PubMed] [Google Scholar]
- 3.Honeyfield DC, Brown SB, Fitzsimons JD, Tillitt DE. Journal of Aquatic Animal Health. 2005;17(1):1. doi: 10.1577/H08-006.1. [DOI] [PubMed] [Google Scholar]
- 4.Earl JW, McCleary BV. Nature. 1994;368(6473):683. doi: 10.1038/368683a0. [DOI] [PubMed] [Google Scholar]
- 5.Talwar D, Davidson H, Cooney J, JO'Reilly St. D. Clin. Chem. 2000;46(5):704. [PubMed] [Google Scholar]
- 6.Soriano EV, Rajashankar KR, Hanes JW, Bale S, Begley TP, Ealick SE. Biochemistry. 2008;47(5):1346. doi: 10.1021/bi7018282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brune M, Hunter JL, Corrie JE, Webb MR. Biochemistry. 1994;33(27):8262. doi: 10.1021/bi00193a013. [DOI] [PubMed] [Google Scholar]
- 8.de Lorimier RM, Smith JJ, Dwyer MA, Looger LL, Sali KM, Paavola CD, Rizk SS, Sadigov S, Conrad DW, Loew L, Hellinga HW. Protein Sci. 2002;11(11):2655. doi: 10.1110/ps.021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanes JW, Kraft CE, Begley TP. Anal. Biochem. 2007;368(1):33. doi: 10.1016/j.ab.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Ausubel FM, Brent F, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biolog. John Wiley and Sons; New York: 1987. [Google Scholar]
- 2.Sambrook J, Fritsch GF, Maniatis T. Molecular Cloning: A Laboratory Guide. Cold Spring Harbor Laboratory Press; USA: 1989. [Google Scholar]
- 3.Soriano EV, Rajashankar KR, Hanes JW, Bale S, Begley TP, Ealick SE. Biochemistry. 2008;47(5):1346. doi: 10.1021/bi7018282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanes JW, Kraft CE, Begley TP. An assay for thiaminase I in complex biological samples. Anal. Biochem. 2007;368(1):33. doi: 10.1016/j.ab.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.