Abstract
Sickle cell disease (SCD) is a disorder known to impact the respiratory system. We sought to identify respiratory muscle force and lung volume relationships in a paediatric SCD population. Thirty-four SCD-SS subjects underwent pulmonary function testing. Height, weight, age, and gender-adjusted percent predicted maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) values were compared to spirometry and lung volumes. Statistical analyses were performed using Pearson’s correlation coefficient and paired two-tailed t-test. The mean ±standard deviation (SD) MIP and MEP was 69.6 ±31.6 cm H2O and 66.9 ±22.9 cm H2O, respectively, and mean ±SD percent predicted MIP (101.3 ±45.9) exceeded MEP (72.1 ±26.0) (p = 0.002). MIP correlated with forced vital capacity (FVC; r = 0.51, p = 0.001) and TLC (r = 0.54, p < 0.0001). MEP also correlated with FVC (r = 0.43, p = 0.011) and total lung capacity (TLC; r = 0.42, p = 0.013). Pearson’s correlation coefficient testing yielded relationships between MIP and MEP (r = 0.64, p < 0.0001). SCD-SS patients showed correlations between respiratory muscle force and lung volume, and reduced percent predicted expiratory muscle force compared to inspiratory muscle force. Respiratory muscle strength may affect lung volumes in these patients, and expiratory muscles may be more susceptible than the diaphragm to SCD-induced vaso-occlusive damage.
Keywords: Sickle cell anaemia, Paediatrics, pulmonary function, muscle strength
Introduction
Sickle cell disease (SCD) is an autosomal recessive disorder resulting in altered haemoglobin configuration. This abnormality in haemoglobin formation causes red cell membrane distortion and impaired microvasculature blood flow, leading to repeated vaso-occlusive events and pain crises (Gladwin and Vichinsky, 2008; Steinberg, 1999; Driscoll, 2007). Over time, these episodes may result in multi-system organ injury. In the respiratory system, vaso-occlusive events cause repeated pain crises and acute chest episodes, damaging the lung parenchyma and pulmonary vasculature (Sedrak et al, 2009; Caboot and Allen, 2008). Studies investigating pulmonary function changes in children with SCD have shown that both restrictive lung processes (Sylvester et al, 2004), and obstructive lung disease may be present, and can worsen over time (Koumbourlis et al, 2007, Koumbourlis et al, 2001; Field et al, 2008). In addition, vaso-occlusive crises also cause rib infarction and severe bone pain (Rucknagel, 2001). However, few studies have evaluated the impact of sickle cell disease on skeletal muscle force in children. One study recently identified significant deficits in peripheral maximal muscle strength in children with SCD compared to healthy controls (Dougherty et al, 2011). This study also revealed strong positive correlations between muscle strength and age and lean body mass in SCD subjects (Dougherty et al, 2011). Interestingly, the deficits seen were out of proportion to the expected effect of these variables, suggesting other unidentified factors were contributing to attenuated strength (Dougherty et al, 2011). Despite these new findings, the effects of SCD on the respiratory muscles of the chest wall, abdomen and diaphragm in children with SCD have not yet been characterized.
Respiratory muscle strength testing is a useful pulmonary function assessment that can identify respiratory muscle abnormalities in both children and adults. The respiratory muscle force tests consist of measuring maximal inspiratory (MIP) and expiratory pressures (MEP) at the mouth from a pre-determined lung volume. In children with chronic respiratory diseases, measurements of respiratory muscle strength have been shown to be useful in evaluating children with neuromuscular disorders (DePalo & McCool, 2002), cystic fibrosis (Dassios et al, 2012), and chronic obstructive pulmonary disease (COPD) (Terzano et al, 2008).
Diminished respiratory muscle strength could be, in part, responsible for the decreased lung volumes and flows seen in patients with SCD. In this study, we hypothesized that respiratory muscle force, defined as percent predicted MIP and MEP, would correlate with pulmonary function measures. In addition, we wished to assess whether there might be differential consequences of SCD on inspiratory versus expiratory muscles.
Methods
This study was approved by The Children’s Hospital of Philadelphia Institutional Review Board (No. 2007-6-5188). All subjects participating in this study were enrolled after obtaining informed consent from their parents, and when appropriate, assent was obtained from the subject.
Subjects
All participants were recruited consecutively over a one-year period as part of a larger prospective study investigating oxyhaemoglobin desaturation and vasculopathy in sickle cell disease, and received care in the Sickle Cell Center at The Children’s Hospital of Philadelphia. Patients were recruited for the study if they were between 6 and 21 years of age, were able to complete pulmonary function testing, and had SCD type SS (SCD-SS) in steady state. Patients were disqualified from enrollment in the study if they received hydroxycarbamide, had a red blood cell transfusion within the last three months, had a pain crisis or acute chest syndrome event within the last month, or had congenital heart disease, neuromuscular disease or a chronic lung condition unrelated to SCD-SS.
Pulmonary Function Testing
Spirometry, body plethysmography, and respiratory muscle force testing were all performed at The Children’s Hospital of Philadelphia’s pulmonary function laboratory and administered by experienced paediatric respiratory therapists. American Thoracic Society protocols were followed for all pulmonary function tests (Green et al, 2002; Wanger et al, 2005; Miller et al, 2005). During spirometry testing, forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) and forced expiratory flow between 25% and 75% of FVC (FEF25-75%) were measured using the SpiroAir PFT system (Morgan Scientific, Haverhill, MA), and values were used to calculate the FEV1/FVC ratio. Static lung volumes via body plethysmography testing and respiratory muscle force testing were obtained using the Morgan Bodybox apparatus (Morgan Scientific). Functional residual capacity (FRC) was measured and used to derive the total lung capacity (TLC), residual volume (RV), and the RV/TLC ratio. Subjects were identified as having obstructive or restrictive spirometry patterns or diffusion capacity defects based on established American Thoracic Society pulmonary function test interpretation guidelines (Pellegrino et al 2005). Subjects were determined to have evidence of restrictive lung disease if TLC was less than the lower limit of normal (LLN) compared to the predicted value, i.e., in or below the lowest 5th percentile, as defined by a standard deviation (z) score of lower than (-) 1.6. This corresponded to a percent predicted value of approximately 80%. Subjects were considered to have evidence of obstructive disease if the FEV1/FVC ratio was less than the lower limit of normal compared to predicted, also defined as a z score of lower than (-) 1.6. Using the Morgan Body Box, muscle respiratory force was determined by measuring the MIP and MEP. To obtain MIP, subjects breathed through a closed, flanged mouthpiece attached to a pressure transducer, and were asked to exhale to RV and then produce a maximal inspiratory effort against a closed shutter. Patients were asked to hold their cheeks in with their hands to reduce buccal interference. Similarly, MEP was obtained by having subjects perform an inspiratory manoeuvre to TLC and produce a maximal expiratory effort. To prevent glottic closure, an adaptor with a 2 mm hole was placed between the patient and microbial filter. Testing was determined to be acceptable if maximal pressure was sustained for at least 1.5 s and the results of three efforts were within 20% of each other. All values were collected and stored in the MorganCompas database (Morgan Scientific). Spirometry and body plethysmography measurements were normalized according to age, height, gender, and ethnicity, and MIP and MEP were normalized according to age, height, and gender based on reference equations, and converted to a percent predicted value (Wilson et al, 1984).
Statistical Analysis
Relationships between respiratory muscle force absolute values, percent predicted values, and spirometry and static lung volumes were evaluated by determining the Pearson’s correlation coefficient (r). Correlation coefficients were tested for significance using a p-value of < 0.05 as the threshold for statistical significance. Statistical analyses were completed using the SAS statistical package.
Comparisons between inspiratory and expiratory muscle strength in subjects with SCD were done using a paired two-tailed t-test. A p-value < 0.05 was considered significant.
Results
Thirty-four subjects aged 6-19 years old with SCD-SS (16 males and 18 females) were evaluated. The average participant age was 12.9 ± 3.5 (mean ± standard deviation [SD]) years. All participants were of African ancestry. Spirometry, static lung volume, and respiratory muscle force results are summarized in Table I. Twenty-seven subjects had normal spirometry, and seven subjects (21%) had evidence of mild airway obstruction (FEV1/FVC < (-) 1.6 SD, but FEV1 > LLN in six, and just below LLN in the 7th. One subject had evidence of mild restriction (TLC = (-) 1.8 SD).
Table I.
SCD Demographic data and pulmonary function test results
| Parameter (n = 34) | Mean ± SD |
|---|---|
| Age (years) | 12.9 ± 3.5 |
| Male (n = 16) | 13.2 ± 3.0 |
| Female (n = 18) | 12.6 ± 3.9 |
| Height (cm) | 150.1 ± 19.8 |
| Weight (kg) | 41.7 ± 16.7 |
| Spirometry | |
| FVC (% predicted) | 99.7 ± 12.5 |
| FEV1 (% predicted) | 94.4 ± 11.6 |
| FEV1/FVC (%) | 84.6 ± 6.6 |
| FEF25-75 (% predicted) | 83.3 ± 22.8 |
| Lung Volumes | |
| TLC (% predicted) | 105.8 ± 11.1 |
| RV (% predicted) | 97.2 ± 31.5 |
| RV/TLC (%) | 25.8 ± 8.4 |
| Respiratory muscle force | |
| MIP (cm H2O) | 69.5 ± 31.6 |
| MEP (cm H2O) | 66.9 ± 22.8 |
| MIP (% predicted) | 101.3 ± 45.9 |
| MEP (% predicted) | 72.2 ± 25.97 |
SCD, sickle cell disease; SD, standard deviation; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF25-75, forced expiratory flow between 25% and 75% of FVC; TLC, total lung capacity; RV, reserve volume; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure.
The absolute MIP and MEP was 69.6 ± 31.6 cm H2O and 66.9 ± 22.9 H2O, respectively. There was no correlation between age and absolute MIP and MEP. The percent predicted MIP (%MIP) was 101.3 ± 45.9, and the percent predicted MEP (%MEP) was 72.1 ± 26.0 (p = 0.002). The %MIP correlated with percent predicted FVC, FEV1, and TLC (Table II, Fig. 1). The %MEP correlated with percent predicted FVC and TLC (Table II, Fig. 2). Although %MIP exceeded %MEP, there was a significant correlation between %MIP and %MEP (Table II, Fig. 3).
Table II.
Relationships between spirometry, lung volumes and respiratory muscle force
| % Predicted MIP | % Predicted MEP | |||
|---|---|---|---|---|
| Measure | Correlation (r) | P-value | Correlation (r) | P-value |
| Spirometry | ||||
| FVC (% predicted) | 0.52 | 0.002 | 0.43 | 0.011 |
| FEV1 (% predicted) | 0.44 | 0.008 | 0.22 | 0.211 |
| FEV1/FVC (%) | -0.10 | 0.573 | -0.30 | 0.085 |
| FEF25-75 (% predicted) | 0.09 | 0.613 | -0.04 | 0.822 |
| Lung Volumes | ||||
| TLC (% predicted) | 0.54 | 0.001 | 0.42 | 0.013 |
| RV (% predicted) | 0.09 | 0.613 | 0.12 | 0.499 |
| RV/TLC (%) | 0.01 | 0.955 | 0.10 | 0.573 |
| Respiratory muscle force | ||||
| MIP (% predicted) | ----- | ----- | 0.64 | <0.0001 |
MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF25-75, forced expiratory flow between 25% and 75% of FVC; TLC, total lung capacity; RV, reserve volume.
Fig. 1. Relationship between mean percent predicted MIP and FVC and TLC.
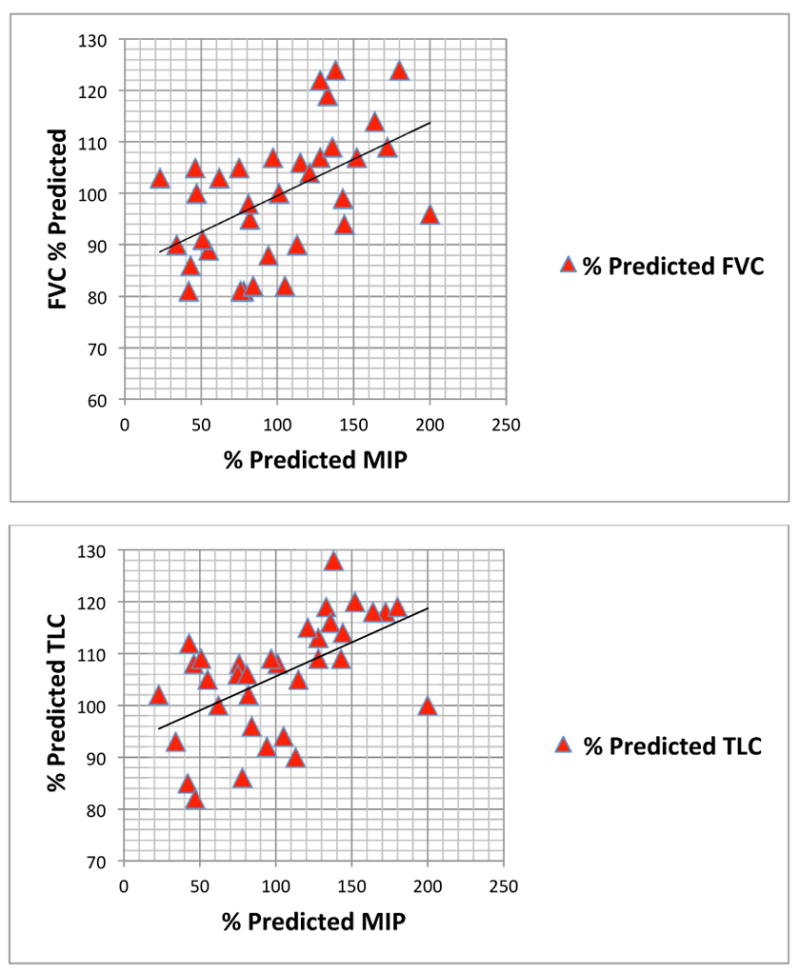
MIP, maximal inspiratory pressure; FVC, forced vital capacity; TLC, total lung capacity.
Fig. 2. Relationship between mean percent predicted MEP and FVC and TLC.
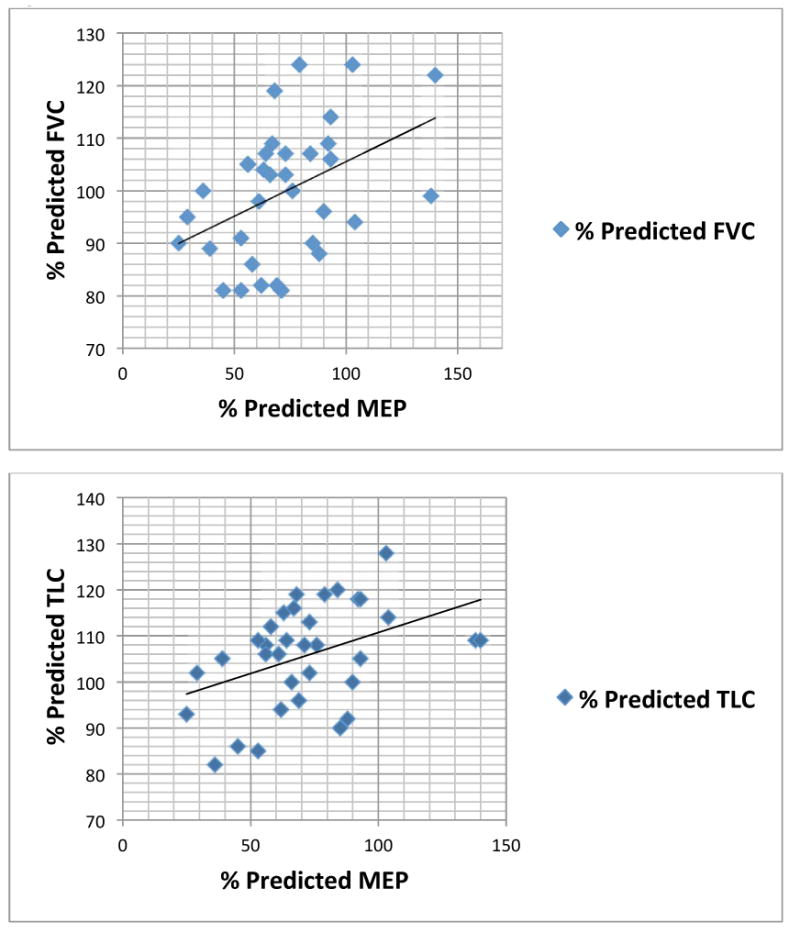
MEP, maximal expiratory pressure; FVC, forced vital capacity; TLC, total lung capacity.
Fig. 3. Relationship between percent predicted MIP and MEP.
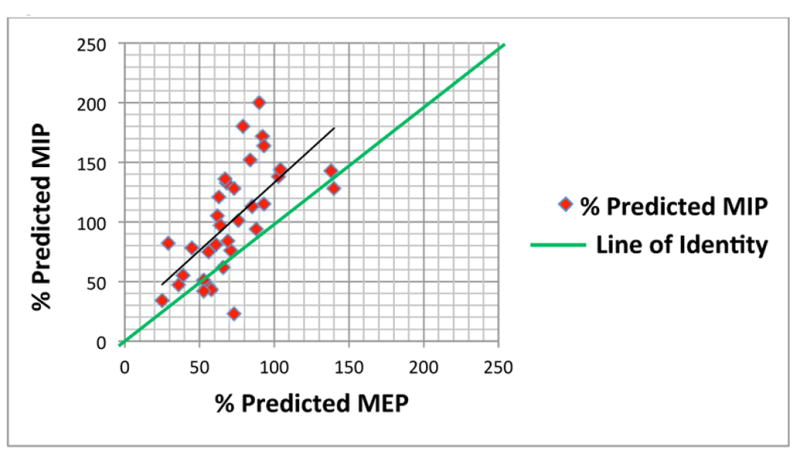
MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure.
Discussion
In this study, significant correlations were seen between %MIP and %MEP and the percent predicted lung volumes FVC and TLC, and both %MIP and %MEP were highly correlated with each other. We also identified evidence of impaired expiratory muscle function in our SCD patients. SCD subjects had similar absolute MIPs and MEPs (Table I). In contrast, multiple studies have established that absolute MEP significantly exceeds absolute MIP in normal adults and children (Wilson et al, 1984; Smyth et al, 1984; Bruschi et al, 1992). Thus, our observation indicates that children with SCD have a selective deficit in percent predicted expiratory muscle force (Table I, Fig. 3). We found no correlation between absolute MIP or absolute MEP and age, in contrast to several studies that have demonstrated positive correlations between absolute respiratory muscle force and age in Caucasian children (Wilson et al, 1984; Gaultier & Zinman, 1983; Heinzmann-Filho et al, 2012; Cox et al, 2012). Whilethere are no studies describing any such correlations in children of African descent to date, we suspect that observed reductions in %MEP in our SCD subjects, combined with the loss of correlation between respiratory muscle strength and age are indicators of a SCD-related process that negatively influences expiratory muscle strength in these children.
It is possible that the discrepancy seen between MIP and MEP values is due to preserved strength of the diaphragm relative to that of the chest wall and abdominal musculature. Maximal inspiratory and expiratory pressures at the mouth are determined by the shortening of separate mutually opposed respiratory muscle groups. MIP is predominantly determined by diaphragmatic contraction, which generates negative intrapleural and airway pressures. Activated external intercostal, sternocleidomastoid and scalene muscles also make minor contributions to this process. In contrast, MEP is due to contraction of the abdominal muscles, such as the external and internal obliques, the transverses abdominis, and the rectus abdominis, as well as the internal intercostals. While inspiratory and expiratory muscles are critical for maximal respiratory muscle force, they have distinctly dissimilar sources of blood supply resulting in different circulatory configurations. The diaphragm has a unique arrangement of arterial supply and venous drainage that is unlike other skeletal muscle (Hussain, 1996). The inferior phrenic, intercostal, and internal mammary arteries provide arterial blood flow to the diaphragm leaflets. The confluence of the phrenic and internal mammary arteries results in an intra-diaphragmatic network of anastomoses that supplies the crural diaphragm and the area of the central tendon. In turn, this circle of arteries anastomoses with branches of the intercostal arteries, forming arterial costophrenic arcades of vessels along the costal segment of the diaphragm (Pearson and Patterson, 2008). This arterial configuration, along with minor contributions from smaller arteries, forms extensive collateralizations critical to effective blood flow (Lockhat et al, 1985). This vascular pattern probably results in muscle fibre resistance to ischaemia, which can protect the diaphragm from hypoxic damage (Hussain, 1996; Viires et al, 1983). In contrast, the abdominal and internal intercostal muscles are primarily supplied by the epigastric and intercostal arteries, respectively, and lack extensive collateralization. Animal models have shown that the diaphragm has approximately seven times more blood flow than the intercostal and abdominal muscles per 100 g of muscle tissue during rest, and that diaphragm blood flow increases to a rate four times the amount of flow to the expiratory muscles during exercise (Poole et al, 1997).
Vascular occlusion causing pain crisis is an established mechanism in SCD, and myonecrosis in adults with SCD has been described (Vicari et al, 2004). Over time, repeated vaso-occlusive events may cause muscle ischaemia. Subclinical expiratory abdominal muscle injury during SCD pain crises may lead to expiratory muscle weakness; we speculate that the diaphragm may be relatively protected from such hypoxic injury due to its extensive collateral blood flow. This, in turn, could explain the difference in MIP and MEP measurements seen in our SCD population.
In a study of maximal handgrip strength in a group of SCD-SS children, Dougherty et al (2011) determined that skeletal muscle strength was attenuated compared to controls. Interestingly, they conclude that the discrepancy was not fully explained by differences in body size or composition (Dougherty et al, 2011). Thus, it is likely that the effect that we observed in expiratory muscle force may also be due to factors other than reduced nutritional state, and in fact may be partly due to vaso-occlusive injury. Furthermore, reduced nutrition would be expected to affect both muscles of inspiration and expiration, and is unlikely to account for selective expiratory muscle weakness. In addition, while it would probably not be responsible for the differential effects we have found on inspiratory and expiratory muscles, anaemia itself could well augment the impaired blood flow mechanisms we discuss by reducing oxygen delivery to the respiratory muscles.
What are the clinical implications of this study, and what does this study suggest for future research? First, pulmonary function testing is widely used in monitoring respiratory status in the SCD population. Mild restricted lung volumes have been reported in children with SCD (Sylvester et al, 2004; Koumbourlis et al, 2007). Although all but one of our subjects had lung volumes within the normal range, there was substantial spread (80-120% predicted). We have shown a direct correlation between percent predicted respiratory muscle strength and percent predicted lung volumes. This study is the first to suggest that lower lung volumes in SCD may be at least partly due to respiratory muscle dysfunction. Respiratory muscle force testing may be an additional method to regularly follow respiratory impairment with disease progression in these patients. Additionally, respiratory muscle force testing may be valuable in detecting early expiratory muscle impairment in SCD children. The most important immediate clinical correlates of such impairment would be 1) exercise limitation; while during tidal breathing, expiration is passive, the expiratory muscles are recruited during exercise, and 2) impaired cough. The latter has obvious implications for airway clearance during times of respiratory illness, such as atelectasis or pneumonia. In more profound cases of expiratory muscle weakness, one might consider assisting cough musculature in various ways, for example, with a cough assist device, and further study is necessary to elucidate the role of such interventions, especially in SCD patients with chronic lung disease, in whom respiratory muscle function could be more severely impaired. Finally, further study is needed to assess the relationship between frequency of vaso-occlusive painful episodes and acute chest syndrome and the severity of respiratory muscle impairment.
Our study has several limitations. The number of subjects is relatively small, a reflection of strict inclusion criteria that included no recent episodes of acute chest syndrome or vaso-occlusive painful episodes, no transfusions within the last 3 months, and no history of treatment with hydroxycarbamide. This led to our studying a milder phenotype, as reflected in the mild lung function abnormalities we report. It may well be that in a more severely affected population, the findings we report could be even more striking.
Our study also highlights the need for normative data for respiratory muscle force testing in children of African descent. Although it is generally presumed that the normative values in this race are similar to Caucasians, this may not be the case (Neder et al, 1999). Thus, further studies are needed to create a reliable normative data set that can be used for comparison studies in all African-Americans undergoing respiratory muscle force testing.
In summary, in children with SCD disease, there is a significant correlation between lung volumes and respiratory muscle strength. The restrictive lung function abnormality previously described in SCD (Sylvester et al 2004) may be, in part, due to changes in the respiratory muscles. Furthermore, expiratory muscle strength is affected disproportionately more than inspiratory muscle strength. This could be due to cumulative vaso-occlusive pain crises affecting the expiratory muscles coupled with the diaphragm’s inherent resistance to hypoxic injury.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute (NHLBI) grant 5R01HL079911-04: Oxyhemoglobin Desaturation and Vasculopathy in Sickle Cell Disease.
Footnotes
Author Contributions: B.A.O., J.C., A.J., J.M., R.A., C.M., K.S.W., T.B.A.M., K.O., and J.A. conceived and designed experiments; B.A.O., J.C., J.M., A.J., and T.J. performed experiments; B.A.O., J.C., A.J., J.M., C.M., and J.A. analysed data; B.A.O., A.J., J.A., and C.M. interpreted experimental results; B.A.O. prepared figures; B.A.O., J.A. and C.M. prepared manuscript. All authors approved final version of the manuscript.
Financial Disclosure: Authors have no financial relationships relevant to this article.
Commercial Affiliations: Authors have no commercial affiliations relevant to this article
Conflict of Interest: Authors have no conflicts of interest to disclose.
References
- Bruschi C, Cerveri I, Zoia MC, Fanfulla F, Fiorentini M, Casali L, Grassi M, Grassi C. Reference values of maximal respiratory mouth pressures: a population-based study. The American review of respiratory disease. 1992;146(3):790–793. doi: 10.1164/ajrccm/146.3.790. [DOI] [PubMed] [Google Scholar]
- Caboot JB, Allen JL. Pulmonary complications of sickle cell disease in children. Current opinion in pediatrics. 2008;20(3):279–287. doi: 10.1097/MOP.0b013e3282ff62c4. [DOI] [PubMed] [Google Scholar]
- Cox DW, Verheggen MM, Stick SM, Hall GL. Characterization of maximal respiratory pressures in healthy children. Respiration; international review of thoracic diseases. 2012;84(6):485–491. doi: 10.1159/000342298. [DOI] [PubMed] [Google Scholar]
- Dassios T, Katelari A, Doudounakis S, Mantagos S, Dimitriou G. Respiratory muscle function in patients with cystic fibrosis. Pediatric pulmonology. 2012 doi: 10.1002/ppul.22709. Epub ahed of print Nov 9 2012. [DOI] [PubMed] [Google Scholar]
- DePalo VA, McCool FD. Respiratory muscle evaluation of the patient with neuromuscular disease. Seminars in respiratory and critical care medicine. 2002;23(3):201–209. doi: 10.1055/s-2002-33028. [DOI] [PubMed] [Google Scholar]
- Driscoll MC. Sickle cell disease. Pediatrics in review / American Academy of Pediatrics. 2007;28(7):259–268. doi: 10.1542/pir.28-7-259. [DOI] [PubMed] [Google Scholar]
- Dougherty KA, Schall JI, Rovner AJ, Stallings VA, Zemel BS. Attenuated maximal muscle strength and peak power in children with sickle cell disease. Journal of pediatric hematology/oncology. 2011;33(2):93–97. doi: 10.1097/MPH.0b013e318200ef49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field JJ, DeBaun MR, Yan Y, Strunk RC. Growth of lung function in children with sickle cell anemia. Pediatric pulmonology. 2008;43(11):1061–1066. doi: 10.1002/ppul.20883. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Zinman R. Maximal static pressures in healthy children. Respiration physiology. 1983;51(1):45–61. doi: 10.1016/0034-5687(83)90101-9. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. The New England journal of medicine. 2008;359(21):2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- Green M, Road J, Sieck GC, Similowski T. Tests of Respiratory muscle strength. In: ATS/ERS Statement on respiratory muscle testing. American journal of respiratory and critical care medicine. 2002;166(4):528–547. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Heinzmann-Filho JP, Vidal PC, Jones MH, Donadio MV. Normal values for respiratory muscle strength in healthy preschoolers and school children. Respiratory medicine. 2012;106(12):1639–1646. doi: 10.1016/j.rmed.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Hussain SN. Regulation of ventilatory muscle blood flow. Journal of applied physiology. 1996;81(4):1455–1468. doi: 10.1152/jappl.1996.81.4.1455. [DOI] [PubMed] [Google Scholar]
- Koumbourlis AC, Lee DJ, Lee A. Longitudinal changes in lung function and somatic growth in children with sickle cell disease. Pediatric pulmonology. 2007 Jun;42(6):483–488. doi: 10.1002/ppul.20601. [DOI] [PubMed] [Google Scholar]
- Koumbourlis AC, Zar HJ, Hurlet-Jensen A, Goldberg MR. Prevalence and reversibility of lower airway obstruction in children with sickle cell disease. The Journal of pediatrics. 2001;138(2):188–192. doi: 10.1067/mpd.2001.111824. [DOI] [PubMed] [Google Scholar]
- Lockhat D, Magder S, Roussos C. Collateral sources of costal and crural diaphragmatic blood flow. Journal of applied physiology. 1985;59(4):1164–1170. doi: 10.1152/jappl.1985.59.4.1164. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 1999;32(6):719–727. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- Pearson FG, Patterson GA. Pearson’s thoracic & esophageal surgery. 3. Churchill Livingstone/Elsevier; Philadelphia, PA, USA: 2008. [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Poole DC, Sexton WL, Farkas GA, Powers SK, Reid MB. Diaphragm structure and function in health and disease. Medicine and science in sports and exercise. 1997 Jun;29(6):738–754. doi: 10.1097/00005768-199706000-00003. [DOI] [PubMed] [Google Scholar]
- Rucknagel DL. The role of rib infarcts in the acute chest syndrome of sickle cell diseases. Pediatric pathology & molecular medicine. 2001;20(2):137–154. [PubMed] [Google Scholar]
- Sedrak A, Rao SP, Miller ST, Hekmat V, Rao M. A prospective appraisal of pulmonary hypertension in children with sickle cell disease. Journal of pediatric hematology/oncology. 2009;31(2):97–100. doi: 10.1097/MPH.0b013e31818e5343. [DOI] [PubMed] [Google Scholar]
- Smyth RJ, Chapman KR, Rebuck AS. Maximal inspiratory and expiratory pressures in adolescents. Normal values. Chest. 1984;86(4):568–572. doi: 10.1378/chest.86.4.568. [DOI] [PubMed] [Google Scholar]
- Steinberg MH. Management of sickle cell disease. The New England journal of medicine. 1999;340(13):1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- Sylvester KP, Patey RA, Milligan P, Dick M, Rafferty GF, Rees D, Thein SL, Greenough A. Pulmonary function abnormalities in children with sickle cell disease. Thorax. 2004;59(1):67–70. [PMC free article] [PubMed] [Google Scholar]
- Terzano C, Ceccarelli D, Conti V, Graziani E, Ricci A, Petroianni A. Maximal respiratory static pressures in patients with different stages of COPD severity. Respiratory research. 20089:8. doi: 10.1186/1465-9921-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari P, Achkar R, Oliveira KR, Miszpupten ML, Fernandes AR, Figueiredo MS, Bordin JO. Myonecrosis in sickle cell anemia: case report and review of the literature. Southern medical journal. 2004;97(9):894–896. doi: 10.1097/01.SMJ.0000125172.95454.B7. [DOI] [PubMed] [Google Scholar]
- Viires N, Sillye G, Aubier M, Rassidakis A, Roussos C. Regional blood flow distribution in dog during induced hypotension and low cardiac output. Spontaneous breathing versus artificial ventilation. The Journal of clinical investigation. 1983;72(3):935–947. doi: 10.1172/JCI111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax. 1984;39(7):535–538. doi: 10.1136/thx.39.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]


