Abstract
Aclidinium bromide inhalation powder (Tudorza) for COPD
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is characterized by prolonged inflammatory changes that occur from exposure of the lungs and airway to noxious gas particles (e.g., tobacco smoke, occupational dust, and chemicals), resulting in limitation of airflow.1,2 Spirometry is typically used to determine airflow limitation (obstruction). During this evaluation, the patient inhales to total lung capacity and then performs forced expiratory maneuvers. Key parameters obtained from spirometry include forced expiratory volume (FEV1) and the total volume of air exhaled during the entire spirometric maneuver (forced vital capacity [FVC]).3
COPD, although preventable and treatable, causes significant extrapulmonary effects that contribute to disease severity. Over the past two decades, there has been a significant increase in the prevalence of COPD, as well as in mortality rates, making it the fourth leading cause of death in the U.S. By 2020, it is estimated that COPD will rank fifth in burden of disease and third as a cause of death throughout the world.1
Symptoms of COPD include dyspnea, chronic cough, and chronic sputum production. Episodes of acute worsening of symptoms often occur.
COPD differs from asthma; in patients with COPD, the airflow limitation is not fully reversible. In a subset of patients with COPD, there is minimal improvement in response to a bronchodilator or to optimal treatment.
The goals of therapy for COPD are the immediate relief and reduction of symptoms and their impact, as well as a decreased risk of future adverse health events. COPD management should be tailored to the needs of each patient. Pharmacological therapy can reduce symptoms, decrease the frequency and severity of exacerbations, and improve health status and exercise tolerance.4,5
Bronchodilators are central to symptom management in COPD. The choice between beta2-agonists, anticholinergic agents, theophylline, or combination therapy depends on availability of these drugs and patient response.4,5 Two long-acting anticholinergic agents are approved for the long-term maintenance treatment of bronchospasm associated with COPD: tiotropium bromide (Spiriva HandiHaler, Boehringer Ingelheim), originally approved in 2004, and aclidinium bromide (Tudorza, Forest), which was approved in July 2012.
CLINICAL PHARMACOLOGY6
Composition6
Aclidinium bromide is a white powder. Its molecular formula is C26H30NO4 S2Br, and it has a molecular mass of 564.56. The chemical structure is shown in Figure 1.
Figure 1.
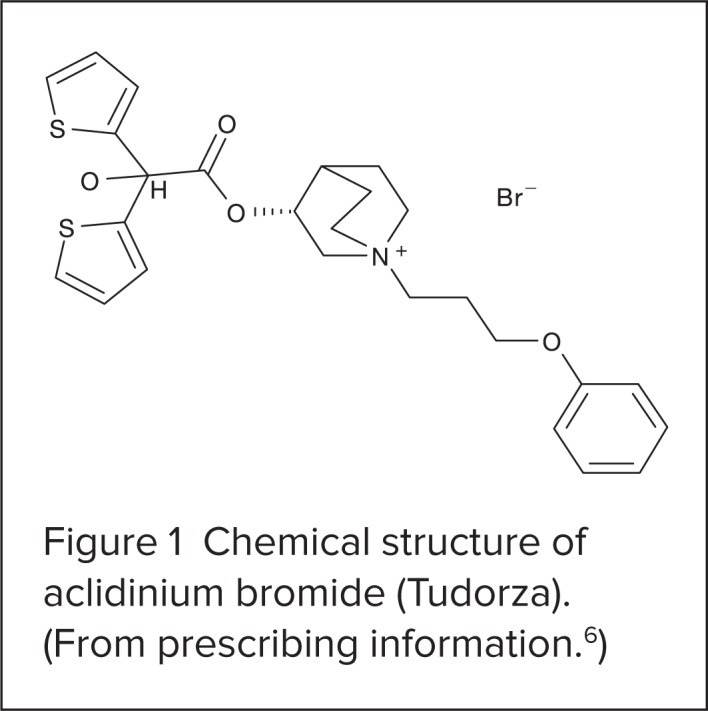
Chemical structure of aclidinium bromide (Tudorza). (From prescribing information.6)
Mechanism of Action6
Aclidinium is a long-acting inhibitor of muscarinic receptors, with a similar affinity to subtypes M1 through M5. M3 receptors are located on bronchial smooth muscle, the vascular endothelium in the lung, and the submucosal mucus glands, where they mediate bronchoconstriction, mucus secretion, and vasodilation. Aclidinium bromide exhibits its pharmacological effects through inhibition of the M3 receptor in smooth muscle, resulting in bronchodilation.
Pharmacodynamics6
The half-life of aclidinium to M3 receptors is six times that of its half-life to M2 receptors, producing bronchodilation via M3 blockade long after its M2 effects, such as tachycardia, diminish. The effect of aclidinium bromide on cardiac rhythm was assessed via a Holter monitor in 336 patients with COPD. No clinically significant effects on cardiac rhythm were observed in 164 patients who received aclidinium 400 mcg twice daily or in 172 patients who received placebo.
Pharmacokinetics6
Absorption. Peak steady-state levels are achieved within 10 minutes of oral inhalation of aclidinium bromide 400 mcg twice daily.
Distribution. Following the intravenous (IV) administration of 400 mcg in humans, aclidinium bromide shows a volume of distribution of approximately 300 L.
Metabolism. Aclidinium undergoes rapid hydrolysis to its alcohol and dithienyl glycolic acid derivatives, neither of which binds to muscarinic receptors. Because of its short plasma half-life, aclidinium bromide and its metabolites are not expected to alter other drugs metabolized through cytochrome P450 (CYP) isoenzymes.
Elimination. IV radiolabeled aclidinium bromide was administered to healthy volunteers and was extensively metabolized, with 1% excreted as unchanged as aclidinium. About 54% and 65% of the radioactivity was excreted in urine, and 20% to 33% of the dose was eliminated in feces. Therefore, almost the entire dose of aclidinium was eliminated by hydrolysis. Renal impairment does not appear to have a significant impact on systemic exposure.
CLINICAL EFFICACY
Jones et al.7
The efficacy and safety of aclidinium bromide were evaluated in patients with moderate-to-severe COPD in the ATTAIN (Aclidinium To Treat Airway obstruction In COPD patieNts) trial. This 24-week, double-blind, randomized, placebo-controlled, parallel-group phase 3 study was conducted in nine European countries and South Africa. Patients were assigned to receive aclidinium 200 mcg, aclidinium 400 mcg, or placebo twice daily. All study treatments were administered via a multiple-dose dry powder inhaler.
The primary endpoint was the change from baseline in morning pre-dose (trough) FEV1 at week 24. Secondary endpoints were the change from baseline in peak FEV1 at week 24 and the percentage of patients achieving clinically significant improvements in health status according to total scores on St. George’s Respiratory Questionnaire (SGRQ) and focal scores on the Transitional Dyspnea Index (TDI) at week 24.
Of the 828 patients, 819 patients were included in the intent-to-treat (ITT) and safety populations. Significant improvements from baseline were observed with aclidinium 200 mcg and 400 mcg compared with placebo for trough FEV1 (99 and 128 mL, respectively; P < 0.0001 for both) and peak FEV1 (185 mL and 209 mL, respectively; P < 0.0001 for both). Improvements in peak and trough FEV1 provided by both doses of aclidinium were statistically superior to placebo at all time points from week 1 to week 24.
Improvements over placebo in baseline-adjusted mean SGRQ total scores were 3.8 units for aclidinium 200 mcg and −4.6 units for aclidinium 400 mcg at week 24.
Patients receiving aclidinium 200 mcg and 400 mcg showed a clinically significant improvement in TDI focal scores (by 1 unit or more) at week 24 compared with placebo (53.3% and 56.9% vs. 45.5%, respectively; odds ratios were 1.47 and 1.68 for 200 mcg and 400 mcg (P < 0.05 and P < 0.01, respectively).
Kerwin et al.8
ACCORD I (AClidinium in Chronic Obstructive Respiratory Disease-I) was a 12-week, double-blind, multicenter phase 3 study that also compared the efficacy and safety of aclidinium with placebo in patients with moderate-to-severe COPD. Patients (n = 561) were randomly assigned, in a 1:1:1 ratio, to receive inhaled twice-daily aclidinium 200 mcg or 400 mcg or placebo.
The primary endpoint was the change from baseline in trough FEV1. The secondary endpoint was the change in baseline from peak FEV1 at week 12. Health status (from SGRQ scores), COPD symptoms (from TDI scores), and safety were also assessed.
At week 12, patients receiving aclidinium 200 mcg and 400 mcg showed significant improvements from baseline in mean 95% confidence interval (CI) trough FEV1 compared with placebo and in peak FEV1 (P ≦ 0.0001) for all measures. Both doses also provided significant improvements in SGRQ scores, TDI scores, and most COPD symptom scores compared with placebo (P < 0.05 for all measures).
Overall, aclidinium bromide 200 mcg and 400 mcg were associated with significant improvements in bronchodilation, health status, and COPD symptoms, with a low incidence of adverse events when compared with placebo.
INDICATION6
Aclidinium bromide is intended for the long-term maintenance treatment of bronchospasm associated with COPD, including chronic bronchitis and emphysema.
SAFETY PROFILE6
Contraindications
There are no contraindications.
Warnings and Precautions
Aclidinium bromide is not indicated for the initial treatment of acute bronchospasms. It is intended only for the maintenance treatment of COPD.
If paradoxical bronchospasm occurs, therapy should be discontinued and other treatment options should be considered.
Aclidinium bromide should be used with caution in patients with narrow-angle glaucoma. Patients should be advised to contact a physician immediately if any eye discomfort or pain, blurred vision, or visual halos occur.
This medication should be used with caution in patients with urinary retention. Patients should be counseled to consult a physician immediately if painful urination occurs; this can be a sign of prostatic hyperplasia or bladder-neck obstruction.
Patients may experience immediate hypersensitivity reactions after administration. If these reactions occur, therapy should be stopped immediately and other treatment options should be considered.
Adverse Drug Reactions
Common drug-induced reactions have occurred in fewer than 3% of patients but with an incidence greater than that with placebo. Reactions have included headache, nasopharyngitis, and cough (Table 1).
Table 1.
Adverse Drug Reactions in Placebo-Controlled Clinical Trials Of Aclidinium Bromide (Tudorza)
| Adverse Reaction |
Tudorza Pressair (N = 636) No. of Patients (%) |
Placebo (N = 640) No. of Patients (%) |
|---|---|---|
| Headache | 42 (6.6) | 32 (5.0) |
| Nasopharyngitis | 35 (5.5) | 25 (3.9) |
| Cough | 19 (3.0) | 14 (2.2) |
| Diarrhea | 17 (2.7) | 9 (1.4) |
| Sinusitis | 11 (1.7) | 5 (0.8) |
| Rhinitis | 10 (1.6) | 8 (1.2) |
| Toothache | 7 (1.1) | 5 (0.8) |
| Fall | 7 (1.1) | 3 (0.5) |
| Vomiting | 7 (1.1) | 3 (0.5) |
Data from aclidinium prescribing information.6
Drug Interactions
The concurrent use of aclidinium bromide with sympathomimetic agents and methyxanthines, as well as other drugs used in the treatment of COPD (e.g., short-acting beta2 agonists and inhaled or oral steroids), showed no increase in adverse drug reactions. Coadministration of aclidinium with other anticholinergic agents should be avoided because of the risk of an increase in anticholinergic effects, such as diplopia, tachycardia, and dry mouth.
Special Populations
Aclidinium bromide is classified as a Pregnancy Category C medication. No adequate or well-controlled studies have been conducted in pregnant women. This medication should be used only if the benefits are judged to outweigh the risk to the fetus. Although no studies have evaluated the effects of aclidinium on breast-fed infants, aclidinium was excreted into the milk of lactating female rats. This drug should be used with caution in nursing women.
Aclidinium is not recommended for use in patients younger than 18 years of age.
No differences in safety and effectiveness were observed in subjects 60 years of age or older or in younger patients. No dosage adjustments are warranted for geriatric patients, but hypersensitivity reactions in older patients should not be ruled out.
No clinically significant differences in the pharmacokinetics of aclidinium were noted among subjects with normal, mild, moderate, or severe renal impairment; therefore, no dosage adjustments are necessary.
The effects of hepatic impairment on the pharmacokinetics of aclidinium have not been studied.
DOSAGE AND ADMINISTRATION6
The recommended dose of aclidinium bromide, available in Tudorza Pressair, is one oral inhalation of 400 mcg twice daily. The breath-actuated multidose dry powder inhaler provides 400 mcg per actuation (Table 2).
Table 2.
Comparison of Dosages and Cost of Inhaled Anticholinergic Agents for the Treatment Of Chronic Obstructive Pulmonary Disease
| Drug | Dose | Cost (Average Wholesale Price) |
|---|---|---|
| Atrovent HFA (ipratropium bromide hydrofluoroalkane) | Two inhalations (17 mcg per actuation) orally four times daily | $239.09 |
| Spiriva HandiHaler (tiotropium bromide) | Two inhalations of the powder capsule (18-mcg capsules) orally once daily | $289.09 |
| Tudorza (aclidinium bromide) | One inhalation (400 mcg per actuation) twice daily | $261.00 |
Data from LexiComp.10
PATIENT COUNSELING6
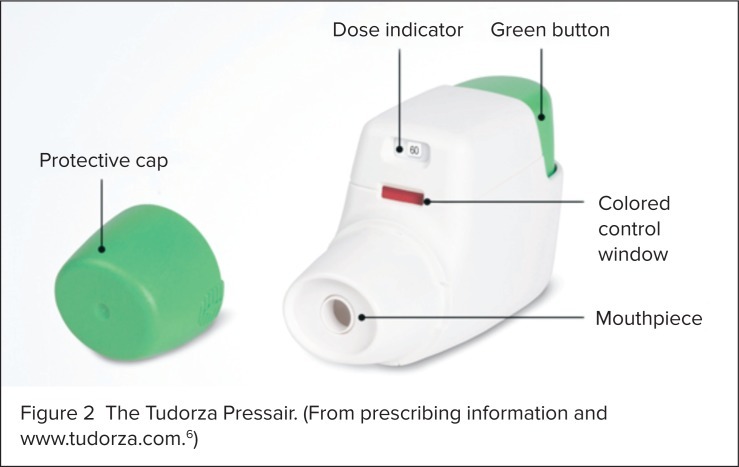
Aclidinium bromide is not a rescue medication, and it should not be used for treating sudden breathing problems. The usual dose is one inhalation two times per day, 12 hours apart. The inhaler should be discarded 45 days after the pouch is opened, after the marking “0” with a red background shows in the middle of the dose indicator, or when the device locks out, whichever comes first. Each inhaler contains 60 doses. The inhaler should be stored at room temperature. It should not be cleaned, but the outside may be wiped with a dry tissue or paper towel only.
An illustration of the Tudorza Pressair is presented in Figure 2. Directions for its use are as follows:6
Step 1: Remove the protective cap.
Step 2: Hold the inhaler with the mouthpiece facing you but not inside your mouth.
Step 3: Before you put the inhaler into your mouth, press the green button all the way down, then release.
Step 4: Check that the control window has changed from red to green.
Step 5: Before you put the inhaler into your mouth, breathe out completely. Put your lips tightly around the mouthpiece. Breathe in quickly and deeply through your mouth until you hear a “click” sound. Keep breathing in, even after the click, to be sure you received the full dose. Do not hold down the green button while you are breathing in.
Step 6: Remove the inhaler from your mouth. Hold your breath for as long as comfortable, then breathe slowly out of your nose.
Step 7: Check the control window to make sure it has turned to red, which means the full dose was received. If window is still green, repeat the inhalation steps.
Step 8: Replace the mouthpiece.
Figure 2.
The Tudorza Pressair. (From prescribing information and www.tudorza.com.6)
P&T COMMITTEE CONSIDERATIONS
Comparison With Other Bronchodilators
Bronchodilators are the mainstay of therapy.4,5,9 Aclidinium bromide is the second long-acting antimuscarinic agent approved for the treatment of COPD; tiotropium (Spiriva HandiHaler) was formerly the only available agent. Patients who had difficulty administering tiotropium correctly had no other option. To administer tiotropium, the patient places a capsule into the center chamber of the Handi-Haler device, then pierces the capsule by pressing and releasing the green button on the side of the device. The tiotropium formulation is dispersed into the air-stream when the patient inhales through the mouthpiece.9 Aclidinium is easier to administer. The patient presses a button only once to release the dose.6 This may be advantageous for many patient populations with impaired dexterity.
Limitations
Some factors may limit the use of aclidinium. For example, the drug must be taken twice daily because of its short half-life, whereas tiotropium is administered once daily, a potential advantage in terms of patient compliance and convenience.6
Cost
The cost can be a consideration, but it is not truly a hindrance. Aclidinium costs $28 less than tiotropium—about 10% less per month (Table 2).10 Although the cost of both agents is comparable and slightly favorable for aclidinium, the increase in dosing frequency can offset the difference in cost.10 However, it is more expensive than ipratropium (Atrovent, Boehringer Ingelheim) (see Table 2).
Efficacy
Several studies have looked at the efficacy of aclidinium, but few were head-to-head comparator studies against another inhaled anticholinergic medication. A double-blind, multicenter, crossover phase 2 trial conducted by Vestbo et al. was designed to compare the onset of action of aclidinium versus tiotropium and placebo.11 This study looked at 115 patients with COPD and an FEV1 between 30% and 60% of predicted. On study days, patients received aclidinium 200 mcg, tiotropium 18 mcg, or placebo. At 30 minutes, at least a 10% increase in FEV1 was noted in patients who received aclidinium (49.5%) and tiotropium (51.8%) compared with those receiving placebo (13.8% of aclidinium), respectively (P < 0.001 for both drugs vs. placebo), indicating a similar onset of action for both drugs.11
A more recent phase 2a double-blind, randomized, double-dummy, crossover study by Fuhr et al. evaluated 30 patients with moderate-to-severe COPD receiving either aclidinium 400 mcg twice daily, tiotropium 8 mcg once daily, or placebo.12 Patients received treatment for 15 days with a 9- to 15-day washout period between regimens. Of the 27 patients who completed the study, the mean change from baseline on day 15 was significantly greater for aclidinium and tiotropium compared with placebo. Bronchodilation with both agents was comparable. However, improvements in FEV1 and FVC were superior for aclidinium, compared with tiotropium, on days 1 and 15. This might have been as a result of the bedtime dose of aclidinium.12 Aclidinium has not been directly compared with ipratropium.
CONCLUSION
Aclidinium bromide offers an alternative to tiotropium for the maintenance treatment of bronchospasm related to COPD. Aclidinium is a long-acting inhibitor of muscarinic receptors with bronchodilatory effects comparable to those of tiotropium.11,12 The device that delivers the medication is breath-activated, potentially making it easier to use compared with the more complex instructions for tiotropium.9 One disadvantage with aclidinium bromide is the need for twice-daily dosing, which may have a discouraging effect on patient compliance.6
Although the cost savings for aclidinium are minimal compared with that for competitor drugs,10 the need for twice-daily dosing is an important consideration in formulary decision-making.
Footnotes
Disclosure: The authors report no commercial or financial relationships in regard to this article.
REFERENCES
- 1.Williams DM, Bourdet SV. Chronic obstructive pulmonary disease. In: DiPiro JT, Talbert RL, Yee GC, et al., editors. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011. pp. 439–464. [Google Scholar]
- 2.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JJ, Silverman EK, Shapiro SD. Chronic obstructive pulmonary disease. In: Fauci AS, Kasper DL, Jameson JL, et al., editors. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2012. [Google Scholar]
- 4.Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease. Revised November 2011. Available at: www.goldcopd.org/uploads/users/files/gold2011_Summary.pdf. Accessed November 12, 2012.
- 5.American Thoracic Society/European Respiratory Society Standards for the Diagnosis and Management of Patients with COPD. Available at: www.archive.thoracic.org/sections/copd/resources/copddoc.pdf. Accessed February 1, 2013.
- 6.Tudorza Pressair, prescribing information. St. Louis, Mo.: Forest; Jul, 2012. Available at: http://frx.com/pi/tudorza_pi.pdf. Accessed March 14, 2013. [Google Scholar]
- 7.Jones P, Singh D, Bateman E, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: The ATTAIN study. Eur Respir J. 2012;40:830–836. doi: 10.1183/09031936.00225511. [DOI] [PubMed] [Google Scholar]
- 8.Kerwin EM, D’Urzo AD, Gelb AF, et al. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I) COPD. 2012;9:90–101. doi: 10.3109/15412555.2012.661492. [DOI] [PubMed] [Google Scholar]
- 9.Spiriva HandiHaler, prescribing information. Ridgefield, Conn.: Boehringer Ingelheim; Oct, 2006. New York: Pfizer. Revised March 2012. Available at: http://bidocs.boehringer-ingelheim.com. Accessed March 14, 2013. [Google Scholar]
- 10.Lexi-Comp Online, Hudson, Ohio. Available at: http://online.lexi.com. Available at: www.crlonline.com. Accessed November 12, 2012.
- 11.Vestbo J, Vogelmeier C, Creemers J, et al. Onset of effect of aclidinium, a novel, long-acting muscarinic antagonist, in patients with COPD. COPD. 2010;7:331–336. doi: 10.3109/15412555.2010.510158. [DOI] [PubMed] [Google Scholar]
- 12.Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 mcg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest. 2012;141:745–752. doi: 10.1378/chest.11-0406. [DOI] [PubMed] [Google Scholar]