Abstract
Background
Sepsis, an inflammatory response to an infection that may lead to severe organ dysfunction and death, is the leading cause of death in medical intensive care units. The Society of Critical Care Medicine has issued guidelines and promoted protocols to improve the management of patients with severe sepsis and septic shock. Generally, the medical community has been slow to adopt these guidelines because of the system challenges associated with protocol implementation. We describe an interdisciplinary team approach to the development and implementation of management protocols for treating patients with severe sepsis and septic shock.
Methods
To determine the effectiveness of the bundled emergency department and critical care order sets developed by the Sepsis Steering Committee, we performed a case review of 1,105 sequential patients admitted to a large academic tertiary referral hospital with a diagnosis of severe sepsis or septic shock between July 2008 and January 2012.
Results
Implementation of the protocol led to improved order set use over time, a significant decrease in the median time to antibiotics of 140 (range 1-820) minutes in 2008 to 72 (range 1-1,020) minutes in 2011 (P≤0.001), and a decrease in median length of stay from 8 days (range 1-54) in 2008 to 7 days (range 1-33) in 2011 (P=0.036).
Conclusion
A multidisciplinary team approach to sepsis management using protocols and early goal-directed therapy is feasible in a large academic medical center to improve the process of care and outcomes.
Keywords: Quality improvement, sepsis, shock–septic
INTRODUCTION
Sepsis is an inflammatory response to an infection that may lead to severe organ dysfunction. Annually, it affects approximately 750,000 people in the United States and is responsible for more than 215,000 deaths.1 The estimated cost of sepsis is more than $17 billion each year.1 The term severe sepsis describes sepsis with acute organ dysfunction, and the term septic shock describes sepsis with organ dysfunction and hypotension refractory to fluid resuscitation.1,2 Together they are the most common cause of death in noncoronary critical care (CC) units, with a mortality of 28%-50%.1 Evidence suggests that early diagnosis, the timely initiation of appropriate antibiotics, and resuscitation to hemodynamic goals improve clinical outcomes.3-12 The Surviving Sepsis Campaign has recommended protocols and bundles to improve timely compliance with evidence-based treatments and to enhance outcomes.3,4 Hospitals have been slow to adopt the recommended protocols because of implementation challenges, financial concerns, and hierarchical systems of care. Challenges include resource allocation for the critically ill in emergency departments (EDs), communication, transitions of care, and multidisciplinary coordination of care. We describe the Ochsner experience with a multidisciplinary team approach and quality improvement tools such as rapid-cycle change to influence the process of care and improve clinical outcomes.
METHODS
The preparation for sepsis project implementation began in summer 2007. The quality improvement project involved a multidisciplinary Sepsis Steering Committee consisting of ED and CC physicians and nurses, a clinical pharmacist, and members of the hospital administration, performance improvement, and medical informatics staffs. The project began with the development of bundled ED and CC orders sets for management of sepsis. These order sets were based on the Society of Critical Care Medicine (SCCM) Surviving Sepsis guidelines for 6-hour (ED) and 24-hour (CC) bundles. The implementation of the order sets in July 2008 was followed by a comprehensive educational campaign targeting the ED and CC nurses and physicians.
Patients enrolled in this study were admitted to the medical intensive care unit (MICU) with a diagnosis of severe sepsis or septic shock. Severe sepsis was defined as sepsis with 2 organ failures or serum lactic acid (LA) level ≥4 mm/L. Septic shock was defined as a mean arterial blood pressure (MABP) <65 mmHg after a 1.5 L bolus of normal saline.2 The clinical pharmacist for the MICU team collected data from paper and electronic charts on a daily basis, deidentified patient information, and stored the data in a password-protected database. Metrics collected included the time of admission, the time of diagnosis, the first serum LA level, the location of the patient before transfer to the MICU, ED order set use, CC order set use, cultures drawn prior to antibiotic administration, the time to antibiotics (if the patient was already on antibiotics, then the time to new antibiotics was recorded), and the achievement of early goal-directed therapy (EGDT) endpoints.3 EGDT endpoints include central venous pressure (CVP) ≥8 cm H2O, MABP ≥65 mmHg, and mixed central venous oxygen saturation (ScvO2) ≥70%.5 The time to antibiotics and the time to meeting EGDT goals were based on the time that severe sepsis and septic shock were diagnosed. In the ED, diagnosis time was defined as the time when the serum LA level was called to the ED or when the ED physician started treating the patient for severe sepsis. Septic shock start time was when the patient's MABP reached <65 mmHg after the initial fluid bolus. On the hospital floor, diagnosis time was defined as the time of the Rapid Response Team (RRT) call or the time of documented vital sign deterioration. The RRT included an experienced CC nurse and respiratory therapist with backup from the MICU resident.
Two administrative committees were formed to review the sepsis data. A small multidisciplinary team met monthly to review individual segments of care processes for all sepsis cases to look for lapses and opportunities to improve. The metrics team meetings allowed for open collegial discussion and rapid-cycle feedback that optimized healthcare delivery. The larger Sepsis Steering Committee met every other month and acted as an advisory board of key stakeholders offering a global evaluation of the sepsis project.
Project Implementation
Early identification of severe sepsis and septic shock in the ED or on the inpatient floors became a critical factor in the process of care. Materials on the signs of sepsis were disseminated widely across the hospital in a variety of formats, including posters, pocket cards, online modules, and lectures. Once sepsis was identified, the use of ED 6-hour and CC 24-hour bundled order sets guided evidence-based standardized care across transitions (Table). In the ED, the multidisciplinary team owned the process for identifying severe sepsis and septic shock early, starting resuscitation, placing the ScvO2 monitoring catheter, and consulting the MICU team. The MICU team committed to assuming care of the patient within 1 hour of consultation to facilitate patient disposition, expedite patient transfer to the CC unit, and assist the timely completion of EGDT. On the inpatient floors, calls about patient deterioration triggered a rapid response call. The RRT assisted with sepsis diagnosis, facilitated notification of the MICU team, initiated EGDT, and transported patients to the CC unit.
Table.
Emergency Department (6-Hour) and Critical Care (24-Hour) Bundled Order Sets
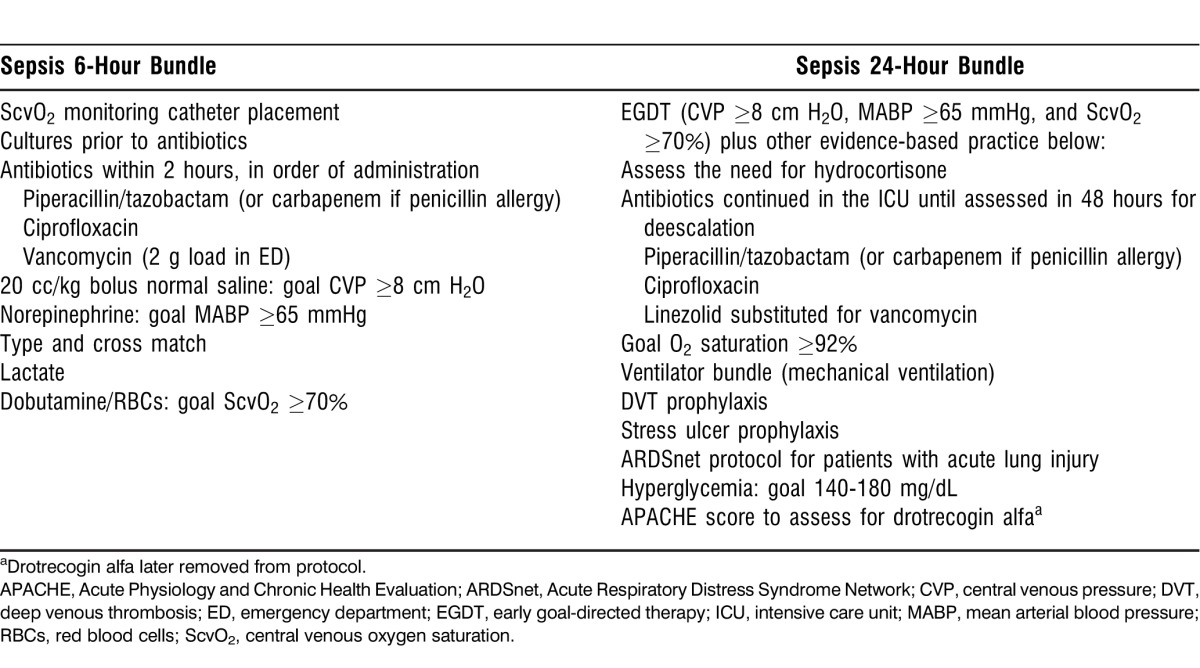
Treatment initiation using the ED 6-hour bundle focused on the immediate goals of placement of an ScvO2 catheter; aggressive volume resuscitation with normal saline to meet resuscitation goals of CVP ≥8 cm H2O, MABP ≥65 mmHg, and ScvO2 ≥70%; the administration of empiric broad-spectrum antibiotics within 2 hours; and blood culture collection prior to antibiotic administration. In the ED, resource allocation made managing patients with EGDT for 6 hours impractical, as the landmark Rivers study found.5 The CC 24-hour bundle focused on longer-term, evidence-based practices for severe sepsis and septic shock, including glucose control, venous thromboembolic prophylaxis with low-molecular-weight heparin, hydrocortisone for patients with vasopressor-dependent shock, stress ulcer prophylaxis, the Acute Respiratory Distress Syndrome Network protocol for patients with acute lung injury requiring mechanical ventilation, and the dosing of antibiotics for continued administration.
A key component of the sepsis guidelines is the timely use of empiric broad-spectrum antimicrobials, facilitated by including broad-spectrum antimicrobials in bundles. Studies have consistently demonstrated that timely administration and appropriate choice of antimicrobials has a positive effect on survival in severe sepsis and septic shock patients.9-11 The correct dosing of antimicrobials in severe sepsis and septic shock patients is complicated by augmented renal clearance, increased volume of distribution, and renal insufficiency.13,14
The Ochsner sepsis management protocol uses piperacillin/tazobactam, ciprofloxacin, and vancomycin for the initial doses of antimicrobials. After the initial loading dose of 2 g of vancomycin administered in the ED, the 24-hour bundled order set then transitions to linezolid because of the latter's ease of administration, absence of renal dose adjustment, and lipophilic qualities. The severity of sepsis has no substantial effect in decreasing the peak plasma concentration and serum concentration curve of linezolid after a 600 mg standard dose.15 In addition, we had concerns that the long-term use of vancomycin at the higher doses required for tissue penetration could lead to renal insufficiency among critically ill patients.16
Data Analysis Methodology and Process of Care Adaptation
Rapid-cycle feedback for this project followed a traditional plan-do-study-act (PDSA) methodology. Initially, rapid-cycle feedback occurred daily to improve the process of care. This activity required the presence of vigilant onsite clinicians to monitor for lapses in the process of care. The clinical pharmacist and attending intensivist reviewed the cases of patients with severe sepsis and septic shock who were admitted to the MICU each morning to provide a mechanism for immediate feedback to the entire team on performance and improvement opportunities. When the intensivist felt that missed steps required immediate attention, the concerns were communicated to multidisciplinary clinical team members to provide feedback within 24 hours. Communication about missed opportunities occurred via the sepsis champion for each healthcare team to promote engagement and improve clinical practice. For example, physician leaders for each respective area provided feedback to ED and CC physicians. Nursing feedback occurred via the clinical nurse specialist and unit director for the MICU and the sepsis nursing champion for the ED.
Early adaptations to the process of care based on the PDSA methodology included the creation of a paper nursing flow sheet to facilitate the transition of nursing care from the ED to CC, RRT training, house officer bedside presence until goal attainment, focused education, individual coaching, and equipment acquisition. The nursing flow sheet was implemented within the first 2 months of the project and led to better documentation and data extraction. Because of dynamic changes in scientific evidence, order sets changed over time. For example, the glucose control changed from tight control to moderate control following the results of the Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation trial.17 Also, drotrecogin alfa (activated protein C)—an antiinflammatory and anticoagulant that had been shown to improve outcomes in patients with severe sepsis and septic shock—was taken off the market after research showed that its use led to increased bleeding complications.18 Additionally, the order sets required adjustment when the hospital pharmacy replaced doripenem with meropenem on the formulary.
In January 2010, the Sepsis Steering Committee set a new goal in the case review process by determining a measure of perfect care. Perfect care was defined as obtaining cultures prior to administering antibiotics, starting antibiotics within 2 hours, using order sets, placing an ScvO2 monitoring catheter, and meeting 6-hour resuscitation goals.3
Four years after project implementation, the Sepsis Steering Committee continues to meet quarterly and the metrics team meets every other month. Data analysis occurs on a continuous basis, and statistical analysis of data is done at least every 6 months. The medical informatics department assists with analytics, including in-hospital mortality indices (determined using administrative discharge data) and external reporting.
RESULTS
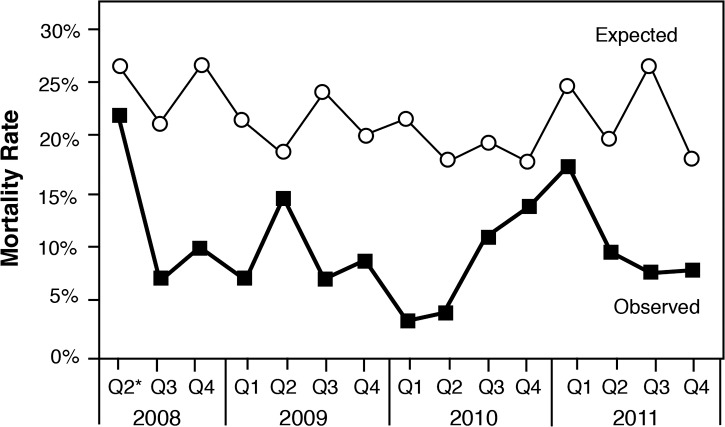
Between July 2008 and January 2012, 1,105 sequential patients with severe sepsis and septic shock were prospectively enrolled in the sepsis management database. Demographics included a median patient age of 63 years and an initial Acute Physiology and Chronic Health Evaluation (APACHE) score range of 21-25. The Ochsner patient population was similar to populations discussed in previous published reports, with pneumonia being the most common cause of severe sepsis and septic shock.8 The most common sources of sepsis in the Ochsner cohort were pneumonia (42%), the urinary tract (24%), and the abdomen (23%). Risk-adjusted in-hospital mortality decreased over time as the range of APACHE scores narrowed to 24-25 by 2012 (Figures 1 and 2). The rise in mortality seen in 2010 may be explained by an increased number of outside transfers of patients with severe sepsis and septic shock to the institution via the Regional Referral Center that opened in mid-2010. These patients were transferred from other institutions where typically they had been septic for prolonged periods of time and were outside the 6-hour window for EGDT.
Figure 1.
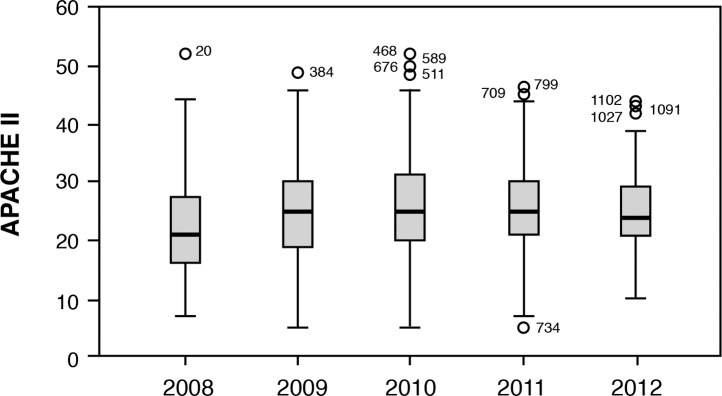
Acute Physiology and Chronic Health Evaluation (APACHE) scores over time.
Figure 2.
Risk-adjusteda mortality over time.
aAdjusted for conditions present on admission. Exclude skilled nursing facility, psychiatry, rehabilitation, pediatrics, and newborns. Compare group: major teaching hospitals nationwide. Data source: Truven CareDiscovery (Truven Health Analytics, Ann Arbor, MI).
*2008 Q2 only includes May and June.
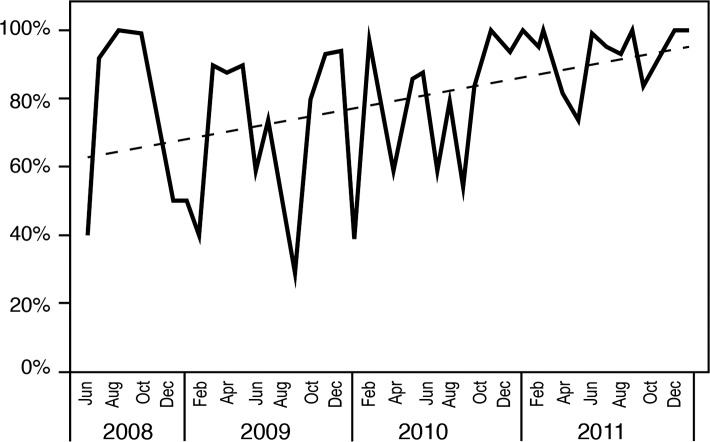
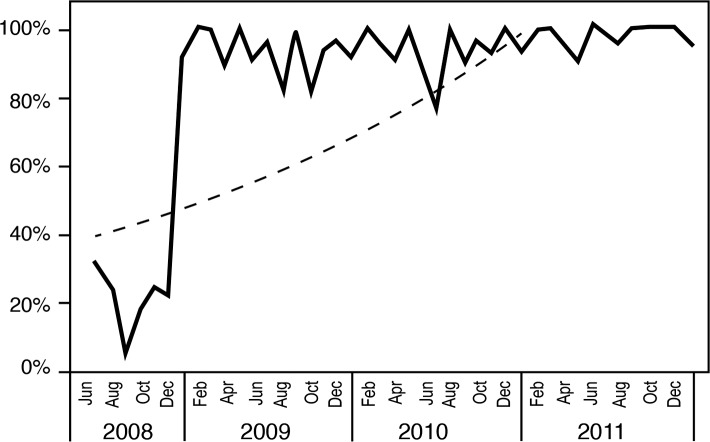
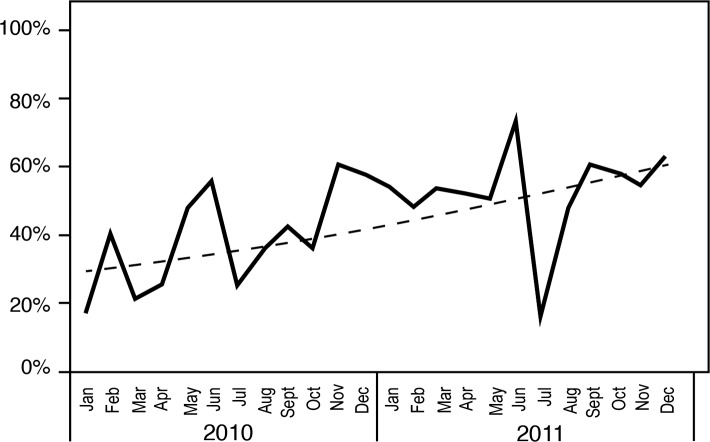
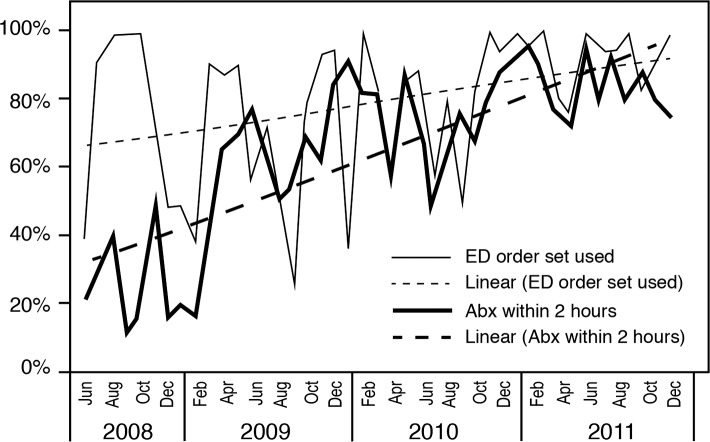
The sepsis project activities resulted in improved compliance with order sets over time (Figures 3 and 4), coinciding with improved attainment of perfect care over time (Figure 5). ED order set use correlated with improved time to antibiotics (Figure 6), a significant decrease in median time to antibiotics from 140 minutes (range 1-820) in 2008 to 72 minutes (range 1-1,020) in 2011 (P≤0.001), and a decrease in median length of stay from 8 days (range 1-54) in 2008 to 7 days (range 1-33) in 2011 (P=0.036). Improvements in time to antibiotics and length of stay over time were analyzed using an independent-samples Kruskal-Wallis test. We calculated the length of stay as the time from sepsis diagnosis until the time of discharge and excluded mortalities.
Figure 3.
Emergency department (6-hour) order set compliance.
Figure 4.
Sepsis critical care (24-hour) order set compliance.
Figure 5.
Perfect care over time.
Figure 6.
Emergency department (ED) order set compliance and time to antibiotics (<2 hours).
Abx, antibiotics.
DISCUSSION
This study revealed that multidisciplinary teamwork using quality improvement principles can improve the process of care and outcomes, with changes that are feasible and sustainable within a large academic tertiary referral hospital. The newly revised SCCM guidelines continue to promote protocols to improve sepsis outcomes. According to a recent metaanalysis, a preponderance of studies supports the use of EGDT-based bundles and the early initiation of antibiotics.19 Despite the recommended guidelines and increasing evidence that supports the guidelines, hospitals have been slow to implement standardized evidence-based protocols to manage severe sepsis and septic shock.20,21 The current healthcare system is not conducive to the interdisciplinary coordinated care that is required to implement time-dependent treatments. For the treatment of severe sepsis and septic shock, hospital administrators, ED physicians, intensivists, nurses, and pharmacists must coordinate care and commit to practice-based learning.
Hewett et al22,23 have proposed that the interprofessional and intergroup climate in hospitals leads to poor communication and impedes system approaches to improving healthcare. An intergroup culture in the hospital setting refers to individuals identifying with their professional groups and valuing their groups over others, leading to poor communication, poor teamwork, competition for scarce resources, and impedance of system protocols.22 Examples of intergroups that are related to sepsis include ED physicians, intensivists, ED nurses, CC nurses, pharmacists, medical informatics professionals, and hospital administrators. The Ochsner team was able to overcome this ingrained hospital intergroup culture by recognizing its existence, forming a multidisciplinary group that included representatives from all key groups, and identifying respected champions to change the culture within each individual group. This approach improved communication and led to compromises in duties between disciplines where traditionally roadblocks had stymied the implementation of treatment protocols. One significant example was the ED physician placing the ScvO2 catheter and the CC team assuming care within 1 hour of being consulted to facilitate EGDT and transfer from the ED.
The rapid-cycle/PDSA feedback was also critical to the Ochsner team's success. The team identified early problems with the ED nursing documentation and the transition of care. In fall 2009, they developed an ED nursing flow sheet that mirrored the current CC nursing flow sheet to provide better documentation in the ED and a more efficient transition of care to CC. Another success of the PDSA cycle was a protocol change that required house officers to maintain bedside attendance until goals were attained. Implemented in early 2010, this change resulted in a dramatic increase in the number of patients receiving perfect care in May and June 2010, and the steady rise continued after that period.
The Ochsner experience demonstrates that a large tertiary referral academic center can implement severe sepsis and septic shock management protocols to standardize the delivery of care, improve the process of care, and enhance outcomes. The success of this quality improvement project stemmed from the multidisciplinary team-based approach that improved communication between medical specialists and other allied healthcare providers.
This research is limited because it represents a nonrandomized study from a single center. Although Ochsner has experienced steady improvement in sepsis process of care measures over the project timeframe, we cannot exclude the idea that education and other unmeasured factors caused this temporal improvement.
CONCLUSION
Hospitals are complex systems that are resistant to change and have been slow to implement best practices for managing severe sepsis and septic shock. A multidisciplinary collaborative approach to the management of severe sepsis and septic shock resulted in improvements in the process of care as measured by a significant improvement in time to antibiotics and a decrease in hospital length of stay for patients. The improvement was achieved in a relatively short period of time and has been maintained over several years.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Systems-Based Practice, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001 Jul;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS. SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003 Apr; 2001;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, et al. International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses; American College of Chest Physicians; American College of Emergency Physicians; Canadian Critical Care Society; European Society of Clinical Microbiology and Infectious Diseases; European Society of Intensive Care Medicine; European Respiratory Society; International Sepsis Forum; Japanese Association for Acute Medicine; Japanese Society of Intensive Care Medicine; Society of Critical Care Medicine; Society of Hospital Medicine; Surgical Infection Society; World Federation of Societies of Intensive and Critical Care Medicine. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008 Jan;3636(1)(4):296–327. 1394–1396. Erratum in: Crit Care Med. 2008 Apr. [Google Scholar]
- 4.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013 Feb;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 5.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Dellinger RP, Townsend SR, et al. Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010 Feb;38(2):367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 7.Castellanos-Ortega A, Suberviola B, García-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010 Apr;38(4):1036–1043. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 8.Winterbottom F, Seoane L, Sundell E, Niazi J, Nash T. Improving sepsis outcomes for acutely ill adults using interdisciplinary order sets. Clin Nurse Spec. 2011 Jul-Aug;25(4):180–185. doi: 10.1097/NUR.0b013e318221f2aa. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Ellis P, Arabi Y, et al. Cooperative Antimicrobial Therapy of Septic Shock Database Research Group. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009 Nov;136(5):1237–1248. doi: 10.1378/chest.09-0087. Epub 2009 Aug 20. [DOI] [PubMed] [Google Scholar]
- 11.Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010 Apr;38(4):1045–1053. doi: 10.1097/CCM.0b013e3181cc4824. [DOI] [PubMed] [Google Scholar]
- 12.Sivayoham N, Rhodes A, Jaiganesh T, van Zyl Smit N, Elkhodhair S, Krishnanandan S. Outcomes from implementing early goal-directed therapy for severe sepsis and septic shock: a 4-year observational cohort study. Eur J Emerg Med. 2012 Aug;19(4):235–240. doi: 10.1097/MEJ.0b013e32834bbea6. [DOI] [PubMed] [Google Scholar]
- 13.Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49(1):1–16. doi: 10.2165/11318140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Pea F, Viale P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock—does the dose matter? Crit Care. 2009;13(3):214. doi: 10.1186/cc7774. Epub 2009 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thallinger C, Buerger C, Plock N, et al. Effect of severity of sepsis on tissue concentrations of linezolid. J Antimicrob Chemother. 2008 Jan;61(1):173–176. doi: 10.1093/jac/dkm431. Epub 2007 Nov 13. [DOI] [PubMed] [Google Scholar]
- 16.Cano EL, Haque NZ, Welch VL, et al. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Study Group. Incidence of nephrotoxicity and association with vancomycin use in intensive care unit patients with pneumonia: retrospective analysis of the IMPACT-HAP Database. Clin Ther. 2012 Jan;34(1):149–157. doi: 10.1016/j.clinthera.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Finfer S, Chittock DR. et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009 Mar 26;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. NICE-SUGAR Study Investigators. Epub 2009 Mar 24. [DOI] [PubMed] [Google Scholar]
- 18.Ranieri VM, Thompson BT, Barie PS, et al. PROWESS-SHOCK Study Group. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012 May 31;366(22):2055–2064. doi: 10.1056/NEJMoa1202290. Epub 2012 May 22. [DOI] [PubMed] [Google Scholar]
- 19.Barochia AV, Cui X, Vitberg D, et al. Bundled care for septic shock: an analysis of clinical trials. Crit Care Med. 2010 Feb;38(2):668–678. doi: 10.1097/CCM.0b013e3181cb0ddf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest. 2010 Sep;138(3):551–558. doi: 10.1378/chest.09-2210. Epub 2010 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reade MC, Huang DT, Bell D, et al. British Association for Emergency Medicine; UK Intensive Care Society; UK Society for Acute Medicine; Australasian Resuscitation in Sepsis Evaluation Investigators; Protocolized Care for Early Septic Shock Investigators. Variability in management of early severe sepsis. Emerg Med J. 2010 Feb;27(2):110–115. doi: 10.1136/emj.2008.070912. [DOI] [PubMed] [Google Scholar]
- 22.Hewett DG, Watson BM, Gallois C. Trust, distrust and communication accommodation among hospital doctors. In: Candlin CN, Crichton J, editors. Discourses of Trust. Basingstoke, UK: Palgrave Macmillan;; 2013. pp. 36–51. In. eds. [Google Scholar]
- 23.Hewett DG, Watson BM, Gallois C, Ward M, Leggett BA. Intergroup communication between hospital doctors: implications for quality of patient care. Soc Sci Med. 2009 Dec;69(12):1732–1740. doi: 10.1016/j.socscimed.2009.09.048. Epub 2009 Oct 21. [DOI] [PubMed] [Google Scholar]