Abstract
Although allogeneic transplantation is a potentially curative therapy for adults with acute lymphoblastic leukemia (ALL), the high risk of the ablative regimen limits its use, especially in older patients where the need for better therapy is greatest. Recent observations suggesting that a donor derived graft vs leukemia effect is important is facilitating cure after an allogeneic transplant has kindled interest in utilizing a reduced-intensity approach, which relies in large part on this effect to control and cure the disease. Several trials have now been reported that suggest that a reduced-intensity conditioning (RIC) approach might be very effective, especially in patients who are in the first remission of the disease from either related, unrelated, or cord blood transplant donors, with approximately 50% of patients alive and in remission two years after transplant. These studies suggest that prospective studies of RIC should be conducted in patients with ALL in first remission who are at high risk for relapse based on age or comorbidity to determine its role in the overall management of adult patients with this disease.
Keywords: Acute lymphoblastic leukemia, graft vs leukemia, pre-B ALL, total body irradiation, reduced intensity transplant
Introduction
Acute lymphoblastic leukemia (ALL) is a hematologic malignancy of the bone marrow characterized by the rapid proliferation and subsequent accumulation of immature lymphocytes. ALL accounts for 20% of all acute leukemias that are seen in adults over the age of 20 years. Over the past two decades, there has been substantial improvement in the understanding of the molecular biology of the disease and in the management of adult patients who have this disorder. The success of therapy in children with ALL has continued to fuel the quest for similar success in adults utilizing the same principles that guide therapy in children, namely intensive induction and consolidation, maintenance therapy, and prevention of disease in extramedullary sites such as the central nervous system (CNS). However, except for some studies focused on the treatment of young adults, the majority of adult patients will relapse and succumb to their disease. Allogeneic fully ablative hematopoietic stem cell transplantation (HCT) has been utilized effectively in the treatment of patients with high-risk disease and in those who have suffered a relapse, but is limited to treatment of younger patients. Laboratory and clinical studies are beginning to refine the decisions concerning both the indication and timing of allogeneic HCT.
The contribution of a donor-derived antitumor response has been the basis for the development of effective reduced-intensity approaches in the treatment of acute myeloid leukemia and low-grade lymphoma, but the role of a reduced-intensity conditioning (RIC) transplant in the treatment of patients with ALL is less clear. This article reviews the role and limitations of allogeneic HCT in the management of adult patients with this disease, the evidence of a graft vs ALL response after allogeneic transplant, and summarizes the findings of early reports of studies in adult patients of reduced-intensity transplantation in the management of the disease.
Prognostic factors in ALL
The major prognostic factors for achieving an initial complete remission (CR) are advancing age, a slow response to treatment, the presence of Philadelphia (Ph) chromosome, and measurable minimal residual disease after completion of induction and consolidation therapy.1-6 These prognostic factors are of even greater importance in predicting the durability of remission and survival and are utilized to assess the need for allogeneic HCT during first remission. Table 1 identifies some of the generally accepted adverse prognostic factors for remission duration in adult ALL that have been identified in previous clinical trials and are often utilized in determining a patient's risk of relapse and the decision to pursue allogeneic transplant. The cure rates for patients with ALL generally fall with age with very few long-term survivors over the age of 50-60 years.
Table 1. High-risk features in adult ALL.
| All | B-Precursor | T-ALL | |
|---|---|---|---|
| At diagnosis | |||
| High WBC | > 30,000/μL (20-50) | > 100,000 | |
| Subtype | pro B | early T | |
| CD10-neg pre B | mature T | ||
| Cytogenetics/ | complex | t(9;22)/BCR-ABL | BAALC+ |
| Molecular genetics | Karyotype | t(4;11)/ALL1-AF4 (pro B) | HOX11L2 |
| t(1:19) | EGR+ | ||
| During course | time to CR (> 4 weeks), more than 1 cycle to CR | ||
| MRD persistence (molecular failure) | |||
| MRD reappearance (molecular relapse) | |||
| Age | > 35 | ||
Abbreviations: CR, complete remission; MRD, minimal residual disease; WBC, white blood cell count
Allogeneic HCT in first complete remission of ALL
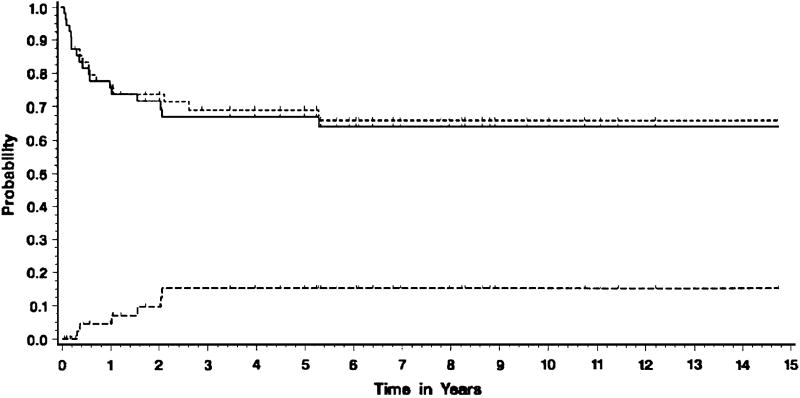
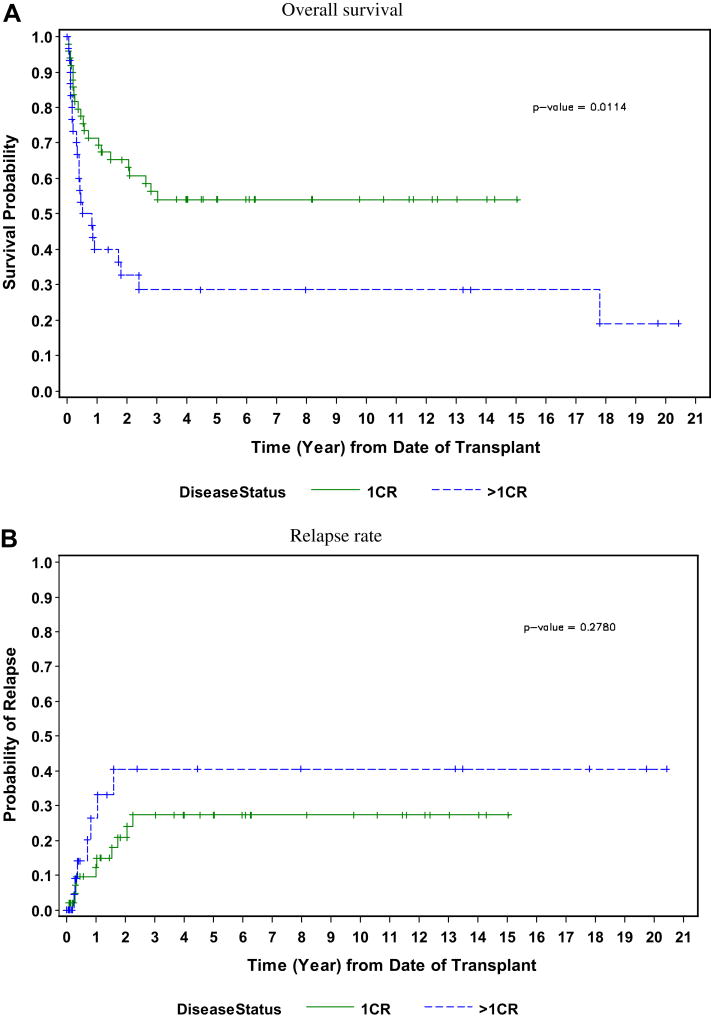
Allogeneic HCT in first CR has generally been reserved for those patients who present with poor risk features. In several phase II studies, patients with high risk disease treated with allogeneic HCT had a disease-free survival (DFS) longer than would have been predicted, especially those with Ph+ ALL. Depending on the number of risk factors present at diagnosis in an individual patient, standard chemotherapy leads to continued remissions ranging from less than 10% to more than 50%.1,7 Studies have been conducted that indicate that HCT offers some groups of high-risk patients long-term disease survival rates of between 40% and 60%.8-14 At the City of Hope and Stanford University, two series of patients with high-risk features who underwent allogeneic HCT in first CR have been recently updated. Selection criteria included white blood cell count (WBC) >25,000/μL, chromosomal translocations t(9;22), t(4;11), t(8;14); age older than 30; extramedullary disease at the time of diagnosis; and/or requiring more than 4 weeks to achieve a CR. Two-thirds of the patients had at least one risk factor, and the remaining patients had two or more high-risk features at presentation. The majority of these patients underwent allogeneic HCT within the first 4 months after achieving a CR. Allogeneic HCT during first remission led to prolonged DFS in this patient population who would otherwise have been expected to fare poorly. At a median follow-up of greater than 5 years, the probability of event-free survival was 64% with a relapse rate of 15% (Figure 1).15 In those patients with Ph+ ALL, disease-free survival following an ablative regimen of total body irradiation (TBI)/etoposide resulted in a 55% disease-free survival with a median follow-up of 8 years in all patients (Figure 2).16
Figure 1.
Probability of event-free survival (EFS), overall survival (OS), and relapse for 55 adult patients with high-risk acute lymphoblastic leukemia transplanted in first remission. Updated with permission.15
Reprinted from Experimental Hematolology, 31, Jamieson CH, Amylon MD, Wong RM, Blume KG. Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience, p 981-986, Copyright 2003, with permission from Elsevier.
Figure 2.
Probability of long-term disease-free survival and risk of relapse in 79 patients with Ph+ ALL following allogeneic HCT in first remission and beyond first remission.19
Reprinted from Biology of Blood and Marrow Transplantation, 15, Larson RA, Allogeneic hematopoietic cell transplantation is not recommended for all adults with standard-risk acute lymphoblastic leukemia in first complete remission, p 11-16, Copyright 2008, with permission from Elsevier.
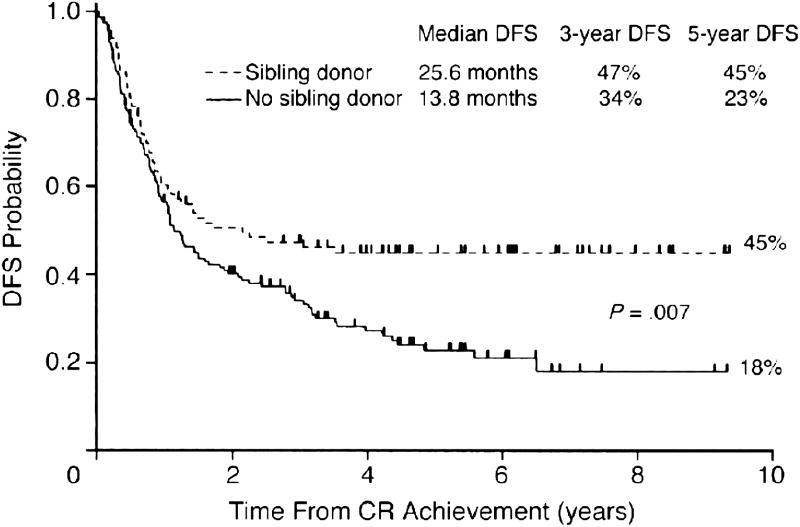
The French Group on Therapy for Adult ALL conducted a study comparing chemotherapy to autologous and allogeneic HCT.7 Although the overall results of treatment did not show a treatment advantage for the group treated with allogeneic HCT, subgroup analysis revealed that those patients with high-risk disease, including patients with a t(9;22) and t(1;19) translocation, had a higher 5-year survival of 44%, as opposed to 20% in the other two groups (Figure 3). These trials have supported the strategy of taking patients with high-risk disease to transplant while in first remission. The recently reported Easter Cooperative Oncology Group (ECOG)-Medical Research Council (MRC) trial comparing chemotherapy and allogeneic and autologous transplant showed that an allogeneic HCT resulted in improved disease control in all adult patients with ALL, but with long-term benefit seen mostly in younger patients with lowerrisk disease.17 Table 2 shows the disease-free survival and response rate for patients on this trial with the risk of relapse reduced following allogeneic HCT when compared to either chemotherapy or autologous HCT, showing the improved outcomes and better leukemia control for those patients undergoing allogeneic transplant in first remission. Although the study clearly demonstrated better disease control, the overall benefit was undermined by the toxicity observed in older patients treated with the fully ablative TBI/etoposide regimen. Table 3 shows a comparison of a number of other trials comparing chemotherapy to HCT. This trial has led to considerable debate about its meaning for the optimal treatment of patients with ALL, with some advocating early transplant for nearly all patients, while others have cautioned a continued individual assessment.18,19 All agree that new approaches to reducing the toxicity of treatment in older patients are necessary.
Figure 3.
Disease-free survival (DFS) according to genetic randomization. The group with a sibling donor comprised 100 patients, whereas that with no sibling donor included 159 patients.7
Abbreviations: CR, complete remission; DFS, disease-free survival
Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. Journal of Clinical Oncololgy 2004; 22: 4075-4086. Reprinted with permission. ©2008 American Society of Clinical Oncology. All rights reserved.
Table 2.
MRC-ECOG UKALLXII/EC2993: outcome after allogeneic hematopoietic stem cell transplantation (HSCT) in Ph− patients who had donors vs those who did not have donors.
| # | 5-year overall survival % | 5-year relapse rates % | 2-year nonrelapse mortality % | |
|---|---|---|---|---|
| High-risk* | 401 | |||
| Donor | 171 | 40 | 39 | 39 |
| No donor | 230 | 36 | 62 | 12 |
| Standard-risk | 512 | |||
| Donor | 218 | 63 | 27 | 20 |
| No donor | 294 | 51 | 50 | 7 |
High-risk is defined as age ≥ 35 years, WBC > 30,000/μL for patients with B-cell disease or WBC > 100,000/μL for patients with T-cell disease, or time to attain CR > 4 weeks.
Table 3.
Comparison of several large trials for acute lymphoblastic leukemia CR1 patient outcome.
| Group Study | No. pts. considered for HCT | Outcome Measure | Chemo- Autologous therapy,% HCT,% | Allogeneic HCT,% | P |
|---|---|---|---|---|---|
| MRC-ECOG | 913 | OS at 5 y | 45 | 53 | 0.02 |
| JALSG-ALL93 | 142 | OS at 6 y | 40 -- | 46 | NS |
| LALA-87 | 257 | OS at 10 y | 31 | 46 | 0.04 |
| LALA-87 high-risk | 156 | OS at 10 y | 11 | 44 | 0.009 |
| LALA-94 | 259 | DFS at 3 y | 34 | 47 | 0.007 |
| LALA-94 high-risk | 211 | OS at 5 y | 21 32 | 51 but median OS not reached | Not stated |
| GOELAMS 02 high-risk | 156 | OS at 6 y | 40 at 6 y | 75 at 6 y | 0.0027 |
| PETHEMA 93 high-risk | 183 | OS at 5 y | 49 | 40 | .56 |
Abbreviations: HCT, hematopoietic cell transplantation; NS, not significant; OS, overall survival
Role of graft-vs.-leukemia effect in patients with ALL
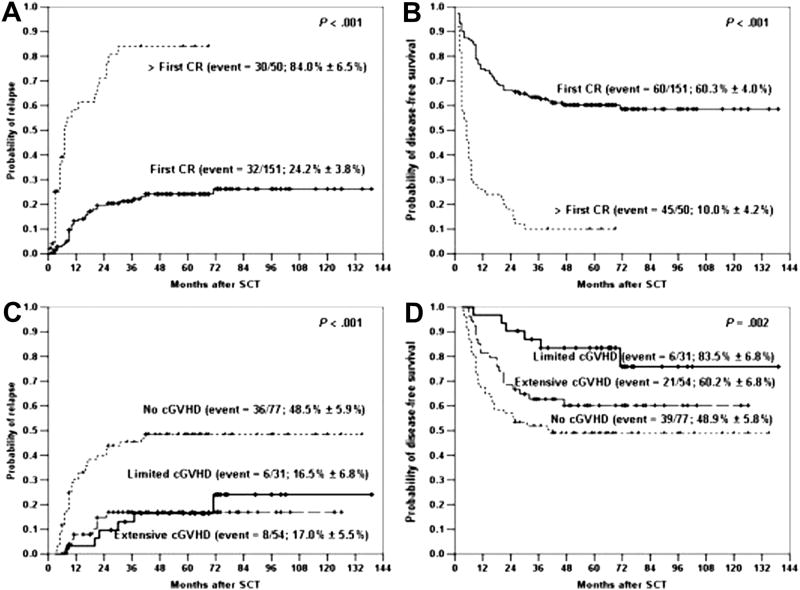
The low response rate in patients with ALL following donor leukocyte infusion (DLI) to treat post-transplant relapse has led to questions about the contribution of the graft-vs-leukemia (GVL) effect in preventing relapse in this disease. The GVL effect is derived from observations of a higher relapse rate after autologous or syngeneic HCT compared to allogeneic HCT, lower incidence of relapse in patients who had graft-vs-host disease (GVHD), as well as increased relapse rates in recipients of T-cell-depleted marrow grafts. The most compelling argument for a strong GVL effect in ALL comes from both single institution and registry data.20,21 These studies show consistent decrease in relapse rates in patients who develop GVHD compared to those patients who do not. Table 4 shows the rate of relapse after HCT for ALL in first CR and the correlation with GVHD. The occurrence of acute, chronic, or both forms of GVHD correlated with the best DFS. A study of 192 patients with ALL, most of whom were transplanted in second remission,22 evaluated the probability of relapse among patients without or with GVHD. Relapse was significantly higher in the group that had less than grade II GVHD. In patients without significant GVHD, the actuarial risk of relapse approached 80% vs 40% in those who developed grade II or higher. An evaluation of 1132 patients with T- or B-lineage ALL supports the observation that both acute and chronic GVHD are associated with a decreased risk of relapse in both of the major immunophenotypes of adult ALL.23 Recent studies have also demonstrated the beneficial impact of chronic GVHD on reducing relapse in patients undergoing allogeneic HCT for ALL, including Ph+ ALL (Figure 4).24,25 In another study of 199 patients with ALL who underwent transplant mostly from a related matched sibling and a TBI/cyclophosphamide regimen, the development of chronic GVHD was associated with a lower incident of relapse (P < .001).26
Table 4. Relapse after transplantation for acute lymphoblastic leukemia in first remission.35.
| Group | Relapse probability at 3 years (%) |
|---|---|
| Allogeneic, non-T depleted | |
| No GVHD | 44 ± 17 |
| Acute only | 17 ± 9 |
| Chronic only | 20 ± 19 |
| Both | 15 ± 10 |
| Syngeneic | 41 ± 32 |
| Allogeneic, T-depleted | 34 ± 13 |
Abbreviations: GVHD, graft-vs-host disease
Figure 4.
Impact of chronic GVHD on incidence of relapse following fully ablative allogeneic transplantation.25
Note to publisher: Permission being applied for.
Although the data support the importance of a GVL effect in mediating a clinically useful antileukemic response in patients with ALL, the reasons for the limited beneficial effect for patients with relapsed ALL treated with DLI are not clear. The different outcomes may reflect differences in the ability of ALL cells to present antigen targets, an inhibitory microenvironment, the low frequency of T-cell precursors reactive with minor antigens presented by ALL cells, the susceptibility of ALL targets to lysis, or kinetic differences in the way leukemic cells grow after HCT. Thus, cytoreduction with chemotherapy prior to infusion of DLI is often utilized as a better strategy for treatment of patients with relapsed ALL. As discussed below, it is for these reasons that HCT after reduced-intensity conditioning is of limited effectiveness in patients with ALL who are not in remission.
Clinical studies of reduced intensity conditioning transplant studies in the treatment of ALL
Although there have been a large number of studies performed in evaluating the role of allogeneic reduced-intensity transplantation in patients with myeloid malignancies, multiple myeloma, and low-grade non-Hodgkin's lymphoma, there have been fewer studies conducted in patients with ALL, and all are retrospective. In general, the consensus has been that for patients with ALL, ablative chemoradiotherapy was required for an improved cure rate, but despite better disease control, this approach is of limited use in patients over the age of 45-50 years, as confirmed in the ECOG-MRL trial.17,27,28 In addition, as described above, an evaluation of outcomes suggests that the graft-versus-tumor effect is more effective against myeloid malignancies, such as acute and chronic myeloid leukemia, and malignancies of mature B cells, such as low-grade non-Hodgkin's lymphoma and multiple myeloma, but less so with a more undifferentiated B-cell disease, such as pre-B ALL, especially if not in remission.29-31 Thus, fewer studies have been conducted in this disease. Nevertheless, a few small studies have been reported that suggest that there may well be a role for reduced-intensity allogeneic transplant even in this disease.
A retrospective analysis from the European Group for Blood and Marrow Transplantation reported the outcome of 97 patients with ALL who received a reduced-intensity transplant and suggested that nonmyeloablative transplant may be effective in early, but not advanced, disease.32 With a median follow-up of 2.8 years, the 2-year overall, leukemia-free, and nonrelapse mortality were better in those patients transplanted in remission (CR1 52%, 42%, 18%), compared to those with advanced disease (P = 0.003, P = 0.002, and P = 0.01, respectively). In their multivariate analysis, remission status (P = 0.001) and chronic GVHD (P = 0.01) were the most important determinants for survival. In a recent report, patients undergoing reduced-intensity HCT at City of Hope utilizing either related, unrelated, or cord blood donor following a fludarabine/melphalan regimen also showed an optimistic outcome.
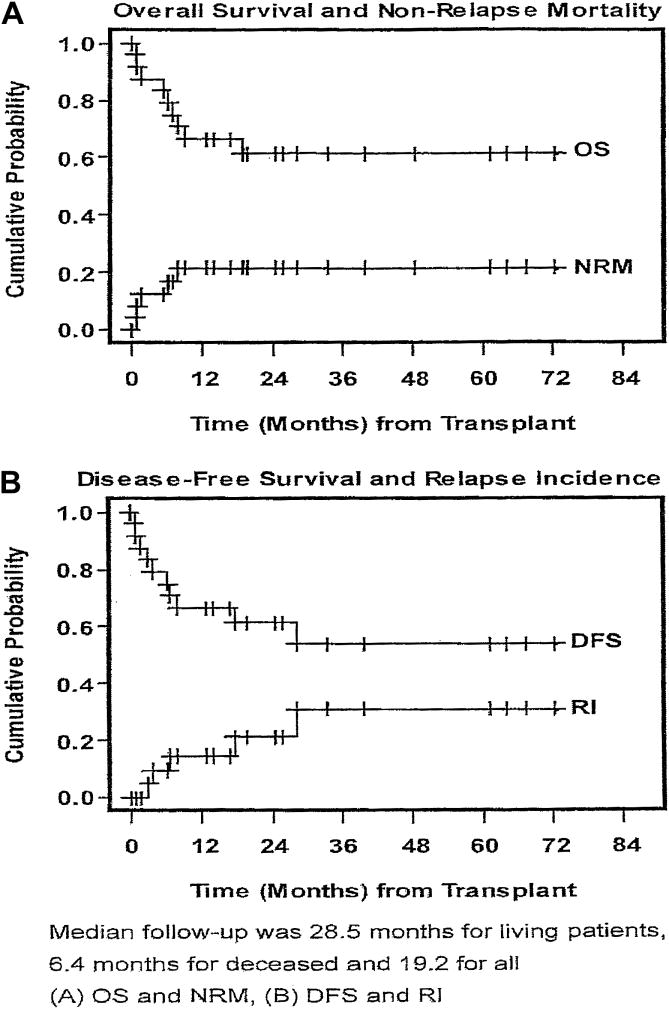
A study of a group of 24 patients either at high risk during first remission or who were transplanted after achieving a second or subsequent remission showed an overall and disease-free survival of 61%, a relapse rate of 21%, and a nonrelapse mortality of 21% at 24 years (Figure 5).33 Indications for transplant were age greater than 50 years (42%), compromised organ function (54%), or a previous transplant (37.5%). The subsequent development of chronic GVHD was observed in 80% of evaluable patients.
Figure 5.
Overall survival of patients with high-risk ALL following reduced-intensity transplant.33
Abbreviations: DFS, disease-free survival; NRM, nonrelapse mortality; OS, overall survival; RI, relapse incidence;
Note to publisher: Permission being applied for.
The Catholic University of Korea conducted a prospective phase II trial of RIC in 37 patients with high-risk ALL who were in first (n = 30) or second CR (n = 7), also utilizing a fludarabine/melphalan regimen.34 The indications for reduced-intensity conditioning allogeneic SCT (RIC-SCT) were: (1) ≥ 50 years, (n = 16; 43.2%) and (2) decreased organ function or active infections, (n = 21; 56.8%). GVHD prophylaxis consisted of calcineurin inhibitor (cyclosporine for sibling and tacrolimus for unrelated transplants) and methotrexate. The cumulative incidence of acute (grades II-IV) and chronic GVHD was 43.2% and 65.6%, respectively. After a median follow-up of 36 months for surviving transplants, the 3-year relapse, nonrelapse mortality, disease-free survival, and overall survival rates were 19.7%, 17.7%, 62.6%, and 64.1%, respectively. Transplants in first CR showed better transplantation outcomes than those in second CR. The potential of antileukemic activity of chronic GVHD was also found.
A recent report from the University of Minnesota evaluated reduced-intensity transplant followed by cord blood transplant in a group of 22 patients and showed both the feasibility of this cell source and good survival (50%) at 3 years after transplant.35
These studies, though nearly all retrospective, are fairly consistent in their outcomes, with approximately 50% of patients disease-free at a median follow-up of at least 2 years, regardless of cell source.
Potential role of RIC in the treatment of adult patients with ALL
The aggregates in these reports suggest that RIC–SCT is a potential therapeutic approach for adults with high-risk ALL in remission who are ineligible for myeloablative transplantation and that it is of limited value in patients with relapsed leukemia not in remission.
The results of the recent UK ALL XII ECOG 2993 study of adult ALL show high toxicity and limited improvement in disease-free survival despite better disease control in older adult patients. Thus, based on the early studies of small groups of patients, there is new increased interest in the development of clinical trials that will explore these reduced-intensity approaches in adult patients with ALL in first remission who would otherwise be candidates for transplantation based on age, cytogenetics, MRD, response to initial treatment, and having an available donor. The poor outcome of older patients with ALL using either standard chemotherapy or transplant makes this an important consideration for treatment in those patients and is now the basis for prospective trials to determine if RIC can improve the cure rate of older adults with ALL in first remission. Such studies in Ph+ ALL would include post-transplant tyrosine kinase inhibitors to further reduce tumor burden and allow maturation of a therapeutic GVL response. Assessment of minimal residual disease as a reflection of the depth of the remission at transplant could also help refine the extent and limits of such an approach in patients with ALL.
Acknowledgments
This work is supported by Grant #PO1 CA 30206, Cancer Center Support Grant #CA 33572, and Grant #U10 CA 46368 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe JM, Buck G, Burnett AK, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 2.Larson RA, Dodge RK, Burns CP, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85:2025–2037. [PubMed] [Google Scholar]
- 3.Hoelzer D, Thiel E, Loffler H, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71:123–131. [PubMed] [Google Scholar]
- 4.Cave H, van der Werff ten Bosch, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer--Childhood Leukemia Cooperative Group. N Engl J Med. 1998;339:591–598. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- 5.Mortuza FY, Papaioannou M, Moreira IM, et al. Minimal residual disease tests provide an independent predictor of clinical outcome in adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:1094–1104. doi: 10.1200/JCO.2002.20.4.1094. [DOI] [PubMed] [Google Scholar]
- 6.Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 7.Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Attal M, Blaise D, Marit G, et al. Consolidation treatment of adult acute lymphoblastic leukemia: a prospective, randomized trial comparing allogeneic versus autologous bone marrow transplantation and testing the impact of recombinant interleukin-2 after autologous bone marrow transplantation. BGMT Group Blood. 1995;86:1619–1628. [PubMed] [Google Scholar]
- 9.Doney K, Fisher LD, Appelbaum FR, et al. Treatment of adult acute lymphoblastic leukemia with allogeneic bone marrow transplantation. Multivariate analysis of factors affecting acute graft-versus-host disease, relapse, and relapse-free survival. Bone Marrow Transplant. 1991;7:453–459. [PubMed] [Google Scholar]
- 10.Blume KG, Forman SJ, Snyder DS, et al. Allogeneic bone marrow transplantation for acute lymphoblastic leukemia during first complete remission. Transplantation. 1987;43:389–392. doi: 10.1097/00007890-198703000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Vernant JP, Marit G, Maraninchi D, et al. Allogeneic bone marrow transplantation in adults with acute lymphoblastic leukemia in first complete remission. J Clin Oncol. 1988;6:227–231. doi: 10.1200/JCO.1988.6.2.227. [DOI] [PubMed] [Google Scholar]
- 12.Chao NJ, Forman SJ, Schmidt GM, et al. Allogeneic bone marrow transplantation for high-risk acute lymphoblastic leukemia during first complete remission. Blood. 1991;78:1923–1927. [PubMed] [Google Scholar]
- 13.Sebban C, Lepage E, Vernant JP, et al. Allogeneic bone marrow transplantation in adult acute lymphoblastic leukemia in first complete remission: a comparative study. French Group of Therapy of Adult Acute Lymphoblastic Leukemia. J Clin Oncol. 1994;12:2580–2587. doi: 10.1200/JCO.1994.12.12.2580. [DOI] [PubMed] [Google Scholar]
- 14.Vey N, Blaise D, Stoppa AM, et al. Bone marrow transplantation in 63 adult patients with acute lymphoblastic leukemia in first complete remission. Bone Marrow Transplant. 1994;14:383–388. [PubMed] [Google Scholar]
- 15.Jamieson CH, Amylon MD, Wong RM, et al. Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience. Exp Hematol. 2003;31:981–986. doi: 10.1016/s0301-472x(03)00231-5. [DOI] [PubMed] [Google Scholar]
- 16.Laport GG, Alvarnas JC, Palmer JM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood. 2008;112:903–909. doi: 10.1182/blood-2008-03-143115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 18.Goldstone AH. Transplants in Adult ALL--? Allo for everyone. Biol Blood Marrow Transplant. 2008;15:7–10. doi: 10.1016/j.bbmt.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Larson RA. Allogeneic hematopoietic cell transplantation is not recommended for all adults with standard-risk acute lymphoblastic leukemia in first complete remission. Biol Blood Marrow Transplant. 2008;15:11–16. doi: 10.1016/j.bbmt.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 21.Appelbaum FR. Graft versus leukemia (GVL) in the therapy of acute lymphoblastic leukemia (ALL) Leukemia. 1997;11(4):S15–S17. [PubMed] [Google Scholar]
- 22.Cornelissen JJ, Carston M, Kollman C, et al. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97:1572–1577. doi: 10.1182/blood.v97.6.1572. [DOI] [PubMed] [Google Scholar]
- 23.Passweg JR, Tiberghien P, Cahn JY, et al. Graft-versus-leukemia effects in T lineage and B lineage acute lymphoblastic leukemia. Bone Marrow Transplant. 1998;21:153–158. doi: 10.1038/sj.bmt.1701064. [DOI] [PubMed] [Google Scholar]
- 24.Esperou H, Boiron JM, Cayuela JM, et al. A potential graft-versus-leukemia effect after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: results from the French Bone Marrow Transplantation Society. Bone Marrow Transplant. 2003;31:909–918. doi: 10.1038/sj.bmt.1703951. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Cho BS, Kim SY, et al. Allogeneic stem cell transplantation in first complete remission enhances graft-versus-leukemia effect in adults with acute lymphoblastic leukemia: antileukemic activity of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1083–1094. doi: 10.1016/j.bbmt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Nordlander A, Mattsson J, Ringden O, et al. Graft-versus-host disease is associated with a lower relapse incidence after hematopoietic stem cell transplantation in patients with acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2004;10:195–203. doi: 10.1016/j.bbmt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Forman SJ. Hematopoietic cell transplantation for acute lymphoblastic leukemia in adults. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' hematopoietic cell transplantation. Chichester, West Sussex, UK: Wiley-Blackwell; 2008. pp. 791–805. [Google Scholar]
- 28.Marks DI. A comparison of two myeloablative regimens for allogeneic hematopoietic cell transplantation for patients with acute lymphoblastic leukemia; Presented at the American Society for Blood and Marrow Transplantation meeting on February 13, 2004; Orlando, Florida. 2004. [Google Scholar]
- 29.Valcarcel D, Martino R, Sureda A, et al. Conventional versus reduced-intensity conditioning regimen for allogeneic stem cell transplantation in patients with hematological malignancies. Eur J Haematol. 2005;74:144–151. doi: 10.1111/j.1600-0609.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 30.Arnold R, Massenkeil G, Bornhauser M, et al. Nonmyeloablative stem cell transplantation in adults with high-risk ALL may be effective in early but not in advanced disease. Leukemia. 2002;16:2423–2428. doi: 10.1038/sj.leu.2402712. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez-Aguirre CH, Gomez-Almaguer D, Cantu-Rodriguez OG, et al. Non-myeloablative stem cell transplantation in patients with relapsed acute lymphoblastic leukemia: results of a multicenter study. Bone Marrow Transplant. 2007;40:535–539. doi: 10.1038/sj.bmt.1705769. [DOI] [PubMed] [Google Scholar]
- 32.Mohty M, Labopin M, Tabrizzi R, et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:303–306. doi: 10.3324/haematol.11960. [DOI] [PubMed] [Google Scholar]
- 33.Stein AS, Palmer JM, O'Donnell MR, et al. Reduced intensity conditioning followed by peripheral blood stem cell transplantation for adult patients with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2009;15:1407–1414. doi: 10.1016/j.bbmt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho BS, Lee S, Kim YJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia. 2009 doi: 10.1038/leu.2009.102. In press. [DOI] [PubMed] [Google Scholar]
- 35.Bachanova V, Verneris MR, DeFor T, et al. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113:2902–2905. doi: 10.1182/blood-2008-10-184093. [DOI] [PubMed] [Google Scholar]