Abstract
ATP-binding cassette transporter G1 (ABCG1) plays a role in the intracellular transport of cholesterol. Invariant NKT (iNKT) cells are a subpopulation of T lymphocytes that recognize glycolipid Ags. In this study, we demonstrate that ABCG1 regulates iNKT cell development and functions in a cell-intrinsic manner. Abcg1–/– mice displayed reduced frequencies of iNKT cells in thymus and periphery. Thymic iNKT cells deficient in ABCG1 had reduced membrane lipid raft content, and showed impaired proliferation and defective maturation during the early stages of development. Moreover, we found that Abcg1–/– mice possess a higher frequency of Vβ7+ iNKT cells, suggesting alterations in iNKT cell thymic selection. Furthermore, in response to CD3ε/CD28 stimulation, Abcg1–/– thymic iNKT cells showed reduced production of IL-4 but increased production of IFN-γ. Our results demonstrate that changes in intracellular cholesterol homeostasis by ABCG1 profoundly impact iNKT cell development and function.
Natural killer T cells are a unique subset of T lymphocytes that share characteristics with both NK cells and conventional T cells. There are two main classes of NKT cells, type I and type II. Type I NKT cells, also referred to as invariant NKT (iNKT) cells, express an invariant TCR (iTCR) α-chain, utilizing Vα14Jα18 in mice and Vα24Jα18 in humans. This iTCR α-chain is paired with a limited TCRβ chain repertoire, predominantly Vβ8, Vβ7, or Vβ2 in mice, and Vβ11 in humans (1). Unlike conventional MHC class I- and class II-reactive T cells, which recognize peptide Ags, iNKT cells recognize glycolipid Ags presented by CD1d, a MHC class I-like molecule (1). At least two classes of glycolipids have been reported to bear antigenic activity for iNKT cells, as follows: glycosphingolipids, as exemplified by Ags found in Sphingomonas bacteria (2, 3), and diacylglycerols, found, for example, in Borrelia bacteria (4). There are reports that other types of lipids, including cholesterol-containing molecules from Helicobacter pylori, are recognized by iNKT cells (5). The best-studied Ag for iNKT cells is the glycosphingolipid, α-galactosylceramide (α-GalCer) (6). Upon activation with α-GalCer, iNKT cells are able to produce large quantities of both Th1 cytokines (IFN-γ) and Th2 cytokines (IL-4, IL-10, and IL-13) (7, 8). Thus, iNKT cells can modulate immunity in a broad range of diseases and conditions, including atherosclerosis, autoimmunity, cancer, diabetes, allergy, and infection (7, 8).
ATP-binding cassette (ABC) transporters are transmembrane proteins that facilitate the transport of specific substrates across the membrane in an ATP-dependent manner (9). ABC transporter G1 (ABCG1) is a member of the ABC transporter family that regulates cholesterol homeostasis in the cell (10). Cholesterol homeostasis is crucial for the growth and survival of cells, because cholesterol is a key component of cell membranes and lipid rafts (11). ABCG1 is expressed in many tissues, including spleen, brain, lung, and kidney, and in many cell types, including lymphocytes, myeloid cells, and endothelial cells (12). Whereas ABCG1 can localize to the plasma membrane, it resides mostly intracellularly. Although initial studies suggested that the main function of ABCG1, similar to the other cholesterol transporter ABC transporter A1 (ABCA1), is to promote cholesterol efflux from cells (12, 13), recent reports by us and others show that ABCG1 is also important for the intracellular transport of cholesterol (14, 15). We previously reported that ABCG1 regulates the transfer of cholesterol from outer to inner membrane leaflets in secretory granules, including insulin granules in the pancreas (14). A recent study by Tarling and Edwards (15) has demonstrated that ABCG1 localizes to endocytic vesicles to facilitate the redistribution of intracellular cholesterol away from the endoplasmic reticulum. Thus, a key function of ABCG1 may be to regulate membrane cholesterol content to facilitate proper membrane fluidity and cholesterol homeostasis.
ABCG1 expression in immune cells, such as macrophages and lymphocytes, impacts their function. ABCG1-deficient macrophages display increased inflammatory activity (16, 17) in response to LPS (18), and when loaded with cholesterol (19). We (20) and others (21) have reported that changes in cholesterol and lipid raft content in the absence of ABCG1 increase TCR signaling and proliferation of CD4+ T cells. Like other lymphocytes, iNKT cells express ABCG1; however, the role of ABCG1 and cholesterol homeostasis in iNKT cell biology is not known. In this study, we demonstrate that changes in intracellular cholesterol homeostasis by ABCG1 profoundly impact thymic iNKT cell development and function.
Materials and Methods
Mice
C57BL/6J mice (000664) and B6.129S7-Rag1tm1Mom/J (002216) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Abcg1–/–/lacZ knock-in mice were purchased from Deltagen (San Mateo, CA) and are congenic on a C57BL/6J background. B6.SJL-Ptprca/BoyAiTac mice (CD45.1 congenic, 004007) were purchased from Taconic Farms (Germantown, NY). Mice were fed a standard rodent chow diet and were housed in microisolator cages in a pathogen-free animal facility of the La Jolla Institute for Allergy and Immunology. All experiments followed guidelines of the La Jolla Institute for Allergy and Immunology Animal Care and Use Committee, and approval for use of rodents was obtained from the La Jolla Institute for Allergy and Immunology according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. Mice were euthanized by CO2 inhalation.
Reagents
Flow cytometry Abs, including anti-mouse CD45.2 (104), CD4 (RM4-5), TCRβ (H57-597), IL-4 (BVD6-24G2), CD44 (IM7), NK1.1 (PK136), and CD1d (1B1), were purchased from eBioscience (San Diego, CA); CD45.1 (A20), IFN-γ (XMG1.2), Vβ7 (TR310), and Vβ8.1/2 (MR5-2) were purchased from BD Biosciences (San Jose, CA); CD19 (6D5) and CD8α (5H10) were purchased from Invitrogen (Carlsbad, CA); Vβ2 (B20.6) was purchased from BioLegend (San Diego, CA); and neuropilin-1 (NRP-1) (polyclonal) was purchased from R&D Systems (Minneapolis, MN). Allophycocyanin-conjugated CD1d tetramers loaded with PBS-57 (an α-GalCer analog) were provided by the National Institutes of Health Tetramer Facility. PE and Pacific blue-conjugated α-GalCer–loaded CD1d tetramers were produced, as previously described (22). Anti-CD3ε (145-2C11), anti-CD28 (37.51), and CD16/CD32 (2.4G2) Abs were purchased from BD Biosciences. RPMI 1640 medium was purchased from Invitrogen (Carlsbad, CA), and PBS was purchased from Thermo Scientific (Rockford, IL).
Primary cell preparation
Single-cell suspensions were prepared from the thymus, spleen, and liver. Spleens and thymi were meshed through a 40-μm strainer (Fisher Scientific, Pittsburgh, PA). RBCs in spleen were lysed in RBC lysis buffer, according to the manufacturer's protocol (BioLegend). Prior to extraction, the liver was perfused with PBS via the portal vein until opaque and meshed through a 100-μm strainer and washed. Total liver cells were then resus-pended in a 37.5% isotonic Percoll solution (Amersham Biosciences, Piscataway, NJ) and centrifuged for 30 min at 850 × g at room temperature. After centrifugation, RBCs were lysed, as described above.
Flow cytometry
Cells were resuspended in 100 μl flow cytometry staining buffer (1% BSA plus 0.1% sodium azide in PBS). FcRγ were blocked with CD16/32 blocking Ab for 10 min, and surface Ags on cells were stained for 30 min at 4°C. Cells were stained with the CD1d tetramer together with the other surface Abs in staining buffer. LIVE/DEAD Fixable Dead Cell Stain (Invitrogen) was used for analysis of viability, and forward- and side-scatter parameters were used for exclusion of doublets from analysis. Ab clones used are listed above.
For measurement of membrane lipid rafts, cells were stained for an additional 10 min at 4°C in PBS with 10 ng Alexa-Fluor 488-labeled cholera toxin B (CT-B) from Vybrant lipid-raft labeling kits (Invitrogen).
For intracellular staining, cells were fixed and permeabilized with the Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD Biosciences) after the cell surface staining. Cells were stained for 30 min at 4°C with directly conjugated fluorescent of IL-4 and IFN-γ Abs.
Calculations of percentages were based on live cells as determined by forward- and side-scatter and viability analysis. Cell fluorescence was assessed using LSR-II (BD Biosciences), and data were analyzed with FlowJo software (Tree Star, Ashland, OR).
In vitro stimulation assays
For activation of iNKT cell hybridomas, 5 × 104 iNKT cell hybridoma DN3A4-1.2 cells were cultured with 5 × 105 thymocytes in the presence of titrated amounts of α-GalCer (1.6, 6.3, 25, 100, 400 ng/ml) in 96-well plates in vitro. After 18 h, IL-2 levels in the supernatant were quantitated using a mouse IL-2 ELISA kit (eBioscience), according to the manufacturer's instructions. For activation of primary iNKT cells, 24-well plates were coated with 5 μg/ml αCD3ε Ab in PBS at 4°C overnight. The next day, the plates were washed twice with PBS, and thymocytes were plated at 2 × 106 cells/well in RPMI 1640 medium supplemented with 5% FBS and 1% penicillin/streptomycin. Soluble αCD28 Ab (2 μg/ml) and GolgiPlug (BD Biosciences) were added, and the cells were incubated at 37°C for 4 h. Thymocytes were stimulated with PMA (1 μg/ml) and ionomycin (200 ng/ml) for 4 h in the presence of GolgiPlug at 37°C. IL-4 and IFN-γ production by iNKT cells was assessed by flow cytometry.
In vivo BrdU proliferation assay and detection of apoptosis
C57BL/6 (B6) and Abcg1–/– mice were injected i.p. with 0.3 mg BrdU (in 100 μl PBS) three times every 4 h. Thymi were harvested the next day, and single-cell suspensions were stained with fluorophore-conjugated Abs and CD1d tetramer. After cell surface staining, cells were analyzed for BrdU incorporation using FITC or allophycocyanin BrdU flow kit (BD Biosciences), according to the manufacturer's instructions. Apoptosis of thymic iNKT cells was measured by flow cytometry using a PE Annexin V Apoptosis Detection Kit 1 (BD Biosciences), according to the manufacturer's instructions.
Generation of bone marrow chimeras
Recipient mice (Rag1–/–) were irradiated in two doses of 450 rad each (for a total of 900 rad) 4 h apart. Bone marrow cells from both femurs and tibias of donor mice (B6.SJL and Abcg1–/–) were collected under sterile conditions. Bones were centrifuged for the collection of marrow, and cells were washed, mixed at 1:1 ratio, and resuspended in PBS for injection. Approximately 5 × 106 bone marrow cells from B6.SJL and Abcg1–/– mice (total 107 cells) in 200 μl PBS were delivered retro-orbitally into each recipient mouse. Recipient mice were housed in a barrier facility under pathogen-free conditions before and after bone marrow transplantation. After bone marrow transplantation, mice were provided autoclaved acidified water with antibiotics (trimethoprim-sulfamethoxazole) and were fed autoclaved food. Mice were analyzed 12 wk after bone marrow reconstitution.
Cholesterol content in iNKT cells
Surface Ags on thymocytes from B6 and Abcg1–/– mice were stained, as described above, followed by iNKT cell (CD8–, TCRβ+, CD1d tetramer+) sorting with a FACSAria cytometer (BD Biosciences). Approximately 2 × 105 events were collected for gas chromatography analysis. Sorted thymic iNKT cells were pelleted by low-spin centrifugation. After several washes with PBS, the cell pellet was extracted with chloroform:methanol (2:1) containing 5-cholestane as internal standard. Total and free cholesterol content was determined by gas–liquid chromatography and normalized to cellular protein, as previously described (23). Cholesteryl ester was calculated as (total cholesterol – free cholesterol) × 1.67. Multiplying by 1.67 corrects for the average fatty acid mass that is lost during saponification.
Quantitative real-time PCR
iNKT cells were FACS sorted from thymus, and total cellular RNA was collected with an RNeasy Plus Micro Kit, according to the manufacturer's protocol (Qiagen, Valencia, CA). RNA purity and quantity were measured with a nanodrop spectrophotometer (Thermo Scientific). Approximately 500 ng RNA was used for synthesis of cDNA with an Iscript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Total cDNA was diluted 1:20 in H2O, and a volume of 9 μl was used for each real-time condition with a MyIQ Single-Color Real-Time PCR Detection System (Bio-Rad) and TaqMan Gene Expression Mastermix and ABCG1 and ABCA1 TaqMan primers (Invitrogen). Data were analyzed and presented on the basis of the relative expression method (24). The formula for this calculation is as follows: relative expression = 2–(SΔCt – CΔCt) where ΔCt is the difference in the threshold cycle between the gene of interest and the housekeeping gene (18S), S is the Abcg1–/– mouse, and C is the B6 control mouse.
Statistical analysis
Data for all experiments were analyzed with Prism software (GraphPad, San Diego, CA). Unpaired Student t test was used for comparison of experimental groups. The p values <0.05 were considered statistically significant.
Results
Abcg1–/– mice display impaired iNKT cell development
To investigate the impact of ABCG1 deficiency on iNKT cell development, iNKT cell frequencies in thymus, liver, and spleen of four- to six-week-old Abcg1–/– and control B6 mice were assessed by flow cytometry (Fig. 1A, 1B). Abcg1–/– mice had significantly lower frequencies of iNKT cells in both thymus and liver compared with control mice (Fig. 1A, 1B). Absolute cell numbers of iNKT cells in thymus and liver were also lower in Abcg1–/– mice compared with control mice (Fig. 1C). A similar tendency was observed in the spleen; however, this did not reach statistical significance in all experiments (Fig. 1A–C). Recently, Milpied et al. (25) reported that recent thymic emigrant iNKT cells express NRP-1 and lack CD69. To determine whether the Abcg1–/– mice had impaired export of iNKT cells from thymus, we examined the expression of NRP-1 and CD69 in splenic iNKT cells of Abcg1–/– and control mice. As shown in Fig. 1D, the frequency of NRP-1+CD69– iNKT cells in spleen of Abcg1–/– mice was significantly lower compared with control mice. Overall, these data suggest that ABCG1 deficiency reduces the production and thymic egress of iNKT cells.
FIGURE 1.
Abcg1–/– mice display reduced iNKT cell frequencies in thymus and liver. (A) Thymocytes, liver mononuclear cells, and splenocytes from four- to six-week-old wild-type B6 and Abcg1–/– mice (n = 9) were stained with fluorophore-conjugated Abs and CD1d tetramer. Representative contour plots show CD19–, CD8α–, TCRβ+, CD1d-tetramer+ iNKT cells. Bar graphs show (B) frequency and (C) total cell number of iNKT cells in thymus, liver, and spleen (iNKT cells [%]: % of live cells). Data are pooled from two to three independent experiments (three to five mice per group for each experiment) with similar results. (D) Graph shows the frequency of NRP-1+, CD69– iNKT cells in spleen. Representative data of two independent experiments with four-week-old mice (five to six mice per group) are shown. (E) Thymocytes, liver mononuclear cells, and splenocytes from B6 (n = 5) and Abcg1–/– mice (n = 5) were stained with fluorophore-conjugated CD19, CD8α, TCRβ, Vβ7, Vβ8.1/2, and Vβ2 Abs, and CD1d tetramer, and analyzed by flow cytometry. Graphs show frequencies of Vβ7+ (top), Vβ8.1/2+ (middle), and Vβ2+ (bottom) B6 and Abcg1–/– iNKT cells in thymus, liver, and spleen. (F) Graphs show absolute cell numbers of Vβ7+ B6 (n = 4) and Abcg1–/– (n = 4) iNKT cells in thymus, liver, and spleen. Data are representative of two independent experiments. Error bars represent means ± SEM. Asterisks denote the significance of differences between groups (*p < 0.05, **p < 0.01, ***p < 0.001, two-tailed Student t test).
iNKT cells can be divided into CD4+ and CD4– subsets, and into subpopulations that express NK1.1, which is associated with functional differences (26). We therefore measured expression of CD4 and NK1.1 on iNKT cells in thymus and spleen of Abcg1–/– mice and B6 control mice by flow cytometry. We did not observe any differences in the percentages of these phenotypic iNKT cell subsets in either organ (Supplemental Fig. 1).
The invariant Vα14Jα18 TCR chain of iNKT cells is paired with a restricted set of β-chains containing mainly Vβ8.1/2, Vβ7, and Vβ2, with diverse CDR3 regions (1). There are data suggesting that Vβ7+ iNKT cells have a higher affinity for the endogenous ligand(s) presented during positive selection of iNKT cells in the thymus (27). Thus, the frequency of Vβ7+ iNKT cells has been used as a surrogate marker for the overall avidity for the iTCR toward its endogenous selecting ligand(s) in the thymus. To determine whether ABCG1 deficiency could impact the selection of iNKT cells in the thymus, we first analyzed Vβ7+ iNKT cells in Abcg1–/– mice. As shown in Fig. 1E, the percentages of Vβ7+ iNKT cells in thymus, liver, and spleen of Abcg1–/– mice were significantly higher than in the control B6 mice. Next, we analyzed Vβ8.1/2+ and Vβ2+ iNKT cells in these organs and found that in thymus and spleen of Abcg1–/– mice, the percentages of Vβ8.1/2+ and Vβ2+ iNKT cells were significantly lower than in the B6 mice, which is in line with the increased frequency of Vβ7+ iNKT cells in the Abcg1–/– mice (Fig. 1E). In liver, the percentage of Vβ8.1/2+ iNKT cells was significantly lower in Abcg1–/– mice compared with B6 mice; however, the percentages of Vβ2+ iNKT cells in Abcg1–/– mice and B6 mice were comparable (Fig. 1E). Next, we determined the absolute cell numbers of Vβ7+ iNKT cells in Abcg1–/– mice. In line with the reduced total iNKT cell frequency in thymus of Abcg1–/– mice compared with control B6 mice (Fig. 1A, 1B), the absolute cell number of Vβ7+ iNKT cells in thymus was also significantly lower in Abcg1–/– mice (Fig. 1F). In liver, the absolute cell number of Vβ7+ iNKT cells tended to be lower in Abcg1–/– mice, but the difference was not statistically significant (p = 0.067) (Fig. 1F). As the frequencies of total iNKT cells in spleen of Abcg1–/– mice and B6 mice were comparable (Fig. 1A, 1B) and Abcg1–/– mice had a higher percentage of Vβ7+ iNKT cells in spleen (Fig. 1E), the absolute cell numbers of Vβ7+ iNKT cells in spleen were significantly higher in Abcg1–/– mice (Fig. 1F). These data demonstrate that thymic selection of Vβ7+ iNKT cells is favored in the absence of ABCG1.
ABCG1 deficiency affects iNKT cell development via a cell-intrinsic mechanism
A critical factor driving iNKT cell development in the thymus is the generation of a CD1d-restricted iTCR by CD4+CD8+ double-positive (DP) cortical thymocytes (28, 29). Once a DP thymocyte expressing an iTCR interacts with a CD1d-expressing DP cortical thymocyte that presents endogenous selecting ligand(s), the iTCR-expressing DP thymocyte is positively selected to enter the thymic iNKT cell precursor pool (30). Subsequently, these iNKT cell precursors undergo a series of proliferation and differentiation/maturation stages (31). Because iNKT cells are positively selected by DP thymocytes, we examined whether DP thymocytes from Abcg1–/– mice had lower CD1d expression. If so, this would be a likely explanation for the observed decrease in iNKT cell frequency. As shown in Fig. 2A, DP thymocytes from Abcg1–/– and B6 mice had similar levels of surface CD1d expression. Next, to investigate whether ABCG1 deficiency affects CD1d-mediated lipid Ag presentation, we incubated the iNKT cell hybridoma DN3A4-1.2 with thymocytes isolated from either Abcg1–/– or B6 mice in the presence of titrated amounts of α-GalCer in vitro. We found that Abcg1–/– thymocytes were as efficient as B6 thymocytes at stimulating IL-2 production by iNKT cell hybridomas (Fig. 2B). Overall, these results show that the impairment in iNKT cell development observed in Abcg1–/– mice was not due to changes in CD1d surface expression on DP thymocytes or their CD1d-mediated Ag presentation.
FIGURE 2.
ABCG1 deficiency affects iNKT cell development via a cell-intrinsic mechanism. (A) Thymocytes from B6 mice (n = 5) and Abcg1–/– mice (n = 4) were stained with CD4, CD8α, and CD1d Abs and analyzed by flow cytometry. Bar graph shows mean fluorescence intensity (MFI) of CD1d on CD4+CD8+ thymocytes from B6 and Abcg1–/– mice. Data are representative of two independent experiments with similar results. (B) iNKT cell hybridoma DN3A4-1.2 cells were cocultured with thymocytes from either B6 (n = 4) or Abcg1–/– (n = 4) mice in the presence of titrated amounts of α-GalCer in vitro. After 18 h, IL-2 production was detected by ELISA. Data are representative of two independent experiments with similar results. (C and D) Bone marrow chimeras were generated by reconstituting irradiated Rag1–/– mice (n = 11) with 1:1 mixed bone marrow cells from CD45.1+ B6.SJL and CD45.2+ Abcg1–/– donor mice. Single-cell suspension from thymus was analyzed by flow cytometry 12 wk following reconstitution. (C) Representative contour plots show CD19–, CD8α–, TCRβ+, CD1d-tetramer+ iNKT cells, which are gated on CD45.1+ and CD45.2+ to identify Abcg1+/+ B6.SJL and Abcg1–/– iNKT cells, respectively. (D) Bar graph shows percentages of Abcg1+/+ B6.SJL and Abcg1–/– iNKT cells in thymus. Data are pooled from three independent experiments (three to four mice per group for each experiment) with similar results. (E) Graph shows frequency of Vβ7+ CD45.1+ B6 and CD45.2+ Abcg1–/– iNKT cells in thymus. Data are pooled from two independent experiments (three mice per group for each experiment) with similar results (**p < 0.01, ***p < 0.001).
To determine whether the impact of ABCG1 deficiency on iNKT cell development is mediated through cell-intrinsic factor(s), we used a mixed bone marrow chimera approach. Irradiated Rag1–/– mice were reconstituted with both CD45.1+ B6.SJL and CD45.2+ Abcg1–/– bone marrow mixed at a 1:1 ratio and analyzed 12 wk after reconstitution. We did not observe any difference in the frequency of Abcg1–/– and B6 DP thymocytes (Supplemental Fig. 2A) or in their surface CD1d expression in the chimeric mice (Supplemental Fig. 2B). However, when analyzing iNKT cells in the thymus, we found that ~3-fold fewer iNKT cells developed from the Abcg1–/– bone marrow than from the B6 bone marrow (Fig. 2C, 2D). These results demonstrate that the impact of ABCG1 deficiency on iNKT cell development in the thymus is cell intrinsic.
To determine whether the increased frequency of Vβ7+ iNKT cells in Abcg1–/– mice (Fig. 1E) was also caused by a cell-intrinsic mechanism, we evaluated the percentage of Vβ7+ iNKT cells in the 1:1 mixed bone marrow chimeric mice. Abcg1–/–-derived iNKT cells in the thymus of the chimeric mice displayed a higher frequency of Vβ7+ population compared with B6-derived iNKT cells (Fig. 2E). These data demonstrate that thymic selection of Vβ7+ iNKT cells is favored in the absence of ABCG1 via an iNKT cell-intrinsic mechanism.
ABCG1 deficiency affects the maturation of iNKT cells in the thymus
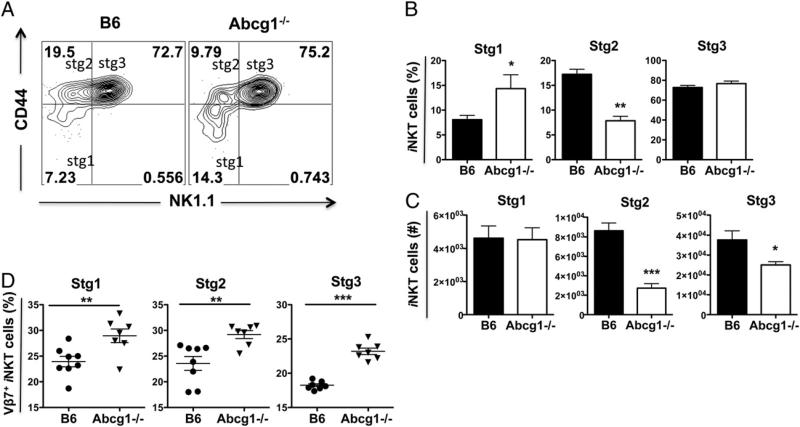
After positive selection in thymus, iNKT cells proliferate and differentiate/mature along stages that are defined by the surface expression of CD44 and NK1.1 (31). Cells with a stage 1 phenotype (CD44low NK1.1–) are followed by stage 2 cells, which have increased CD44 expression (CD44high NK1.1–). Stage 1 and stage 2 iNKT cells are highly proliferative (32, 33). The upregulation of NK1.1 expression by iNKT cells marks stage 3 (CD44high NK1.1+) cells, which are mature but less proliferative (32, 34). Whereas most iNKT cells exit the thymus at stage 2 and complete their maturation in the periphery, some enter stage 3 in the thymus and remain as long-term thymus-resident iNKT cells (31). To determine where during iNKT cell development ABCG1 plays a role, we evaluated the developmental stages of iNKT cells in the 1:1 mixed bone marrow chimeric mice. The percentage of Abcg1–/– iNKT cells in stage 1 (CD44low NK1.12) was ~2-fold larger compared with B6 iNKT cells, whereas the percentage of iNKT cells in stage 2 (CD44high NK1.1–) was reduced by 33%. There were no differences in the percentages of stage 3 (CD44high NK1.1+) iNKT cells between both genotypes (Fig. 3A, 3B). We calculated the absolute cell numbers of Abcg1–/– and B6 iNKT cells at these three maturation stages, and, in line with the reduced relative frequency of Abcg1–/– iNKT cells in the thymus of the chimeric mice (Fig. 2C, 2D), the total number of Abcg1–/– iNKT cells was dramatically reduced at stages 2 and 3 compared with B6 iNKT cells (Fig. 3C). Subsequently, we analyzed Vβ7+ iNKT cells in stage 1–3 of thymic development in Abcg1–/– mice. Similar to the observed increase in the frequency of total thymic Vβ7+ iNKT cells in Abcg1–/– mice (Fig. 1E), the frequencies of Vβ7+ iNKT cells in all three maturation stages were significantly higher in Abcg1–/– mice than in B6 mice (Fig. 3D). These data indicate that ABCG1 deficiency impairs the proper maturation of iNKT cells at early stages of iNKT cell development.
FIGURE 3.
ABCG1 deficiency affects the maturation of iNKT cells in thymus. Single-cell suspensions from thymi of B6:Abcg1–/– 1:1 mixed chimeric mice (n = 10) were stained with fluorophore-conjugated CD45.1, CD45.2, TCRβ, CD44, NK1.1 Ab, and CD1d tetramer and analyzed by flow cytometry. TCRβ+ and CD1d-tetramer+ cells were further gated to distinguish stage 1 (stg1, CD44low NK1.1–), stage 2 (stg2, CD44high NK1.1–), and stage 3 (stg3, CD44high NK1.1+) iNKT cells. (A) Representative contour plots. Bar graphs show (B) percentages and (C) absolute cell numbers of CD45.1+ B6 and CD45.2+ Abcg1–/– iNKT cells of indicated maturation stages. Results are representative of two independent experiments with similar results. (D) Graphs show frequencies of Vβ7+ thymic iNKT cells at stage 1–3 in B6 (n = 8) and Abcg1–/– (n = 7) mice. Data are pooled from two independent experiments (three to four mice per group for each experiment) with similar results (*p < 0.05, **p < 0.01, ***p < 0.001).
Abcg1–/– iNKT cells have reduced proliferation
To determine whether the reduced frequency of iNKT cells in the absence of ABCG1 is due to a decrease in proliferation, we measured proliferation of thymic iNKT cells at different maturation stages in vivo in Abcg1–/– and B6 control mice by BrdU incorporation. In Abcg1–/– mice, the frequency of BrdU+ iNKT cells at stages 1 and 2 was significantly reduced compared with iNKT cells from B6 mice (Fig. 4A, 4B). In stage 3, the frequency of BrdU+ iNKT cells also tended to be lower in Abcg1–/– mice, but the difference was not statistically significant (p = 0.051) (Fig. 4A, 4B). Next, we measured BrdU incorporation of Vβ7+ iNKT cells in Abcg1–/– and B6 mice. In line with the reduced proliferation of total iNKT cells in Abcg1–/– mice (Fig. 4A, 4B), the frequency of BrdU+ Vβ7+ iNKT cells was significantly lower in Abcg1–/– mice compared with B6 mice (Supplemental Fig. 4). To investigate whether increased cell death also contributes to the reduced iNKT cell numbers in the absence of ABCG1, we examined apoptosis of Abcg1–/– and B6 iNKT cells at different stages of thymic development by annexin V staining. We found that the percentages of apoptotic (annexin V+ live) iNKT cells at stage 1–3 in Abcg1–/– and B6 mice were comparable (Fig. 4C, 4D). These results indicate that the reduced frequency of iNKT cells in thymus in the absence of ABCG1 is not due to increased cell death but reduced proliferation, particularly during the early stages of development.
FIGURE 4.
Abcg1–/– iNKT cells display reduced proliferation in early stages of development. B6 (n = 7) and Abcg1–/– (n = 8) mice were injected with BrdU three times every 4 h. The next day, thymi were harvested and single-cell suspensions were stained with fluorophore-conjugated CD8α, TCRβ, CD44, NK1.1, BrdU Ab, and CD1d tetramer and analyzed by flow cytometry. TCRβ+ and CD1d-tetramer+ cells were further gated to distinguish stage 1 (stg1, CD44low NK1.1–), stage 2 (stg2, CD44high NK1.1–), and stage 3 (stg3, CD44high NK1.1+) iNKT cells. (A) Representative contour plots and (B) bar graphs show BrdU incorporation by iNKT cells at each stage. Data are pooled from two independent experiments (three to four mice per group for each experiment) with similar results. (C and D) Thymocytes from B6 (n = 3) and Abcg1–/– (n = 3) mice were cultured overnight and the next day stained with fluorophore-conjugated CD8α, TCRβ, CD44, NK1.1, annexin V Ab, CD1d tetramer, and a live/dead marker and analyzed by flow cytometry. (C) Representative contour plots and (D) bar graphs show percentages of apoptotic (annexin V+ live) iNKT cells at stage 1–3. Data are representative of two independent experiments with similar results (**p < 0.01, ***p < 0.001).
ABCG1 deficiency affects lipid raft content in iNKT cells
We next measured the cholesterol content in iNKT cells isolated from thymus of B6 and Abcg1–/– mice using gas chromatography. We found no significant changes in the cholesteryl ester, free cholesterol, or total cholesterol content in Abcg1–/– iNKT cells (Fig. 5A). However, as ABCG1 is important in intracellular cholesterol transport (14, 15) and cholesterol is an important component of membrane lipid rafts (11), we measured lipid raft content in thymic iNKT cells. Lipid rafts are specialized regions of the cell membrane that are rich in cholesterol and gangliosides and act as platforms to colocalize proteins involved in intracellular signaling pathways (35, 36). In contrast to the comparable cholesterol levels, the lipid raft content of Abcg1–/– iNKT cells in thymus was 43% lower than in B6 iNKT cells (Fig. 5B, 5C). These results suggest that ABCG1 plays a role in regulation of membrane lipid raft content in thymic iNKT cells, which is most likely important for their proper development.
FIGURE 5.
Abcg1–/– iNKT cells display no change in cholesterol content, but have lower lipid raft content. (A) iNKT cells were FACS sorted from thymus of B6 and Abcg1–/– mice (n = 9; 27 mice, 3 mice were pooled for each sample per group), and free cholesterol (FC), cholesteryl ester (CE), and total cholesterol (TC) were measured by gas chromatography. Data are pooled from three independent experiments (3 samples per group for each experiment) with similar results. (B and C) Thymocytes from B6 (n = 4) and Abcg1–/– (n = 4) mice were stained with fluorophore-conjugated Abs, CD1d tetramer, and CT-B, and analyzed by flow cytometry. (B) Representative plot shows lipid raft staining (CT-B) of iNKT cells in thymus. (C) Graph shows mean fluorescence intensity (MFI) of CT-B of iNKT cells in thymus. Data are representative of two independent experiments with similar results (**p < 0.01).
ABCG1 deficiency affects cytokine production of iNKT cells
Because Abcg1–/– iNKT cells have reduced lipid raft content (Fig. 5B, 5C) and the TCR is associated with lipid rafts (37), we next investigated whether deficiency of ABCG1 in iNKT cells would affect their TCR-driven activation. We stimulated thymocytes from B6:Abcg1–/– 1:1 mixed bone marrow chimeric mice with plate-bound aCD3ε and soluble costimulatory αCD28 Ab in vitro and measured IL-4 and IFN-γ production of thymic iNKT cells by intracellular staining. We found that CD45.2+ Abcg1–/– iNKT cells had reduced IL-4 production, but enhanced IFN-γ production compared with CD45.1+ B6 iNKT cells (Fig. 6A, 6B). Previous studies have shown that stage 1 and stage 2 iNKT cells produce abundant IL-4, but less IFN-γ, whereas stage 3 iNKT cells make more IFN-γ, but less IL-4 (32, 34, 38). Our results demonstrate that the frequency and the total cell number of stage 2 iNKT cells are reduced in the absence of ABCG1 (Fig. 3A–C). Therefore, the diminished production of IL-4 by Abcg1–/– iNKT cells might be due to the lower percentages of the potent IL-4 producer stage 2 iNKT cells in the absence of ABCG1. To address this possibility, we stimulated thymocytes from Abcg1–/– and B6 mice with αCD3ε and αCD28 Abs in vitro and measured IL-4 and IFN-γ production of thymic iNKT at different stages by intracellular staining. Stage 2 and stage 3 Abcg1–/– iNKT cells produced significantly less IL-4 and more IFN-γ compared with B6 control (Fig. 6C), demonstrating that the altered cytokine production of Abcg1–/– iNKT cells is not due to the reduced percentages of stage 2 iNKT cells in the absence of ABCG1. Furthermore, to elucidate that the changes in the cytokine production by iNKT cells in the absence of ABCG1 are related to TCR triggering, we stimulated thymocytes from Abcg1–/– and B6 mice in vitro with PMA/ionomycin, which bypasses TCR triggering to induce activation. We then measured IL-4 and IFN-γ production of thymic iNKT cells by intracellular staining. As seen in Fig. 6D, PMA/ionomycin stimulation induced IL-4 and IFN-γ production by Abcg1–/– iNKT cells to a similar degree as in the B6 iNKT cells, indicating that Th1-biased cytokine production we observed in Abcg1–/– iNKT cells is indeed related to TCR triggering. Overall, these results demonstrate that deficiency of ABCG1 in iNKT cells skews their cytokine production, leading to a Th1 bias following TCR-driven activation.
FIGURE 6.
Abcg1–/– iNKT cells display reduced IL-4 and increased IFN-γ production following TCR-driven activation. (A and B) Thymocytes from B6:Abcg1–/– 1:1 mixed chimeric mice (n = 8) were stimulated with plate-bound αCD3ε and soluble αCD28 Ab for 4 h in vitro and stained with fluorophore-conjugated surface Abs and CD1d tetramer, followed by intracellular staining with IL-4 and IFN-γ Ab, and analyzed by flow cytometry. (A) Representative contour plots and (B) bar graph show percentages of IL-4– and IFN-γ–producing CD45.1+ B6 and CD45.2+ Abcg1–/– iNKT cells based on CD8–, TCRβ+, CD1d-tetramer+ gating. Data are pooled from two independent experiments (4 mice per group for each experiment) with similar results. (C) Thymocytes from B6 (n = 4) and Abcg1–/– (n = 4) mice were stimulated with aCD3ε/αCD28 Abs and analyzed, as described above. Bar graphs show IL-4 (left) and IFN-γ (right) producing stage 1 (stg1, CD44low NK1.1–), stage 2 (stg2, CD44high NK1.1–), and stage 3 (stg3, CD44high NK1.1+) iNKT cells. (D) Thymocytes from B6 (n = 4) and Abcg1–/– (n = 4) mice were stimulated with PMA/ionomycin for 4 h in vitro and stained with fluorophore-conjugated surface Abs and CD1d tetramer, followed by intracellular staining with IL-4 and IFN-γ Ab, and analyzed by flow cytometry. Bar graph shows percentages of IL-4– and IFN-γ–producing iNKT cells based on CD8–, TCRβ+, CD1d-tetramer+ gating. Data are representative of two independent experiments with similar results (*β < 0.05, **p < 0.01, ***p < 0.001).
Discussion
In this study, we demonstrate that changes in cholesterol homeostasis caused by the absence of ABCG1 impair iNKT cell development. ABCG1-deficient thymic iNKT cells displayed reduced proliferation in vivo and defective maturation during the early stages of development. Considering the number of thymic iNKT cells, the developmental block was particularly evident at stage 2, although the reduced proliferation of stage 1 cells most likely contributes to the deficit. Moreover, in the absence of ABCG1, thymic iNKT cells had reduced membrane lipid raft content, which was accompanied by a Th1-biased cytokine production in response to TCR stimulation. The defects were cell intrinsic, meaning that they occur in the iNKT cell precursor rather than in the DP thymocyte responsible for iNKT cell-positive selection. Therefore, although it is theoretically possible that a cholesterol-containing compound is an important self-ligand mediating positive selection, our results rule out differences in self-ligand presentation as the responsible factor. In addition, thymic selection of Vβ7+ iNKT cells was favored in the absence of ABCG1 via an iNKT cell-intrinsic mechanism. Importantly, to our knowledge, our work illustrates for the first time an iNKT cell-intrinsic modulation of the TCR Vβ repertoire during thymic selection. Furthermore, to our knowledge, our work is the first to demonstrate that changes in intra-cellular cholesterol homeostasis profoundly impact iNKT cell development and function.
We (20) and Bensinger et al. (21) have previously reported that alterations in intracellular cholesterol homeostasis in the absence of ABCG1 lead to a hyperproliferative phenotype of conventional T cells. In our previous study, we showed that ABCG1-deficient CD4+ T cells displayed enhanced TCR signaling and proliferation as a result of increased cholesterol and lipid raft content (20). In contrast, this study reveals an opposite role of ABCG1 in iNKT cells, as ABCG1-deficient iNKT cells displayed decreased proliferation and lower membrane lipid raft content. iNKT cells differ from naive CD4+ T cells in that they have an activated/memory phenotype and they produce large amounts of both Th1 and Th2 cytokines following activation with glycolipid Ags (8). Therefore, it is plausible that iNKT cells have differences in cholesterol metabolism and, as such, different requirements for cholesterol homeostasis than do CD4+ T cells. Excess cholesterol is exported out of the cell by the cholesterol transporters ABCA1 and ABCG1 (39, 40). ABCA1 effluxes cholesterol to lipid-poor apolipoprotein AI (41), whereas ABCG1 promotes cholesterol efflux to mature high-density lipoprotein particles (13). iNKT cells express both ABCG1 and ABCA1 (Supplemental Fig. 3A, 3B). ABCA1 expression is enhanced in Abcg1–/– iNKT cells compared with B6 iNKT cells (Supplemental Fig. 3B). This is not surprising, because many previous studies have shown that genetic deletion of one cholesterol transporter, either ABCA1 or ABCG1, is compensated for by an upregulation of the other transporter (20, 42, 43). Nonetheless, neither ABCA1 nor ABCG1 can fully compensate for the loss of the other (10). However, we cannot rule out the possibility that changes in ABCA1 expression contributed to our observed findings of altered iNKT cell development and function in Abcg1–/– mice. Whereas, in the absence of ABCG1, the total cholesteryl ester content of CD4+ T cells increases (20), the total cholesteryl ester of iNKT cells is unchanged (Fig. 5A). These results suggest that, in terms of regulating cholesteryl ester content, ABCA1 can substitute for the lack of ABCG1 in iNKT cells, but not in T cells. Future studies of the role of ABCA1 in iNKT cells using floxed mice may be useful to delineate the different roles of these two transporters in iNKT cell development.
Apart from cholesterol efflux, ABCG1 is also important for intracellular cholesterol transport (14, 15). Cholesterol is an essential component of membrane lipid rafts (11). Because iNKT cells have a reduction in the lipid raft content (Fig. 5B, 5C) without any changes in the overall cholesterol content (Fig. 5A) in the absence of ABCG1, the critical role of ABCG1 in iNKT cells seems to be the regulation of the intracellular transport of cholesterol. Miguel et al. (44) have recently shown that the amount of membrane lipid rafts present in CD4+ T cells correlates closely with immunological synapse formation and CD4+ T cell proliferation and activation. Therefore, based on our data, we hypothesize that ABCG1 deficiency causes a decrease in the transport of cholesterol to the cell membrane, affecting lipid rafts, which impacts iNKT cell development and function.
Our results demonstrate that, in the absence of ABCG1, thymic selection of Vβ7+ iNKT cells is favored in a cell-intrinsic manner. Previous studies have shown that Vβ7+ iNKT cells have a lower affinity toward α-GalCer (45), whereas, based on the analysis of CD1d+/– heterozygous mice, they may have a higher affinity for the endogenous ligand(s) for the positive selection of iNKT cells in thymus (27). Therefore, the frequency of thymic Vβ7+ iNKT cells may be a surrogate marker for the overall avidity of the iTCR toward its endogenous selecting ligand(s) in the thymus. In previous studies, which showed that the thymic selection of the Vβ7+ iNKT cells was favored, the reduced avidity of the iTCR toward the selecting ligand(s) was due to reduced expression of CD1d on DP thymocytes (27). However, in our study, the surface expression levels of CD1d on DP thymocytes in Abcg1–/– mice were unchanged (Fig. 2A). Furthermore, the expression levels of the TCR/CD3 complex were similar in the Abcg1–/– iNKT cells compared with control mice (data not shown). As the effects we observed were iNKT cell intrinsic (Fig. 2E), this altogether suggests that the differences in the avidity of the iTCR–CD1d interaction in the Abcg1–/– mice are due to changes in the iTCR itself. Based on our data, we hypothesize that the alterations in the lipid raft content in the absence of ABCG1 modify the distribution of the TCR in iNKT cells so that it is less colocalized in lipid rafts. These changes may make the iTCR less sensitive to the endogenous ligand(s) presented by CD1d for the positive selection of iNKT cells in thymus. Such a reduction may favor the selection of relatively high-affinity Vβ7+ iNKT cells in the thymus and lead to reduced export of iNKT cells to the periphery. Changes in the composition/organization of the iTCR also alter cytokine production of iNKT cells. More work is needed, however, to examine how ABCG1 deficiency leads to a Th1-biased cytokine production.
In summary, we demonstrate a novel role for ABCG1-mediated cholesterol homeostasis in iNKT cell development. iNKT cells have been implicated in the development of atherosclerosis, rheumatoid arthritis, several forms of allergy, as well as autoimmunity (7, 8). All of these diseases have known or proposed lipid constituents that increase risk of disease development. For example, ABCG1-deficient (43, 46), as well as iNKT cell-deficient mice are protected from atherosclerosis development (47–51). Therefore, linking the role of lipid transporters and glycolipid-sensitive iNKT cells could lead to the development of entirely new therapeutic approaches for diseases that have a hyperlipidemic component, such as atherosclerosis.
Supplementary Material
Acknowledgments
We thank Amy Blatchley, Archana Khurana, the Department of Laboratory Animal Care, and the flow cytometry facility at the La Jolla Institute for Allergy and Immunology for excellent technical assistance. We also thank Dr. Isaac Engel and Dr. Meng Zhao for valuable scientific contributions.
This work was supported by National Institutes of Health Grants R01 HL097368 and R01 HL085790 (to C.C.H.), R01 HL094525 (to J.S.P.), and RO1 AI45053 and R37 AI71922 (to M.K.).
Abbreviations used in this article
- ABC
ATP-binding cassette
- ABCA1
ABC transporter A1
- ABCG1
ABC transporter G1
- CT-B
cholera toxin B
- DP
double-positive
- α-GalCer
α-galactosylceramide
- iNKT
invariant NKT
- iTCR
invariant TCR
- NRP-1
neuropilin-1
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat. Rev. Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 2.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 3.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, III, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 5.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J. Clin. Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of Valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu. Rev. Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 8.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins CF. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 10.Tarr PT, Tarling EJ, Bojanic DD, Edwards PA, Baldán A. Emerging new paradigms for ABCG transporters. Biochim. Biophys. Acta. 2009;1791:584–593. doi: 10.1016/j.bbalip.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy MA, Barrera GC, Nakamura K, Baldán A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturek JM, Castle JD, Trace AP, Page LC, Castle AM, Evans-Molina C, Parks JS, Mirmira RG, Hedrick CC. An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells. J. Clin. Invest. 2010;120:2575–2589. doi: 10.1172/JCI41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl. Acad. Sci. USA. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldán A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J. Immunol. 2008;180:3560–3568. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 17.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J. Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 18.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via Toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J. Immunol. 2010;184:173–183. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J. Exp. Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD. Dietary monounsaturated fatty acids promote aortic atherosclerosis in LDL receptor-null, human ApoB100-overexpressing transgenic mice. Arterioscler. Thromb. Vasc. Biol. 1998;18:1818–1827. doi: 10.1161/01.atv.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Milpied P, Massot B, Renand A, Diem S, Herbelin A, Leite-de-Moraes M, Rubio MT, Hermine O. IL-17-producing invariant NKT cells in lymphoid organs are recent thymic emigrants identified by neuropilin-1 expression. Blood. 2011;118:2993–3002. doi: 10.1182/blood-2011-01-329268. [DOI] [PubMed] [Google Scholar]
- 26.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD42K1.12 NKT cell population. Proc. Natl. Acad. Sci. USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schümann J, Mycko MP, Dellabona P, Casorati G, MacDonald HR. Cutting edge: influence of the TCR Vbeta domain on the selection of semi-invariant NKT cells by endogenous ligands. J. Immunol. 2006;176:2064–2068. doi: 10.4049/jimmunol.176.4.2064. [DOI] [PubMed] [Google Scholar]
- 28.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat. Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 30.Kronenberg M, Engel I. On the road: progress in finding the unique pathway of invariant NKT cell differentiation. Curr. Opin. Immunol. 2007;19:186–193. doi: 10.1016/j.coi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat. Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 32.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 33.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J. Exp. Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1(–)CD4(+) CD1d-dependent precursor stage. J. Exp. Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 37.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin. Cell Dev. Biol. 2007;18:608–615. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J. Immunol. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 39.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 42.Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 43.Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1–/– bone marrow. Arterioscler. Thromb. Vasc. Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 44.Miguel L, Owen DM, Lim C, Liebig C, Evans J, Magee AI, Jury EC. Primary human CD4+ T cells have diverse levels of membrane lipid order that correlate with their function. J. Immunol. 2011;186:3505–3516. doi: 10.4049/jimmunol.1002980. [DOI] [PubMed] [Google Scholar]
- 45.Schümann J, Voyle RB, Wei BY, MacDonald HR. Cutting edge: influence of the TCR V beta domain on the avidity of CD1d:alpha-galactosylceramide binding by invariant V alpha 14 NKT cells. J. Immunol. 2003;170:5815–5819. doi: 10.4049/jimmunol.170.12.5815. [DOI] [PubMed] [Google Scholar]
- 46.Baldán A, Pei L, Lee R, Tarr P, Tangirala RK, Weinstein MM, Frank J, Li AC, Tontonoz P, Edwards PA. Impaired development of atherosclerosis in hyperlipidemic Ldlr–/– and ApoE–/– mice transplanted with Abcg1–/– bone marrow. Arterioscler. Thromb. Vasc. Biol. 2006;26:2301–2307. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 47.Aslanian AM, Chapman HA, Charo IF. Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2005;25:628–632. doi: 10.1161/01.ATV.0000153046.59370.13. [DOI] [PubMed] [Google Scholar]
- 48.Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- 49.Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, et al. Natural killer T cells accelerate atherogenesis in mice. Blood. 2004;104:2051–2059. doi: 10.1182/blood-2003-10-3485. [DOI] [PubMed] [Google Scholar]
- 50.Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L, Ramsamy TA, Whitman SC. Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc. Res. 2008;78:167–174. doi: 10.1093/cvr/cvn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.