Abstract
Chronic fibrotic liver diseases such as viral hepatitis eventually develop liver cirrhosis, which causes occurrence of hepatocellular carcinoma (HCC). Given the limited therapeutic efficacy in advanced HCC, prevention of HCC development could be an effective strategy for improving patient prognosis. However, there is still no established therapy to meet the goal. Studies have elucidated a wide variety of molecular mechanisms and signaling pathways involved in HCC development. Genetically-engineered or chemically-treated experimental models of cirrhosis and HCC have been developed and shown their potential value in investigating molecular therapeutic targets and diagnostic biomarkers for HCC prevention. In this review, we overview potential targets of prevention and currently available experimental models, and discuss strategies to translate the findings into clinical practice.
Keywords: Animal model, chemoprevention, clinical trial, hepatocellular carcinoma, liver cirrhosis, prevention
INTRODUCTION
Liver cancer, predominantly HCC, is the second and sixth most lethal cancer in men and women, respectively, and occurred in about 750,000 new patients and caused 700,000 death in 2008 [1]. More than 80% of the cases occur in developing countries in the Asia-Pacific regions and sub-Saharan Africa, and China alone accounts for more than 50% [2]. In the US, HCC is the most rapidly increasing cause of cancer-related mortality, and is ranked as third in men ages 40 to 60, clearly indicating its large socioeconomic impact [2,3]. The incidence of HCC tripled between 1975 and 2005 [4], and is assumed to increase in the next few years and remain high for the next two decades [5,6].
HCC occurs in liver affected with chronic predisposing conditions such as liver cirrhosis, which is seen in about 80% of HCC patients [7,8]. It is estimated that roughly 1~2% of population is affected with liver cirrhosis, and the risk of HCC increases according to severity of liver fibrosis and dysfunction [8-11]. Regular tumor surveillance for cirrhotic patients has enabled efficient early detection of HCC tumors to be eligible for potentially curative treatments: surgical resection, local ablation, and liver transplantation [12]. However, complete tumor removal is not equivalent to a cure because of persisting cirrhosis in remnant liver. In fact, repeated tumor recurrences are observed in nearly 80% of the patients, limiting improvement of 5-year survival up to 70-80% [7]. Once the tumor gets into advanced stage, there is no curative treatment option; even the recently introduced multi-kinase inhibitor, sorafenib, could yield survival benefit only up to several months [13,14].
The refractory nature of HCC suggests that prevention of its development in high-risk patients is the most effective strategy to substantially improve the mortality [15]. In addition, the high prevalence of liver cirrhosis has further highlighted the impact of HCC prevention on global health [16]. In fact, HCC is a more attractive target of preventive intervention compared to other cancer types such as prostate cancer [17], breast cancer [18], and keratinocyte carcinoma [19] because it is easier to identify individuals at risk (i.e., cancer-susceptible cohort) and to monitor treatment response due to the high incidence rate.
RISK FACTORS AND MOLECULAR MECHANISMS OF HCC DEVELOPMENT
A wide range of etiological agents contributes to hepatocarcinogenesis through specific molecular mechanisms, clearly indicating the necessity to develop strategies of HCC prevention accordingly.
Hepatitis B virus
Chronic infection of hepatitis B virus (HBV) is the most common global cause of HCC, affecting more than 350 million individuals (6% of world population) and being the dominant etiology especially in China and Africa [20]. HBV proteins as well as HBV DNA integration into host genome have been suggested to possess direct carcinogenic effect by activating cis and trans oncogenic signals in host hepatocytes [21]. In consistent, high serum HBV DNA level, indicative of increased viral replication, is predictive of HCC development, which is not necessarily accompanied with advanced liver fibrosis [22]. It has been suggested that certain HBV strains, e.g., genotype C in Asian population and genotype F in Alaskan population [23,24] or mutations in HBV genome, e.g., within precore and basal core promoter regions [23] increase the risk of HCC. Superinfection with hepatitis delta virus (HDV) is associated with severer hepatitis, accelerated fibrosis progression, and increased risk of HCC [25].
Hepatitis C virus
Hepatitis C virus (HCV) affects 170 million individuals worldwide, has been the major risk factor of HCC in many industrialized countries, and is contributing to the increasing HCC incidence in the US [4,26]. It is estimated that more than one million patients could develop HCV-related cirrhosis, hepatic decompensation, or HCC by 2020 in the US [27]. Clinically, incidence of HCV-related HCC increases according to severity of liver fibrosis, and in contrast to HBV-related HCC, patients with minimal fibrosis rarely develop HCC, suggesting that cirrhotic microenvironment is the major driver [8,11,28]. There are conflicting evidences that HCV viral factors such as serum RNA level increase HCC risk [29,30]. Some studies suggested that genotype 1 is associated with increased risk of HCC, although it may simply reflect that this genotype is refractory to antiviral therapies and has more chance to progress into advanced disease.
Exposure to environmental carcinogens
Dietary contamination with the potent hepatocarcinogen, aflatoxin B1 (AFB1, a natural mycotoxin produced by Aspergillus fungi) is observed in some (sub)tropical regions with warm and humid climate especially in Eastern and Southeastern Asia and sub-Saharan Africa, where HBV infection is prevalent [20]. AFB1 causes a loss-of-function mutation in the “hotspot” codon 249 (G to T transversion) of exon 7 in the TP53 tumor suppressor gene [31], and exhibits remarkable synergistic hepatocarcinogenic effect with HBV [32,33]. Food or water contamination with other fungal toxins such as fumonisin, blue-green algae-derived microcystins, nitrosamine, and inorganic arsenic, and betel quid chewing are also suggested to increase HCC risk [20,34,35].
Alcohol
Liver cirrhosis induced by long-term, excessive intake of alcohol is a well-established risk factor of HCC, and is one of the major causes in North America and Western Europe [20,36]. It is likely that alcohol has an indirect carcinogenic effect through establishment of cirrhosis [36]. Heavy alcohol consumption shows synergism with HCV and possibly with HBV infection in promoting HCC development supposedly by accelerating cirrhosis progression.
Non-alcoholic fatty liver disease, obesity, and type 2 diabetes
Recent studies have suggested that many HCC cases developed in so-called cryptogenic cirrhosis may be attributable to non-alcoholic fatty liver disease (NAFLD), including non-alcoholic steatohepatitis (NASH) [37]. Epidemiological data have suggested tight association of NAFLD/NASH with visceral obesity and (mainly type 2) diabetes accompanied with insulin resistance, which were also independently reported as risk factors of HCC development [38,39].
Hepatic iron overload disorders: hemochromatosis
Genetic hemochromatosis (GH) is mostly caused by homozygous mutations in the hemochromatosis (HFE) gene, especially C282Y (more common in Caucasian) and to lesser extent H63D, which abnormally increase iron absorption and accumulation especially in the liver, heart, and pancreas. Established cirrhosis is an HCC risk factor in GH. A Swedish population-based cohort study reported an increased HCC incidence especially in male GH patients, mostly harboring the C282Y mutation [40].
In patients with chronic hepatitis C and advanced fibrosis, positive association between liver iron deposition and higher incidence of HCC and poor prognosis was observed [41]. Hepatic iron overload was associated with elevated levels of 8-hydroxy-2’-deoxyguanosine (8-OHdG), which signifies hepatic oxidative DNA damage in patients with chronic hepatitis C [42]. With an excess iron diet, transgenic mice expressing HCV polyprotein showed development of hepatic steatosis, ultrastructural alterations of mitochondria, and HCC accompanied with elevated levels of hepatic 8-OHdG [43].
Genetic risk factors
Recent studies have identified etiology-specific or independent host genetic polymorphisms associated with increased risk of HCC in patients with chronic liver diseases. A single nucleotide polymorphism (SNP) in epidermal growth factor (EGF) gene (SNP rs4444903) was associated with increased EGF expression and elevated risk of HCC development in cirrhotic patients [44,45]. Genome-wide association study (GWAS) have revealed a SNP (rs 17401966) possibly associated with altered expression and function of several potential tumor suppressor genes in 1p36.22 namely KIF1B, UBE4B, and PGD in HBV-related Chinese HCC patients [46] and SNPs in MICA (rs rs2596542) [47] and DEPDC5 (rs1012068) [48] in HCV-related Japanese HCC patients. These findings await future validation. Given the magnitude of risk for the loci are generally low (odds ratio well below 1.5), it may be more clinically meaningful to measure them simultaneously to evaluate their collective effect.
Other risk factors
HCC risk in primary biliary cirrhosis (PBC) with stage 4 cirrhosis is about the same as in HCV-related cirrhosis [49]. HCC occurs less frequently in autoimmune hepatitis (AIH) only after establishment of cirrhosis, suggesting that inflammation alone is not sufficient [50]. Only scarce epidemiological evidence exists for other rare forms of chronic liver diseases such as alpha 1-antitrypsin deficiency [51]. There are conflicting evidences regarding association of smoking with HCC risk, for which confounding effect of alcohol abuse cannot be fully excluded [2,20,52]. Human immunodeficiency virus (HIV) co-infection increases risk of HCC in patients with chronic viral hepatitis [53].
Heterogeneous distribution of HCC risk factors
There is a large disparity in the distribution of the risk factors and patient demographics across geographic sites; the majority of cases in developing countries in Eastern Asia and Africa are caused by HBV with or without exposure to AFB1, whereas HCV-related HCC is more frequent in developed countries in the West and Japan [2]. In addition, in developing countries, the patients do not visit clinics until tumors get into advanced stage because early-stage HCC is generally asymptomatic. By contrast, in developed countries, HCC is diagnosed at earlier stage under the regular tumor surveillance program, and more likely to be treated with the curative therapies [7]. Thus, the strategy of patient management should be designed with specific consideration to the dominant etiologies, stage of disease, and available medical and socioeconomic resources available at each site. HBV and HCV contribute to development of more than 80% of HCC worldwide [1].
Altered molecular pathways in cirrhosis
Irrespective of the etiology, established cirrhosis serves as a milieu/microenvironment that fosters initiation and promotion of carcinogenesis by facilitating genetic aberrations and cellular transformation, which is often referred to as “field cancerization” or “field effect” [54-57]. For the majority of etiological agents, hepatocarcinogenesis is tightly associated with repeated cycles of hepatocyte death followed by regeneration and assumedly with accumulating potentially oncogenic mutations. This process could take several decades (typically 20-40 years) before establishment of liver cirrhosis, which may reflect the organ's resistance to replication errors [58]. Severity of liver fibrosis is correlated with increasing risk of HCC especially in HCV-infected patients [8,11].
Oxidative stress and inflammatory response are considered to play a central role in fibrosis progression and HCC development in NAFLD [59] and alcoholic hepatitis [60]. More frequent epigenomic alteration, impaired immune surveillance, and increased genetic susceptibility were suggested in alcoholic cirrhosis. Viral products such as HCV core protein may have direct carcinogenic effect by inducing generation of reactive oxygen species and transactivation of intracellular signaling cascades such as mitogen-activated protein kinase (MAPK) and activator protein (AP)-1 pathways [61].
Hepatocyte proliferation is generally decreased at the stage of cirrhosis after many rounds of regeneration accompanied with telomere shortening that induces chromosomal instability, impaired hepatocyte proliferation, and abnormality in cellular senescence [2]. Several molecular dysregulations observed in HCC tumors could be involved in hepatocarcinogenesis: telomerase (hTERT) reactivation [62], loss of cell cycle checkpoints by inactivation of p53/p21 pathway [63], loss of heterozygosity in insulin-like growth factor 2 receptor (IGF2R) gene [64], disruption of Rb/p16 pathway [65], increased resistance to apoptosis [66], activation of cellular growth/proliferation/development-related pathways such as MAPK pathway, ErbB receptor pathway, beta-catenin/Wnt pathway, and PI3K/Akt pathway [67,68]. However, it is still controversial whether these are early or late events in HCC initiation and progression.
microRNA (miRNA) profiling studies have identified dysregulated miRNAs in HCC tumors, and some of them are assumed to be involved in the process of hepatocarcinogenesis [69]. miR-122, down-regulated due to DNA methylation-mediated silencing, is assumed to be involved in dedifferentiation and tumorigenesis by activating CCNG1 [70]. Silencing of miR-122 was associated with increased tumor invasiveness, elevated alpha-fetoprotein expression, and higher HCC grade [71]. Restoration of miR-122 expression suppressed tumorigenicity of HCC and aflatoxin-transformed cells [72]. Therapeutic delivery of miR-26a suppressed tumorigenesis in a murine liver cancer model [73].
Preneoplastic lesions in cirrhotic liver
Relative difficulty in imaging-based follow-up and sampling of preneoplastic lesions in the liver has made molecular assessment of the sequential carcinogenic process more challenging. In addition, there has been diversity and inconsistency in histological diagnostic criteria and terminology (such as adenomatous and atypical adenomatous hyperplasia) for preneoplastic lesions and early HCC between pathologists, especially between East and West [74,75]. Recently emerging international guideline classifies clinically detectable preneoplastic lesions into low-grade dysplastic nodule (L-DN or LGDN) or high-grade dysplastic nodule (H-DN or HGDN) [76], which provides the basis of molecular interrogation [77,78]. L-DN, occasionally accompanied with large cell change, shows more benign clinical behavior, whereas H-DN, as a potential HCC precursor, could exhibit cytologic atypia called small cell change and nodule-in-nodule appearance, in which the subnodule represents dedifferentiation of parent nodule (i.e., well-differentiated/early HCC). Histological features of early HCC include “stromal invasion”, invading tumor cells into intra-nodule portal tract. It may be helpful to clarify corresponding neoplastic lesions in animal models of hepatocarcinogenesis to better understand the mechanism of sequential malignant transformation of hepatocytes.
APPROACHES TO HCC PREVENTION
Cancer prevention is grouped into the following categories: (1) “primary prevention” to prevent exposure to risk factors, (2) “secondary prevention” to prevent cancer development in patients with risk factors, and (3) “tertiary prevention” to prevent cancer recurrence in patients curatively treated for initial cancer but not for the risk factor [79-82] (Table 1). In the field of hepatology, “primary prevention” and “secondary prevention” sometimes also refer to prevention of initial and recurrent HCC, respectively [16,83].
Table 1.
Approaches to HCC Prevention
| Disease etiology | Type of prevention |
|||
|---|---|---|---|---|
| Primary | Secondary | Tertiary (targeting “early” recurrence) | Tertiary (targeting “late” recurrence) | |
| Hepatitis B virus | Universal vaccination | Blood screening, HCC surveillance | Cytotoxic drug? | Antiviral therapy (interferon, nucreos(t)ide analogs) |
| Prevention of new infection | Antiviral therapy (interferon, nucreos(t)ide analogs) | Transarterial embolization? | ||
| (Screening of blood products, safe injection practice) | Antiinflammatory/cytoprotective therapy (glycyrrhizin, UDCA)? Antifibrosis therapy? Acyclic retinoid? Vitamin K2? | Molecular targeted drug (gefitinib)? Immune modulator (sirolimus?, thymopentin?, adoptive immunothepaty?, HCC vaccination?) | Antiinflammatory/cytoprotective therapy (glycyrrhizin, UDCA)? Antifibrosis therapy? Acyclic retinoid? Vitamin K2? | |
| Hepatitis C virus | Prevention of new infection | Blood screening, HCC surveillance | Same as above | Antiviral therapy (interferon, interferon + ribavirion?) |
| (Screening of blood products, safe injection practice) | Antiviral therapy (interferon, interfron + ribavirion?) Antiinflammatory/cytoprotective therapy (glycyrrhizin, UDCA)? Antifibrosis therapy? Iron depletion? Acyclic retinoid? Vitamin K2? S-adenosylmethionine (SAMe)? | Antiinflammatory/cytoprotective therapy (glycyrrhizin, UDCA)? Antifibrosis therapy? Iron depletion? Acyclic retinoid? Vitamin K2? S-adenosylmethionine (SAMe)? | ||
| Dietary carcinogens (mainly aflatoxin B1) | Reduction of food/water pollution (establishment of water and sanitation infrastructure) | Antioxidant, inducer of cytoprotective enzymes (chlorophyllin?, oltipraz?, 3H1,2-dithiole-3-thione?) | Same as above | Antioxidant, inducer of cytoprotective enzymes (chlorophyllin?, oltipraz?, 3H1,2-dithiole-3-thione?) |
| Alcohol | Prevention of alcohol dependence/abuse | Abstinence of alcohol intake (not effective in cirrhosis?) Correction of nutritional deficiencies | Same as above | Abstinence of alcohol intake (not effective in cirrhosis?) Correction of nutritional deficiencies |
| Non-alcoholic metabolic disorders | Education on diet and physical activity | Life style/diet modification | Same as above | Life style/diet modification |
| (non-alcoholic fatty liver disease, obesity, type 2 diabetes) | Policy for healthy diet | Weight loss (diet, aerobic exercise) Control of diabetes Cytoprotective drug (UDCA?) | Weight loss (diet, aerobic exercise) Control of diabetes Cytoprotective drug (UDCA?) | |
| Genetic hemochromatosis | Genetic screening for high risk population | Iron depletion | Same as above | Iron depletion |
| Excess dietary iron | Education on dietary/drinking habit | Modification of dietary/drinking habit | Same as above | Modification of dietary/drinking habit |
| Iron depletion | Iron depletion | |||
| Primary biliary cirrhosis | Cytoprotective drug (UDCA?) | Same as above | Cytoprotective drug (UDCA?) | |
| Autoimmune hepatitis | Corticosteroids, immunosuppressive drugs | Same as above | Corticosteroids, immunosuppressive drugs | |
HCC: hepatocellular carcinoma, UDCA: ursodeoxycholic acid
“Early” recurrence: dissemination of primary tumor cells, usually observed within 1~2 years after curative treatment.
“Late” recurrence: de novo multicentric hepatocarcinogenesis independent from completely removed primary tumor, usually observed more than 1~2 years after curative treatment.
Primary prevention has been proven as an effective measure, whereas there are still no established secondary and tertiary prevention therapies. Interferon has been extensively tested in viral hepatitis-related HCC as proof-of-principle therapy, although there is still need for more potent and less toxic treatment.
Primary prevention
Primary prevention aims to eliminate or reduce exposure to etiological agents, and could be immediately effective especially in developing countries [84].
Viral hepatitis-related HCC
One prominent success is the population-based universal infant vaccination for HBV, which has shown to be effective in preventing neonatal HBV infection from infected mother (vertical transmission). A cross-sectional study enrolling 1515 healthy Taiwanese children revealed that the overall prevalence of HBV surface antigen seropositivity decreased from 9.8% to 1.3% over a decade after introducing the vaccination program [85]. Furthermore, annual HCC incidence in children 6 to 9 years old declined from 0.52 to 0.13 [86]. HCV vaccination is as of yet unavailable due to the high variability in the viral genomic structure, the large number of quasispecies, and the lack of a neutralizing antibody [35]. Therefore, current efforts focus on preventing new infections by educating on safe injection practice, screening donated blood products, and systematically identifying mostly asymptomatic HBV- or HCV-infected individuals with screening.
Non-viral hepatitis-related HCC
Elimination of the food contamination may be an effective public health strategy to reduce HCC [87]. Post-harvest intervention was effective to reduce AFB1 intake in west Africa [88]. However, such intervention, which requires water and sanitation infrastructure, may be practically infeasible in many resource-poor countries [34,82,87]. Strategies to reduce other risk factors such as alcohol abuse, obesity, and diabetes are also needed especially in developed countries. Dietary iron overload as a result of drinking habit may also be preventable [35].
Secondary prevention
Secondary prevention aims to prevent HCC in individuals chronically affected with the etiological agents. This approach is grouped into two categories: (1) eradication of the etiological agents (curative treatment) and (2) blockade of carcinogenesis progression in the presence of etiological agents (non-curative treatment). Complete eradication of HBV and HCV is still challenging once chronic infection is established, and there are already more than a half billion people with chronic infection, rationalizing the non-curative approach as a practical option.
Secondary prevention should be “less toxic” to be well tolerated for long-term treatment to asymptomatic patients, and “inexpensive” considering long duration of the treatment and large size of target patient population mainly residing in poor countries. Regular tumor surveillance in patients at high-risk of HCC is a vital component of secondary prevention [49], and has been shown to improve patient survival [89,90]. However, implementation of the program in the US is still unsatisfactory [91]. Secondary and tertiary prevention will play more roles in developed countries, where exposure to etiological agents is well controlled by means of primary prevention.
HBV-related HCC
Alpha interferon and nucleoside and nucleotide analogs, suppressing HBV replication, are clinically available as antiviral therapies [92]. A series of studies collectively suggested that interferon therapy lessens the risk of HCC development [93-97]. Prolonged suppression of HBV replication by a nucleoside analog, lamivudine, reduced the risk of HBV-related HCC: the incidence of HCC was reduced from 7.4% to 3.9% (hazard ratio 0.49) in a prospective trial enrolling 651 Taiwanese patients, and from 13.3% to 1.1% in a retrospective survey of 2795 Japanese patients [98-100].
HCV-related HCC
Meta-analyses of retrospective and relatively small prospective clinical trials of interferon-based treatment have shown that sustained viral response (SVR) is consistently associated with lower risk of HCC [80,101-105] and improved patient survival [106], although SVR is achieved in only up to 50-60% of patients. Irrespective of viral clearance, it has been suggested that suppression of hepatic inflammation could delay disease progression and reduce HCC risk; biochemical response, i.e., normalization of liver enzymes such as alanine aminotransferase (ALT), achieved by either interferon, glycyrrhizin, or ursodeoxycholic acid (UDCA), have been suggested to reduce HCC risk [80,105,107-112]. HCC risk was reduced in patients with more advanced fibrosis/cirrhosis in large randomized controlled trials of maintenance low-dose interferon (hepatitis C long-term antiviral treatment against cirrhosis [HALT-C] trial and Evaluation of PegIntron in Control of hepatitis C Cirrhosis [EPIC3] trial) [113,114]. Despite the plausible chemopreventive effect of interferon, the modest effect, poor tolerability (nearly 40% of participants could not complete full regimen in the HALT-C trial), and excess mortality [115] have drawn concern for its wide applicability for chemoprevention especially in Western countries. In some Asian countries such as Japan, antiviral therapy mainly with interferon is recommended as preventive therapy in the practice guideline [116]. HFE gene mutations, in particular H63D, were associated with increased SVR [117]. Of note, SVR does not preclude the necessity of HCC surveillance because the patients are still at risk of developing HCC [118].
Aflatoxin B1-related HCC
Chlorophyllin, water-soluble salts of natural chlorophylls, acts as an interceptor molecule forming tight molecular complexes and reduces carcinogenic activity of AFB1 in vivo [119], acts as an antioxidant [120], and serves as a potent inhibitor of cytochrome P450 enzymes from bioactivation of the carcinogen. A clinical trial in Qidong, China confirmed that chlorophyllin reduced urinary level of aflatoxin-N7-guanine adducts, a biomarker of biological effectiveness of the therapy [121], warranting further assessment of its long-term effect and safety profile in a larger patient cohort. Supplementary diet with chlorophylls-rich foods such as spinach and leafy green vegetables may be an alternative. Oltipraz, a dithiolethione originally developed as an anti-schistosomal drug, potently induces enzymes detoxifying AFB1 such as glutathione-S-transferase that enhance phase II detoxification [122-126], and inhibits phase I enzymes such as CYP1A2 and CYP3A4, that produce carcinogenic metabolite [127]. Clinical trials of oltipraz conducted in Qidong showed a 2.6-fold increase in urinary excretion of aflatoxin-mercapturic acid, a detoxification product of reactive, DNA-damaging metabolite [128]. However, a follow-up study failed to show any change in the urinary marker [129]. In addition, the high cost of its synthesis and safety issues raised concerns with its further development, and a second generation dithiolethione analog, 3H1,2-dithiole-3-thione (D3T) is now under evaluation [35,126,130].
Alcohol- and metabolic disorder-related HCC
It is not clear whether termination of alcohol abuse reduces HCC risk in alcoholic cirrhosis despite survival benefit [36,131]. There is also no clear evidence that correction of obesity and diabetes reduces HCC, suggesting the need to determine benefit of lifestyle adjustment, weight loss, bariatric surgery, and treatment of diabetes on HCC risk. Several pharmaceutical interventions have also been considered, including insulin-sensitizing agents (e.g., troglitazone, rosiglitazone, and pioglitazone) that upregulate peroxisome proliferator-activated receptor gamma (PPAR-gamma), lipid-lowering drugs (e.g., 3-hydroxy-3-methylglutaryl coenzyme A reductase, fibrates), cytoprotective agents (e.g., UDCA), and anti-oxidants (e.g., vitamin E, iron depletion, betaine, S-adenosyl-methionine, N-acetylcystein, and probucol) [132].
Iron overload-related HCC
A cohort study with long-term clinical observation showed that phlebotomy/venesection (iron depletion therapy) could be effective to reduce HCC incidence and prolong survival in GH [133]. A population-based study suggested that HCC preventive effect of phlebotomy is limited in patients with advanced fibrosis [40]. Homozygous HFE gene C282Y mutation, the risk allele of GH, is readily detectable with standard molecular assays, and targeted screening in combination with measurement of plasma transferrin has been proposed to identify the risk population subjected to HCC surveillance and phlebotomy [134-136]. In patients with chronic hepatitis C, long-term phlebotomy also lowered serum ALT level and HCC [137-139].
HCC associated with other risk factors
In PBC with stage 4 cirrhosis, UDCA may lower HCC incidence [111,140,141]. Lack of biochemical response to UDCA was associated with relatively high risk of HCC [142]. AIH is largely responsive to corticosteroids and other immunosuppressive drugs, but no study has been conducted in the context of HCC prevention [143].
Tertiary prevention
HCC recurrence targeted by tertiary prevention is categorized into two types: (1) dissemination of primary tumor cells mostly observed within 1-2 years after curative treatment as “early” recurrence, and (2) de novo carcinogenesis arising from remnant cirrhotic liver that appears as “late” recurrence independently from the treated primary tumor [54,69,144,145]. Theoretically, the latter is divided into (2)-a promotion/growth of (pre)neoplastic clones that already exist but are not detected at the time of curative treatment, and (2)-b de novo initiation of (pre)neoplastic clones that are not present at the time of treatment.
Albeit diverse molecular mechanisms are involved in the process of carcinogenesis according to the etiological agents, fully developed HCC tumor generally present similar histological, biological, and clinical characteristics, and uniform treatments are applied irrespective of the etiology [49]. Accordingly, attempts of tertiary prevention have been made mostly on mixtures of various different etiologies mainly consisting of HBV and HCV. Several strategies have been evaluated as adjuvant (i.e., post-surgical) or neoadjuvant (i.e., pre-surgical) treatment in combination with surgical resection. In theory, all the secondary prevention measures may be utilized for tertiary prevention of “late” recurrences.
Cytotoxic agents
Cytotoxic drugs, including doxorubicin, epirubicin, 5-fluorouracil (5-FU), mitomycin C, cisplatin, uracil-tegafur (UFT), and 1-hexylcarbamoyl-5-fluorouracil (HCFU), have been assessed with systemic or transarterial administration for unresectable or advanced-stage HCC without showing any remarkable clinical benefit despite considerable toxicity in both adjuvant and neoadjuvant settings [146-149]. A tumor-selective prodrug of 5-FU, capecitabine, was well tolerated in patients with advanced HCC [150], and is being tested in an on-going clinical trial as adjuvant therapy after resection. Combination of gemcitabine (a cytotoxic nucleoside analog) and oxaliplatin (a platinum agent) are also under evaluation (Table 2).
Table 2.
On-going Clinical Trials of HCC Prevention
| Agent/intervention | Type of agent/intervention | Type of prevention | Participants | Phase | Completion date | NCT ID |
|---|---|---|---|---|---|---|
| S-adenosylmethionine (SAMe) | Nutritional supplement | Secondary | Advanced chronic hepatitis C | 2 | Dec 2012 | NCT005134 61 |
| Phlebotomy | Iron depletion | Secondary | Compensated alcoholic cirrhosis | 3 | Jun 2017 | NCT013427 05 |
| PEG interferon alpha-2b + ribavirin | Immune modulator, anti-viral | Tertiary | HCV-related HCC after resection | 4 | Feb 2013 | NCT003756 61 |
| Interferon-alpha | Immune modulator | Tertiary | p48-positive HCC after resection | - | Jan 2011 | NCT008389 68 |
| Sirolimus | Immune modulator | Tertiary | HCC (exceeding Milan criteria) after liver transplantation | 3 | Aug 2013 | NCT005541 25 |
| Sorafenib (STORM trial) | Kinase inhibitor | Tertiary | HCC after resection | 3 | Oct 2014 | NCT006927 70 |
| Gefitinib | Kinase inhibitor | Tertiary | HCC after resection | 2 | Dec 2012 | NCT002821 00 |
| Thymopentin | Immune modulator | Tertiary | HBV-related HCC after resection | 3 | Feb 2012* | NCT004606 81 |
| Sustained released 5-FU ± cisplatin (TACE) | Cytotoxic agent | Tertiary | HCC after resection | - | Dec 2010* | NCT008178 95 |
| Epirubicin and lipiodol (TACE) | Cytotoxic agent | Tertiary | HCC after resection | 2 | Dec 2010* | NCT008200 53 |
| Capecitabine (systemic) | Cytotoxic agent | Tertiary | HCC after resection | 2/3 | Jul 2012* | NCT005615 22 |
| Gemcitabine + Oxaliplatin (systemic) (vs. doxorubicin + 5-FU + cisplatin) | Cytotoxic agent | Tertiary | HCC after liver transplantation | - | Dec 2010* | NCT011250 20 |
| Gemcitabine + Oxaliplatin (sytemic) (vs. arterial lipiodol) | Cytotoxic agent | Tertiary | HCC after resection/ablation | 3 | Jun 2014 | NCT004703 40 |
HCC: hepatocellular carcinoma, HCV: hepatitis C virus, HBV: hepatitis B virus, TACE: transarterial chemoembolization, NCT ID: National Clinical Trials Identifier
Current recruitment status is unknown in the database From www.ClinicalTrials.gov accessed June 2012
Transarterial embolization
As adjuvant therapy, transarterial chemoembolization (TACE) demonstrated modest improvement in disease-free survival and/or overall survival [151-153]. However, application as neoadjuvant therapy could worsen clinical outcome because it could increase risk of liver failure, delay surgery, and compromise subsequent treatment by promoting tumor dissemination and development of collateral tumor arteries [148].
Transarterial radioembolization (TARE) with iodine-131 or rhenium-188 labeled lipiodol or yttrium-90 labeled microspheres (glass/resin beads) is an alternative to TACE with comparable effectiveness and better tolerability for unresectable HCC [154,155]. Small clinical trials of adjuvant therapy with 131I-lipiodol-based TARE has shown improved disease-free and/or overall survival [156-159], warranting further clinical evaluation.
Adjuvant immunotherapy: interferon and others
Interferon has been intensively assessed as adjuvant therapy for surgical or ablative therapy in multiple randomized controlled trials [160-166]. These studies consistently showed a trend of reducing post-treatment recurrence or death with or without statistical significance. Interestingly, Mazzaferro et al. [165] reported positive effect of adjuvant interferon therapy only on “late” recurrence in HCV-infected individuals, suggesting that interferon suppressed initiation and/or promotion of de novo (pre)neoplastic clones rather than regrowth of disseminated primary tumor cells. A recent systematic review reported statistically significant benefit of interferon on tumor recurrence and patient survival, although a concern on adverse side effects was again raised [167]. Despite the limitation, tertiary prevention with interferon may be cost-effective in managing HCV-related HCC [168].
Immunosuppression after liver transplantation with sirolimus, also known as an mTOR inhibitor rapamycin, has been reported to reduce HCC recurrence and improve survival [169,170]. Adoptive immunotherapy, utilizing the patient's own lymphocytes activated with interleukin-2 and an antibody to CD3 in vitro, reduced post-surgical recurrence and recurrence-free/disease-specific survival, although further assessment is lacking [171]. Cancer vaccination with autologous HCC fragments has been tried in small phase 1/2 trials, and resulted in a decrease in tumor recurrence [172,173]. Purified thymus extracts (pTE) and synthetic thymic peptides (sTP) are thought to enhance anti-tumor and anti-infectious immunity in cancer patients despite limited clinical evidence [174]. A clinical trial of a peptide representing residues 32-36 of a nuclear protein, thymopoietin (thymopentin, TP5) is currently on-going (Table 2).
Vitamin A analog
As an adjuvant therapy, a synthetic acyclic retinoid (vitamin A analogue), polyprenoic acid (peretinoin, NIK-333) lowered incidence of second primary tumors (i.e., “late” recurrence) after surgical or ablative treatment of HCC [175] in a larger phase 2/3 trial1.
Interestingly, peretinoin showed dose-dependent recurrence suppressive effect 2 years after the therapy was started. Similarly, the HCC suppressive effect of interferon was not apparent for the first several years in retrospective and prospective trials [113,176]. These observations may suggest that both peretinoin and interferon suppress very early step of carcinogenesis, i.e., initiation of (pre)neoplastic clones, and have no substantial effect on (pre)neoplastic clones that already pass a certain step of malignant transformation. The several years of lag time required to see the HCC suppressive effect may reflect a latent period for the initiated clones to become detectable by clinical diagnostic modalities. This may be worth noting in the design of HCC prevention trials.
POTENTIAL TARGETS OF HCC PREVENTION
The process of hepatocarcinogenesis includes initial exposure to etiological agents, establishment of chronic diseased condition, progression of liver fibrosis, “initiation” of (pre)neoplastic lesion with irreversible somatic alterations, and “promotion” of the initiated lesion into clinically recognizable HCC tumor (Fig. 1). It is also important to note that “initiation” and “promotion” are assumed to take place under persisting chronic irritation and inflammation with chemicals, hormones, or other mediators produced in association with underlying chronic liver disease. Each of these processes could be regarded as a preventive target. Pharmaceutical intervention with natural or synthetic agents to block, retard or reverse any step of the carcinogenic process is referred to as chemoprevention [15].
Fig. (1).
Mechanisms of hepatocarcinogenesis. Molecular pathways involved in HCC tumor development are summarized in upper panel. HCC tumor often develops as subnodule within high-grade dysplastic nodule accompanied with various molecular aberrations. Lower panel summarizes molecular alterations observed during the course of progressive liver fibrosis that leads to establishment of cirrhosis. Cirrhotic microenvironment in the liver is assumed to support initiation and promotion of hepatocarcinogenesis (so called “field effect” or “field cancerization”). HCC: hepatocellular carcinoma, HBV: hepatitis B virus, HCV: hepatitis C virus, NAFLD: non-alcoholic fatty liver disease, HSC: hepatic stellate cell
Both curative (i.e., elimination of etiological agents) and non-curative (i.e., prevention of HCC in the presence of etiological agents) treatments could be considered as secondary or tertiary prevention therapy depending on potency, mode of administration, toxicity profile, and cost. Non-curative treatment could be directed at counteracting specific carcinogenic pathways such as cellular growth signaling, regulators of cell cycle or apoptosis, oxidative stress response, and inflammatory response, or preventing liver cirrhosis, the milieu promoting and supporting hepatocarcinogenesis. Several molecular pathways are involved in the processes of both carcinogenesis and fibrogenesis, and these pathways diversely function across different types of resident liver cells and infiltrating leokocytes, and/or even different populations of the same cell types, interacting with each other via autocrine and paracrine mediators.
To date, only anti-viral/inflammatory and iron depletion therapies are likely to exhibit clinically meaningful effect as non-curative HCC prevention therapies. Molecular targets such as miR-122 and platelet-derived growth factor pathway on activated hepatic stellate cells seem to be promising [177], although all of these experimental observations await clinical evaluation.
Therapies targeting hepatitis viruses
Therapy of hepatitis B
Interferon (interferon alpha-2a and peginterferon alpha-2a) and several nucleoside/nucleotide analogs (lamivudine, adefovir, entecavir, telbivudine, and tenofovir) are clinically available [178]. Frequent emergence of viral strains resistant to lamivudine is now well recognized, and the search for optimal regimen with other available drugs such as adefovir and entecavir is currently underway [92].
Therapy of hepatitis C
Growing molecular understanding of the HCV lifecycle has led to the development of direct acting antivirals (DAAs) targeting viral proteins, processes of entry to host cells, replication, virion assembly, and secretion, as opposed to modifying host immunity like interferon. HCV protease NS3/4A inhibitors such as telaprevir improved viral response in combination with the standard interferon-based regimen [179]. Inhibitors of other HCV proteins like NS5A (essential for viral replication and assembly) and NS5B (RNA polymerase) are in phase 1-2 clinical trials [180]. In addition, a miR-122 inhibitor [181] and a phytochemical, silymarin [182], among others are under clinical evaluation as anti-HCV agents. Host genes involved in the HCV lifecycle could be another class of preventive targets. An inhibitor of cyclophilin B (CyPB), a host factor interacting with NS5B, showed anti-HCV effect [183] and is currently being evaluated in a phase 2 trial. Recently discovered SNPs in IL28B gene (e.g., rs12979860) showed strong association with SVR [184].
Targets in liver fibrosis
Resolution/reversal of human liver fibrosis/cirrhosis has been clinically observed when etiological agents were successfully eliminated, suggesting that therapies for liver fibrogenesis could be used for secondary or tertiary prevention [185]. SVR improved histological fibrosis [186,187] and hepatic venous pressure gradient (HVPG) [188], a well-known prognostic factor and predictor of HCC development [189]. Weight loss in patients with NAFLD also results in improvement of liver fibrosis [190]. UDCA was shown to reduce liver fibrosis in patients with PBC [191]. Rapidly expanding knowledge of the etiology-specific molecular mechanisms in liver fibrosis has revealed many potential targets, although there is no established therapy yet [9,10,192-195].
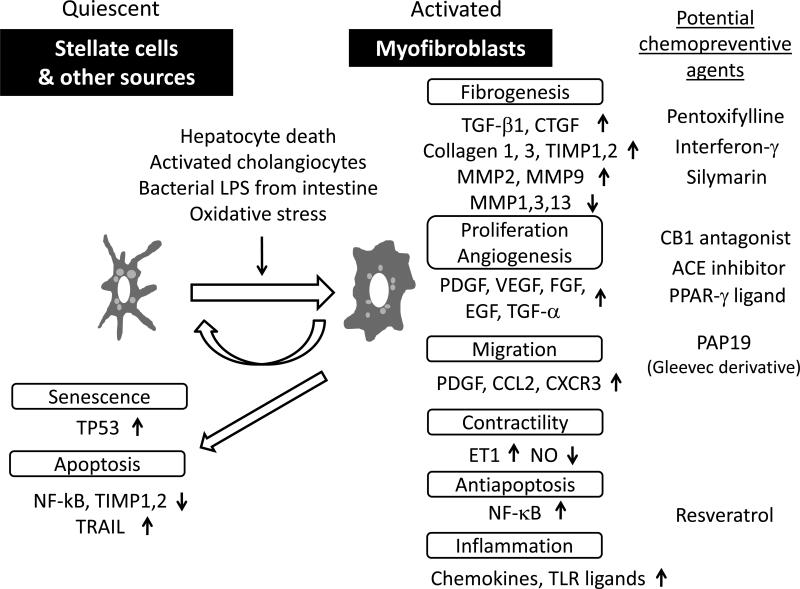
Liver fibrosis is an excessive wound healing response to chronic liver injury that results in increased production and deposition of scar tissue, extracellular matrix (ECM). Dynamic balancing between fibrogenesis (synthesis of and deposition of ECM) and fibrolysis (removal of ECM) determines liver fibrosis under complex interplay between various cell types in the liver, including hepatic stellate cells (HSCs, liver-specific pericytes), Kupffer cells (liver-specific macrophages), hepatocytes, cholangiocytes, sinusoidal endothelial cells, and infiltrating immune cells. Hepatic myofibroblasts (HMFs) play the central role as the key fibrogenic effector cells by producing ECM such as collagens (Fig. 2). Transdifferentiation of quiescent lipid/vitamin A-storing HSCs is the major cellular source of HMFs [192,193], together with other less frequent sources such as periportal/perivascular fibroblasts [196], bone marrow [197], and epithelial-mesenchymal transition [198,199].
Fig. (2).
Molecular pathways in hepatic myofibroblast. Hepatic myofibroblasts, mainly derived from transdifferentiated hepatic stellate cells and periportal/perivascular fibroblasts, play major role in liver fibrogenesis. Various paracrine stimuli trigger the activation characterized by several phenotypic changes that involve specific genes and pathways (see text for details).
Pathways of stellate cell activation
HSC activation is initiated by liver injury, not necessarily accompanied with inflammation as seen in GH, and subsequent release of the following fibrogenic mediators. Reactive oxygen species (ROS) are generated through lipid peroxidation by HSCs, hepatocytes, Kupffer cells, and inflammatory cells with enhancement by ethanol, unsaturated fatty acids, and iron [200,201]. Hepatocyte death, including apoptosis and necrosis, serves as a stimuli activating HSCs [202], and is a potential therapeutic target [203]. Bacterial lipoploysaccharide (LPS) permeabilized from intestinal microbiota and endogenous ligands elicit fibrogenic response through Toll-like receptor 4 (TLR4) expressed on HSCs by inducing the major fibrogenic cytokine, transforming growth factor (TGF)-beta [204]. HCV infection induces TGF-beta through ROS production, p38 MAPK, C-Jun NH2-terminal kinase (JNK), ERK, and nuclear factor kappaB (NF-kappaB) pathways [205], although toxicity concern has been raised to target TGF-beta pathway itself [194]. Platelets are important source of paracrine stimuli, including platelet-derived growth factor (PDGF), TGF-beta, and EGF. Activated, proliferating cholangiocytes (reactive cholangiocytes), showing characteristic ductular formation known as “ductular reaction”, stimulate HSC activation by secreting profibrogenic cytokines and growth factors such as TGF-beta, connective tissue growth factor (CTGF), PDGF, and hedgehog ligands [206,207]. Dynamic changes in ECM composition also contribute to initiate HSC activation and create positive feedback pathways by increasing deposition of collagen type I and III, fibronectin, and altering growth signaling through membrane receptors such as integrins [208,209].
Autocrine loop of PDGF is the most potent mitogenic signal, which induces expression of beta PDGF receptor. Transgenic mice expressing either PDGF-B or PDGF-C develop liver fibrosis [210,211]. Notably, the latter also developed hepatic steatosis and HCC, suggesting that the model is suitable for assessment of HCC preventive therapies. In fact, peretinoin was recently shown to repress fibrosis and HCC development in PDGF-C transgenic mice [212]. Monoclonal antibody targeting PDGF-B ligand (AbyD3263) reduced liver fibrosis in bile duct ligated mice [213]. Activated HSC-specific delivery of PDGF kinase inhibitor PAP19 (an imatinib derivative) with mannose-6-phosphate modified human serum albumin (M6PHSA) reduced collagen production in bile duct ligated rats [214]. Targeted delivery of interferon-gamma, an anti-fibrotic cytokine, was attempted using a cyclic peptide recognizing beta PDGF receptor to reduce fibrosis in carbon tetrachloride-treated mice [215]. CTGF also serves as a potent fibrogenic stimulus in TGF-beta-dependent and independent manners, and is potentially associated with hyperglycemia and hyperinsulinemia [193]. Vascular endothelial growth factor (VEGF) is induced by hypoxia due to impaired blood circulation accompanying progressive fibrotic change, and contributes to angiogenesis [216]. HSC migration to liver injury site can be driven by chemoattractants such as PDGF, MCP-1, and CXCR3 and inhibited by adenosine.
Activated HSCs/HMFs express alpha-smooth muscle actin (alpha-SMA) and myosin that increase contractility of sinusoid and entire liver, in which endothelin-1 (ET-1), nitric oxide (NO), angiotensinogen II, eicosanoids, somatostatin, and carbon monoxide play roles as regulators [217]. Matrix-degrading matrix metalloproteinases such as MMP-1 are inactivated by tissue inhibitors of metalloproteinases including TIMP-1 produced by activated HSCs/HMFs [218,219], providing a rationale to target TIMP-1 as anti-fibrotic therapy. Collagen type I, fibronectin, proteoglycans, and other matrix constituents are produced by activated HSCs/HMFs with paracrine and autocrine TGF-beta as the most potent stimulus activating downstream Smad signaling cascade [220]. Chemokine ligands and receptors mediate both fibrogenic (CCR1, CCR2, and CCR5) and anti-fibrogenic (CXCL9 and CXCR3) signals [10].
NF-kappaB activation in HSCs/HMFs promotes liver fibrosis by direct fibrogenic effect, antiapoptotic effect, and secretion of inflammatory chemokines such as CCL2, CCL3, CXCL2, and CXCL5 attracting macrophages that contribute to further activation of HSCs [221-223]. It is suspected that NF-kappaB activation is a common theme linking liver injury, fibrosis, and initiation and promotion of HCC by creating tumor-friendly microenvironment [221]. Interleukin (IL)-32 expressed by hepatocytes was recently reported to be associated with hepatic inflammation and fibrosis in HCV infection [224]. A CCL5 receptor antagonist, Met-CCL5, reduced liver fibrosis in mice [225].
Adipokines including leptin, adiponectin, and resistin are implicated in liver fibrogenesis in NAFLD and hepatitis C [226,227]. Neuroendocrine signaling mediated by cannabinoid (CB)1 receptor is fibrogenic [228], and an antagonist CP-945598 is under clinical evaluation. Other neuroendocrine such as neurotrophins also promote fibrogenesis [229]. Suppression of heat shock protein (Hsp) 47, a collagen-specific chaperon, by siRNA delivered with vitamin A-coupled liposome, targeting retinol binding protein on HSCs, reduced fibrosis in rat models [230].
Pathways of liver fibrosis resolution
The molecular process of fibrolysis is accompanied with reversal of activated HSCs/HMFs to quiescent state or clearance of them through apoptosis or senescence. NK cell is considered to be important in the clearance with both mechanisms [231]. An angiotensin receptor inhibitor may reduce liver fibrosis by inhibiting expression of NADPH oxidase and collagen type I [232]. An angiotensin-converting enzyme inhibitor regressed fibrosis by reducing survival of activated HSCs by inhibiting NF-kappaB pathway [233]. PPAR-gamma negatively regulates HSC activation through epigenetic modification [234]. Recent studies have suggested involvement of microRNA such as miR-199, miR-200, miR-29, miR-150, miR-194, miR-132, miR-16, and miR-27 in HSC activation and inactivation [234-238].
Genome-wide transcriptomic and proteomic profiling results revealed difference between the genes involved in HSC activation in vitro and in vivo [222,239-243]. A gene-expression signature of culture-activated HSCs was enriched in human cirrhosis with poor clinical outcome, and conversely, a gene-expression signature of lethal human cirrhosis was induced in chemically-induced rodent model of cirrhosis and HCC [244,245]. This suggests that genomic profiling is a useful and convenient tool to evaluate clinical relevance of molecular dysregulations in experimental models of liver fibrosis and HCC.
Targets in inflammatory pathways
Several inflammatory pathways have been implicated in development of HCC [246,247]. Involvement of NF-kappaB pathway, especially in later stage (i.e., promotion) of hepatocarcinogenesis, has been pointed out in experimental models, although the strategy to antagonize the pathway is still not clear yet [221]. JNK, a MAPK, pathway is activated by stress and proinflammatory signals such as ROS, and suggested to be associated with promotion of HCC development [248]. A JNK inhibitor, SP600125, suppressed HCC development in chemically-treated (DEN) rats by shifting hepatocytic Smad3-mediated signal from oncogenesis to tumor suppression [249]. JNK activation in non-parenchymal liver cells generated an inflammatory hepatic microenvironment that support HCC development [250], and JNK1 knockout resulted in decreased liver fibrosis [251]. JNK pathway exhibits cross talks with growth factor (e.g., EGF)-related signaling pathways [252]. Selective inhibition of cyclooxygenase-2 (COX2) prevents HCC in an experimental animal model [253]. Liver-specific expression of lymphotoxin (LT)-alpha and beta in mice caused hepatic inflammation and HCC, which was suppressed by inhibition of LT beta receptor [254].
Targets in cytoprotective enzyme-related pathways
Oxidative stress is often linked to HCC development in chronic viral hepatitis, alcoholic and non-alcoholic chronic liver injury. Phase 2 enzymes, including conjugating and antioxidative enzymes such as glutathione S-transferase (GST), UDP-glucuronosyltransferase (UGT), and NAD(P)H:quinone oxidoreductase (NQO1) exert cytoprotective effect by eliminating carcinogens and mutagens and enhancing cellular resistance to oxidative stress [126]. Many genes encoding such cytoprotective enzymes have antioxidant response element (ARE) in the regulatory regions, which is activated by a transcription factor, nuclear factor-E2-related factor 2 (Nrf2) [125]. Nrf2 is sequestered in cytoplasm by Kelch ECH-associating protein 1 (Keap1). Oxidative stress or activation of kinases such as protein kinase C (PKC), MAPK, PI3K could cause dissociation and nuclear translocation of Nrf2 followed by activation of the target genes. Several chemical agents are known to induce Nrf2 signaling and prevent chemically-induced HCC in animal models: phenolic antioxidant, e.g., butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and ethoxyquin; dithiolethiones, e.g., oltipraz and 3H-1,2-dithiole-3-thione (D3T); sulforaphane, an isothiocyanate derived from cruciferous vegetables; triterpenoids, a synthetic derivatives of oleanolic acid [126]. Deficiency of a pentose phosphate pathway (PPP) enzyme, TAL (encoded by Taldo1 gene) induced oxidative stress, hepatic inflammation and HCC in mice, and these phenotypes were prevented by N-acetylcysteine (NAC) [255].
Depletion of (pre)neoplastic clones: retinoid (vitamin A) and radioembolization
Retinoid X receptor-alpha (RXR-alpha) is a nuclear receptor for retinoids, vitamin A and its functional analogs, and ligand-dependent transcription factor involved in regulation of epithelial cell growth, differentiation, development, and apoptosis. Upon ligand binding, RXR-alpha homodimarizes or heterodimarizes with retinoic acid receptors (RARs) or PPARs, and transactivates target genes regulating normal cell proliferation and differentiation [256]. RXR-alpha is phosphorylated by activation of Raf-MAPK signaling, sequestered from ubiquitin/proteosome-mediated degradation, and accumulates in liver cells to promote carcinogenesis. Use of acyclic retinoid is proposed to counteract this process, induce apoptosis, and eventually eliminate the (pre)neoplastic clones or inhibit “initiation” as “clonal deletion” therapy. In the clinical setting, an acyclic retinoid, peretinoin, has shown to reduce serum level of lectin-reactive alpha-fetoprotein factor 3, an assumed marker of hypothetical latent malignant clones [257]. Radioembolization and adoptive immunotherapy are assumed to eliminate clinically undetectable “initiated” tumor clones. A small molecule EGFR tyrosine kinase inhibitor, gefitinib, suppressed growth of initiated HCC tumors in vivo [258] and is currently under clinical evaluation in the setting of tertiary prevention (Table 2).
Other molecular targets
A multi-kinase antagonist, sorafenib, inhibits VEGFR-2/3, PDGF receptor, and B-Raf kinase, and is prescribed as a chemotherapeutic drug [13,14]. Recent experimental evidence has suggested that the drug may also have antifibrotic effects [259,260]. In addition, it has been shown to improve portal hypertention, a clinical manifestation of severe cirrhosis and a predictor of HCC development [261], supposedly due to its antiangiogenic activity [262]. Sorafenib's role in HCC tertiary prevention is currently under clinical evaluation in a large-scale phase 3 trial [263]. This class of drugs may have potential to block activation of MAPK signaling in hepatice stellate cells.
Renin-angiotensin system (RAS) is suggested to be involved in hepatocarcinogenesis [264]. Inhibition of angiotensin-II (AT-II) by angiotensin-converting enzyme inhibitor (ACE-I) down regulates angiogenic factors such as VEGF, and ACE-I administration combined with branched-chain amino acids (BCAA) has been shown to attenuate insulin resistance-related hepatocaricnogenesis in a diabetic rat model [265].
PHYTOCHEMICALS AND NUTRITIONAL SUPPLEMENTS
Phytochemicals, plant-derived bioactive chemicals, and other dietary substances have been increasingly recognized as potential treatment options in HCC prevention [266,267]. Together with other food-derived agents and nutritional supplements, this class of drugs are referred to as complementary and alternative medicine (CAM). Some of them exert pleiotropic effect in preventing carcinogenesis, although mechanisms are not fully known. A few of them such as glycyrrhizin are incorporated in clinical practice in specific countries [267].
One challenge for this class of drug is to capture its assumedly mild effect as suggested by a large-scale controlled trial testing combinations of vitamins and minerals (retinol and zinc; riboflavin and niacin; ascorbic acid and molybdenum; beta-carotene, alpha-tocopherol, and selenium) that failed to demonstrate improvement of HCC-related mortality [268,269].
Glycyrrhizin
Glycyrrhizin, an extract of licorice root (glycyrrhizae radix), has similar chemical structure to cortisone. The aqueous preparation, Stronger Neo-Minophargen C (SNMC), has been used for over 35 years in Japan, and shown to lower serum aminotransferases, improve liver histology, suppress HCC development (i.e., secondary prevention) and recurrence after surgery (i.e., tertiary prevention) despite the lack of apparent antiviral activity [110,112,270,271]. Glycyrrhizin is considered to have cytoprotective effects through both antioxidative properties and immune modulating activities such as induction of interferon-gamma and stimulation of NK cells, and thereby suppress hepatic necroinflammation that leads to liver fibrogenesis and HCC development [272,273]. Glycyrrhizin is one of ingredients of a traditional Chinese herbal medicine, Sho-saiko-to (TJ-9), which also prevented HCC development and prolong patient survival in a clinical trial [274].
Vitamin K
Vitamin K is a lipid-soluble vitamin, and classified into naturally occurring vitamin K1 and K2 and chemically synthesized vitamin K3 which demonstrates the most potent anti-proliferative activity in tumor cells in vitro. Vitamin K is required to synthesize coagulation factors, including prothrombin. Des-gamma-carboxy prothrombin (DCP, also known as protein induced by vitamin K absence II [PIVKA-II]) is an abnormal prothrombin induced by the absence of vitamin K2 and is one of clinically used HCC tumor markers together with alpha-fetoprotein. DCP has been associated with aggressive behavior of HCC tumor such as more frequent post-treatment recurrence [275]. Metatetrenone (vitamin K2) caused cell-cycle arrest, induced apoptosis, and inhibited growth and invasiveness of HCC cell lines in vitro and in vivo [276]. Clinically, metatetrenone suppressed HCC development in cirrhotic women and recurrence after curative treatment in small clinical trials without serious adverse effect [277-279]. The safety profile and relatively low cost would further warrant evaluation in larger clinical trial.
S-adenosylmethionine
S-adenosylmethionine (SAMe), which is available as a nutritional supplement for liver, joint, and mental health, is a major methyl donor ubiquitously involved in transmethylation reactions in the body [280]. SAMe is synthesized from methionine by the enzyme, methionine adenosyl transferase (MAT), mainly in the liver, and regulates hepatocyte growth and apoptosis [280]. In cirrhotic liver, activity of MAT is reduced accompanied with hypermethioninemia [281]. Matla gene knockout mice exhibited reduced SAMe biosynthesis, and developed steatohepatitis with oxidative liver injury and HCC [282]. SAMe inhibits mitogenic effect of hepatocyte growth factor (HGF), induced apoptosis in cancer cells (but not in normal liver cells), and prevented initiation of chemically-induced HCC in rats [283]. No effect was observed on growth of already establish tumor cells. A phase 2 clinical trial of SAMe is on-going with the primary endpoint assessing change in serum alpha-fetoprotein level (Table 2).
Carotenoids
Natural carotenoids such as beta-carotene are obtained from fruits and vegetables, and have been evaluated for cancer prevention as antioxidants, although clear benefit has not been observed yet [284]. Beta-carotene suppressed chemically-induced HCC in rats [285]. Mixture of cartenoids, including beta-carotene, alpha-carotene, lycopene, and orange-derived beta-cryptoxanthin, was tested in small clinical trials, and showed moderate effect to reduce HCC incidence [16].
Green tea and coffee
Epigallocatechin gallate (EGCG) is the most abundant green tea catechin polyphenol, and exerts potent antioxidative activity, inhibits tumor growth, and induces apoptosis in vitro [266]. The proapoptotic effect of EGCG was attributed to up-regulation of miR-16 in HCC cells [286]. In rodent HCC models induced with chemicals such as aflatoxin and diethylnitrosamine (DEN), green tea or EGCG reduced the size and number of tumor and placental glutathione S-transferase (GST-P)-positive preneoplastic foci [287,288]. EGCG reduced the number of spontaneous liver tumors in C3H/HeNCrj mice [289]. In a phase 2a trial of green tea polyphenols for individuals infected with HBV and exposed to aflatoxin, urine 8-hydroxydeoxyguanosine (8-OHdG), an oxidative DNA damage biomarker, were significantly reduced after 3 months of treatment [290]. Habit of drinking green tea reduced the risk of liver cancer development in alcohol drinkers [291].
Epidemiological studies have suggested modest effect of coffee drinking in reducing HCC risk [292,293]. Administeration of both coffee and caffeine, a methylxanthine, reduced chemically induced hepatocarcinogenesis in animal models [294,295]. Coffee induced UDP glucuronosyltransferases (UGT1A), an enzyme having antioxidative, cytoprotective, and genoprotective capabilities in vitro and in vivo in caffeine independent manner, and up-regulated glucuronidation by aryl hydrocarbon receptor (AhR) signaling and Nrf2 binding to antioxidant and xenobiotic response elements (ARE/XRE) in transgenic UGT1A mice [292].
Silymarin
Silymarin is a herbal flavonoid extracted from Lady's thistle's seeds, in which silibinin is the major active constituent. Silibinin showed anti-tumor effect through induction of cell cycle arrest and apoptosis in HCC cells [296]. Silymarin suppressed N-nitrosodiethylamine (NDEA)-induced hepatocarcinogenesis in rats [297]. An observational study in the participants of HALT-C trial showed that silymarin use was associated with reduced fibrosis progression, but effect of clinical outcomes such as development of cirrhosis complication and HCC, and death was not evident during 5.5 years of median follow-up [298]. Silymarin is also evaluated as an anti-HCV agent.
Resveratrol
Resveratrol (3,4’,5-trihydroxy-trans-stilbene) is a polyphenol found in peanuts, grapes, berries, and red wine, which has been studied for multiple chemotherapeutic and chemopreventive activities, including antioxidant effect by modulating expression and activity of inducible nitric oxide synthase (iNOS), antiproliferative effect accompanied with decreased HGF activity and cell cycle arrest, antiangiogenic effect through suppression of HIF-1a and VEGF expression, and antiinflammatory effect inhibiting NF-kappaB pathway [266,299]. Resveratrol also has proapoptotic effect through p38 MAPK and caspase-3 expression, which was enhanced by Caveolin-1 (CAV1) [300]. A carcinogen-activating enzyme, cytochrome P450 1A1 is inhibited by resveratrol [301]. In rodent models of HCC induced by DEN, grape extracts (assumed to be resveratrol-rich) inhibited development of preneoplastic loci positive for GST-P [302]. COX-2 activity, which is linked to overexpression of antiapoptotic Bcl-2, was suppressed by resveratrol without cardiotoxicity [303].
Miscellaneous agents
Other agents that have been evaluated in preclinical and clinical settings include genistein (phytoestrogen isoflavone), selenium (an essential mineral), curcumin (a polyfenol derived from spice turmeric), capsaicin (phenolic compound contained in hot red peppers), glucosinolate and indole-3-carbinol (I3C) (extract from cruciferous vegetables), and N-acetylcysteine (NAC) (a precursor of glutathione) [266,267,304]. Dithiolethiones are known as more potent inducers of conjugating and detoxification enzymes than phenolic antioxidants, and synthesized dithiolethiones, oltipraz and D3T, have been evaluated for the treatment of AFB1-related HCC.
EXPERIMENTAL MODELS FOR HCC PREVENTION STUDY
With increasing recognition that the complex interplay between multiple cell types in the physiological hepatic microenvironment is critical in HCC development, it becomes more important to develop experimental models recapitulating relevant biological and/or clinical contexts. Whereas genetic engineering provides mechanistic insight in understanding pathogenic molecular aberrations, non-physiological dysregulation of engineered genes/pathways raises a concern. Traditional chemical- or diet-induced animal models may be more preferable in this regard, and the recent advent of genomic assays has enabled the interrogation of pathogenic molecular mechanisms even in these models. More sophisticated in vitro co-culture systems are also a promising tool.
In vitro models
In vitro models hold value for their relatively low costs and the high reproducibility of the results, and they can be easily adopted for high-throughput testing. Several in vitro models have been employed in the assessment of antifibrotic drugs: culture-activated rat or human HSCs [192,194] and immortalized human HSC cell lines such as LX-1/LX-2 [305] and TWNT-4 [306]. To better capture the multicellular nature in physiological microenvironment of the liver, precision-cut sliced liver tissue culture has also been utilized to study HSC biology associated with liver fibrogenesis [307-309] and to test antifibrotic drugs such as pentoxifylline, imatinib (Gleevec, PDGF receptor antagonist), and dexamethasone [310], and antioxidant/hepatoprotective activity of lichen Usnea ghattensis [311]. Sliced tissue culture model also allowed genomic assessment of molecular targeted drug [312]. Emerging micro- and nano-scale bioengineering technologies have enabled the construction of 2-dimensional or 3-dimensional multicellular hepatic microenvironment in vitro that could be used in future studies [313,314].
In vivo models
It is still challenging to create animal models faithfully mimicking or recapitulating at least part of the natural history of HCC development: sequential development of chronic fibrosing hepatitis, progressive cirrhosis, and initiation and promotion of HCC. Rodent models are frequently used with the use of chemicals, diets, and genetic engineering (Table 3) [315-317]. More attention has been paid to the hepatic microenvironment which supports not only hepatocarcinogenesis, but also the maintenance and progression of established cancer nodules [246,318-320].
Table 3.
Experimental Models for HCC Prevention Research
| Model | Presence of Liver Disease | HCC development | Preventive intervention | References |
|---|---|---|---|---|
| Chemically-induced models | ||||
| Diethylnitrosamine (DEN) | When dosed repeatedly, bridging fibrosis by 8 weeks, cirrhosis by 12 weeks | Varies based on species, dose, route, and frequency of administration | Gefitinib, SP600125, silymarin, resveratrol, curcumin, acyclic retinoid and peretinoin inhibit HCC development; EGCG and resveratrol reduce preneoplastic GST-P foci | 249, 258, 288, 297, 302, 317, 323, 326, 327, 328, 330, 373 |
| Carbon tetrachloride (CCl4) | Panlobular fibrosis | HCC develops after prolonged treatment | Sunitinib decreases inflammation and fibrosis; EGCG decreases fibrosis | 194, 316, 370, 371, 372 |
| Diet-induced models | ||||
| Methionine and choline-deficient (MCD) diet | Periportal steatosis; cirrhosis rarely | HCC develops rarely | 316 | |
| Choline-deficient, L-amino acid-defined (CDAA) diet | Steatosis by 1 week, bridging fibrosis by 12 weeks and cirrhosis by 30 weeks | 100% incidence by 52 weeks | 4-HPR decreases GST-P-positive foci; ASA decreases GGT-positive foci | 355, 356, 357 |
| CDAA diet combined with DEN | Steatohepatitis and cirrhosis | HCC develops by 16 weeks | 358 | |
| Surgical Models | ||||
| Bile duct ligation (BDL) | Biliary fibrosis/cirrhosis | HCC develops by 1 month | Sorafenib reduces inflammation and fibrosis; rapamycin decreases extracellular matrix accumulation | 376, 377 |
| Orthotopic implantation | Fibrosis can be induced by concurrent treatment with thioacetamide (TAA) and oral alcohol | Injected HCCs can be either hepatoma cell lines or primary tumors from patients | Sorafenib suppressed intrahepatic recurrences and prolonged survival after tumor resection | 383, 388, 389 |
| Genetically engineered models | ||||
| EGF transgenic | 100% incidence by 7 months; enhanced by Myc | 332 | ||
| TGF-alpha transgenic | Little fibrosis or steatosis | 75% incidence by 12-15 months; enhanced by Myc | 324, 331, 333 | |
| E2F 1 transgenic | 33% incidence by 12 months; enhanced by Myc | 324, 334, 335 | ||
| TGF-beta 1 transgenic | Fibrosis by 12 weeks | No spontaneous HCC; increases TAA-induced HCC development | 379, 380 | |
| PDGF-C transgenic | Steatosis and fibrosis by 9 months; no cirrhosis | 80% incidence by 12 months | Peretinoin | 211, 212 |
| FGF19 transgenic | Dysplastic/neoplastic nodules | HCC develops after 10 months | FGFR4 knockout | 340, 341 |
| miR-221 transgenic | HCC development accelerated by DEN | Anti-miR-221 oligo | 338 | |
| NEMO knockout | NASH characterized by steatohepatitis and periportal liver fibrosis by 8 weeks, steatosis by 6 months | HCC develops by 12 months | 343, 344 | |
| TAK1 knockout | Intrahepatic cholestasis and periportal fibrosis by 6 weeks | 88% incidence by 33 weeks | 344 | |
| PTEN knockout | Perisinusoidal fibrosis and steatohepatitis by 40 weeks | 66% incidence by 78 weeks | Eicosapentaenoic acid reduces steatohepatitsis and HCC development | 345, 346, 347 |
| IL-6 knockout | HCC incidence in response to DEN dramatically decreased | 348 | ||
| Acox1 knockout | Steatohepatitis | HCC develops by 15 months | 324, 367 | |
| Mdr2 knockout | Biliary fibrosis by 4 weeks with disease progressing similar to primary sclerosing cholangitis | HCC develops after 8-10 months | 374, 375 | |
| Mst1/2 knockout | Hepatomegaly | HCC develops after 7-15 months | 350 | |
| Nf2 knockout | Expansion of liver progenitor cells by 6-8 weeks, hepatomegaly, no inflammation or fibrosis | HCC develops after 30 weeks, HCC lung metastases after 1 year | 351 | |
| KLF6 knockout | Increased HCC development in response to DEN | 325 | ||
| FXR knockout | Hepatic inflammation and regeneration, elevated bile acids | HCC develops after 15 months | 352, 353 | |
HCC: hepatocellular carcinoma
Viral component-induced models are detailed in [315].
It is critical for a model to capture relevant biological and clinical context with regard to affected genes and molecular pathways, and dosing and schedule of the treatment ideally specific to assumed etiology [315]. Chemically-treated rats have been popular because of their higher propensity for developing fibrosis and cirrhosis. In contrast, mice have recently garnered more attention because of their small size and ease in genetic modification [194]. Some of the genetic perturbations were not sufficient to induce HCC development by themselves, but could increase susceptibility to additional carcinogenic stimuli. One potential limitation of genetic engineering models is that they may not reflect physiological dysregulation of the genes in human.
Genomics studies on human specimens have revealed that non-tumor liver tissues harbor molecular information associated with higher risk of “early” or “late” recurrence [69,145]. Propensity to “early” recurrence (dissemination of primary tumor cells) could be modulated by antitumor immunity in the liver such as a shift from Th1 to Th2 cytokines [321]. Anti-tumor inflammatory response was correlated with poorer clinical outcome [322]. Genomic and other molecular information predictive of “late” recurrence (de novo carcinogenesis) was also identified [245]. These data may provide potential targets for secondary and/or tertiary prevention to be evaluated in relevant animal models.
Animal models of HCC development
Chemically-induced models of HCC
A series of antioxidants and phytochemicals have been tested on rodent models treated with chemicals such as diethylnitrosamine (DEN), dimethylnitrosamine (DMN), thioacetamide (TAA), carbon tetrachloride (CCl4), and aflatoxin to induce HCC [266]. These chemicals are usually administered in drinking water, by oral gavage, by inhaled gases or by intraperitoneal injection.
DEN is a genotoxic chemical carcinogen that causes HCC formation in both mice and rats with a big diversity in the protocol, e.g., dosage, age, sex, and strain of the animals. For example, in mice, a single bolus injection of DEN into a 15-day-old mice, when hepatocyes are still proliferating, caused HCC development in 10 months [316]. DEN has traditionally been used in the “two-step” (initiation/promotion) model of hepatocarcinogensis whereby DEN is administered at a relatively high dose (typically ~200 mg/kg) as an initiating agent and phenobarbital or 2-acetylaminoflouren (2-AAF) is given as the promoter. Limitations of the “two-step” approach include (i) HCC development is slow ranging from 12 to 18 months, and (ii) HCC tumors do not develop in a setting of cirrhosis as is typically observed in human. Repeated administration of DEN however induces cirrhosis as well as HCC [323]. Interestingly, HCCs that develop in response to DEN in mice have a global gene expression signature that is similar to a molecular subclass of human HCC with poor prognosis [324,325].
DEN-treated rodent models have been frequently used to examine potential preventive agents [16,266,267]. C3H/HeN mice fed a 0.2% curcumin-containing diet had a reduction in HCC multiplicity and incidence induced by DEN [326]. Acyclic retinoid inhibited liver tumorigenesis induced by DEN given in drinking water at 40 ppm for 2 weeks in C57BLKS/J- +Lepr(db)/+Lepr(db) obese mice [327]. The synthetic retinoid, peretinoin, inhibited carcinogenesis dose-dependently by reducing TGF-alpha expression in the surrounding liver tissue and HCC in DEN rats [328]. Peretinoin also inhibited proliferation of TGF-alpha-expressing oval-like cells and activated HSCs in 3’-methyl-4-dimethylaminoazobenzene (3’-MeDAB)-treated rats [329]. Resveratrol dose-dependently reduced total number and multiplicity of tumor nodules in “two-step” DEN rats [330]. A small molecule EGFR tyrosine kinase inhibitor, gefitinib, suppressed growth of HCC tumors initiated by DEN [258].
Genetically engineered models of HCC – transgenic models
In TGF-alpha transgenic CD1 mice under the control of the metallothionein promoter, around 75% of the mice developed multifocal, well-differentiated HCCs after 12 to 15 months [331]. The surrounding liver tissue in this model exhibited little fibrosis or steatosis and therefore was not very reminiscent of human disease. All CD2F1 transgenic mice that express a secretable form of EGF developed HCC by 7.1 months [332]. In these mice, combination of the Myc gene with either TGF-alpha or EGF dramatically increased the development of HCC, although Myc transgene alone developed only hepatocellular adenomas [333].
HCC tumors in double Myc TGF-alpha transgenic mice were characterized by high genetic instability, high proliferation rates, low levels of beta-catenin alterations, and a gene expression pattern most similar to that of poorer survival group of human HCC [324]. Myc also enhanced HCC development in E2F1 transgenic mice. Single transgenic mice carrying the E2F1 gene under the albumin promoter developed HCC at a 33% incidence after 12 months [334], but when combined with Myc under the metallothionein promoter, HCC developed in 100% of mice after 9 months [335]. Interestingly, Myc E2F1 transgenic mice developed HCC that had very little genetic instability, low proliferation rates, activation of beta-catenin and a gene expression pattern most similar to those of better survival group of human HCC [324]. Therapeutic delivery of miR-26a in Myc transgenic mice reduced proliferation of liver cancer cells and induced tumor-specific apoptosis [73]. These models might be useful to study how to prevent “early” recurrence from each of the human HCC tumor subclasses.
miR-221 is up-regulated in 70-80% of HCCs [336] where its expression is correlated with higher tumor stage and metastasis [337]. In miR-221 transgenic B6D2F2 mice under the control of the alpha1 anti-trypsin promoter, 50% of males develop HCCs after 9 months [338]. Administration of DEN caused tumorigenesis in both transgenic and wild-type male mice after 6 months, but the transgenic mice developed more and larger tumors. Interestingly, no spontaneous tumors were observed in female transgenic mice, but administration of DEN did cause tumor development in the transgenic females, but not the wild-type controls, after 9 months. Tumor development in transgenic animals could be inhibited with anti-miR-221 oligonucleotides.
The fibroblast growth factor (FGF) pathway has recently been implicated in HCC [339] and a dual VEGFR and FGF receptor (FGFR) inhibitor, brivanib, is being tested in clinical trials of HCC. Interestingly, transgenic FVB mice overexpressing FGF19 predominantly in skeletal muscle under the control of the myosin light chain promoter develop HCC in 10 months [340]. FGF19 uniquely binds to FGFR4 so the progeny of FGF19 transgenic mice bred with FGFR4 knockout mice do not develop HCC [341]. Since FGF19 expression is associated with poor prognosis in human HCC patients [342], this might be a useful model for future studies as well.
Genetically engineered models of HCC – knockout models
Underscoring the role of inflammation in HCC development, inhibitory kappa B kinase (IKK) subunit IKK-gamma (NEMO)-mediated NF-kB activation was shown to prevent spontaneous development of steatohepatitis and HCC [343]. Liver parenchyma-specific deletion of NEMO caused NASH and HCC. These authors have also shown that MAP3-kinase TGF-beta-activated kinase 1 (TAK1) suppressed hepatocarcinogenesis by activating NF-kappaB [344]. Interestingly, in mice with liver parenchyma-specific deletion of TAK1, NEMO promoted HCC development independent of NF-kappaB.
Hepatocyte-specific knockout of Pten induced perisinusoidal fibrosis and steatohepatitis characterized by macrovesicular steatosis, ballooning hepatocytes and lobular inflammatory cell infiltration after 40 weeks and HCC after 78 weeks in mice [345,346]. When the mice were fed a 5% eicosapentaenoic acid (EPA)-supplemented standard chow, steatohepatitis was ameliorated and HCC development was decreased [347]. Since PTEN loss activates AKT, AKT inhibitors could also be tested for their efficacy in inhibiting HCC development in this model.
Gender disparity of HCC development in mice treated with DEN was due to decreased IL-6 production by Kupffer cells in females, which may also explain why HCC incidence is higher in male patients [348]. Male and female IL6 knockout mice develop HCC at roughly the same rate and estrogen treatment reduces IL-6 production by Kupffer cells in male mice treated with DEN. Knockout of Dicer1 induced impaired hepatocyte survival and hepatocarcinogenesis in cooperation with additional oncogenic stimuli in mice [349].
The Hippo-Lats-Yorkie pathway is linked to tissue overgrowth and tumorigenesis in Drosophila. Mst1 and Mst2, the mammalian Hippo orthologs, are constitutively active in normal mouse liver where they suppress proliferative signals from Yap1, the mammalian Yorkie ortholog. The inhibitory Ser127 phosphorylation of Yap1 was lost in Mst1/2 knockout mice causing liver overgrowth and HCC. Mst1/2 controlled Yap1 phosphorylation through a kinase other than Lats1/2 in the mouse liver. Mst1 was more important in suppressing HCC development as HCCs developed in all of Mst1−/−Mst2+/− mice after 15 months, whereas HCCs developed in 25% of Mst1+/− Mst2−/− mice. Loss of activated Mst1 was also observed in human HCC [350].
Liver stem/progenitor cells (historically called oval cells) are also important in the development of HCC. Liver-specific deletion of the neurofibromatosis type 2 (Nf2 or Merlin) tumor suppressor gene caused massive expansion of progenitor cells throughout the liver which eventually developed into HCCs and cholangiocarcinomas. Despite the link between Nf2 and the Hippo-Lats-Yorkie pathway in Drosophila, Nf2 is not a regulator of Yap1 in liver progenitor cells. Proliferation of Nf2−/− liver progenitor cells was instead driven by increased EGFR activation. Therefore, Nf2 knockout mice might be another model to study the preventive effects of EGFR inhibitors [351].
Inactivation of the tumor suppressor KLF6 was associated with decreased survival in HCC patients. In consistent, KLF6+/− mice developed significantly more tumors in response to DEN. Downregulation of KLF6 was associated with increased Mdm2 expression and decreased p53 expression in both human and mouse tumors establishing a link between the KLF6 and p53 tumor suppressors [325].
The Farnesoid X receptor (FXR) belongs to a nuclear hormone receptor superfamily and plays a vital role in liver metabolism. Its expression has been reported to be down-regulated in human HCC [352] and both male and female FXR knockout C57BL/6 mice develop HCCs after 15 months [353]. Further analysis of these animals demonstrated progressive liver disease preceding HCC development that was characterized by steatosis after 6 months, inflammatory infiltration after 9 months and fibrosis between 9 and 12 months [352]. Not surprisingly, gene expression analysis demonstrated metabolic malfunction in these animals. This model seems particularly suitable for prevention studies given the progressive nature of disease.
Diet-induced models of HCC and genetic models of NAFLD and HCC
Choline-deficient diets are known to cause steatosis, fibrosis and HCC. A methionine and choline-deficient (MCD) diet causes periportal steatosis, hepatocyte necrosis and oval cell proliferation in both mice and rats [315]. However, cirrhosis and HCC develop infrequently in rodents fed this diet so its utility for HCC prevention studies may be limited. A choline-deficient, L-amino acid-defined (CDAA) diet leads to fatty liver and HCC development more reliably and has been used in rats to study NASH [354]. Hepatocyte death and regeneration are observed after two weeks and oval cell proliferation begins after 4 weeks. Bridging fibrosis results from stellate cell activation and borderline cirrhosis is recognized by 12 weeks with frank cirrhosis developing by 30 weeks. HCCs start to develop around 30 weeks and reach 100% incidence by 52 weeks [355]. N-(4-hydroxyphenyl)retinamide (4-HPR) decreased the number and size of placental GST-P-positive foci [356] and acetylsalicylic acid (ASA, aspirin) decreases the number and size of gamma-glutamyltransferase (GGT)-positive nodules [357], although direct measurement of tumor formation is more ideal than these surrogate markers [266].
Neither 4-HPR nor ASA had any effect on steatosis in the CDAA model, indicating studies aimed at determining whether inhibiting steatohepatitis can prevent HCC development are still needed. One disadvantage though is the amount of time it takes for HCC to develop. Adult Sprague-Dawley rats fed a choline-deficient, high trans-fat diet with DEN in drinking water, however, developed steatohepatitis, cirrhosis and HCC in just 16 weeks [358]. Recent studies have revealed important role of dysregulated miRNA expression in HCC development in CDAA-fed mice: TGF-beta-mediated upregulation of miR-181b promoted hepatocarcinogenesis by targeting TIMP3 [359], and miR-155, miR-221/222, and miR-21 were up-regulated and miR-122 was down-regulated at early stages of hepatocarcinogenesis [360]. A methyl-deficient diet caused down-regulation of miR-34a, miR-127, and miR-16a, assumedly targeting E2F3, NOTCH1, BCL6, ZFHX1B, and BCL2, as early changes in the process of carcinogenesis in rats [361].
Several genetic factors predisposing to NAFLD/NASH have also been studied in rodent models, including deletion of leptin (ob/ob mice), agouti gene (KK-Ay/a mice), SREBP-1c, IKK-gamma (NEMO), and PTEN [343,362-366]. Acyl-CoA oxidase knockout mice developed steatohepatitis and HCC by 15 months [367]. HCC tumors in Acox1−/− mice had gene expression patterns that were not very similar to human HCC [324]. Both dietary and genetic obesity promoted DEN-induced HCC in mice through induction of IL6 and TNF and increased hepatic inflammation and oncogenic Stat3 activation [368]. Deletion of glycine N-methyltransferase (GNMT), involved in S-adenosylmethionine (SAM) catabolism, induced fatty liver and fibrosis in mice, which was prevented by nicotinamide (NAM) [369].
Animal models of liver fibrosis
Animal models of liver fibrosis could be valuable tools to study carcinogenic microenvironment in the liver (“field effect”). However, commonly used rodent models do not completely mimic liver fibrosis and formation of cirrhosis as seen in human with respect to the pattern and amount of extracellular matrix deposition and alterations in the vascular and histological architecture of the liver. Therefore, it is advisable that promising therapies be tested in multiple mechanistically distinct models in order to exclude model-specific artifacts [10,194].
Models of parenchymal fibrosis
Hepatic toxins such as CCl4, TAA, and DEN induce liver injury, hepatocyte necrosis, and regeneration that lead to panlobular parenchymal liver fibrosis [194,315]. CCl4-treated animals were originally intended to study HCC development, but they are more frequently used today as a model of fibrosis. The severe hepatocyte necrosis observed in this model is not reminiscent of human chronic liver disease; however, the fibrosis observed closely resembles the pattern observed in human [194]. Sunitinib given daily at 40 mg/kg could decrease inflammation, HSC activation and fibrosis in Wistar rats treated with inhalation of CCl4 [370]. Similarly, the green tea polyphenol, EGCG, decreased liver injury, HSC activation and fibrosis in mice and rats treated with CCl4 [371,372]. These studies were not carried out long enough to assess the effect of sunitinib and EGCG on HCC development. EGCG given in drinking water inhibited spontaneous hepatocarcinogenesis in C3H/HeNCrj mice [289].
Repeated exposure to low-dose DEN elicits a chronic liver disease that progresses similar to humans. DEN administered weekly at 50 mg/kg caused inflammation after 2 weeks and periportal fibrosis with occasional bridging fibrosis after 8 weeks. Regenerative nodules started to form after 10 weeks and distinct cirrhosis was apparent after 12 weeks and progressed to HCC after 14 weeks [373]. The low-dose administration of carcinogen could also be regarded as better mimicking actual clinical setting [15].
Models of biliary fibrosis
Extrahepatic bile duct ligation (BDL) has been used to generate biliary fibrosis/cirrhosis in rodent. Mortality is high in the BDL model and therefore it is relatively difficult to assess the effect of intervention on end-stage cirrhosis complications including carcinogenesis. Mdr2 (Abcb4, mouse homolog of the human MDR3) −/− mice accumulated bile salts in intrahepatic bile ducts and developed biliary fibrosis as a result of cholangiocyte proliferation after 4 weeks [374,375]. Fibrosis progression in this model resembled human primary sclerosing cholangitis, and tumor developed after 8-10 months. In BDL model, a multikinase inhibitor sorafenib given at 40 mg/kg daily has been shown to reduce inflammation and decrease intrahepatic fibrosis [376]. Similarly, rapamycin given at 0.5 mg/kg daily decreased ECM accumulation and activated HSCs [377]. Alpha-naphthyl-isothiocyanate (ANIT)-treated rat is another model of biliary fibrosis [378].
Genetically engineered models of liver fibrosis
Liver-targeted TGF-beta1 transgenic mice developed fibrosis after 12 weeks, but not HCC [379]. However, when given TAA in drinking water at 300 mg/L, HCC incidence increased from 40% in wild-type controls to 100% [380]. PDGF-C transgenic mouse model more resembled disease progression in human: the model exhibited HSC activation after 3-5 weeks and steatosis and fibrosis by 9 months. The pericellular and perivenular fibrosis in this model was reminiscent of alcoholic liver disease and NASH in human, and preneoplastic foci developed after 6 months, and 80% of the mice had HCC by 12 months [211]. However, little hepatocyte injury in this model as demonstrated by the low levels of serum aminotransferases and absence of cirrhosis and regenerative nodule may be limitations. PDGF-B transgenic mice similarly developed hepatic fibrosis, but not HCC [210]. One concern on these models is the transgenes were expressed in hepatocytes, whereas physiologically they are mainly produced by activated HSCs and inflammatory cells [194].
Senescence of activated HSC is known to limit the development of liver fibrosis [231,381]. Interestingly, IL-22 transgenic C57BL/6 mice were protected from CCL4-induced liver fibrosis through increased senescence of HSC [382]. IL-22 induces senescence by binding to its receptors, IL-10R2 and IL-22R1, on HSC and activating STAT3 and up-regulating SOCS3 which lead to increased expression of p53. The ability of compounds that induce HSC senescence to prevent HCC development and not just liver fibrosis remains to be tested.
Animal models to assess tumor microenvironment
Orthotopic xenograft models may allow studying tumor microenvironment that affects biological behavior of the tumor such as growth and intra- and extra-hepatic metastasis [383]. Genomic studies have identified common molecular subclasses of primary human HCC tumors that are linked to certain molecular abnormalities and associated with clinical outcome such as survival and tumor recurrence after surgery [69,384], some of which seem to be preserved in hepatoma cell lines [385-387]. Xenograft of these cells therefore may present an opportunity to study molecular subclass-specific prevention targets. Fresh human HCC samples can also be orthotopically implanted into the livers of BALB/c nude mice [388]. After 10 days, the resultant HCC tumors were resected and the mice were treated with or without sorafenib daily at 40 mg/kg. Sorafenib suppressed postsurgical intrahepatic recurrences and prolonged postoperative survival.
It is also critical to model cellular and tissue context in the liver, in which tumors develop. An orthotopic mouse model, whereby mouse hepatoma cells Hepa129 were implanted in syngenic C3H mice, was treated with or without intraperitoneal injections of TAA and oral alcohol to induce liver fibrosis [389]. Tumors that developed in the fibrotic livers were 3.7-fold larger than those in non-fibrotic controls, suggests that underlying fibrotic disease has dramatic effects on tumor progression.
CLINICAL CHALLENGE AND FUTURE PERSPECTIVE
Despite all of these potential preventive approaches, very few of them have been moved to clinical evaluation assumedly due to several obstacles (Table 2). First, clinical trials of preventive therapies may be less attractive to the pharmaceutical industry due to their longer length and the requirement for a larger trial size because a slower occurrence of clinical endpoint is expected compared to trials of chemotherapeutic drugs. In addition, the requirement for less toxicity and cost to enable long-term administration for mostly asymptomatic individuals further raises the bar. As a potential solution in general, novel trial design, namely “exploratory investigational new drug studies” or “phase 0” clinical trials, have been proposed [390,391]. Particularly in HCC prevention, it seems to be reasonable to start the assessment of promising therapy in the setting of tertiary prevention because HCC recurrence is more frequent than initial HCC occurrence [7,95]. In fact, many experimental approaches, e.g., interferon, retinoid, radioembolization, and adoptive immune therapy, were first tested as tertiary prevention therapies. In addition, higher toxicity and cost may be more justifiable compared to the situation of secondary prevention because the participants are regarded as “cancer” patients.
Second, clinical trial endpoints are uncertain especially for antifibrosis therapies. This has extra relevance because liver fibrosis-related trial endpoints may be used as surrogate markers of HCC occurrence in HCC prevention trials. Liver biopsy has been regarded as gold standard in the evaluation of hepatic fibrosis, but the problem of sampling variability and invasiveness are considered critical drawbacks [10,392,393]. Clear and widely applicable definition of clinical endpoint, as was proposed for assessment of anti-HCC drugs, is urgently needed to establish the drug development pipeline for HCC prevention [10,394]. Surrogate biomarker of clinical outcome will be extremely useful. For example, in the field of antifibrosis drug development, substantial efforts have been taken to develop non-invasive methods, including ultrasound-based elastography, laboratory test-based scoring methods, magnetic resonance spectroscopy, and metabolic breath test, some of which are under clinical evaluation [395,396]. A panel of 7 genetic polymorphisms (Cirrhosis Risk Score) was shown to be associated with the risk of fibrosis progression in male Caucasian patients with mild chronic hepatitis C [397,398]. However, none of them has been reliably connected with long-term clinical outcomes such as HCC development (most relevant endpoint in HCC prevention) and cirrhosis complication, hepatic decompensation, and death. Genomic profiling of archived tissue specimen has been suggested as a promising approach to overcome this issue by directly linking molecular information with long-term clinical outcomes [244,245]. Although such tissue-based approach will require invasive tissue acquisition procedure, one may argue that it is well rationalized in establishing the therapy's benefit in the setting of a clinical trial [269].
One practical issue in tertiary prevention is how to distinguish between “early” and “late” recurrences, either of which is assumed to be targeted by each specific therapeutic approach. Genetic assessment of tumor clonality will provide more reliable biological evidence for this distinction, although no such test is clinically available [245,399-401]. Clinical studies have suggested that radiological and/or histological assessment perform reasonably well to discriminate “early” recurrence (intrahepatic metastasis of treated primary tumor) form “late” recurrence (de novo multicentric cancer) based on the assumption that the latter is hypovascular and well-differentiated while tumor size is small [402,403]. Multidisciplinary efforts to establish standardized clinical endpoints for HCC preventive trials will also need to address this type of clinically assessable endpoint.
Third, there is no systematic way to reassess antiviral or antifibrotic drugs that did not meet their primary endpoint (i.e., complete viral clearance or resolution of fibrosis) for their potential value as non-curative preventive therapy. A point of trade-off between effectiveness and toxicity/cost of the drugs will be different between applications such as antiviral/antifibrotic and preventive therapies. Such a system may facilitate efficient allocation/relocation of drugs in the management of the entire clinical course of chronic liver diseases aiming at controlling critical outcomes such as HCC development and death.
CONCLUSION
HCC prevention involves almost all fields of clinical and basic hepatology research. Therefore, efficient and functional integration of multidisciplinary efforts will be the key in translating basic science-oriented discovery to clinical practice. To this end, it will be extremely important to establish widely applicable clinical trial endpoint(s) and develop reliable biomarkers to measure clinical outcome.
ACKNOWLEDGEMENTS
Yujin Hoshida is supported by European Commission's 7th Framework Programme (FP7-Health 2010, Heptromic), Bryan C. Fuchs is supported by a K-award from NCI (5 K01CA140861).
ABBREVIATIONS
- AFB1
aflatoxin B1
- alpha-SMA
alpha smooth muscle actin
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- CTGF
connective tissue growth factor
- DAA
direct acting antiviral
- DEN
diethylnitrosamine
- DMN
dimethylnitrosamine
- ECM
extracellular matrix
- EGF
epidermal growth factor
- GH
genetic hemochromatosis
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- H-DN
high-grade dysplastic nodule
- HGF
hepatocyte growth factor
- HMF
hepatic myofibroblast
- HSC
hepatic stellate cell
- IKK
inhibitory kappa B kinase
- iNOS
inducible nitric oxide synthase
- JNK
C-Jun NH2-terminal kinase
- L-DN
low-grade dysplastic nodule
- MAPK
mitogen-activated protein kinase
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NF-kappaB
nuclear factor kappa B
- PDGF
platelet-derived growth factor
- PPAR-gamma
peroxisome proliferator-activated receptor gamma
- RAR
retinoic acid receptor
- ROS
reactive oxigen species
- TAA
thioacetamide
- TACE
transarterial chemoembolization
- TARE
transarterial radioembolization
- TGF
transforming growth factor
- UDCA
ursodeoxycholic acid
- VEGF
vascular endothelial growth factor
Footnotes
Okita et al., ASCO Meeting Abstract 2010, 28, 4024
CONFLICT OF INTEREST
Declared none.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 521, e511–516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 6.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S, Nishiguchi S, Kuroki T, Imazeki F, Yokosuka O, Kinoyama S, Yamada G, Omata M. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174–181. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 200848(Suppl 1):S20–37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 15.Sporn MB, Suh N. Chemoprevention: an essential approach to controlling cancer. Nat Rev Cancer. 2002;2:537–543. doi: 10.1038/nrc844. [DOI] [PubMed] [Google Scholar]
- 16.Morgan TR. Chemoprevention of hepatocellular carcinoma in chronic hepatitis C. Recent Results Cancer Res. 2011;188:85–99. doi: 10.1007/978-3-642-10858-7_7. [DOI] [PubMed] [Google Scholar]
- 17.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr., Baker LH, Coltman CA., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 19.Dore DD, Lapane KL, Trivedi AN, Mor V, Weinstock MA. Association between statin use and risk for keratinocyte carcinoma in the veterans affairs topical tretinoin chemoprevention trial. Ann Intern Med. 2009;150:9–18. doi: 10.7326/0003-4819-150-1-200901060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu MC, Yuan JM. Environmental factors and risk for hepatocellular carcinoma. Gastroenterology. 2004;127:S72–78. doi: 10.1016/j.gastro.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Kremsdorf D, Soussan P, Paterlini-Brechot P, Brechot C. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene. 2006;25:3823–3833. doi: 10.1038/sj.onc.1209559. [DOI] [PubMed] [Google Scholar]
- 22.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, Liaw YF, Chen CJ. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134–1143. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livingston SE, Simonetti JP, McMahon BJ, Bulkow LR, Hurlburt KJ, Homan CE, Snowball MM, Cagle HH, Williams JL, Chulanov VP. Hepatitis B virus genotypes in Alaska Native people with hepatocellular carcinoma: preponderance of genotype F. J Infect Dis. 2007;195:5–11. doi: 10.1086/509894. [DOI] [PubMed] [Google Scholar]
- 25.Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40. doi: 10.1038/nrgastro.2009.205. [DOI] [PubMed] [Google Scholar]
- 26.Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson IM, Davis GL, El-Serag H, Negro F, Trepo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8:924–933. doi: 10.1016/j.cgh.2010.06.032. quiz e117. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N, Kumada H. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–938. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 29.Ishiguro S, Inoue M, Tanaka Y, Mizokami M, Iwasaki M, Tsugane S. Impact of viral load of hepatitis C on the incidence of hepatocellular carcinoma: A population-based cohort study (JPHC Study). Cancer Lett. 2011;300:173–179. doi: 10.1016/j.canlet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Lee MH, Yang HI, Lu SN, Jen CL, Yeh SH, Liu CJ, Chen PJ, You SL, Wang LY, Chen WJ, Chen CJ. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol. 2010;28:4587–4593. doi: 10.1200/JCO.2010.29.1500. [DOI] [PubMed] [Google Scholar]
- 31.Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 32.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 33.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 34.Fan JG, Farrell GC. Prevention of hepatocellular carcinoma in nonviral-related liver diseases. J Gastroenterol Hepatol. 2009;24:712–719. doi: 10.1111/j.1440-1746.2009.05776.x. [DOI] [PubMed] [Google Scholar]
- 35.Kew MC. Prevention of hepatocellular carcinoma. Ann Hepatol. 2010;9:120–132. [PubMed] [Google Scholar]
- 36.La Vecchia C. Alcohol and liver cancer. Eur J Cancer Prev. 2007;16:495–497. doi: 10.1097/CEJ.0b013e3280145b5d. [DOI] [PubMed] [Google Scholar]
- 37.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 38.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 39.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Elmberg M, Hultcrantz R, Ekbom A, Brandt L, Olsson S, Olsson R, Lindgren S, Loof L, Stal P, Wallerstedt S, Almer S, Sandberg-Gertzen H, Askling J. Cancer risk in patients with hereditary hemochromatosis and in their first-degree relatives. Gastroenterology. 2003;125:1733–1741. doi: 10.1053/j.gastro.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Ko C, Siddaiah N, Berger J, Gish R, Brandhagen D, Sterling RK, Cotler SJ, Fontana RJ, McCashland TM, Han SH, Gordon FD, Schilsky ML, Kowdley KV. Prevalence of hepatic iron overload and association with hepatocellular cancer in end-stage liver disease: results from the National Hemochromatosis Transplant Registry. Liver Int. 2007;27:1394–1401. doi: 10.1111/j.1478-3231.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 42.Fujita N, Sugimoto R, Ma N, Tanaka H, Iwasa M, Kobayashi Y, Kawanishi S, Watanabe S, Kaito M, Takei Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008;15:498–507. doi: 10.1111/j.1365-2893.2008.00972.x. [DOI] [PubMed] [Google Scholar]
- 43.Furutani T, Hino K, Okuda M, Gondo T, Nishina S, Kitase A, Korenaga M, Xiao SY, Weinman SA, Lemon SM, Sakaida I, Okita K. Hepatic iron overload induces hepatocellular carcinoma in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2006;130:2087–2098. doi: 10.1053/j.gastro.2006.02.060. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe KK, Lemoine A, Finkelstein DM, Kawasaki H, Fujii T, Chung RT, Lauwers GY, Kulu Y, Muzikansky A, Kuruppu D, Lanuti M, Goodwin JM, Azoulay D, Fuchs BC. Epidermal growth factor gene functional polymorphism and the risk of hepatocellular carcinoma in patients with cirrhosis. Jama. 2008;299:53–60. doi: 10.1001/jama.2007.65. [DOI] [PubMed] [Google Scholar]
- 45.Abu Dayyeh BK, Yang M, Fuchs BC, Karl DL, Yamada S, Sninsky JJ, O'Brien TR, Dienstag JL, Tanabe KK, Chung RT. A Functional Polymorphism in the Epidermal Growth Factor Gene Is Associated With Risk for Hepatocellular Carcinoma. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Zhai Y, Hu Z, Wu C, Qian J, Jia W, Ma F, Huang W, Yu L, Yue W, Wang Z, Li P, Zhang Y, Liang R, Wei Z, Cui Y, Xie W, Cai M, Yu X, Yuan Y, Xia X, Zhang X, Yang H, Qiu W, Yang J, Gong F, Chen M, Shen H, Lin D, Zeng YX, He F, Zhou G. Genome-wide association study identifies 1p36.22 as a new susceptibility locus for hepatocellular carcinoma in chronic hepatitis B virus carriers. Nat Genet. 2010;42:755–758. doi: 10.1038/ng.638. [DOI] [PubMed] [Google Scholar]
- 47.Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H, Koike K, Kamatani N, Kubo M, Nakamura Y, Matsuda K. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455–458. doi: 10.1038/ng.809. [DOI] [PubMed] [Google Scholar]
- 48.Miki D, Ochi H, Hayes CN, Abe H, Yoshima T, Aikata H, Ikeda K, Kumada H, Toyota J, Morizono T, Tsunoda T, Kubo M, Nakamura Y, Kamatani N, Chayama K. Variation in the DEPDC5 locus is associated with progression to hepatocellular carcinoma in chronic hepatitis C virus carriers. Nat Genet. 2011 doi: 10.1038/ng.876. [DOI] [PubMed] [Google Scholar]
- 49.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, Bomford A, O'Grady JG, Harrison PM, Heneghan MA. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for follow-up and screening. Hepatology. 2008;48:863–870. doi: 10.1002/hep.22432. [DOI] [PubMed] [Google Scholar]
- 51.Villanueva A, Newell P, Hoshida Y. Inherited hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2010;24:725–734. doi: 10.1016/j.bpg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C, Morlat P, Cacoub P, Chene G. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalite 2005 study. J Hepatol. 2009;50:736–745. doi: 10.1016/j.jhep.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Sherman M. Recurrence of hepatocellular carcinoma. N Engl J Med. 2008;359:2045–2047. doi: 10.1056/NEJMe0807581. [DOI] [PubMed] [Google Scholar]
- 55.Yasui H, Hino O, Ohtake K, Machinami R, Kitagawa T. Clonal growth of hepatitis B virus-integrated hepatocytes in cirrhotic liver nodules. Cancer Res. 1992;52:6810–6814. [PubMed] [Google Scholar]
- 56.Aihara T, Noguchi S, Sasaki Y, Nakano H, Imaoka S. Clonal analysis of regenerative nodules in hepatitis C virus-induced liver cirrhosis. Gastroenterology. 1994;107:1805–1811. doi: 10.1016/0016-5085(94)90824-9. [DOI] [PubMed] [Google Scholar]
- 57.Ochiai T, Urata Y, Yamano T, Yamagishi H, Ashihara T. Clonal expansion in evolution of chronic hepatitis to hepatocellular carcinoma as seen at an X-chromosome locus. Hepatology. 2000;31:615–621. doi: 10.1002/hep.510310311. [DOI] [PubMed] [Google Scholar]
- 58.Kawai H, Suda T, Aoyagi Y, Isokawa O, Mita Y, Waguri N, Kuroiwa T, Igarashi M, Tsukada K, Mori S, Shimizu T, Suzuki Y, Abe Y, Takahashi T, Nomoto M, Asakura H. Quantitative evaluation of genomic instability as a possible predictor for development of hepatocellular carcinoma: comparison of loss of heterozygosity and replication error. Hepatology. 2000;31:1246–1250. doi: 10.1053/jhep.2000.7298. [DOI] [PubMed] [Google Scholar]
- 59.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 60.McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29:222–232. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- 61.Koike K. Hepatitis C virus contributes to hepatocarcinogenesis by modulating metabolic and intracellular signaling pathways. J Gastroenterol Hepatol. 2007;22(Suppl 1):S108–111. doi: 10.1111/j.1440-1746.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- 62.Oh BK, Jo Chae K, Park C, Kim K, Jung Lee W, Han KH, Nyun Park Y. Telomere shortening and telomerase reactivation in dysplastic nodules of human hepatocarcinogenesis. J Hepatol. 2003;39:786–792. doi: 10.1016/s0168-8278(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 63.Plentz RR, Park YN, Lechel A, Kim H, Nellessen F, Langkopf BH, Wilkens L, Destro A, Fiamengo B, Manns MP, Roncalli M, Rudolph KL. Telomere shortening and inactivation of cell cycle checkpoints characterize human hepatocarcinogenesis. Hepatology. 2007;45:968–976. doi: 10.1002/hep.21552. [DOI] [PubMed] [Google Scholar]
- 64.Yamada T, De Souza AT, Finkelstein S, Jirtle RL. Loss of the gene encoding mannose 6-phosphate/insulin-like growth factor II receptor is an early event in liver carcinogenesis. Proc Natl Acad Sci U S A. 1997;94:10351–10355. doi: 10.1073/pnas.94.19.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higashitsuji H, Itoh K, Nagao T, Dawson S, Nonoguchi K, Kido T, Mayer RJ, Arii S, Fujita J. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med. 2000;6:96–99. doi: 10.1038/71600. [DOI] [PubMed] [Google Scholar]
- 66.Higashitsuji H, Nagao T, Nonoguchi K, Fujii S, Itoh K, Fujita J. A novel protein overexpressed in hepatoma accelerates export of NF-kappa B from the nucleus and inhibits p53-dependent apoptosis. Cancer Cell. 2002;2:335–346. doi: 10.1016/s1535-6108(02)00152-6. [DOI] [PubMed] [Google Scholar]
- 67.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 68.Farazi PA, DePinho RA. The genetic and environmental basis of hepatocellular carcinoma. Discov Med. 2006;6:182–186. [PubMed] [Google Scholar]
- 69.Hoshida Y, Toffanin S, Lachenmayer A, Villanueva A, Minguez B, Llovet JM. Molecular Classification and Novel Targets in Hepatocellular Carcinoma: Recent Advancements. Semin Liver Dis. 2010;30:35–51. doi: 10.1055/s-0030-1247131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E, Grazi GL, Croce CM, Bolondi L, Negrini M. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 71.Kojima K, Takata A, Vadnais C, Otsuka M, Yoshikawa T, Akanuma M, Kondo Y, Kang YJ, Kishikawa T, Kato N, Xie Z, Zhang WJ, Yoshida H, Omata M, Nepveu A, Koike K. MicroRNA122 is a key regulator of alpha-fetoprotein expression and influences the aggressiveness of hepatocellular carcinoma. Nat Commun. 2011;2:338. doi: 10.1038/ncomms1345. [DOI] [PubMed] [Google Scholar]
- 72.Zeng C, Wang R, Li D, Lin XJ, Wei QK, Yuan Y, Wang Q, Chen W, Zhuang SM. A novel GSK-3 beta-C/EBP alpha-miR-122-insulin-like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma. Hepatology. 2010;52:1702–1712. doi: 10.1002/hep.23875. [DOI] [PubMed] [Google Scholar]
- 73.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Desmet VJ. East-West pathology agreement on precancerous liver lesions and early hepatocellular carcinoma. Hepatology. 2009;49:355–357. doi: 10.1002/hep.22681. [DOI] [PubMed] [Google Scholar]
- 75.Sakamoto M. Early HCC: diagnosis and molecular markers. J Gastroenterol. 2009;44(Suppl 19):108–111. doi: 10.1007/s00535-008-2245-y. [DOI] [PubMed] [Google Scholar]
- 76.Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 77.Nam SW, Lee JH, Noh JH, Lee SN, Kim SY, Lee SH, Park CK, Ahn YM, Park WS, Yoo NJ, Lee JY. Comparative analysis of expression profiling of early-stage carcinogenesis using nodule-in-nodule-type hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2006;18:239–247. doi: 10.1097/00042737-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Nam SW, Park JY, Ramasamy A, Shevade S, Islam A, Long PM, Park CK, Park SE, Kim SY, Lee SH, Park WS, Yoo NJ, Liu ET, Miller LD, Lee JY. Molecular changes from dysplastic nodule to hepatocellular carcinoma through gene expression profiling. Hepatology. 2005;42:809–818. doi: 10.1002/hep.20878. [DOI] [PubMed] [Google Scholar]
- 79.Forns X, Sanchez Tapias JM, Pares A, Llovet JM, Bruix J, Rodes J. Expected developments in hepatology. Best Pract Res Clin Gastroenterol. 2002;16:957–970. doi: 10.1053/bega.2002.0341. [DOI] [PubMed] [Google Scholar]
- 80.Craxi A, Camma C. Does chemotherapy prevent HCV-related hepatocellular carcinoma? Cons. Dig Liver Dis. 2010;42(Suppl 3):S287–292. doi: 10.1016/S1590-8658(10)60518-X. [DOI] [PubMed] [Google Scholar]
- 81.Greenberg D, Earle C, Fang CH, Eldar-Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost-utility analyses in oncology. J Natl Cancer Inst. 2010;102:82–88. doi: 10.1093/jnci/djp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kensler TW, Qian GS, Chen JG, Groopman JD. Translational strategies for cancer prevention in liver. Nat Rev Cancer. 2003;3:321–329. doi: 10.1038/nrc1076. [DOI] [PubMed] [Google Scholar]
- 83.Colombo M, Donato MF. Prevention of hepatocellular carcinoma. Semin Liver Dis. 2005;25:155–161. doi: 10.1055/s-2005-871195. [DOI] [PubMed] [Google Scholar]
- 84.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen HL, Chang MH, Ni YH, Hsu HY, Lee PI, Lee CY, Chen DS. Seroepidemiology of hepatitis B virus infection in children: Ten years of mass vaccination in Taiwan. Jama. 1996;276:906–908. [PubMed] [Google Scholar]
- 86.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 87.Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol. 2010;25:657–663. doi: 10.1111/j.1440-1746.2009.06167.x. [DOI] [PubMed] [Google Scholar]
- 88.Turner PC, Sylla A, Gong YY, Diallo MS, Sutcliffe AE, Hall AJ, Wild CP. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study. Lancet. 2005;365:1950–1956. doi: 10.1016/S0140-6736(05)66661-5. [DOI] [PubMed] [Google Scholar]
- 89.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 90.Colombo M. Screening and diagnosis of hepatocellular carcinoma. Liver Int. 2009;29(Suppl 1):143–147. doi: 10.1111/j.1478-3231.2008.01938.x. [DOI] [PubMed] [Google Scholar]
- 91.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 93.Lok AS. Prevention of hepatitis B virus-related hepatocellular carcinoma. Gastroenterology. 2004;127:S303–309. doi: 10.1053/j.gastro.2004.09.045. [DOI] [PubMed] [Google Scholar]
- 94.Lin SM, Yu ML, Lee CM, Chien RN, Sheen IS, Chu CM, Liaw YF. Interferon therapy in HBeAg positive chronic hepatitis reduces progression to cirrhosis and hepatocellular carcinoma. J Hepatol. 2007;46:45–52. doi: 10.1016/j.jhep.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 95.Ikeda K, Kobayashi M, Saitoh S, Someya T, Hosaka T, Akuta N, Suzuki Y, Suzuki F, Tsubota A, Arase Y, Kumada H. Significance of hepatitis B virus DNA clearance and early prediction of hepatocellular carcinogenesis in patients with cirrhosis undergoing interferon therapy: long-term follow up of a pilot study. J Gastroenterol Hepatol. 2005;20:95–102. doi: 10.1111/j.1440-1746.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- 96.Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta-analysis. J Viral Hepat. 2009;16:265–271. doi: 10.1111/j.1365-2893.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 97.Sung JJ, Tsoi KK, Wong VW, Li KC, Chan HL. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28:1067–1077. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- 98.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 99.Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295–1303. [PubMed] [Google Scholar]
- 100.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, Tanikawa K. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 101.Papatheodoridis GV, Papadimitropoulos VC, Hadziyannis SJ. Effect of interferon therapy on the development of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a meta-analysis. Aliment Pharmacol Ther. 2001;15:689–698. doi: 10.1046/j.1365-2036.2001.00979.x. [DOI] [PubMed] [Google Scholar]
- 102.Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, Sood GK. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192–199. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 103.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288. 288, e281. doi: 10.1016/j.cgh.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 104.Velosa J, Serejo F, Marinho R, Nunes J, Gloria H. Eradication of Hepatitis C Virus Reduces the Risk of Hepatocellular Carcinoma in Patients with Compensated Cirrhosis. Dig Dis Sci. 2011 doi: 10.1007/s10620-011-1621-2. [DOI] [PubMed] [Google Scholar]
- 105.Watanabe S, Enomoto N, Koike K, Izumi N, Takikawa H, Hashimoto E, Moriyasu F, Kumada H, Imawari M. Cancer preventive effect of pegylated interferon alpha-2b plus ribavirin in a real-life clinical setting in Japan: PERFECT interim analysis. Hepatol Res. 2011 doi: 10.1111/j.1872-034X.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 106.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A Sustained Virologic Response Reduces Risk of All-Cause Mortality in Patients with Hepatitis C. Clin Gastroenterol Hepatol. 2011 doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 107.Ikeda K, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Arase Y, Murashima N, Chayama K, Kumada H. Long-term interferon therapy for 1 year or longer reduces the hepatocellular carcinogenesis rate in patients with liver cirrhosis caused by hepatitis C virus: a pilot study. J Gastroenterol Hepatol. 2001;16:406–415. doi: 10.1046/j.1440-1746.2001.02450.x. [DOI] [PubMed] [Google Scholar]
- 108.Craxi A, Camma C. Prevention of hepatocellular carcinoma. Clin Liver Dis. 2005;9:329–346. viii. doi: 10.1016/j.cld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 109.Arase Y, Ikeda K, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, Hosaka T, Sezaki H, Yatsuji H, Kawamura Y, Kumada H. Interferon-induced prolonged biochemical response reduces hepatocarcinogenesis in hepatitis C virus infection. J Med Virol. 2007;79:1485–1490. doi: 10.1002/jmv.20925. [DOI] [PubMed] [Google Scholar]
- 110.Kumada H. Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neominophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology. 2002;62(Suppl 1):94–100. doi: 10.1159/000048283. [DOI] [PubMed] [Google Scholar]
- 111.Tarao K, Fujiyama S, Ohkawa S, Miyakawa K, Tamai S, Hirokawa S, Masaki T, Tanaka K. Ursodiol use is possibly associated with lower incidence of hepatocellular carcinoma in hepatitis C virus-associated liver cirrhosis. Cancer Epidemiol Biomarkers Prev. 2005;14:164–169. [PubMed] [Google Scholar]
- 112.van Rossum TG, Vulto AG, de Man RA, Brouwer JT, Schalm SW. Review article: glycyrrhizin as a potential treatment for chronic hepatitis C. Aliment Pharmacol Ther. 1998;12:199–205. doi: 10.1046/j.1365-2036.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 113.Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim HY, Sterling RK, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Morgan TR. Maintenance Peginterferon Therapy and Other Factors Associated With Hepatocellular Carcinoma in Patients With Advanced Hepatitis C. Gastroenterology. 2011 doi: 10.1053/j.gastro.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bruix J, Poynard T, Colombo M, Schiff E, Burak K, Heathcote EJ, Berg T, Poo JL, Mello CB, Guenther R, Niederau C, Terg R, Bedossa P, Boparai N, Griffel LH, Burroughs M, Brass CA, Albrecht JK. Maintenance therapy with peginterferon alfa-2b does not prevent hepatocellular carcinoma in cirrhotic patients with chronic hepatitis C. Gastroenterology. 2011;140:1990–1999. doi: 10.1053/j.gastro.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Di Bisceglie AM, Stoddard AM, Dienstag JL, Shiffman ML, Seeff LB, Bonkovsky HL, Morishima C, Wright EC, Snow KK, Lee WM, Fontana RJ, Morgan TR, Ghany MG, Group H-CT. Excess mortality in patients with advanced chronic hepatitis C treated with long-term peginterferon. Hepatology. 2011;53:1100–1108. doi: 10.1002/hep.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clinical Practice Guidelines for Hepatocellular Carcinoma - The Japan Society ofHepatology 2009 update. Hepatol Res. 2010;40(Suppl 1):2–144. doi: 10.1111/j.1872-034X.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 117.Bonkovsky HL, Naishadham D, Lambrecht RW, Chung RT, Hoefs JC, Nash SR, Rogers TE, Banner BF, Sterling RK, Donovan JA, Fontana RJ, Di Bisceglie AM, Ghany MG, Morishima C. Roles of iron and HFE mutations on severity and response to therapy during retreatment of advanced chronic hepatitis C. Gastroenterology. 2006;131:1440–1451. doi: 10.1053/j.gastro.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 118.Nagaoki Y, Aikata H, Miyaki D, Murakami E, Hashimoto Y, Katamura Y, Azakami T, Kawaoka T, Takaki S, Hiramatsu A, Waki K, Imamura M, Kawakami Y, Takahashi S, Chayama K. Clinical features and prognosis in patients with hepatocellular carcinoma that developed after hepatitis C virus eradication with interferon therapy. J Gastroenterol. 2011 doi: 10.1007/s00535-011-0384-z. [DOI] [PubMed] [Google Scholar]
- 119.Dashwood R, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Chemopreventive properties of chlorophylls towards aflatoxin B1: a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat Res. 1998;399:245–253. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- 120.Kamat JP, Boloor KK, Devasagayam TP. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim Biophys Acta. 2000;1487:113–127. doi: 10.1016/s1388-1981(00)00088-3. [DOI] [PubMed] [Google Scholar]
- 121.Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, Qian GS, Kuang SY, Gange SJ, Jacobson LP, Helzlsouer KJ, Bailey GS, Groopman JD, Kensler TW. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc Natl Acad Sci U S A. 2001;98:14601–14606. doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sudakin DL. Dietary aflatoxin exposure and chemoprevention of cancer: a clinical review. J Toxicol Clin Toxicol. 2003;41:195–204. doi: 10.1081/clt-120019137. [DOI] [PubMed] [Google Scholar]
- 123.Gupta E, Olopade OI, Ratain MJ, Mick R, Baker TM, Berezin FK, Benson AB, 3rd, Dolan ME. Pharmacokinetics and pharmacodynamics of oltipraz as a chemopreventive agent. Clin Cancer Res. 1995;1:1133–1138. [PubMed] [Google Scholar]
- 124.Kensler TW, Egner PA, Wang JB, Zhu YR, Zhang BC, Lu PX, Chen JG, Qian GS, Kuang SY, Jackson PE, Gange SJ, Jacobson LP, Munoz A, Groopman JD. Chemoprevention of hepatocellular carcinoma in aflatoxin endemic areas. Gastroenterology. 2004;127:S310–318. doi: 10.1053/j.gastro.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 125.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yates MS, Kensler TW. Keap1 eye on the target: chemoprevention of liver cancer. Acta Pharmacol Sin. 2007;28:1331–1342. doi: 10.1111/j.1745-7254.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 127.Sofowora GG, Choo EF, Mayo G, Shyr Y, Wilkinson GR. In vivo inhibition of human CYP1A2 activity by oltipraz. Cancer Chemother Pharmacol. 2001;47:505–510. doi: 10.1007/s002800000245. [DOI] [PubMed] [Google Scholar]
- 128.Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, Jacobson LP, Munoz A, Helzlsouer KJ, Groopman JD, Kensler TW. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- 129.Glintborg B, Weimann A, Kensler TW, Poulsen HE. Oltipraz chemoprevention trial in Qidong, People's Republic of China: unaltered oxidative biomarkers. Free Radic Biol Med. 2006;41:1010–1014. doi: 10.1016/j.freeradbiomed.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 130.Dimitrov NV, Bennett JL, McMillan J, Perloff M, Leece CM, Malone W. Clinical pharmacology studies of oltipraz--a potential chemopreventive agent. Invest New Drugs. 1992;10:289–298. doi: 10.1007/BF00944183. [DOI] [PubMed] [Google Scholar]
- 131.Day CP. Treatment of alcoholic liver disease. Liver Transpl. 2007;13:S69–75. doi: 10.1002/lt.21336. [DOI] [PubMed] [Google Scholar]
- 132.Schuppan D, Gorrell MD, Klein T, Mark M, Afdhal NH. The challenge of developing novel pharmacological therapies for non-alcoholic steatohepatitis. Liver Int. 2010;30:795–808. doi: 10.1111/j.1478-3231.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- 133.Niederau C, Fischer R, Purschel A, Stremmel W, Haussinger D, Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 134.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, McLaren CE, Bahlo M, Nisselle AE, Vulpe CD, Anderson GJ, Southey MC, Giles GG, English DR, Hopper JL, Olynyk JK, Powell LW, Gertig DM. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358:221–230. doi: 10.1056/NEJMoa073286. [DOI] [PubMed] [Google Scholar]
- 135.Allen KJ, Nisselle AE, Collins VR, Williamson R, Delatycki MB. Asymptomatic individuals at genetic risk of haemochromatosis take appropriate steps to prevent disease related to iron overload. Liver Int. 2008;28:363–369. doi: 10.1111/j.1478-3231.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 136.Phatak PD, Bonkovsky HL, Kowdley KV. Hereditary hemochromatosis: time for targeted screening. Ann Intern Med. 2008;149:270–272. doi: 10.7326/0003-4819-149-4-200808190-00009. [DOI] [PubMed] [Google Scholar]
- 137.Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y, Takayama T, Niitsu Y. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830–836. doi: 10.1007/s00535-007-2095-z. [DOI] [PubMed] [Google Scholar]
- 138.Price L, Kowdley KV. The role of iron in the pathophysiology and treatment of chronic hepatitis C. Can J Gastroenterol. 2009;23:822–828. doi: 10.1155/2009/290383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T, Ikeda T, Niitsu Y. Normalization of elevated hepatic 8-hydroxy-2'-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697–8702. [PubMed] [Google Scholar]
- 140.Jackson H, Solaymani-Dodaran M, Card TR, Aithal GP, Logan R, West J. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study. Hepatology. 2007;46:1131–1137. doi: 10.1002/hep.21795. [DOI] [PubMed] [Google Scholar]
- 141.Gong Y, Huang Z, Christensen E, Gluud C. Ursodeoxycholic acid for patients with primary biliary cirrhosis: an updated systematic review and meta-analysis of randomized clinical trials using Bayesian approach as sensitivity analyses. Am J Gastroenterol. 2007;102:1799–1807. doi: 10.1111/j.1572-0241.2007.01235.x. [DOI] [PubMed] [Google Scholar]
- 142.Kuiper EM, Hansen BE, Adang RP, van Nieuwkerk CM, Timmer R, Drenth JP, Spoelstra P, Brouwer HT, Kuyvenhoven JP, van Buuren HR. Relatively high risk for hepatocellular carcinoma in patients with primary biliary cirrhosis not responding to ursodeoxycholic acid. Eur J Gastroenterol Hepatol. 2010;22:1495–1502. doi: 10.1097/MEG.0b013e32834059e7. [DOI] [PubMed] [Google Scholar]
- 143.Yeoman AD, Longhi MS, Heneghan MA. Review article: the modern management of autoimmune hepatitis. Aliment Pharmacol Ther. 2010;31:771–787. doi: 10.1111/j.1365-2036.2010.04241.x. [DOI] [PubMed] [Google Scholar]
- 144.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 145.Hoshida Y, Villanueva A, Llovet JM. Molecular profiling to predict hepatocellular carcinoma outcome. Expert Rev Gastroenterol Hepatol. 2009;3:101–103. doi: 10.1586/egh.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamamoto M, Arii S, Sugahara K, Tobe T. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83:336–340. doi: 10.1002/bjs.1800830313. [DOI] [PubMed] [Google Scholar]
- 147.Hasegawa K, Takayama T, Ijichi M, Matsuyama Y, Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44:891–895. doi: 10.1002/hep.21341. [DOI] [PubMed] [Google Scholar]
- 148.Lau WY, Lai EC, Lau SH. The current role of neoadjuvant/adjuvant/chemoprevention therapy in partial hepatectomy for hepatocellular carcinoma: a systematic review. Hepatobiliary Pancreat Dis Int. 2009;8:124–133. [PubMed] [Google Scholar]
- 149.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 150.von Delius S, Lersch C, Mayr M, Stock K, Schulte-Frohlinde E, Schmid RM, Eckel F. Capecitabine for treatment of advanced hepatocellular carcinoma. Hepatogastroenterology. 2007;54:2310–2314. [PubMed] [Google Scholar]
- 151.Izumi R, Shimizu K, Iyobe T, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Postoperative adjuvant hepatic arterial infusion of Lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology. 1994;20:295–301. [PubMed] [Google Scholar]
- 152.Li JQ, Zhang YQ, Zhang WZ, Yuan YF, Li GH. Randomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomy. J Cancer Res Clin Oncol. 1995;121:364–366. doi: 10.1007/BF01225689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ueno S, Tanabe G, Yoshida A, Yoshidome S, Takao S, Aikou T. Postoperative prediction of and strategy for metastatic recurrent hepatocellular carcinoma according to histologic activity of hepatitis. Cancer. 1999;86:248–254. [PubMed] [Google Scholar]
- 154.Van de Wiele C. Radioembolization of hepatocellular carcinoma. Curr Drug Discov Technol. 2010;7:247–252. doi: 10.2174/157016310793360701. [DOI] [PubMed] [Google Scholar]
- 155.Raoul JL, Boucher E, Rolland Y, Garin E. Treatment of hepatocellular carcinoma with intra-arterial injection of radionuclides. Nat Rev Gastroenterol Hepatol. 2010;7:41–49. doi: 10.1038/nrgastro.2009.202. [DOI] [PubMed] [Google Scholar]
- 156.Lau WY, Leung TW, Ho SK, Chan M, Machin D, Lau J, Chan AT, Yeo W, Mok TS, Yu SC, Leung NW, Johnson PJ. Adjuvant intra-arterial iodine-131-labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet. 1999;353:797–801. doi: 10.1016/s0140-6736(98)06475-7. [DOI] [PubMed] [Google Scholar]
- 157.Lau WY, Lai EC, Leung TW, Yu SC. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann Surg. 2008;247:43–48. doi: 10.1097/SLA.0b013e3181571047. [DOI] [PubMed] [Google Scholar]
- 158.Boucher E, Bouguen G, Garin E, Guillygomarch A, Boudjema K, Raoul JL. Adjuvant intraarterial injection of 131I-labeled lipiodol after resection of hepatocellular carcinoma: progress report of a case-control study with a 5-year minimal follow-up. J Nucl Med. 2008;49:362–366. doi: 10.2967/jnumed.107.044750. [DOI] [PubMed] [Google Scholar]
- 159.Tabone M, Vigano L, Ferrero A, Pellerito R, Carbonatto P, Capussotti L. Prevention of intrahepatic recurrence by adjuvant (131)iodine-labeled lipiodol after resection for hepatocellular carcinoma in HCV-related cirrhosis. Eur J Surg Oncol. 2007;33:61–66. doi: 10.1016/j.ejso.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 160.Ikeda K, Arase Y, Saitoh S, Kobayashi M, Suzuki Y, Suzuki F, Tsubota A, Chayama K, Murashima N, Kumada H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228–232. doi: 10.1053/jhep.2000.9409. [DOI] [PubMed] [Google Scholar]
- 161.Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Shuto T, Kinoshita H. Randomized clinical trial of long-term outcome after resection of hepatitis C virus-related hepatocellular carcinoma by postoperative interferon therapy. Br J Surg. 2002;89:418–422. doi: 10.1046/j.0007-1323.2001.02054.x. [DOI] [PubMed] [Google Scholar]
- 162.Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299–306. doi: 10.7326/0003-4819-138-4-200302180-00008. [DOI] [PubMed] [Google Scholar]
- 163.Lin SM, Lin CJ, Hsu CW, Tai DI, Sheen IS, Lin DY, Liaw YF. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376–382. doi: 10.1002/cncr.20004. [DOI] [PubMed] [Google Scholar]
- 164.Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J, Zhou XD, Zhou J, Qiu SJ, Shen YF. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G, Tagger A, Colombo M, Bonino F, Majno P, Llovet JM. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 166.Lo CM, Liu CL, Chan SC, Lam CM, Poon RT, Ng IO, Fan ST, Wong J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Breitenstein S, Dimitroulis D, Petrowsky H, Puhan MA, Mullhaupt B, Clavien PA. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. Br J Surg. 2009;96:975–981. doi: 10.1002/bjs.6731. [DOI] [PubMed] [Google Scholar]
- 168.Hoshida Y, Shiratori Y, Omata M. Cost-effectiveness of adjuvant interferon therapy after surgical resection of Hepatitis C-related hepatocellular carcinoma. Liver. 2002;22:479–485. doi: 10.1034/j.1600-0676.2002.01736.x. [DOI] [PubMed] [Google Scholar]
- 169.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, Onaca N, Goldstein R, Levy M, Klintmalm GB. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 170.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 171.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 172.Kuang M, Peng BG, Lu MD, Liang LJ, Huang JF, He Q, Hua YP, Totsuka S, Liu SQ, Leong KW, Ohno T. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574–1579. doi: 10.1158/1078-0432.ccr-03-0071. [DOI] [PubMed] [Google Scholar]
- 173.Peng BG, Liang LJ, He Q, Kuang M, Lia JM, Lu MD, Huang JF. Tumor vaccine against recurrence of hepatocellular carcinoma. World J Gastroenterol. 2005;11:700–704. doi: 10.3748/wjg.v11.i5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wolf E, Milazzo S, Boehm K, Zwahlen M, Horneber M. Thymic peptides for treatment of cancer patients. Cochrane Database Syst Rev. 2011;2:CD003993. doi: 10.1002/14651858.CD003993.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Muto Y, Moriwaki H, Ninomiya M, Adachi S, Saito A, Takasaki KT, Tanaka T, Tsurumi K, Okuno M, Tomita E, Nakamura T, Kojima T. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group. N Engl J Med. 1996;334:1561–1567. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 176.Effect of interferon-alpha on progression of cirrhosis to hepatocellular carcinoma: a retrospective cohort study. International Interferon-alpha Hepatocellular Carcinoma Study Group. Lancet. 1998;351:1535–1539. [PubMed] [Google Scholar]
- 177.Poelstra K, Schuppan D. Targeted therapy of liver fibrosis/cirrhosis and its complications. J Hepatol. 2011 doi: 10.1016/j.jhep.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 178.Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol. 2011 doi: 10.1038/nrgastro.2011.33. [DOI] [PubMed] [Google Scholar]
- 179.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, Ferenci P, Nevens F, Mullhaupt B, Pockros P, Terg R, Shouval D, van Hoek B, Weiland O, Van Heeswijk R, De Meyer S, Luo D, Boogaerts G, Polo R, Picchio G, Beumont M. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 180.Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O'Boyle DR, 2nd, Lemm JA, Wang C, Knipe JO, Chien C, Colonno RJ, Grasela DM, Meanwell NA, Hamann LG. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schoniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 183.Watashi K, Ishii N, Hijikata M, Inoue D, Murata T, Miyanari Y, Shimotohno K. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell. 2005;19:111–122. doi: 10.1016/j.molcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 184.Afdhal NH, McHutchison JG, Zeuzem S, Mangia A, Pawlotsky JM, Murray JS, Shianna KV, Tanaka Y, Thomas DL, Booth DR, Goldstein DB. Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology. 2011;53:336–345. doi: 10.1002/hep.24052. [DOI] [PubMed] [Google Scholar]
- 185.Mallet V, Gilgenkrantz H, Serpaggi J, Verkarre V, Vallet-Pichard A, Fontaine H, Pol S. Brief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis C. Ann Intern Med. 2008;149:399–403. doi: 10.7326/0003-4819-149-6-200809160-00006. [DOI] [PubMed] [Google Scholar]
- 186.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, Fujiyama S, Yoshida H, Omata M. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 187.Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–1313. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 188.Roberts S, Gordon A, McLean C, Pedersen J, Bowden S, Thomson K, Angus P. Effect of sustained viral response on hepatic venous pressure gradient in hepatitis C-related cirrhosis. Clin Gastroenterol Hepatol. 2007;5:932–937. doi: 10.1016/j.cgh.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 189.Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 190.Dixon JB, Bhathal PS, Hughes NR, O'Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 191.Degott C, Zafrani ES, Callard P, Balkau B, Poupon RE, Poupon R. Histopathological study of primary biliary cirrhosis and the effect of ursodeoxycholic acid treatment on histology progression. Hepatology. 1999;29:1007–1012. doi: 10.1002/hep.510290444. [DOI] [PubMed] [Google Scholar]
- 192.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 195.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 197.Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 198.Nitta T, Kim JS, Mohuczy D, Behrns KE. Murine cirrhosis induces hepatocyte epithelial mesenchymal transition and alterations in survival signaling pathways. Hepatology. 2008;48:909–919. doi: 10.1002/hep.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Popov Y, Schuppan D. Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive? Gastroenterology. 2010;139:722–725. doi: 10.1053/j.gastro.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 200.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 201.De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007;462:266–272. doi: 10.1016/j.abb.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402–410. doi: 10.1055/s-0030-1267540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, Makhviladze M, Huyghe M, Hecht D, Oltersdorf T, Shapiro DA. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007;46:324–329. doi: 10.1002/hep.21664. [DOI] [PubMed] [Google Scholar]
- 204.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 205.Lin W, Tsai WL, Shao RX, Wu G, Peng LF, Barlow LL, Chung WJ, Zhang L, Zhao H, Jang JY, Chung RT. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology. 2010;138:2509–2518. 2518, e2501. doi: 10.1053/j.gastro.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sedlaczek N, Jia JD, Bauer M, Herbst H, Ruehl M, Hahn EG, Schuppan D. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol. 2001;158:1239–1244. doi: 10.1016/S0002-9440(10)64074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Patsenker E, Popov Y, Stickel F, Jonczyk A, Goodman SL, Schuppan D. Inhibition of integrin alphavbeta6 on cholangiocytes blocks transforming growth factor-beta activation and retards biliary fibrosis progression. Gastroenterology. 2008;135:660–670. doi: 10.1053/j.gastro.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shafiei MS, Rockey DC. The role of integrin-linked kinase in liver wound healing. J Biol Chem. 2006;281:24863–24872. doi: 10.1074/jbc.M513544200. [DOI] [PubMed] [Google Scholar]
- 209.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 210.Czochra P, Klopcic B, Meyer E, Herkel J, Garcia-Lazaro JF, Thieringer F, Schirmacher P, Biesterfeld S, Galle PR, Lohse AW, Kanzler S. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–428. doi: 10.1016/j.jhep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 211.Campbell JS, Hughes SD, Gilbertson DG, Palmer TE, Holdren MS, Haran AC, Odell MM, Bauer RL, Ren HP, Haugen HS, Yeh MM, Fausto N. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2005;102:3389–3394. doi: 10.1073/pnas.0409722102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Okada H, Honda M, Campbell JS, Sakai Y, Yamashita T, Takebuchi Y, Hada K, Shirasaki T, Takabatake R, Nakamura M, Sunakozaka H, Tanaka T, Fausto N, Kaneko S. Acyclic retinoid targets platelet-derived growth factor signaling in the prevention of hepatic fibrosis and hepatocellular carcinoma development. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0028. [DOI] [PubMed] [Google Scholar]
- 213.Ogawa S, Ochi T, Shimada H, Inagaki K, Fujita I, Nii A, Moffat MA, Katragadda M, Violand BN, Arch RH, Masferrer JL. Anti-PDGF-B monoclonal antibody reduces liver fibrosis development. Hepatol Res. 2010;40:1128–1141. doi: 10.1111/j.1872-034X.2010.00718.x. [DOI] [PubMed] [Google Scholar]
- 214.Gonzalo T, Beljaars L, van de Bovenkamp M, Temming K, van Loenen AM, Reker-Smit C, Meijer DK, Lacombe M, Opdam F, Keri G, Orfi L, Poelstra K, Kok RJ. Local inhibition of liver fibrosis by specific delivery of a platelet-derived growth factor kinase inhibitor to hepatic stellate cells. J Pharmacol Exp Ther. 2007;321:856–865. doi: 10.1124/jpet.106.114496. [DOI] [PubMed] [Google Scholar]
- 215.Bansal R, Prakash J, Post E, Beljaars L, Schuppan D, Poelstra K. Novel engineered targeted interferon-gamma blocks hepatic fibrogenesis in mice. Hepatology. 2011 doi: 10.1002/hep.24395. [DOI] [PubMed] [Google Scholar]
- 216.Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16:281–288. doi: 10.3748/wjg.v16.i3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4–12. doi: 10.1053/jhep.2003.50044. [DOI] [PubMed] [Google Scholar]
- 218.Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069–11076. doi: 10.1074/jbc.M111490200. [DOI] [PubMed] [Google Scholar]
- 219.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002;36:850–860. doi: 10.1053/jhep.2002.35625. [DOI] [PubMed] [Google Scholar]
- 220.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Luedde T, Schwabe RF. NF-kappaB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007;132:1937–1946. doi: 10.1053/j.gastro.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 223.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Moschen AR, Fritz T, Clouston AD, Rebhan I, Bauhofer O, Barrie HD, Powell EE, Kim SH, Dinarello CA, Bartenschlager R, Jonsson JR, Tilg H. IL-32: A new proinflammatory cytokine involved in HCV-related liver inflammation and fibrosis. Hepatology. 2011 doi: 10.1002/hep.24285. [DOI] [PubMed] [Google Scholar]
- 225.Berres ML, Koenen RR, Rueland A, Zaldivar MM, Heinrichs D, Sahin H, Schmitz P, Streetz KL, Berg T, Gassler N, Weiskirchen R, Proudfoot A, Weber C, Trautwein C, Wasmuth HE. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J Clin Invest. 2010;120:4129–4140. doi: 10.1172/JCI41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 227.Parola M, Marra F. Adipokines and Redox Signaling: Impact on Fatty Liver Disease. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3848. [DOI] [PubMed] [Google Scholar]
- 228.Teixeira-Clerc F, Julien B, Grenard P, Tran Van Nhieu J, Deveaux V, Li L, Serriere-Lanneau V, Ledent C, Mallat A, Lotersztajn S. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med. 2006;12:671–676. doi: 10.1038/nm1421. [DOI] [PubMed] [Google Scholar]
- 229.Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- 230.Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, Takimoto R, Takada K, Miyanishi K, Matsunaga T, Takayama T, Niitsu Y. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 231.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Moreno M, Gonzalo T, Kok RJ, Sancho-Bru P, van Beuge M, Swart J, Prakash J, Temming K, Fondevila C, Beljaars L, Lacombe M, van der Hoeven P, Arroyo V, Poelstra K, Brenner DA, Gines P, Bataller R. Reduction of advanced liver fibrosis by short-term targeted delivery of an angiotensin receptor blocker to hepatic stellate cells in rats. Hepatology. 2010;51:942–952. doi: 10.1002/hep.23419. [DOI] [PubMed] [Google Scholar]
- 233.Oakley F, Teoh V, Ching ASG, Bataller R, Colmenero J, Jonsson JR, Eliopoulos AG, Watson MR, Manas D, Mann DA. Angiotensin II activates I kappaB kinase phosphorylation of RelA at Ser 536 to promote myofibroblast survival and liver fibrosis. Gastroenterology. 2009;136:2334–2344. e2331. doi: 10.1053/j.gastro.2009.02.081. [DOI] [PubMed] [Google Scholar]
- 234.Mann J, Chu DC, Maxwell A, Oakley F, Zhu NL, Tsukamoto H, Mann DA. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. 714, e701–704. doi: 10.1053/j.gastro.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Murakami Y, Toyoda H, Tanaka M, Kuroda M, Harada Y, Matsuda F, Tajima A, Kosaka N, Ochiya T, Shimotohno K. The Progression of Liver Fibrosis Is Related with Overexpression of the miR-199 and 200 Families. PLoS One. 2011;6:e16081. doi: 10.1371/journal.pone.0016081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Venugopal SK, Jiang J, Kim TH, Li Y, Wang SS, Torok NJ, Wu J, Zern MA. Liver fibrosis causes downregulation of miRNA-150 and miRNA-194 in hepatic stellate cells, and their overexpression causes decreased stellate cell activation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G101–106. doi: 10.1152/ajpgi.00220.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Guo CJ, Pan Q, Jiang B, Chen GY, Li DG. Effects of upregulated expression of microRNA-16 on biological properties of culture-activated hepatic stellate cells. Apoptosis. 2009;14:1331–1340. doi: 10.1007/s10495-009-0401-3. [DOI] [PubMed] [Google Scholar]
- 238.Ji J, Zhang J, Huang G, Qian J, Wang X, Mei S. Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett. 2009;583:759–766. doi: 10.1016/j.febslet.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 239.Drews F, Knobel S, Moser M, Muhlack KG, Mohren S, Stoll C, Bosio A, Gressner AM, Weiskirchen R. Disruption of the latent transforming growth factor-beta binding protein-1 gene causes alteration in facial structure and influences TGF-beta bioavailability. Biochim Biophys Acta. 2008;1783:34–48. doi: 10.1016/j.bbamcr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 240.Bosselut N, Housset C, Marcelo P, Rey C, Burmester T, Vinh J, Vaubourdolle M, Cadoret A, Baudin B. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 2010;10:1017–1028. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- 241.Kim PK, Kim MR, Kim HJ, Yoo HS, Kim JS, Cho EH, Kim CW. Proteome analysis of the rat hepatic stellate cells under high concentrations of glucose. Proteomics. 2007;7:2184–2188. doi: 10.1002/pmic.200700051. [DOI] [PubMed] [Google Scholar]
- 242.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 243.Guo CJ, Pan Q, Cheng T, Jiang B, Chen GY, Li DG. Changes in microRNAs associated with hepatic stellate cell activation status identify signaling pathways. Febs J. 2009;276:5163–5176. doi: 10.1111/j.1742-4658.2009.07213.x. [DOI] [PubMed] [Google Scholar]
- 244.Hoshida Y, Villanueva A, Sangiovanni A, Sole M, Gould J, Gupta S, Gabriel S, Peix J, Colombo M, Llovet JM, Golub TR. Gene-expression signature predicts outcome of liver cirrhosis. Hepatology. 2009;50:312A. [Google Scholar]
- 245.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, Reich M, Chan JA, Glickman JN, Ikeda K, Hashimoto M, Watanabe G, Daidone MG, Roayaie S, Schwartz M, Thung S, Salvesen HB, Gabriel S, Mazzaferro V, Bruix J, Friedman SL, Kumada H, Llovet JM, Golub TR. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Maeda S. NF-kappaB, JNK, and TLR Signaling Pathways in Hepatocarcinogenesis. Gastroenterol Res Pract. 2010;2010:367694. doi: 10.1155/2010/367694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nagata H, Hatano E, Tada M, Murata M, Kitamura K, Asechi H, Narita M, Yanagida A, Tamaki N, Yagi S, Ikai I, Matsuzaki K, Uemoto S. Inhibition of c-Jun NH2-terminal kinase switches Smad3 signaling from oncogenesis to tumor- suppression in rat hepatocellular carcinoma. Hepatology. 2009;49:1944–1953. doi: 10.1002/hep.22860. [DOI] [PubMed] [Google Scholar]
- 250.Das M, Garlick DS, Greiner DL, Davis RJ. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kluwe J, Pradere JP, Gwak GY, Mencin A, De Minicis S, Osterreicher CH, Colmenero J, Bataller R, Schwabe RF. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–359. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Weston CR, Wong A, Hall JP, Goad ME, Flavell RA, Davis RJ. The c-Jun NH2-terminal kinase is essential for epidermal growth factor expression during epidermal morphogenesis. Proc Natl Acad Sci U S A. 2004;101:14114–14119. doi: 10.1073/pnas.0406061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nagahara T, Okano J, Fujise Y, Abe R, Murawaki Y. Preventive effect of JTE-522, a selective cyclooxygenase-2 inhibitor, on DEN-induced hepatocarcinogenesis in rats. Biomed Pharmacother. 2010;64:319–326. doi: 10.1016/j.biopha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 254.Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA, Thimme R, Blum H, Nedospasov SA, Zatloukal K, Ramzan M, Ciesek S, Pietschmann T, Marche PN, Karin M, Kopf M, Browning JL, Aguzzi A, Heikenwalder M. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16:295–308. doi: 10.1016/j.ccr.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hanczko R, Fernandez DR, Doherty E, Qian Y, Vas G, Niland B, Telarico T, Garba A, Banerjee S, Middleton FA, Barrett D, Barcza M, Banki K, Landas SK, Perl A. Prevention of hepatocarcinogenesis and increased susceptibility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J Clin Invest. 2009;119:1546–1557. doi: 10.1172/JCI35722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shimizu M, Takai K, Moriwaki H. Strategy and mechanism for the prevention of hepatocellular carcinoma: phosphorylated retinoid X receptor alpha is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci. 2009;100:369–374. doi: 10.1111/j.1349-7006.2008.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Moriwaki H, Yasuda I, Shiratori Y, Uematsu T, Okuno M, Muto Y. Deletion of serum lectin-reactive alpha-fetoprotein by acyclic retinoid: a potent biomarker in the chemoprevention of second primary hepatoma. Clin Cancer Res. 1997;3:727–731. [PubMed] [Google Scholar]
- 258.Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V, Rosmorduc O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–314. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- 259.Chen YL, Lv J, Ye XL, Sun MY, Xu Q, Liu CH, Min LH, Xu LM, Li HP, Liu P, Ding X. Sorafenib suppresses TGF-beta1-induced epithelial-to-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology. 2011 doi: 10.1002/hep.24254. [DOI] [PubMed] [Google Scholar]
- 260.Wang Y, Gao J, Zhang D, Zhang J, Ma J, Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53:132–144. doi: 10.1016/j.jhep.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 261.Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923–928. doi: 10.1016/j.jhep.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Coriat R, Gouya H, Mir O, Ropert S, Vignaux O, Chaussade S, Sogni P, Pol S, Blanchet B, Legmann P, Goldwasser F. Reversible decrease of portal venous flow in cirrhotic patients: a positive side effect of sorafenib. PLoS One. 2011;6:e16978. doi: 10.1371/journal.pone.0016978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM). Cancer. 2009;115:4646. doi: 10.1002/cncr.24673. [DOI] [PubMed] [Google Scholar]
- 264.Yoshiji H, Noguchi R, Ikenaka Y, Kaji K, Aihara Y, Fukui H. Impact of reninangiotensin system in hepatocellular carcinoma. Curr Cancer Drug Targets. 2011;11:431–441. doi: 10.2174/156800911795538084. [DOI] [PubMed] [Google Scholar]
- 265.Yoshiji H, Noguchi R, Kaji K, Ikenaka Y, Shirai Y, Namisaki T, Kitade M, Tsujimoto T, Kawaratani H, Fukui H. Attenuation of insulin-resistance-based hepatocarcinogenesis and angiogenesis by combined treatment with branched-chain amino acids and angiotensin-converting enzyme inhibitor in obese diabetic rats. J Gastroenterol. 2010;45:443–450. doi: 10.1007/s00535-009-0158-z. [DOI] [PubMed] [Google Scholar]
- 266.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. Eur J Cancer Prev. 2009;18:13–25. doi: 10.1097/CEJ.0b013e3282f0c090. [DOI] [PubMed] [Google Scholar]
- 267.Glauert HP, Calfee-Mason K, Stemm DN, Tharappel JC, Spear BT. Dietary antioxidants in the prevention of hepatocarcinogenesis: a review. Mol Nutr Food Res. 2010;54:875–896. doi: 10.1002/mnfr.200900482. [DOI] [PubMed] [Google Scholar]
- 268.Qu CX, Kamangar F, Fan JH, Yu B, Sun XD, Taylor PR, Chen BE, Abnet CC, Qiao YL, Mark SD, Dawsey SM. Chemoprevention of primary liver cancer: a randomized, double-blind trial in Linxian, China. J Natl Cancer Inst. 2007;99:1240–1247. doi: 10.1093/jnci/djm084. [DOI] [PubMed] [Google Scholar]
- 269.Lu SC. Antioxidants in the treatment of chronic liver diseases: why is the efficacy evidence so weak in humans? Hepatology. 2008;48:1359–1361. doi: 10.1002/hep.22463. [DOI] [PubMed] [Google Scholar]
- 270.Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 271.Tarao K, Takemiya S, Tamai S, Sugimasa Y, Ohkawa S, Akaike M, Tanabe H, Shimizu A, Yoshida M, Kakita A. Relationship between the recurrence of hepatocellular carcinoma (HCC) and serum alanine aminotransferase levels in hepatectomized patients with hepatitis C virus-associated cirrhosis and HCC. Cancer. 1997;79:688–694. doi: 10.1002/(sici)1097-0142(19970215)79:4<688::aid-cncr5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 272.Abe N, Ebina T, Ishida N. Interferon induction by glycyrrhizin and glycyrrhetinic acid in mice. Microbiol Immunol. 1982;26:535–539. doi: 10.1111/j.1348-0421.1982.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 273.Kimura M, Watanabe H, Abo T. Selective activation of extrathymic T cells in the liver by glycyrrhizin. Biotherapy. 1992;5:167–176. doi: 10.1007/BF02171049. [DOI] [PubMed] [Google Scholar]
- 274.Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Kim SR, Monna T, Kobayashi K, Tango T. Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9). Cancer. 1995;76:743–749. doi: 10.1002/1097-0142(19950901)76:5<743::aid-cncr2820760506>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 275.Bertino G, Ardiri AM, Calvagno GS, Bertino N, Boemi PM. Prognostic and diagnostic value of des-gamma-carboxy prothrombin in liver cancer. Drug News Perspect. 2010;23:498–508. doi: 10.1358/dnp.2010.23.8.1444236. [DOI] [PubMed] [Google Scholar]
- 276.Otsuka M, Kato N, Shao RX, Hoshida Y, Ijichi H, Koike Y, Taniguchi H, Moriyama M, Shiratori Y, Kawabe T, Omata M. Vitamin K2 inhibits the growth and invasiveness of hepatocellular carcinoma cells via protein kinase A activation. Hepatology. 2004;40:243–251. doi: 10.1002/hep.20260. [DOI] [PubMed] [Google Scholar]
- 277.Mizuta T, Ozaki I, Eguchi Y, Yasutake T, Kawazoe S, Fujimoto K, Yamamoto K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer. 2006;106:867–872. doi: 10.1002/cncr.21667. [DOI] [PubMed] [Google Scholar]
- 278.Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. Jama. 2004;292:358–361. doi: 10.1001/jama.292.3.358. [DOI] [PubMed] [Google Scholar]
- 279.Tamori A, Habu D, Shiomi S, Kubo S, Nishiguchi S. Potential role of vitamin K(2) as a chemopreventive agent against hepatocellular carcinoma. Hepatol Res. 2007;37(Suppl 2):S303–307. doi: 10.1111/j.1872-034X.2007.00202.x. [DOI] [PubMed] [Google Scholar]
- 280.Lu SC, Mato JM. S-Adenosylmethionine in cell growth, apoptosis and liver cancer. J Gastroenterol Hepatol. 2008;23(Suppl 1):S73–77. doi: 10.1111/j.1440-1746.2007.05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kinsell LW, Harper HA, Barton HC, Michaels GD, Weiss HA. Rate of Disappearance From Plasma of Intravenously Administered Methionine in Patients With Liver Damage. Science. 1947;106:589–590. doi: 10.1126/science.106.2763.589. [DOI] [PubMed] [Google Scholar]
- 282.Martinez-Chantar ML, Corrales FJ, Martinez-Cruz LA, Garcia-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 283.Lu SC, Ramani K, Ou X, Lin M, Yu V, Ko K, Park R, Bottiglieri T, Tsukamoto H, Kanel G, French SW, Mato JM, Moats R, Grant E. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–471. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 285.Bishayee A, Sarkar A, Chatterjee M. Further evidence for chemopreventive potential of beta-carotene against experimental carcinogenesis: diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis is prevented more effectively by beta-carotene than by retinoic acid. Nutr Cancer. 2000;37:89–98. doi: 10.1207/S15327914NC3701_12. [DOI] [PubMed] [Google Scholar]
- 286.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–146. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 287.Qin G, Ning Y, Lotlikar PD. Chemoprevention of aflatoxin B1-initiated and carbon tetrachloride-promoted hepatocarcinogenesis in the rat by green tea. Nutr Cancer. 2000;38:215–222. doi: 10.1207/S15327914NC382_11. [DOI] [PubMed] [Google Scholar]
- 288.Tamura K, Nakae D, Horiguchi K, Akai H, Kobayashi Y, Satoh H, Tsujiuchi T, Denda A, Konishi Y. Inhibition by green tea extract of diethylnitrosamine-initiated but not choline-deficient, L-amino acid-defined diet-associated development of putative preneoplastic, glutathione S-transferase placental form-positive lesions in rat liver. Jpn J Cancer Res. 1997;88:356–362. doi: 10.1111/j.1349-7006.1997.tb00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nishida H, Omori M, Fukutomi Y, Ninomiya M, Nishiwaki S, Suganuma M, Moriwaki H, Muto Y. Inhibitory effects of (−)-epigallocatechin gallate on spontaneous hepatoma in C3H/HeNCrj mice and human hepatoma-derived PLC/PRF/5 cells. Jpn J Cancer Res. 1994;85:221–225. doi: 10.1111/j.1349-7006.1994.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, Zhang L, Wang JS. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 291.Mu LN, Zhou XF, Ding BG, Wang RH, Zhang ZF, Chen CW, Wei GR, Zhou XM, Jiang QW, Yu SZ. [A case-control study on drinking green tea and decreasing risk of cancers in the alimentary canal among cigarette smokers and alcohol drinkers]. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:192–195. [PubMed] [Google Scholar]
- 292.Kalthoff S, Ehmer U, Freiberg N, Manns MP, Strassburg CP. Coffee induces expression of glucuronosyltransferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology. 2010;139:1699–1710. 1710, e1691–1692. doi: 10.1053/j.gastro.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 293.Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, Franceschi S, La Vecchia C. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430–435. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 294.Tanaka T, Nishikawa A, Shima H, Sugie S, Shinoda T, Yoshimi N, Iwata H, Mori H. Inhibitory effects of chlorogenic acid, reserpine, polyprenoic acid (E-5166), or coffee on hepatocarcinogenesis in rats and hamsters. Basic Life Sci. 1990;52:429–440. doi: 10.1007/978-1-4615-9561-8_45. [DOI] [PubMed] [Google Scholar]
- 295.Hosaka S, Kawa S, Aoki Y, Tanaka E, Yoshizawa K, Karasawa Y, Hosaka N, Kiyosawa K. Hepatocarcinogenesis inhibition by caffeine in ACI rats treated with 2-acetylaminofluorene. Food Chem Toxicol. 2001;39:557–561. doi: 10.1016/s0278-6915(00)00175-7. [DOI] [PubMed] [Google Scholar]
- 296.Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11:8441–8448. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- 297.Ramakrishnan G, Raghavendran HR, Vinodhkumar R, Devaki T. Suppression of N-nitrosodiethylamine induced hepatocarcinogenesis by silymarin in rats. Chem Biol Interact. 2006;161:104–114. doi: 10.1016/j.cbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 298.Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE. Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. Aliment Pharmacol Ther. 2011;33:127–137. doi: 10.1111/j.1365-2036.2010.04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36:43–53. doi: 10.1016/j.ctrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 300.Yang HL, Chen WQ, Cao X, Worschech A, Du LF, Fang WY, Xu YY, Stroncek DF, Li X, Wang E, Marincola FM. Caveolin-1 enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J Transl Med. 2009;7:22. doi: 10.1186/1479-5876-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P-450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999;56:760–767. [PubMed] [Google Scholar]
- 302.Kweon S, Kim Y, Choi H. Grape extracts suppress the formation of preneoplastic foci and activity of fatty acid synthase in rat liver. Exp Mol Med. 2003;35:371–378. doi: 10.1038/emm.2003.49. [DOI] [PubMed] [Google Scholar]
- 303.Luther DJ, Ohanyan V, Shamhart PE, Hodnichak CM, Sisakian H, Booth TD, Meszaros JG, Bishayee A. Chemopreventive doses of resveratrol do not produce cardiotoxicity in a rodent model of hepatocellular carcinoma. Invest New Drugs. 2011;29:380–391. doi: 10.1007/s10637-009-9332-7. [DOI] [PubMed] [Google Scholar]
- 304.Darvesh AS, Bishayee A. Selenium in the prevention and treatment of hepatocellular carcinoma. Anticancer Agents Med Chem. 2010;10:338–345. doi: 10.2174/187152010791162252. [DOI] [PubMed] [Google Scholar]
- 305.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, Omoto K, Yamamoto S, Tanaka N, Kobayashi N. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499–507. doi: 10.3727/000000003108747064. [DOI] [PubMed] [Google Scholar]
- 307.Guyot C, Combe C, Balabaud C, Bioulac-Sage P, Desmouliere A. Fibrogenic cell fate during fibrotic tissue remodelling observed in rat and human cultured liver slices. J Hepatol. 2007;46:142–150. doi: 10.1016/j.jhep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 308.Guyot C, Lepreux S, Combe C, Sarrazy V, Billet F, Balabaud C, Bioulac-Sage P, Desmouliere A. Fibrogenic cell phenotype modifications during remodelling of normal and pathological human liver in cultured slices. Liver Int. 2010;30:1529–1540. doi: 10.1111/j.1478-3231.2010.02342.x. [DOI] [PubMed] [Google Scholar]
- 309.van de Bovenkamp M, Groothuis GM, Draaisma AL, Merema MT, Bezuijen JI, van Gils MJ, Meijer DK, Friedman SL, Olinga P. Precision-cut liver slices as a new model to study toxicity-induced hepatic stellate cell activation in a physiologic milieu. Toxicol Sci. 2005;85:632–638. doi: 10.1093/toxsci/kfi127. [DOI] [PubMed] [Google Scholar]
- 310.van de Bovenkamp M, Groothuis GM, Meijer DK, Olinga P. Precision-cut fibrotic rat liver slices as a new model to test the effects of anti-fibrotic drugs in vitro. J Hepatol. 2006;45:696–703. doi: 10.1016/j.jhep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 311.Verma N, Behera BC, Makhija U. Antioxidant and hepatoprotective activity of a lichen Usnea ghattensis in vitro. Appl Biochem Biotechnol. 2008;151:167–181. doi: 10.1007/s12010-008-8164-9. [DOI] [PubMed] [Google Scholar]
- 312.Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, Snyder E, Faversani A, Coggi G, Flavin R, Bosari S, Loda M. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci U S A. 2010;107:8352–8356. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 314.Chen AA, Thomas DK, Ong LL, Schwartz RE, Golub TR, Bhatia SN. Humanized mice with ectopic artificial liver tissues. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1101791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–879. doi: 10.1016/j.jhep.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Fausto N, Campbell JS. Mouse models of hepatocellular carcinoma. Semin Liver Dis. 2010;30:87–98. doi: 10.1055/s-0030-1247135. [DOI] [PubMed] [Google Scholar]
- 317.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 319.Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: Current status and therapeutic targets. Semin Cancer Biol. 2011;21:35–43. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 321.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY, Wang XW. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 322.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Huang KW, Huang YC, Tai KF, Chen BH, Lee PH, Hwang LH. Dual therapeutic effects of interferon-alpha gene therapy in a rat hepatocellular carcinoma model with liver cirrhosis. Mol Ther. 2008;16:1681–1687. doi: 10.1038/mt.2008.160. [DOI] [PubMed] [Google Scholar]
- 324.Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 325.Tarocchi M, Hannivoort R, Hoshida Y, Lee UE, Vetter D, Narla G, Villanueva A, Oren M, Llovet JM, Friedman SL. Carcinogen-induced hepatic tumors in KLF6+/− mice recapitulate aggressive human hepatocellular carcinoma associated with p53 pathway deregulation. Hepatology. 2011;54:522–531. doi: 10.1002/hep.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000;21:331–335. doi: 10.1093/carcin/21.2.331. [DOI] [PubMed] [Google Scholar]
- 327.Shimizu M, Sakai H, Shirakami Y, Iwasa J, Yasuda Y, Kubota M, Takai K, Tsurumi H, Tanaka T, Moriwaki H. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J- +(db)/+Lepr(db) mice. Cancer Prev Res (Phila) 2011;4:128–136. doi: 10.1158/1940-6207.CAPR-10-0163. [DOI] [PubMed] [Google Scholar]
- 328.Kagawa M, Sano T, Ishibashi N, Hashimoto M, Okuno M, Moriwaki H, Suzuki R, Kohno H, Tanaka T. An acyclic retinoid, NIK-333, inhibits N-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of TGF-alpha expression and cell proliferation. Carcinogenesis. 2004;25:979–985. doi: 10.1093/carcin/bgh093. [DOI] [PubMed] [Google Scholar]
- 329.Sano T, Kagawa M, Okuno M, Ishibashi N, Hashimoto M, Yamamoto M, Suzuki R, Kohno H, Matsushima-Nishiwaki R, Takano Y, Tsurumi H, Kojima S, Friedman SL, Moriwaki H, Tanaka T. Prevention of rat hepatocarcinogenesis by acyclic retinoid is accompanied by reduction in emergence of both TGF-alpha-expressing oval-like cells and activated hepatic stellate cells. Nutr Cancer. 2005;51:197–206. doi: 10.1207/s15327914nc5102_10. [DOI] [PubMed] [Google Scholar]
- 330.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis: inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179:131–144. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 331.Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell. 1990;61:1137–1146. doi: 10.1016/0092-8674(90)90076-q. [DOI] [PubMed] [Google Scholar]
- 332.Tonjes RR, Lohler J, O'Sullivan JF, Kay GF, Schmidt GH, Dalemans W, Pavirani A, Paul D. Autocrine mitogen IgEGF cooperates with c-myc or with the Hcs locus during hepatocarcinogenesis in transgenic mice. Oncogene. 1995;10:765–768. [PubMed] [Google Scholar]
- 333.Murakami H, Sanderson ND, Nagy P, Marino PA, Merlino G, Thorgeirsson SS. Transgenic mouse model for synergistic effects of nuclear oncogenes and growth factors in tumorigenesis: interaction of c-myc and transforming growth factor alpha in hepatic oncogenesis. Cancer Res. 1993;53:1719–1723. [PubMed] [Google Scholar]
- 334.Conner EA, Lemmer ER, Omori M, Wirth PJ, Factor VM, Thorgeirsson SS. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene. 2000;19:5054–5062. doi: 10.1038/sj.onc.1203885. [DOI] [PubMed] [Google Scholar]
- 335.Calvisi DF, Conner EA, Ladu S, Lemmer ER, Factor VM, Thorgeirsson SS. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J Hepatol. 2005;42:842–849. doi: 10.1016/j.jhep.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 336.Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM, Bolondi L, Negrini M. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27:5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 337.Fu X, Wang Q, Chen J, Huang X, Chen X, Cao L, Tan H, Li W, Zhang L, Bi J, Su Q, Chen L. Clinical significance of miR-221 and its inverse correlation with p27Kip(1) in hepatocellular carcinoma. Mol Biol Rep. 2011;38:3029–3035. doi: 10.1007/s11033-010-9969-5. [DOI] [PubMed] [Google Scholar]
- 338.Callegari E, Elamin BK, Giannone F, Milazzo M, Altavilla G, Fornari F, Giacomelli L, D'Abundo L, Ferracin M, Bassi C, Zagatti B, Corra F, Miotto E, Lupini L, Bolondi L, Gramantieri L, Croce CM, Sabbioni S, Negrini M. Liver tumorigenicity promoted by microRNA-221 in a mouse transgenic model. Hepatology. 2012 doi: 10.1002/hep.25747. [DOI] [PubMed] [Google Scholar]
- 339.Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L, French DM, Finn RS, Lowe SW, Powers S. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell. 2011;19:347–358. doi: 10.1016/j.ccr.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan JP, Stinson J, Stewart T, French DM. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.French DM, Lin BC, Wang M, Adams C, Shek T, Hotzel K, Bolon B, Ferrando R, Blackmore C, Schroeder K, Rodriguez LA, Hristopoulos M, Venook R, Ashkenazi A, Desnoyers LR. Targeting FGFR4 Inhibits Hepatocellular Carcinoma in Preclinical Mouse Models. PLoS One. 2012;7:e36713. doi: 10.1371/journal.pone.0036713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M, Kato A, Shida T, Okamura D, Miyazaki M. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56. doi: 10.1186/1471-2407-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 344.Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, Muller M, de Vos R, Wolf MJ, Boege Y, Seleznik GM, Zeller N, Erny D, Fuchs T, Zoller S, Cairo S, Buendia MA, Prinz M, Akira S, Tacke F, Heikenwalder M, Trautwein C, Luedde T. TAK1 suppresses a NEMO-dependent but NF-kappaB-independent pathway to liver cancer. Cancer Cell. 2010;17:481–496. doi: 10.1016/j.ccr.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 345.Watanabe S, Horie Y, Suzuki A. Hepatocyte-specific Pten-deficient mice as a novel model for nonalcoholic steatohepatitis and hepatocellular carcinoma. Hepatol Res. 2005;33:161–166. doi: 10.1016/j.hepres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 346.Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–1783. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Ishii H, Horie Y, Ohshima S, Anezaki Y, Kinoshita N, Dohmen T, Kataoka E, Sato W, Goto T, Sasaki J, Sasaki T, Watanabe S, Suzuki A, Ohnishi H. Eicosapentaenoic acid ameliorates steatohepatitis and hepatocellular carcinoma in hepatocyte-specific Pten-deficient mice. J Hepatol. 2009;50:562–571. doi: 10.1016/j.jhep.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 348.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 349.Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y, Hebrok M. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology. 2009;136:2304–2315. e2301–2304. doi: 10.1053/j.gastro.2009.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Liu N, Meng Z, Lou G, Zhou W, Wang X, Zhang Y, Zhang L, Liu X, Yen Y, Lai L, Forman BM, Xu Z, Xu R, Huang W. Hepatocarcinogenesis in FXR-/- mice mimics human HCC progression that operates through HNF1alpha regulation of FXR expression. Mol Endocrinol. 2012;26:775–785. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 354.Nakae D. Endogenous liver carcinogenesis in the rat. Pathol Int. 1999;49:1028–1042. doi: 10.1046/j.1440-1827.1999.00990.x. [DOI] [PubMed] [Google Scholar]
- 355.Nakae D, Yoshiji H, Mizumoto Y, Horiguchi K, Shiraiwa K, Tamura K, Denda A, Konishi Y. High incidence of hepatocellular carcinomas induced by a choline deficient L-amino acid defined diet in rats. Cancer Res. 1992;52:5042–5045. [PubMed] [Google Scholar]
- 356.Tamura K, Nakae D, Horiguchi K, Akai H, Kobayashi Y, Andoh N, Satoh H, Denda A, Tsujiuchi T, Yoshiji H, Konishi Y. Inhibition by N-(4-hydroxyphenyl)retinamide and all-trans-retinoic acid of exogenous and endogenous development of putative preneoplastic, glutathione S-transferase placental form-positive lesions in the livers of rats. Carcinogenesis. 1997;18:2133–2141. doi: 10.1093/carcin/18.11.2133. [DOI] [PubMed] [Google Scholar]
- 357.Denda A, Tang Q, Endoh T, Tsujiuchi T, Horiguchi K, Noguchi O, Mizumoto Y, Nakae D, Konishi Y. Prevention by acetylsalicylic acid of liver cirrhosis and carcinogenesis as well as generations of 8-hydroxydeoxyguanosine and thiobarbituric acid-reactive substances caused by a choline-deficient, L-amino acid-defined diet in rats. Carcinogenesis. 1994;15:1279–1283. doi: 10.1093/carcin/15.6.1279. [DOI] [PubMed] [Google Scholar]
- 358.de Lima VM, Oliveira CP, Alves VA, Chammas MC, Oliveira EP, Stefano JT, de Mello ES, Cerri GG, Carrilho FJ, Caldwell SH. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol. 2008;49:1055–1061. doi: 10.1016/j.jhep.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 359.Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010;29:1787–1797. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Wang B, Majumder S, Nuovo G, Kutay H, Volinia S, Patel T, Schmittgen TD, Croce C, Ghoshal K, Jacob ST. Role of microRNA-155 at early stages of hepatocarcinogenesis induced by choline-deficient and amino acid-defined diet in C57BL/6 mice. Hepatology. 2009;50:1152–1161. doi: 10.1002/hep.23100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Tryndyak VP, Ross SA, Beland FA, Pogribny IP. Down-regulation of the microRNAs miR-34a, miR-127, and miR-200b in rat liver during hepatocarcinogenesis induced by a methyl-deficient diet. Mol Carcinog. 2009;48:479–487. doi: 10.1002/mc.20484. [DOI] [PubMed] [Google Scholar]
- 362.Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–213. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 363.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 364.Nakayama H, Otabe S, Ueno T, Hirota N, Yuan X, Fukutani T, Hashinaga T, Wada N, Yamada K. Transgenic mice expressing nuclear sterol regulatory element-binding protein 1c in adipose tissue exhibit liver histology similar to nonalcoholic steatohepatitis. Metabolism. 2007;56:470–475. doi: 10.1016/j.metabol.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 365.Beraza N, Malato Y, Vander Borght S, Liedtke C, Wasmuth HE, Dreano M, de Vos R, Roskams T, Trautwein C. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut. 2008;57:655–663. doi: 10.1136/gut.2007.134288. [DOI] [PubMed] [Google Scholar]
- 366.Watanabe S, Horie Y, Kataoka E, Sato W, Dohmen T, Ohshima S, Goto T, Suzuki A. Non-alcoholic steatohepatitis and hepatocellular carcinoma: lessons from hepatocyte-specific phosphatase and tensin homolog (PTEN)-deficient mice. J Gastroenterol Hepatol. 2007;22(Suppl 1):S96–S100. doi: 10.1111/j.1440-1746.2006.04665.x. [DOI] [PubMed] [Google Scholar]
- 367.Fan CY, Pan J, Usuda N, Yeldandi AV, Rao MS, Reddy JK. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- 368.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Varela-Rey M, Martinez-Lopez N, Fernandez-Ramos D, Embade N, Calvisi DF, Woodhoo A, Rodriguez J, Fraga MF, Julve J, Rodriguez-Millan E, Frades I, Torres L, Luka Z, Wagner C, Esteller M, Lu SC, Martinez-Chantar ML, Mato JM. Fatty liver and fibrosis in glycine N-methyltransferase knockout mice is prevented by nicotinamide. Hepatology. 2010;52:105–114. doi: 10.1002/hep.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, Rodes J, Friedman SL, Carmeliet P, Jimenez W, Morales-Ruiz M. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 371.Tipoe GL, Leung TM, Liong EC, Lau TY, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 372.Yasuda Y, Shimizu M, Sakai H, Iwasa J, Kubota M, Adachi S, Osawa Y, Tsurumi H, Hara Y, Moriwaki H. (−)-Epigallocatechin gallate prevents carbon tetrachloride-induced rat hepatic fibrosis by inhibiting the expression of the PDGFRbeta and IGF-1R. Chem Biol Interact. 2009;182:159–164. doi: 10.1016/j.cbi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 373.Lee TY, Kim KT, Han SY. Expression of ErbB receptor proteins and TGF-alpha during diethylnitrosamine-induced hepatocarcinogenesis in the rat liver. Korean J Hepatol. 2007;13:70–80. [PubMed] [Google Scholar]
- 374.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 375.Katzenellenbogen M, Pappo O, Barash H, Klopstock N, Mizrahi L, Olam D, Jacob-Hirsch J, Amariglio N, Rechavi G, Mitchell LA, Kohen R, Domany E, Galun E, Goldenberg D. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–4010. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 376.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 377.Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786–796. doi: 10.1016/j.jhep.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 378.Lichtman SN, Wang J, Clark RL. A microcholangiographic study of liver disease models in rats. Acad Radiol. 1995;2:515–521. doi: 10.1016/s1076-6332(05)80410-6. [DOI] [PubMed] [Google Scholar]
- 379.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Schnur J, Nagy P, Sebestyen A, Schaff Z, Thorgeirsson SS. Chemical hepatocarcinogenesis in transgenic mice overexpressing mature TGF beta-1 in liver. Eur J Cancer. 1999;35:1842–1845. doi: 10.1016/s0959-8049(99)00224-5. [DOI] [PubMed] [Google Scholar]
- 381.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 382.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis. Hepatology. 2012 doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Yao X, Hu JF, Daniels M, Yien H, Lu H, Sharan H, Zhou X, Zeng Z, Li T, Yang Y, Hoffman AR. A novel orthotopic tumor model to study growth factors and oncogenes in hepatocarcinogenesis. Clin Cancer Res. 2003;9:2719–2726. [PubMed] [Google Scholar]
- 384.Thorgeirsson SS, Lee JS, Grisham JW. Functional genomics of hepatocellular carcinoma. Hepatology. 2006;43:S145–150. doi: 10.1002/hep.21063. [DOI] [PubMed] [Google Scholar]
- 385.Lee JS, Thorgeirsson SS. Functional and genomic implications of global gene expression profiles in cell lines from human hepatocellular cancer. Hepatology. 2002;35:1134–1143. doi: 10.1053/jhep.2002.33165. [DOI] [PubMed] [Google Scholar]
- 386.Kawai HF, Kaneko S, Honda M, Shirota Y, Kobayashi K. alpha-fetoprotein-producing hepatoma cell lines share common expression profiles of genes in various categories demonstrated by cDNA microarray analysis. Hepatology. 2001;33:676–691. doi: 10.1053/jhep.2001.22500. [DOI] [PubMed] [Google Scholar]
- 387.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Feng YX, Wang T, Deng YZ, Yang P, Li JJ, Guan DX, Yao F, Zhu YQ, Qin Y, Wang H, Li N, Wu MC, Wang HY, Wang XF, Cheng SQ, Xie D. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology. 2011;53:483–492. doi: 10.1002/hep.24075. [DOI] [PubMed] [Google Scholar]
- 389.Kornek M, Raskopf E, Tolba R, Becker U, Klockner M, Sauerbruch T, Schmitz V. Accelerated orthotopic hepatocellular carcinomas growth is linked to increased expression of pro-angiogenic and prometastatic factors in murine liver fibrosis. Liver Int. 2008;28:509–518. doi: 10.1111/j.1478-3231.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 390.Greenwald P, Dunn BK. Do we make optimal use of the potential of cancer prevention? Recent Results Cancer Res. 2009;181:3–17. doi: 10.1007/978-3-540-69297-3_1. [DOI] [PubMed] [Google Scholar]
- 391.Kensler TW, Groopman JD. Is it time to advance the chemoprevention of environmental carcinogenesis with microdosing trials? Cancer Prev Res (Phila) 2009;2:1003–1007. doi: 10.1158/1940-6207.CAPR-09-0232. [DOI] [PubMed] [Google Scholar]
- 392.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 393.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 394.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, Gores GJ. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 395.Patel KD, Abeysekera KW, Marlais M, McPhail MJ, Thomas HC, Fitzpatrick JA, Lim AK, Taylor-Robinson SD, Thomas EL. Recent advances in imaging hepatic fibrosis and steatosis. Expert Rev Gastroenterol Hepatol. 2011;5:91–104. doi: 10.1586/egh.10.85. [DOI] [PubMed] [Google Scholar]
- 396.Pinzani M, Vizzutti F, Arena U, Marra F. Technology Insight: noninvasive assessment of liver fibrosis by biochemical scores and elastography. Nat Clin Pract Gastroenterol Hepatol. 2008;5:95–106. doi: 10.1038/ncpgasthep1025. [DOI] [PubMed] [Google Scholar]
- 397.Huang H, Shiffman ML, Friedman S, Venkatesh R, Bzowej N, Abar OT, Rowland CM, Catanese JJ, Leong DU, Sninsky JJ, Layden TJ, Wright TL, White T, Cheung RC. A 7 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis C. Hepatology. 2007;46:297–306. doi: 10.1002/hep.21695. [DOI] [PubMed] [Google Scholar]
- 398.Marcolongo M, Young B, Dal Pero F, Fattovich G, Peraro L, Guido M, Sebastiani G, Palu G, Alberti A. A seven-gene signature (cirrhosis risk score) predicts liver fibrosis progression in patients with initially mild chronic hepatitis C. Hepatology. 2009;50:1038–1044. doi: 10.1002/hep.23111. [DOI] [PubMed] [Google Scholar]
- 399.Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, Miyamoto A, Kondo M, Arai I, Yamamoto T, Ota H, Dono K, Umeshita K, Nakamori S, Sasaki Y, Ishikawa O, Imaoka S, Monden M. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39:215–221. doi: 10.1016/s0168-8278(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 400.Chen YJ, Yeh SH, Chen JT, Wu CC, Hsu MT, Tsai SF, Chen PJ, Lin CH. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119:431–440. doi: 10.1053/gast.2000.9373. [DOI] [PubMed] [Google Scholar]
- 401.Ng IO, Guan XY, Poon RT, Fan ST, Lee JM. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol. 2003;199:345–353. doi: 10.1002/path.1287. [DOI] [PubMed] [Google Scholar]
- 402.Sakon M, Nagano H, Nakamori S, Dono K, Umeshita K, Murakami T, Nakamura H, Monden M. Intrahepatic recurrences of hepatocellular carcinoma after hepatectomy: analysis based on tumor hemodynamics. Arch Surg. 2002;137:94–99. doi: 10.1001/archsurg.137.1.94. [DOI] [PubMed] [Google Scholar]
- 403.Ikeda K, Arase Y, Kobayashi M, Saitoh S, Someya T, Hosaka T, Suzuki Y, Suzuki F, Tsubota A, Akuta N, Kumada H. Significance of multicentric cancer recurrence after potentially curative ablation of hepatocellular carcinoma: a longterm cohort study of 892 patients with viral cirrhosis. J Gastroenterol. 2003;38:865–876. doi: 10.1007/s00535-003-1163-2. [DOI] [PubMed] [Google Scholar]