Abstract
Myalgic encephalomyelitis (ME) is a debilitating illness of unknown etiology characterized by neurocognitive dysfunction, inflammation, immune abnormalities and gastrointestinal distress. An increasing body of evidence suggests that disruptions in the gut may contribute to the induction of neuroinflammation. Therefore, reports of human endogenous retroviral (HERV) expression in association with neuroinflammatory diseases prompted us to investigate the gut of individuals with ME for the presence of HERV proteins. In eight out of 12 individuals with ME, immunoreactivity to HERV proteins was observed in duodenal biopsies. In contrast, no immunoreactivity was detected in any of the eight controls. Immunoreactivity to HERV Gag and Env proteins was uniquely co-localized in hematopoietic cells expressing the C-type lectin receptor CLEC4C (CD303/BDCA2), the co-stimulatory marker CD86 and the class II major histocompatibility complex HLA-DR, consistent with plasmacytoid dendritic cells (pDCs). Although the significance of HERVs present in the pDCs of individuals with ME has yet to be determined, these data raise the possibility of an involvment of pDCs and HERVs in ME pathology. To our knowledge, this report describes the first direct association between pDCs and HERVs in human disease.
Keywords: Myalgic encephalomyelitis, ME, human endogenous retrovirus, HERV, plasmacytoid dendritic cell, pDC
Myalgic encephalomyelitis (ME) is a debilitating disorder characterized by multi-systemic neuropathology, gastrointestinal (GI) dysfunction, inflammation, and innate immune dysregulation (1). Immunological symptoms often include viral reactivation, cytokine and chemokine irregularities, and decreased natural killer (NK) cell function (2–7). Additionally, reports of individuals with ME-expressing autoantibodies (8, 9), and the successful treatment of ME with the B-cell-depleting drug rituximab (10, 11), suggest that a subset of these individuals may suffer from an uncharacterized antibody-mediated autoimmunity.
Little is known regarding the pathophysiology of ME; therefore, diseases with similar or overlapping symptoms often serve as useful guides when exploring new experimental concepts. For instance, autoimmune diseases such as multiple sclerosis (MS) and systemic lupus erythematosus (SLE) have many symptoms that overlap with those of ME. Neurological manifestations often associated with ME (12), are analogous to the neuroinflammation and cognitive abnormalities associated with MS and SLE (13, 14). Additionally, GI aberrations, which are common to individuals with MS and SLE (15–17), are among the most frequent symptoms reported by those with ME (18, 19).
The gut-associated lymphoid tissue represents the largest immune compartment in the body. In fact, it has been estimated that more than 60% of all T-cells may reside within the small intestine (20), emphasizing the potential contribution of the gut to systemic immunity. Indeed, increases in serum bacterial by-products, particularly lipopolysaccharides resulting from bacterial translocation in the gut, are found to be associated with systemic immune activation in many diseases such as HIV/AIDS, inflammatory bowel disease (IBD), and acute graft-versus-host disease (21–23). Extraintestinal immune dysregulation originating within the gut is described in such diseases as HIV/AIDS and idiopathic lymphocytopenia (24, 21). Although, its contribution to neuroimmune disease in humans is largely unknown, recent studies using animal models support a connection between GI immunity and neuroinflammation. Lee and colleagues reported intestinal microbiota to significantly influence the balance between pro-inflammatory and anti-inflammatory immune responses during the induction of experimental autoimmune encephalomyelitis, an animal model for MS (25). While limitations always exist when using animal models to study human disease, these data clearly support the concept of neuroinflammation associated with alterations in the gut.
Emerging evidence supports a role for human endogenous retroviruses (HERVs) in the etiopathology of autoimmune diseases such as MS and SLE (26). HERVs are remnants of ancient retroviral infections that integrated into the germ line and are now transmitted vertically (27). Not including the long interspersed elements (LINE) and other retrotransposons, HERVs constitute approximately 8% of the human genome (28). Although HERV proteins are self-antigens and should not induce an immune response, HERV proteins and serum antibodies against HERVs have been associated with a number of autoimmune diseases, including MS and SLE (29–32). These associations have motivated researchers to search for a role of HERV proteins in human pathology. Although associations are often difficult to render into definitive causation, it is widely believed that the humoral immune response against HERV proteins leads to autoimmunity through a process of molecular mimicry (33). HERV proteins are also known to act as superantigens, which have the ability to cause polyclonal T-cell activation and massive cytokine production (34). In 2001, Perron and colleagues reported the potential immunopathogenic properties of the HERV-W envelope protein, as a major proinflammatory and superantigenic determinant associated with MS (35).
In summary, individuals with ME have a significant number of symptoms that are similar to those described in autoimmune diseases such as MS and SLE. Additionally, the expression of HERV proteins has been observed in the lymphoid tissue of individuals with autoimmune disease. Finally, the gut represents the largest lymphoid compartment and is a significant site of ME-related pathology. For these reasons, we sought to determine if endogenous retroviral proteins could be detected in GI tissue of those with ME.
Materials and Methods
Clinical evaluation
Biopsies were acquired as existing de-identified surplus clinical diagnostic specimens, under an exemption to institutional review board (IRB) approval as detrmined by the University of Nevada, Reno Office of Human Research Protection. Therefore, patients’ information was limited to a general pathological description by which the specimens were grouped prior to de-identification. ME specimens were from 12 individuals fulfilling both the Canadian consensus criteria of ME (36), as well as the less restrictive and overlapping Fukuda criteria for chronic fatigue syndrome (CFS) (37). Fecal microbial analysis established the presence of substantial disruption of gut microbiota composition, with occurrence of a higher load of microbes with higher pathogenic potential in all ME cases, particularly of the Enterococcus, Streptococcus, and Prevotella genera. Gastritis (mainly antritis) was present in all cases and routine histological examination showed a lympho-plasmatic infiltrate in the sub-mucosa in all specimens. All cases tested negative for Helicobacter pylori and all individuals with ME tested negatively for HIV. Controls were eight anonymous individuals without symptoms of ME who underwent routine gastroscopy for epigastric pain.
Tissues and preparation
Punch biopsies were obtained from the duodenum and stomach of ME cases and controls. Fresh tissues were fixed in 4% paraformaldehyde for 4 h at 4°C and cryoprotected with a 30% sucrose solution in phosphate-buffered saline (PBS).
Immunohistochemical analysis
Immunohistochemical staining was performed on 0.5-µm-thick tissue sections. Tissue slides were de-paraffinized with xylene and rehydrated through a graded alcohol series. Antigen retrieval was carried out by boiling slides in sodium citrate (0.01 M, pH 6.0) at 95°C for 10 min. The slides were next rinsed in PBS and incubated in cold methanol for 20 min at –20°C. Tissue sections were then incubated with serum (matching the host of the secondary antibody) to block non-specific staining (1 h at 37°C) and then incubated with the primary antibody overnight at 4°C in a humidified chamber. After washing 3 times with PBS containing 0.1% Tween 20, the sections were incubated with the secondary antibody for 1 h at 37°C. All cases and controls were analyzed for the presence of HERV and gamma-retroviral Env and Gag proteins. Isotype-matched controls or secondary-only controls were included with all experiments. A summary of each antibody and the concentration at which it was used are presented in Table I. Slides were examined using a Zeiss LSM 7000 scanning laser confocal microscope (Carl Zeiss Microscopy, Thornwood, NY, USA) and images were captured with the Zeiss Zen 2009 analysis software.
Table I.
Antibodies and dilutions.
| Antibody | Source | Dilution used |
|---|---|---|
| Mouse anti-HERV-K-Gag IgG1 (clone 5i75) mAb | US Biological, Salem, MA, USA | 1:500 |
| Mouse anti-HERV-K18.1-Env IgG pAb | Ab Cam, Cambridge, MA, USA | 1:500 |
| Rabbit anti-HERV-R-Env IgG pAb | Ab Cam, Cambridge, MA, USA | 1:500 |
| Rabbit anti-HERV-FRD-Env IgG pAb | Ab Cam, Cambridge, MA, USA | 1:500 |
| Rat anti-SFFV-gp52 IgG1κ (clone 7C10) mAb1 | S. Ruscetti, NCI, Frederick, MD, USA | 1:500 |
| Goat anti-MLV-Gag IgG pAb | S. Ruscetti, NCI, Frederick, MD, USA | 1:500 |
| FITC Mouse anti-Human CD303 (clone AC144) mAb | MiltenyBiotec, Auburn, CA, USA | 1:500 |
| Mouse anti-Human CD45 IgG1 (clone Ros220) mAb | Beckman Coulter, Brea, CA, USA | 1:500 |
| FITC Mouse anti-Human CD86 IgG1κ (clone 2231) | BD Pharmigen, San Jose, CA, USA | 1:500 |
| FITC Mouse anti-Human HLA-DR (clone AC122) | MiltenyBiotec, Auburn, CA, USA | 1:500 |
| Rat anti-HHV-8 ORF73 (clone LN-53) mAb | Advanced Bio., Columbia, MD, USA | 1:1000 |
| Alexa647 Rat IgG1κ (clone R3-34) mAb isotype control | BD Pharmigen, San Jose, CA, USA | 1:500 |
| Rat IgG1κ mAb isotype control | eBiosciences, San Diego, CA, USA | 1:500 |
| Mouse IgG1 isotype control | Ab Cam, Cambridge, MA, USA | 1:500 |
| APC Mouse IgG1κ isotype control | BioLegdend, San Diego, CA, USA | 1:500 |
| Rhodamine Donkey anti-Rat IgG pAb2 | American Qualex, San Clemente, CA, USA | 1:1000 |
| FITC Donkey anti-Mouse IgG pAb3 | Jackson Immuno., West Grove, PA, USA | 1:1000 |
| Dylight594 Donkey anti-Mouse IgG pAb3 | Jackson Immuno., West Grove, PA, USA | 1:500 |
| Dylight488 Bovine anti-Goat IgG pAb3 | Jackson Immuno., West Grove, PA, USA | 1:500 |
| Rhodamine Goat anti-Rabbit IgG pAb2 | American Qualex, San Clemente, CA, USA | 1:500 |
| Normal goat serum | Invitrogen, Carlsbad, CA, USA | 1:2000 |
Conjugated to rhodamine by American Qualex
Absorbed to cross-reactive species
Minimum cross-reactivity reported by manufacturer; mAb (monoclonal antibody), pAb (polyclonal antibody).
Statistical analysis
Statistical analysis of ME cases and controls for the presence of HERV proteins was performed using the Chi-square method. The non-parametric Mann Whitney method was used to analyze for the absolute differences in gut-associated plasmacytoid dendritic cells (pDCs).
Results
Detection of immunoreactive proteins in gut biopsies
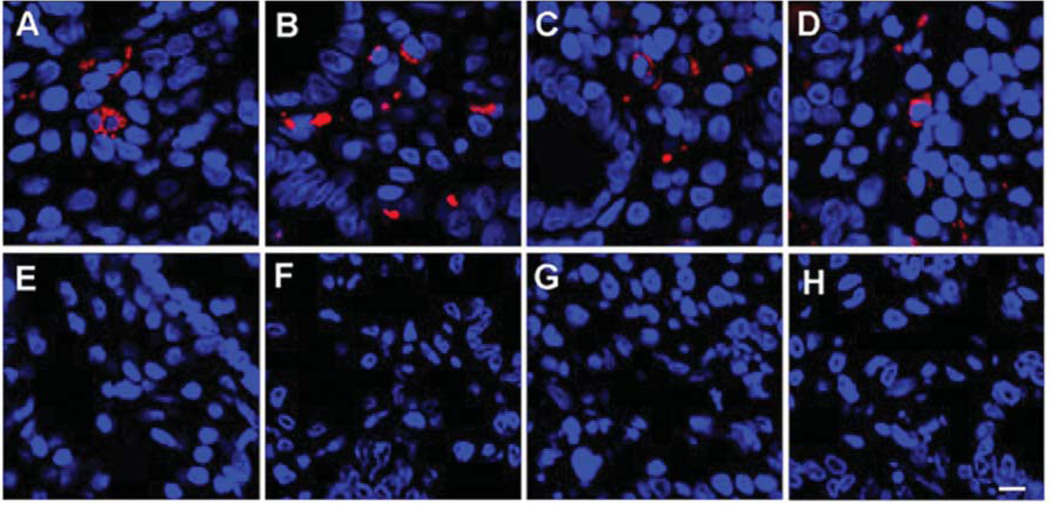
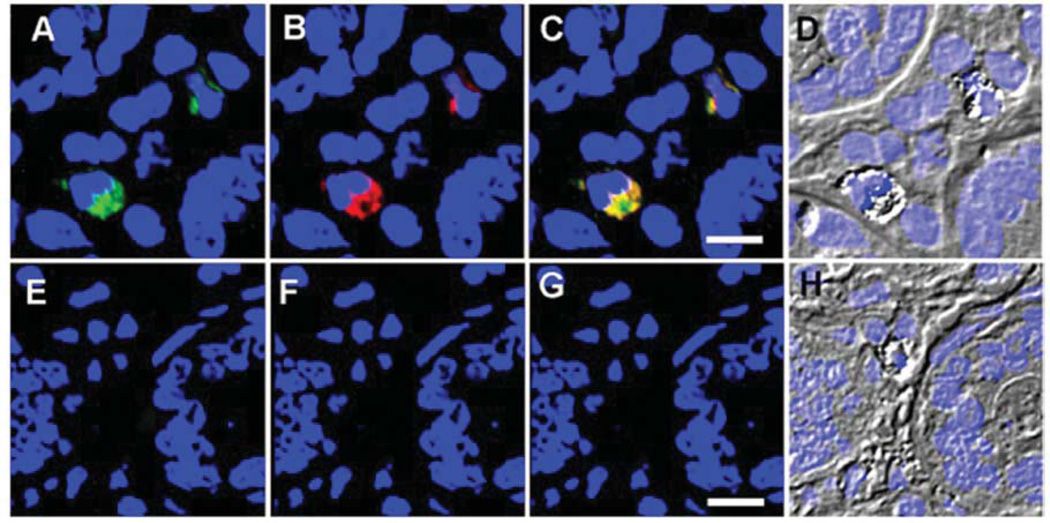
Immunochemical analyses of 12 ME gut biopsies probed for viral antigens showed that eight samples of the duodenum were immunoreactive to antibodies raised against HERV proteins (Figure 1A–D). In contrast, no immunoreactivity was observed in any of the control duodenum samples (Figure 1E–H, p=0.003 by Chi-square). Additional analysis was conducted using two anti-gammaretroviral antibodies: goat polyclonal IgG antibody raised against the Gag protein of murine leukemia virus (Figure 2A) and a rat monoclonal IgG1κ antibody (clone 7C10) raised against the Env protein of spleen focus forming virus (Figure 2B). The observed immunoreactivity was reproducibly consistent with the previous anti-HERV results, suggesting that the antigammaretroviral antibodies were cross-reactive with the HERV antigen(s). Additionally, the immunoreactivity was observed to co-localize (Figure 2C) in cells with an eccentric nucleus and granular inclusions (Figure 2D). Consistent with previous observations using antibodies to HERVs, no immunoreactivity was observed in the control biopsies using either anti-gammaretroviral antibody (Figure 2E–G). Matched stomach biopsies collected from ME cases and controls were also analyzed, but were consistently nonreactive when probed with the same anti-HERV and antigammaretroviral antibodies (data not shown).
Figure 1.
Immunoreactivity to monoclonal (mAb) and polyclonal (pAb) antibodies against human endogenous retrovirus (HERV) in a duodenal biopsy of representative individuals. A: Immunoreactivity to antiHERVK Gag IgG1 mAb (Dylight594 donkey antimouse IgG secondary) in a duodenal biopsy of an ME case. B: Immunoreactivity to anti-HERVK18.1 Env IgG pAb (Dylight594 donkey anti-mouse IgG secondary) in a duodenal biopsy of an ME case. C: Immunoreactivity to antiHERVFRD Env IgG pAb (rhodamine goat antirabbit IgG secondary in a duodenal biopsy of an ME case. D: Immunoreactivity to antiHERVR Env (rhodamine goat anti-rabbit IgG secondary) in a duodenal biopsy of an ME case. E: Immunoreactivity to antiHERV-K Gag IgG1 mAb (Dylight594 donkey anti-mouse IgG secondary) in a duodenal biopsy of a control. F: Immunoreactivity to antiHERVK18.1 Env IgG pAb (Dylight594 donkey antimouse IgG secondary) in a duodenal biopsy of a control. G: Immunoreactivity to antiHERVFRD Env IgG pAb (rhodamine goat anti-rabbit IgG secondary) in a duodenal biopsy of a control. H: Immunoreactivity to antiHERVR Env (rhodamine goat antirabbit IgG secondary) in a duodenal biopsy of a control subject. (Bar represents 20 µm). TOPO3 was used to indicate nucleus localization in all images.
Figure 2.
Immunoreactivity to rat antiSFFVgp52 Env and goat antiMLV Gag (gammaretroviral) antibodies. A: Immunoreactivity to goat anti-Gag IgG pAb (Dylight488 bovine anti-goat IgG pAb secondary) in a duodenal biopsy of an ME case. B: Immunoreactivity to rat antiEnv IgG1κ mAb (rhodamine donkey anti-rat IgG pAb secondary) in a duodenal biopsy of an ME case. C: Overlay of images A and B (Bar represents 20 µm). D: Morphology of the immunoreactive cells of an ME case. E: Immunoreactivity to goat antiGag IgG pAb (Dylight488 bovine antigoat IgG pAb secondary) in a duodenal biopsy of a control. F: Immunoreactivity to rat anti-Env IgG1κ mAb (rhodamine donkey antirat IgG pAb secondary) in a duodenal biopsy of a control. G: Overlay of images E and F (Bar represents 20 µm). H: Morphology of the immunoreactive cells of a control. TOPO3 was used to indicate nucleus localization in all images.
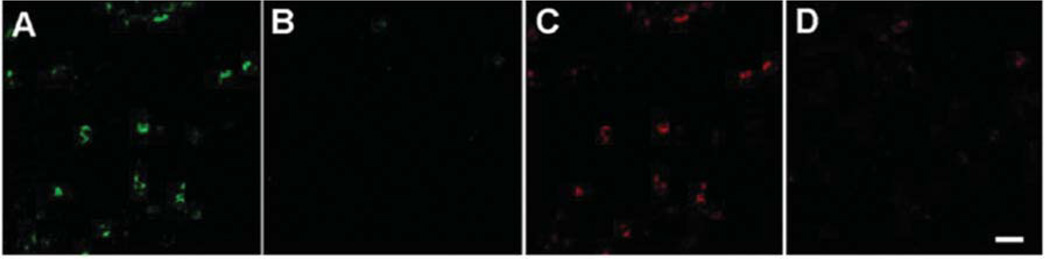
Specificity of the primary anti-gammaretroviral antibodies was established when their ability to detect Env and Gag proteins was ablated by pre-incubation with purified and heatdeactivated virus derived from 22Rv1 (Figure 3). In order to rule out non-specific binding of the secondary antibody, the rat monoclonal IgG1κ antibody to Env (7C10), was directly labeled with rhodamine and used to probe the tissue sections (Figure 4A). Potential non-specific binding of the secondary antibody was additionally excluded by using the rhodamineconjugated donkey anti-rat IgG and the Alexa647-conjugated donkey anti-goat IgG secondary antibodies in the absence of the primary antibody (Figure 4B). Non-specific binding of the primary rat monoclonal antibody was further ruled out by the lack of immunoreactivity by using an irrelevant rat monoclonal antibody (rat anti-human herpes virus-8 UL84 IgG) and a rat IgG1κ isotype control (Figure 4C and D). Potential nonspecific binding of the secondary antibody was excluded by replacing the primary antibody with normal goat serum (Figure 4E). Finally, to address the potential cross-reactivity of the secondary antibody with the incorrect primary antibody (for example, donkey anti-goat IgG secondary antibody crossreacting with the rat IgG1κ primary antibody to Env), biopsies were probed with a primary antibody followed by incubation with the mismatched secondary antibody (Figure 4F and G).
Figure 3.
Specificity of the rat antiSFFVgp52 Env and goat antiMLV Gag antibodies established by antibody depletion. Antibodies were pre-incubated (1 h, 25°C, constant shaking) with purified and heatinactivated retrovirus derived from 22Rv1, prior to probing duodenal biopsy sections. A: Duodenal biopsies were probed with polyclonal goat antibodies to MLV Gag. B: Duodenal biopsies were probed with polyclonal goat antibodies against MLV Gag preincubated with heatinactivated 22Rv1. C: Duodenal biopsies were probed with monoclonal rat antibody against SFFVgp52 Env. D: Duodenal biopsies were probed with monoclonal rat antibody against SFFVgp52 Env, preincubated with heat-inactivated 22Rv1 (Bar represents 20 µm).
Figure 4.

Evaluation of the specificity and potential crossreactivity of the antibodies used. A: Duodenal biopsies were probed with rhodomine-conjugated anti-SFFV-gp-52 Env IgG1κ monoclonal antibody (mAb) (1500). B: Duodenal biopsies were probed with rhodamine-conjugated donkey anti-rat IgG polyclonal antibody (pAb) (1:1000) and Dylight488-conjugated bovine anti-goat IgG pAb (1500). C: Duodenal biopsies were probed with rat anti-HHV8 ORF73 mAb (11,000), followed by incubation with rhodamine-conjugated donkey anti-rat IgG pAb (11000). D: Duodenal biopsies were probed with rat IgG1κ mAb isotype control (1:500), followed by incubation with rhodamine-conjugated donkey anti-rat IgG pAb (1:1,000) (Bar represents 20 µm). E: Duodenal biopsies were probed with normal goat serum (1:2,000), followed by incubation with Dylight488-conjugated bovine anti-goat IgG pAb (1:500). F: Duodenal biopsies were probed with rat anti-SFFV-gp-52 Env IgG1κ mAb, followed by incubation with Dylight488-conjugated bovine anti-goat IgG pAb (1:500). G: Duodenal biopsies were probed with goat anti-MLV-Gag IgG pAb followed by incubation with rhodamin-conjugated donkey anti-rat IgG pAb (1:1,000). TOPO3 was used to indicate nucleus localization in all images.
Phenotype of the immunoreactive cells
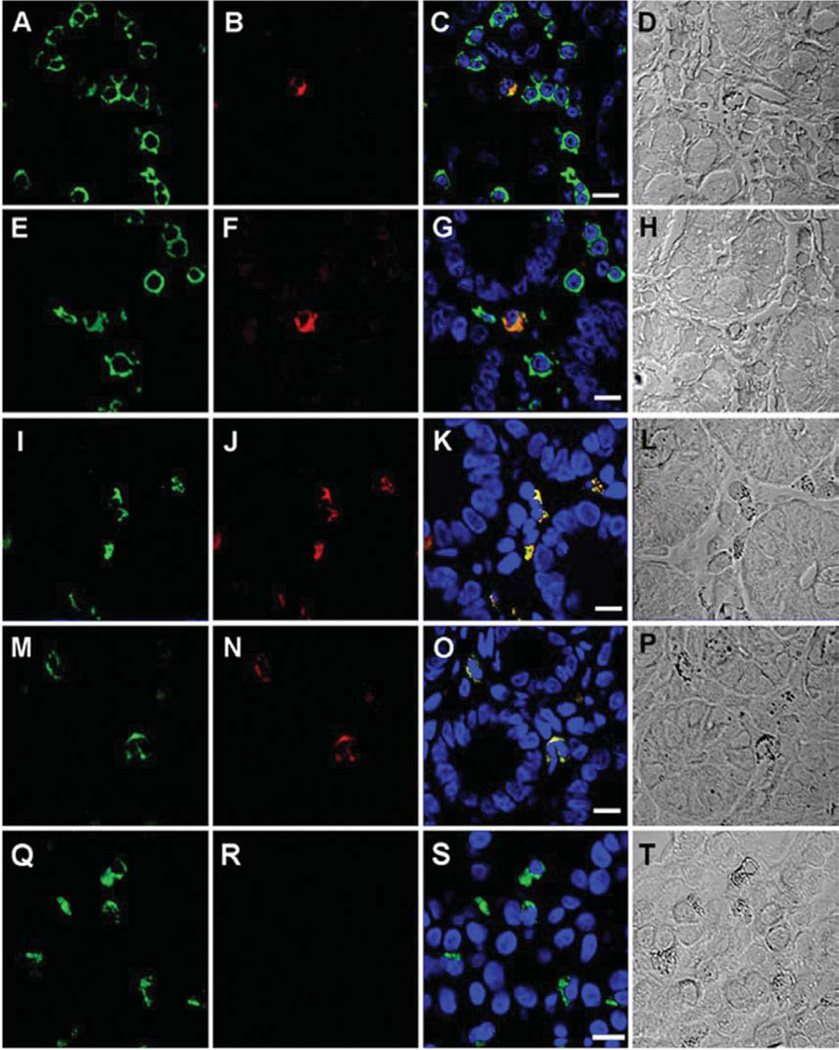
In order to determine the phenotype of the immunoreactive cells, we first performed an immunohistochemical analysis using the pan-lymphocyte marker CD45 (Figure 5A) in combination with the rhodamine-conjugated rat IgG1κ monoclonal antibody against Env (Figure 5B). Consistent with a hematopoietic lineage, only cells expressing CD45 proved immunoreactive with the antibody against Env (Figure 5C). We next sought to determine if the immunoreactive cells were pDCs. pDCs are professional antigen-presenting cells (APCs) that express CD45 and exclusively express the surface marker CD303 (38– 40). Our analysis revealed that CD303 expression (Figure 5E) and immunoreactivity to the rhodamine-conjugated rat monoclonal antibody to Env (Figure 5F) uniquely co-localized in the same cells (Figure 5G), suggesting that the observed immunoreactivity was specific for pDCs. In order to provide further evidence that the immunoreactive cells were APCs, immunohistochemical analysis was performed using the co-stimulatory marker CD86 and the major histocompatibility (MHC) class II antigen HLA-DR, both of which are expressed by APCs, including pDCs (41, 42). Again, our analysis confirmed that expression of CD86 (Figure 5I) and immunoreactivity to the rhodamine-conjugated rat monoclonal antibody against Env (Figure 5J) co-localized in the same cell (Figure 5K). Likewise, expression of HLA-DR (Figure 5M) and immunoreactivity to the rat monoclonal antibody to Env (Figure 5N) also co-localized in the same cells (Figure 5O). Lastly, the possibility of non-specific binding of the antibodies to CD86 and HLA-DR was addressed by the inclusion of matched isotype controls (Figure 5R). When taken together; these observations support the premise that the observed anti-HERV immunoreactivity is uniquely present in pDCs.
Figure 5.
Phenotype of duodenal-associated cells immunoreactive to rat monoclonal antibody to SFFVgp52 Env. A: Expression of CD45 (FITC) in a duodenal biopsy of an ME case. B: Immunoreactivity of rhodamineconjugated monoclonal rat antibody to SFFVgp52 Env in duodenal biopsy of an ME case. C: Merged images A and B, (Bar represents 20 µm). D: Morphology of the immunoreactive cells. E: Expression of CD303 (FITC) in a duodenal biopsy of an ME case. F: Immunoreactivity of rhodamine-conjugated monoclonal rat antibody to SFFVgp52 Env in a duodenal biopsy of an ME case. G: Merged images E and F (Bar represents 20 µm). H: Morphology of the immunoreactive cells. I: Expression of CD86 (FITC) in duodenal a biopsy of an ME case. J: Immunoreactivity of a rhodamine-conjugated monoclonal rat antibody to SFFV-gp-52 Env in a duodenal biopsy of an ME case. K: Merged images I and J, (Bar represents 20 µm). L: Morphology of the immunoreactive cells. M: Expression of HLADR (FITC) in a duodenal biopsy of an ME case. N: Immunoreactivity of rhodamine-conjugated monoclonal rat antibody to SFFV-gp-52 Env in duodenal biopsy of an ME case. O: Merged images M and N, (Bar represents 20 µm). P: Morphology of the immunoreactive cells. Q: Expression of CD303 (FITC) in a duodenal biopsy of an ME case. R: Immunoreactivity of APC-conjugated mouse IgG1 isotype control in duodenal biopsy of an ME case. S: Merged images Q and R (Bar represents 20 µm). T: Morphology of the immunoreactive cells. TOPO3 was used to a indicate nucleus localization in images C, G, K, O and S.
Quantitative analysis of pDCs
Finally, in order to evaluate if the relative number of duodenum-associated pDCs differed between ME cases and controls, we analyzed five random microscopic fields of view for eight individuals from each group (Table II). We also determined the frequency at which the pDCs of ME cases were immunoreactive to the HERV antibodies. ME cases were found to have approximately 4.7-times as many pDCs per field, when compared to the controls (35.6±10.9 vs. 7.5±3.5, respectively, p<0.001 by Mann Whitney). Additionally, we observed that approximately 44% (15.7/35.6) of the duodenum-associated pDCs in the eight ME cases were immunoreactive to antibodies against HERVs.
Table II.
Quantitative analysis of plasmacytoid dendritic cells (pDCs). Five microscopic fields were randomly chosen and used to count total pDCs in the duodenum of 8 controls (top) and 8 cases (bottom). No Env-positive pDCs were observed in any controls. Cases are reported as a ratio of Env-positive pDCs to total pDCs.
| Field | Controls | Group | Standard | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1 | 7 | 10 | 6 | 8 | 7 | 12 | 7 | 8 | ||
| 2 | 6 | 13 | 1 | 9 | 6 | 13 | 6 | 9 | ||
| 3 | 4 | 11 | 4 | 10 | 5 | 10 | 3 | 12 | ||
| 4 | 0 | 13 | 7 | 13 | 0 | 11 | 0 | 13 | ||
| 5 | 9 | 10 | 9 | 0 | 3 | 15 | 0 | 10 | ||
| Average | 5.2 | 11.4 | 5.4 | 8 | 4.2 | 12.2 | 3.2 | 10.4 | 7.5 | 3.5 |
| Cases | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1 | 26/76 | 17/35 | 19/48 | 10/30 | 18/28 | 12/25 | 18/25 | 10/16 | ||
| 2 | 16/50 | 17/47 | 16/28 | 11/29 | 12/25 | 15/45 | 20/55 | 11/17 | ||
| 3 | 18/52 | 12/25 | 12/40 | 12/31 | 10/18 | 19/40 | 30/56 | 14/30 | ||
| 4 | 25/40 | 17/26 | 16/45 | 12/29 | 8/19 | 16/41 | 19/51 | 10/19 | ||
| 5 | 26/52 | 13/30 | 16/40 | 12/36 | 15/20 | 21/50 | 13/27 | 12/27 | ||
| Average | 22.2/54 | 15.2/32.6 | 15.8/40.2 | 11.4/31 | 12.6/22 | 16.6/40.2 | 20/42.8 | 11.4/21.8 | 15.7/35.6 | 3.9/10.9 |
Discussion
In this study, we have shown that gut biopsies from 8 out of 12 individuals with ME displayed immunoreactivity consistent with the presence of HERV proteins. However, the same immunoreactivity was not observed in the biopsies of the controls. Additionally, we have shown that the immunoreactivity was observed in cells with a phenotype that is consistent with pDCs. These observations suggest that the presence of the HERV protein in pDCs may be associated with a pathological manifestation in at least a subset of individuals with ME.
Our detection of proteins that react with monoclonal antibodies to HERVs is consistent with HERV expression. Nevertheless, it could be argued that an exogenous retroviral infection may potentially account for the observed results. The present study was conducted using surplus clinical biopsies; therefore, the proper matched specimens were not available to conduct a rigorous transcriptional analysis on all cases. This issue was compounded by the de-identified nature of the specimens, thus preventing the collection of additional biological material. Nonetheless, cryopreserved lymphocytes and preserved RNA from a duodenal biopsy were available from two HERV-positive ME cases. Therefore, we performed unbiased next-generation sequencing (NGS) on both RNA derived from the duodenum and purified pDCs from these individuals. Multiple contigs of known HERV genes were observed; however, no open reading frames were identified that could account for an infectious retrovirus (unpublished data). We also attempted to identify an infectious murine leukemia virus (MLV)-related virus by co-culturing lymphocytes and purified pDCs using the DERSE indicator cell line (43). The DERSE indicator cell line (a kind gift from Dr. Vineet KewalRamani) is derived from the prostate cancer cell line LNCaP and stably transfected with an MLV vector containing the green fluorescent protein (GFP) gene in reverse orientation. Only after rescue and transfer to new cells through reverse transcriptase and integrase enzymatic activity can the GFP be detected. In previous experiments, the sensitivity of this assay was established by detecting infectious MLV-related virus derived from two individual 22Rv1 cells per culture (unpublished data). In spite of using this sensitive detection method, we were unable to identify an infectious MLV-related retrovirus. Although we cannot definitively rule out the possibility that our observations were the result of an infectious retrovirus, our results using HERV-specific monoclonal and polyclonal antibodies, as well as NGS and co-culture methods, strongly argue against this.
A number of immunological observations have been described in relation to ME; however, the data presented here, to our knowledge, represent the first report of an association of pDCs with this disease. While the expression of endogenous retroviral proteins in the pDCs of ME cases does not intrinsically explain pathology, the observation that the immunoreactive proteins are only observed in pDCs is supportive of this concept. This supposition is further supported by our previous report of the dysregulation of inflammatory cytokines in a cohort of ME cases (44). One subset of this cohort was characterized by elevated interleukin-8 (CXCL8), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), interferon gamma-induced protein-10 (CXCL10), macrophage inflammatory proteins-alpha and beta (CCL3 and CCL4) and depressed interferon-alpha (IFN-α) production, all of which are produced in significant quantities by pDCs (45). The production of type-I IFN by pDCs is a result of the engagement of Toll-like receptors (TLR) 7 and 9 in early endosomes (46), whereas inflammatory cytokine production occurs as a result of their engagement in late endosomes (47). A deviation in the spatiotemporal regulation of pDCs favoring TLR-activation in late endosomes over early endosomes would be consistent with our previous observations of cytokine dysregulation in those with ME. Similar abnormalities in the pDC function and distribution has also been reported in individuals with IBD. Brumgard et al. observed that isolated pDCs from patients with flaring Crohn’s disease and ulcerative colitis overproduce inflammatory cytokines while underproducing type-I IFN in response to the synthetic TLR-9 agonist ODN 2216 (48). Additionally, they reported on a significantly higher frequency of pDC in the inflamed colonic mucosa of patients with IBD. These data suggest that our observations in subjects with ME may not be unique to this disease but may, in fact, be common to diseases characterized by chronic inflammation.
A potential dysregulation of pDCs may suggest an explanation for a number of other clinical observations associated with ME. As stated previously, pDCs are most remarkable for their ability to produce copious amounts of type-I IFN. In 1978, Trinchieri and colleagues indirectly identified pDCs by their ability to activate natural killer (NK) cell-mediated cytotoxicity through the production of IFN-α (49) and several subsequent studies have expanded upon the importance of type-I IFN in modulating NK cell function (50). Decreased NK cell activity is a commonly reported observation associated with ME (51); therefore, an aberrant pDC response leading to a decrease in IFN-α would be consistent with dysregulation of NK cells. Inflammatory cytokine and chemokine abnormalities have also been reported in association with ME by other researchers. For instance, Natelson et al. reported elevated levels of CXCL8 and IL-10 in the spinal fluid of individuals with influenzalike onset of ME (52, 53). Moreover, Vernon et al. and others reported that CXCL8 gene transcription was elevated in those with ME (52, 53). Finally, Chao et al. reported IL-6 to be up-regulated in subsets of ME cases (54).
In our previous report, the expression of inflammatory cytokines was more prevalent in a subset of individuals characterized by a gamma-delta T-cell clonality (44). A similar clonality has been observed in autoimmune diseases characterized by the expression of HERVs, such as rheumatoid arthritis and MS (55, 56). Additionally, the expansion of gamma-delta T-cells in response to the expression of endogenous gamma retroviruses has been reported in animal models (57). Some HERV proteins act as superantigens (34), promoting the expansion of T-cell populations and, therefore, the observation of gamma-delta T-cell clonality associated with ME, as well as other diseases, may be the result of HERV superantigen stimulation.
Although the Canadian consensus criteria for ME and the Fukuda criteria for CFS do not include symptoms of autoimmunity, the recent study by Fluge et al. supports the notion that at least a subset of individuals with ME may have an autoimmune element associated with their disease (11). Autoimmune diseases, such as SLE, MS and rheumatoid arthritis, have several symptoms that overlap with those of ME and all have been associated with the pDC dysfunction. Moreover, the same autoimmune diseases are also reported to be associated with the expression of HERVs, although a physical connection between HERVs and pDCs has not yet been reported. As Stoye points out in his recent review (58), the role of HERVs in autoimmunity remains an unproven hypothesis; however, an increasing number of studies suggest that HERVs may have the capacity to contribute to disease pathology (59, 60). It is noteworthy that work conducted in the laboratory of Dr. Bridget Huber showed that HERV-K18 expression could be induced by herpes viruses such as Epstein-Barr virus and human herpes virus 6 (HHV-6) (34,61). Consistent with that work and with the data presented here, both of these viruses have been observed in the duodenum of individuals with ME (62).
The expression of HERV proteins in autoimmune diseases such as SLE, MS and Sjögren’s syndrome, is also evident by reports of antibodies to retroviral proteins in those who are not found to be infected with an exogenous retrovirus (26). If the expression of HERV proteins in pDCs is found to be associated with these autoimmune diseases, it may help explain the presence of such antibodies. Inflammation is known to increase HERV expression (63, 64); therefore, if pDC-associated inflammation drives the expression of endogenous retroviruses, it is also conceivable that dysregulated expression of other proteins in pDCs may occur. Consequently, the antigen-presenting abilities of pDCs may contribute to the production of auto-reactive antibodies, as is observed in ME (65, 66, 8, 67–69). The etiopathology associated with autoimmune diseases, HERV expression, and pDCs is clearly not a simple relationship. Nevertheless, the prospect of an involvement of HERVs and pDCs in ME pathology provides an opportunity for more directed studies into the immune dysregulation associated with this disease.
The expression of HERV proteins in pDCs may lead to ME-related pathology. Conversely, their expression might merely be the result of the inflammation associated with the disease, or perhaps a combination of both. Nevertheless, the presence of these proteins in the pDCs of individuals with ME but not in controls does support an involvement of pDCs in ME. The results presented in this report, if confirmed and extended by other studies, may provide a greater understanding over the etiopathology of ME.
Acknowledgements
We are indebted to Drs. Kenneth Hunter, Doug Redelman and Gregory Pari for their constructive input and suggestions during the course of this work and to Dr. David Schooley for his careful review of this manuscript. We are also grateful to Alicia Lange for her histotechnological assistance, Dr. Pravinkumar Purushothaman for his technical microscopy assistance and to Daniel Moothart for his assistance in isotyping and conjugating the 7C10 rat-monoclonal antibody. This work was supported in part by NIH grant AI078234.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R, Bateman L, Baumgarten-Austrheim B, Bell DS, Carlo-Stella N, Chia J, Darragh A, Jo D, Lewis D, Light AR, Marshall-Gradisbik S, Mena I, Mikovits JA, Miwa K, Murovska M, Pall ML, Stevens S. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270:327–338. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choppa PC, Vojdani A, Tagle C, Andrin R, Magtoto L. Multiplex PCR for the detection of Mycoplasma fermentans, M. hominis and M. penetrans in cell cultures and blood samples of patients with chronic fatigue syndrome. Mol Cell Probes. 1998;12:301–308. doi: 10.1006/mcpr.1998.0186. [DOI] [PubMed] [Google Scholar]
- 3.Klimas NG, Salvato FR, Morgan R, Fletcher MA. Immunologic abnormalities in chronic fatigue syndrome. J Clin Microbiol. 1990;28:1403–1410. doi: 10.1128/jcm.28.6.1403-1410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caligiuri M, Murray C, Buchwald D, Levine H, Cheney P, Peterson D, Komaroff AL, Ritz J. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol. 1987;139:3306–3313. [PubMed] [Google Scholar]
- 5.Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–392. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 6.De Meirleir K, Suhadolnik RJ, Lebleu B, Englebienne P. Antiviral pathway activation in chronic fatigue syndrome and acute infection. Clin Infect Dis. 2002;34:1420–1421. doi: 10.1086/339949. author reply 1421–l1422. [DOI] [PubMed] [Google Scholar]
- 7.Suhadolnik RJ, Reichenbach NL, Hitzges P, Sobol RW, Peterson DL, Henry B, Ablashi DV, Muller WE, Schroder HC, Carter WA, et al. Upregulation of the 2-5A synthetase/RNase L antiviral pathway associated with chronic fatigue syndrome. Clin Infect Dis. 1994;18(Suppl 1):S96–104. doi: 10.1093/clinids/18.supplement_1.s96. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinov K, von Mikecz A, Buchwald D, Jones J, Gerace L, Tan EM. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. J Clin Invest. 1996;98:1888–1896. doi: 10.1172/JCI118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Mikecz A, Konstantinov K, Buchwald DS, Gerace L, Tan EM. High frequency of autoantibodies to insoluble cellular antigens in patients with chronic fatigue syndrome. Arthritis Rheum. 1997;40:295–305. doi: 10.1002/art.1780400215. [DOI] [PubMed] [Google Scholar]
- 10.Fluge O, Bruland O, Risa K, Storstein A, Kristoffersen EK, Sapkota D, Naess H, Dahl O, Nyland H, Mella O. Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study. PLoS One. 2011;6:e26358. doi: 10.1371/journal.pone.0026358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fluge O, Mella O. Clinical impact of B-cell depletion with the anti-CD20 antibody rituximab in chronic fatigue syndrome: a preliminary case series. BMC Neurol. 2009;9:28. doi: 10.1186/1471-2377-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchwald D, Cheney PR, Peterson DL, Henry B, Wormsley SB, Geiger A, Ablashi DV, Salahuddin SZ, Saxinger C, Biddle R, et al. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann Intern Med. 1992;116:103–113. doi: 10.7326/0003-4819-116-2-103. [DOI] [PubMed] [Google Scholar]
- 13.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 14.Utset TO, Fink J, Doninger NA. Prevalence of neurocognitive dysfunction and other clinical manifestations in disabled patients with systemic lupus erythematosus. J Rheumatol. 2006;33:531–538. [PubMed] [Google Scholar]
- 15.Apperloo-Renkema HZ, Bootsma H, Mulder BI, Kallenberg CG, van der Waaij D. Host-microflora interaction in systemic lupus erythematosus (SLE): colonization resistance of the indigenous bacteria of the intestinal tract. Epidemiol Infect. 1994;112:367–373. doi: 10.1017/s0950268800057770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert EC, Hagspiel KD. Gastrointestinal and hepatic manifestations of systemic lupus erythematosus. J Clin Gastroenterol. 45:436–441. doi: 10.1097/MCG.0b013e31820f81b8. [DOI] [PubMed] [Google Scholar]
- 17.Ghezzi A, Zaffaroni M. Neurological manifestations of gastrointestinal disorders, with particular reference to the differential diagnosis of multiple sclerosis. Neurol Sci. 2001;22(Suppl 2):S117–122. doi: 10.1007/s100720100048. [DOI] [PubMed] [Google Scholar]
- 18.Devanur LD, Kerr JR. Chronic fatigue syndrome. J Clin Virol. 2006;37:139–150. doi: 10.1016/j.jcv.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Maes M, Mihaylova I, Leunis JC. Increased serum IgM antibodies directed against phosphatidyl inositol (Pi) in chronic fatigue syndrome (CFS) and major depression: evidence that an IgM-mediated immune response against Pi is one factor underpinning the comorbidity between both CFS and depression. Neuro Endocrinol Lett. 2007;28:861–867. [PubMed] [Google Scholar]
- 20.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Curr Opin Immunol. 1993;5:247–252. doi: 10.1016/0952-7915(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 21.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 22.Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- 23.Cooke KR, Olkiewicz K, Erickson N, Ferrara JL. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8:441–448. doi: 10.1179/096805102125001046. [DOI] [PubMed] [Google Scholar]
- 24.Lee PI, Ciccone EJ, Read SW, Asher A, Pitts R, Douek DC, Brenchley JM, Sereti I. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J Infect Dis. 2009;199:1664–1670. doi: 10.1086/598953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balada E, Vilardell-Tarres M, Ordi-Ros J. Implication of human endogenous retroviruses in the development of autoimmune diseases. Int Rev Immunol. 29:351–370. doi: 10.3109/08830185.2010.485333. [DOI] [PubMed] [Google Scholar]
- 27.Belshaw R, Dawson AL, Woolven-Allen J, Redding J, Burt A, Tristem M. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K(HML2): implications for present-day activity. J Virol. 2005;79:12507–12514. doi: 10.1128/JVI.79.19.12507-12514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss RA. The discovery of endogenous retroviruses. Retrovirology. 2006;3:67. doi: 10.1186/1742-4690-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefebvre S, Hubert B, Tekaia F, Brahic M, Bureau JF. Isolation from human brain of six previously unreported cDNAs related to the reverse transcriptase of human endogenous retroviruses. AIDS Res Hum Retroviruses. 1995;11:231–237. doi: 10.1089/aid.1995.11.231. [DOI] [PubMed] [Google Scholar]
- 30.Blomberg J, Nived O, Pipkorn R, Bengtsson A, Erlinge D, Sturfelt G. Increased antiretroviral antibody reactivity in sera from a defined population of patients with systemic lupus erythematosus. Correlation with autoantibodies and clinical manifestations. Arthritis Rheum. 1994;37:57–66. doi: 10.1002/art.1780370109. [DOI] [PubMed] [Google Scholar]
- 31.Bengtsson A, Blomberg J, Nived O, Pipkorn R, Toth L, Sturfelt G. Selective antibody reactivity with peptides from human endogenous retroviruses and nonviral poly (amino acids) in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:1654–1663. doi: 10.1002/art.1780391007. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen HB, Geny C, Deforges L, Perron H, Tourtelotte W, Heltberg A, Clausen J. Expression of endogenous retroviruses in blood mononuclear cells and brain tissue from multiple sclerosis patients. Acta Neurol Scand Suppl. 1997;169:38–44. doi: 10.1111/j.1600-0404.1997.tb08148.x. [DOI] [PubMed] [Google Scholar]
- 33.Query CC, Keene JD. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987;51:211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- 34.Sutkowski N, Conrad B, Thorley-Lawson DA, Huber BT. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity. 2001;15:579–589. doi: 10.1016/s1074-7613(01)00210-2. [DOI] [PubMed] [Google Scholar]
- 35.Perron H, Jouvin-Marche E, Michel M, Ounanian-Paraz A, Camelo S, Dumon A, Jolivet-Reynaud C, Marcel F, Souillet Y, Borel E, Gebuhrer L, Santoro L, Marcel S, Seigneurin JM, Marche PN, Lafon M. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287:321–332. doi: 10.1006/viro.2001.1045. [DOI] [PubMed] [Google Scholar]
- 36.Carruthers B, Jain AK, De Meirleir K, Peterson D, Klimas N, Lerner A, Bested A, Flor-Henry P, Joshi P, Powles A, Sherkey J, van de Sande M. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J Chronic Fatigue Syndrome. 2003;11:1–12. [Google Scholar]
- 37.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 39.Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63:1133–1148. doi: 10.1016/s0198-8859(02)00752-8. [DOI] [PubMed] [Google Scholar]
- 40.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chehimi J, Starr SE, Kawashima H, Miller DS, Trinchieri G, Perussia B, Bandyopadhyay S. Dendritic cells and IFN-alpha-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology. 1989;68:486–490. [PMC free article] [PubMed] [Google Scholar]
- 42.Laupeze B, Fardel O, Onno M, Bertho N, Drenou B, Fauchet R, Amiot L. Differential expression of major histocompatibility complex class Ia, Ib, and II molecules on monocytes-derived dendritic and macrophagic cells. Hum Immunol. 1999;60:591–597. doi: 10.1016/s0198-8859(99)00025-7. [DOI] [PubMed] [Google Scholar]
- 43.Lee K, Ruscetti F, Lloyd P, Rein A, Fanning-Heidecke G, KewalRamani V. Development of a GFP-indicator Cell Line for the Detection of XMRV. Abstracts Presented at the 18th Conference on Retroviruses and Opportunistic Infections Paper # 2152011 [Google Scholar]
- 44.Lombardi VC, Hagen KS, Hunter KW, Diamond JW, Smith-Gagen J, Yang W, Mikovits JA. Xenotropic murine leukemia virus-related virus-associated chronic fatigue syndrome reveals a distinct inflammatory signature. In Vivo. 25:307–314. [PubMed] [Google Scholar]
- 45.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 47.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee KD, Coffman RL, Barrat FJ. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumgart DC, Metzke D, Guckelberger O, Pascher A, Grotzinger C, Przesdzing I, Dorffel Y, Schmitz J, Thomas S. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn’s disease and ulcerative colitis. Clin Exp Immunol. 166:46–54. doi: 10.1111/j.1365-2249.2011.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinchieri G, Santoli D, Dee RR, Knowles BB. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Identification of the anti-viral activity as interferon and characterization of the human effector lymphocyte subpopulation. J Exp Med. 1978;147:1299–1313. doi: 10.1084/jem.147.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiteside TL, Friberg D. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. Am J Med. 1998;105:27S–34S. doi: 10.1016/s0002-9343(98)00155-7. [DOI] [PubMed] [Google Scholar]
- 52.Natelson BH, Weaver SA, Tseng CL, Ottenweller JE. Spinal fluid abnormalities in patients with chronic fatigue syndrome. Clin Diagn Lab Immunol. 2005;12:52–55. doi: 10.1128/CDLI.12.1.52-55.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vernon SD, Unger ER, Dimulescu IM, Rajeevan M, Reeves WC. Utility of the blood for gene expression profiling and biomarker discovery in chronic fatigue syndrome. Dis Markers. 2002;18:193–199. doi: 10.1155/2002/892374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao CC, Gallagher M, Phair J, Peterson PK. Serum neopterin and interleukin-6 levels in chronic fatigue syndrome. J Infect Dis. 1990;162:1412–1413. doi: 10.1093/infdis/162.6.1412-a. [DOI] [PubMed] [Google Scholar]
- 55.Olive C, Gatenby PA, Serjeantson SW. Persistence of gamma/delta T cell oligoclonality in the peripheral blood of rheumatoid arthritis patients. Immunol Cell Biol. 1994;72:7–11. doi: 10.1038/icb.1994.2. [DOI] [PubMed] [Google Scholar]
- 56.Bieganowski P, Bieganowska K, Zaborski J, Czlonkowska A. Oligoclonal expansion of gamma delta T cells in cerebrospinal fluid of multiple sclerosis patients. Mult Scler. 1996;2:78–82. doi: 10.1177/135245859600200203. [DOI] [PubMed] [Google Scholar]
- 57.Sim GK, Augustin A. The presence of an endogenous murine leukemia virus sequence correlates with the peripheral expansion of gamma delta T cells bearing the BALB invariant delta (BID) T cell receptor delta. J Exp Med. 1993;178:1819–1824. doi: 10.1084/jem.178.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoye JP. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat Rev Microbiol. 2012;10:395–406. doi: 10.1038/nrmicro2783. [DOI] [PubMed] [Google Scholar]
- 59.Wang-Johanning F, Radvanyi L, Rycaj K, Plummer JB, Yan P, Sastry KJ, Piyathilake CJ, Hunt KK, Johanning GL. Human endogenous retrovirus K triggers an antigen-specific immune response in breast cancer patients. Cancer Res. 2008;68:5869–5877. doi: 10.1158/0008-5472.CAN-07-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ariza ME, Williams MV. A human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis? J Invest Dermatol. 131:2419–2427. doi: 10.1038/jid.2011.217. [DOI] [PubMed] [Google Scholar]
- 61.Tai AK, Luka J, Ablashi D, Huber BT. HHV-6A infection induces expression of HERV-K18-encoded superantigen. J Clin Virol. 2009;46:47–48. doi: 10.1016/j.jcv.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 62.Fremont M, Metzger K, Rady H, Hulstaert J, De Meirleir K. Detection of herpesviruses and parvovirus B19 in gastric and intestinal mucosa of chronic fatigue syndrome patients. In Vivo. 2009;23:209–213. [PubMed] [Google Scholar]
- 63.Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006;176:7636–7644. doi: 10.4049/jimmunol.176.12.7636. [DOI] [PubMed] [Google Scholar]
- 64.Kelleher CA, Wilkinson DA, Freeman JD, Mager DL, Gelfand EW. Expression of novel-transposon-containing mRNAs in human T cells. J Gen Virol. 1996;77(Pt 5):1101–1110. doi: 10.1099/0022-1317-77-5-1101. [DOI] [PubMed] [Google Scholar]
- 65.Hokama Y, Empey-Campora C, Hara C, Higa N, Siu N, Lau R, Kuribayashi T, Yabusaki K. Acute phase phospholipids related to the cardiolipin of mitochondria in the sera of patients with chronic fatigue syndrome (CFS), chronic Ciguatera fish poisoning (CCFP), and other diseases attributed to chemicals, Gulf War, and marine toxins. J Clin Lab Anal. 2008;22:99–105. doi: 10.1002/jcla.20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klein R, Berg PA. High incidence of antibodies to 5-hydroxytryptamine, gangliosides and phospholipids in patients with chronic fatigue and fibromyalgia syndrome and their relatives: evidence for a clinical entity of both disorders. Eur J Med Res. 1995;1:21–26. [PubMed] [Google Scholar]
- 67.Tanaka S, Kuratsune H, Hidaka Y, Hakariya Y, Tatsumi KI, Takano T, Kanakura Y, Amino N. Autoantibodies against muscarinic cholinergic receptor in chronic fatigue syndrome. Int J Mol Med. 2003;12:225–230. [PubMed] [Google Scholar]
- 68.Vernon SD, Reeves WC. Evaluation of autoantibodies to common and neuronal cell antigens in Chronic Fatigue Syndrome. J Autoimmune Dis. 2005;2:5. doi: 10.1186/1740-2557-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wheatland R. Chronic ACTH autoantibodies are a significant pathological factor in the disruption of the hypothalamic-pituitary-adrenal axis in chronic fatigue syndrome, anorexia nervosa and major depression. Med Hypotheses. 2005;65:287–295. doi: 10.1016/j.mehy.2005.02.031. [DOI] [PubMed] [Google Scholar]