SUMMARY
Soft tissue sarcomas (STS) represent a diverse group of histologic subtypes with targetable molecular alterations, often treated as a single disease. Sunitinib malate is a multitargeted receptor tyrosine kinase inhibitor active in other solid tumors carrying similar alterations (i.e., imatinib mesylate-refractory gastrointestinal stromal tumors). This single-institution phase II study investigated the safety and efficacy of sunitinib malate in three common STS subtypes. Patients with documented unresectable or metastatic STS (liposarcoma, leiomyosarcoma, and malignant fibrous histiocytoma [MFH]), measurable disease, and 3 or less prior lines of therapy were eligible. Treatment consisted of sunitinib malate, 50 mg daily, for 4 weeks every 6 weeks. Forty-eight patients were enrolled, and 35% were heavily pretreated (≥2 prior lines of chemotherapy). The safety profile resembled previously known sunitinib malate toxicities. Median progression-free and overall survivals for liposarcoma, leiomyosarcoma, and MFH were 3.9 and 18.6, 4.2 and 10.1, and 2.5 and 13.6 months, respectively. The 3-month progression-free rates in the untreated and pretreated (chemotherapy) patients with liposarcoma, leiomyosarcoma, and MFH were 75% and 69.2%, 60%, and 62.5%, and and 25% and 44.4%, respectively. With the caveats that a minority of patients with potentially indolent or low-grade disease could have been included and the small numbers, a 3-month progression-free rate of >40% suggests activity for sunitinib malate at least in liposarcomas and leiomyosarcomas. Thus, we believe that further investigation in these susceptible STS subtypes is warranted.
Keywords: soft tissue sarcoma, targeted therapy, sunitinib malate, progression-free rate, survival, tyrosine kinase inhibitor
INTRODUCTION
Soft tissue sarcomas (STS) are rare malignancies that are of mesenchymal origin. With over 50 distinct subtypes, STS display considerable heterogeneity.1 Effective systemic therapies for this broad range of rare diseases have been lacking, consequently leading to poor prognosis for patients with advanced disease.
Traditionally, doxorubin has been the backbone of chemotherapy for patients with metastatic disease, with response rates from 10% to 30%.2-4 Doxorubin in combination with ifosfamide has been shown to produce higher response rates but without improvement in 1-year survival.5 Median survival for those with metastatic disease remains poor at 8 to 12 months.6
A greater molecular understanding of the distinct subtypes of STS has led to the development and introduction of novel, molecularly targeted therapies for this disease. The best example is the discovery of the gain-of-function mutation in the c-KIT cell surface transmembrane receptor in gastrointestinal stromal tumors (GIST). Treatment strategies that target such mutations (for example, imatinib mesylate, a tyrosine kinase inhibitor (TKI) of the c-KIT receptor) have shown remarkably efficacious results.7
Many subtypes of STS have been found to express various tyrosine kinase receptors and pro-angiogenic growth factors, which are considered to play a role in tumor pathogenesis and angiogenesis. For example, 45% of synovial sarcomas express platelet-derived growth factor beta (PDGF-β).8 Tumors producing vascular endothelial growth factor (VEGF) have been found in numerous STS, with one study suggesting an association between serum VEGF levels and tumor grade and survival.9
Sunitinib malate is a multi-targeted TKI shown to target PDGF receptor-alpha and -beta; VEGF receptor-1, -2, and 3; c-KIT; and FLT3. Sunitinib malate has been shown to be safe and effective both in patients with imatinib-resistant GIST and in those with metastatic renal cell carcinoma, with FDA approval for both indications. Dosing of 50 mg daily for 28 days with 2 weeks off between cycles has been shown to deliver an acceptable toxicity profile.10, 11
In this phase II study, our objective was to determine the efficacy and safety of sunitinib malate in patients with metastatic or unresectable STS. We studied some of the most frequent STS histologies (liposarcoma, leiomyosarcoma, and malignant fibrous histiocytoma [MFH]) and included fibrosarcomas, in an attempt to generate data general enough to extrapolate to the majority of STS and specific enough to drive future hypotheses.
METHODS
Patients
All patients provided signed informed consent prior to enrollment; the study was approved by our Institutional Review Board. Patients 18 years or older with unresectable and/or metastatic liposarcoma, leiomyosarcoma, fibrosarcoma, or MFH were eligible for this study. Other inclusion criteria included ECOG performance status ≤ 2, life expectancy > 16 weeks, resolution of all acute toxic effects from prior therapies (chemotherapy, radiotherapy, or surgery) to NCI CTCAE version 3.0 grade ≤ 1, AST and ALT levels ≤ 2.5 times the institutional upper limit of normal (ULN) or < 5 times ULN if malignant liver involvement is present, total serum bilirubin < 1.5 times ULN, absolute neutrophil count ≥ 1500/μL, platelet count ≥ 100,000/μL, hemoglobin concentration ≥ 9.0 g/dL, serum calcium concentration < 12.0 mg/dL, and serum creatinine < 1.5 times ULN. Additionally, patients had to display measurable disease as defined by RECIST12 and could not have had more than 3 prior chemotherapy regimens.
Key exclusion criteria included prior TKI therapy; major surgery, radiation therapy, or chemotherapy within 4 weeks of starting the study treatment; NCI CTCAE version 3 grade 3 hemorrhage within 4 weeks of starting study treatment; history of or known brain metastases or evidence of symptomatic brain or leptomeningeal disease; cardiac dysrrhythmias of NCI CTCAE grade 2 or greater; hypertension that cannot be controlled by medications (>150/100 mmHg despite optimal medical therapy); and concomitant use of agents known to induce or inhibit CYP3A4 or to be metabolized by CYP450.
Study Design
This single-institution, open-label phase II clinical trial followed a single-stage design in which the primary objective was to individually estimate the overall response rate (ORR) of previously treated patients with each of the four chosen STS cohorts to sunitinib malate. Secondary objectives included determination of progression-free survival (PFS), overall survival (OS), and safety (toxicities) of sunitinib malate in the studied STS cohorts. This trial was amended to exclude fibrosarcoma after only one patient was accrued while the other cohorts had completed 75% or more of their planned accrual. Only patients with liposarcoma, leiomyosarcoma, or MFH were enrolled after this amendment.
Patients received 50 mg of sunitinib malate orally once daily on days 1-28 of a planned 42-day cycle. Stepwise dose modifications (reductions) to 37.5 mg and 25 mg were planned by design. Grade 3 and 4 non-hematologic toxicities were treated by holding therapy until toxicity was grade ≤ 1 plus one level of dose reduction. Grade 3 hematologic toxicity (excluding lymphopenia) was treated by holding treatment until toxicity was grade ≤ 2, after which patients resumed dose at the same level. Grade 4 hematologic toxicity was treated by holding treatment until toxicity was grade ≤ 2 plus one level of dose reduction.
Efficacy Assessments
Baseline evaluations were conducted with computed tomography scans or magnetic resonance imaging, physical examination, complete blood count and differential, comprehensive metabolic panel, and ECG. Patients were re-evaluated every 6 weeks while on study treatment. Response assessments were based on the longest diameter tumor measurements in accordance with RECIST. Patients assigned the status of partial response (PR) or complete response (CR) required repeat confirmatory assessments 4-5 weeks later. Patients assigned the status of stable disease (SD) were required to meet the SD criteria at least once after study entry at a minimum interval of no less than 6 weeks.
Statistical Methods
A multiple cohort, single-stage study design was used to maximize the chance at identifying a histology in which this therapy could be efficacious. We planned to reject the null hypothesis that the response rate (CR + PR) was at ≤5% if at least four responses out of 48 patients were observed. With an alpha level of 0.10, there was a 97% chance of rejecting the null hypothesis if the true response rate was 20% and 86% power if the true response rate was 15%. Due to the diversity in the histologic presentation of STS and given that activity of sunitinib malate against one type does not necessarily imply activity against another STS type, we attempted to enroll four cohorts of size ≥ 12 patients, each representing the most common histopathologies (with a total of 48 patients to be enrolled in this study). In addition to the individual ORR result, we also planned to note the observed response rates for the four cohorts together (general response of STS to sunitinib malate).
The median survival times for PFS and OS were estimated using the Kaplan-Meier (K-M) method, but also assuming an exponential distribution, as this estimation has a smaller expected variance than when the K-M method is used.13, 14 In our study, the efficiency of the K-M fit to the exponential fit for estimating the median was 0.48, which means that it would take a sample twice as large with the K-M method (1/0.48 ≈ 2/1) to obtain an estimate as precise as with the exponential method. The Hollander-Proschan preliminary test was used to assess the suitability of the exponential distribution assumption, using α = 0.10.15 Survival comparisons between subtypes were made by the log-rank test.
RESULTS
Patient Characteristics
From September 2006 to August 2007, a total of 48 patients were enrolled on the study by the Moffitt Cancer Center Sarcoma program (ClinicalTrials.gov identifier NCT00400569). In general, the patients had been heavily pretreated previously, with a significant number receiving more than one prior chemotherapy regimen (35%) and most with multiple metastatic sites. Not surprisingly, the most common site of metastases was the lungs, but liver and bones were also frequently involved (Table 1). The median times from diagnosis to enrollment ranged from 20 to 22 months for the three subgroups.
Table 1.
Patient characteristics
| Liposarcoma | Leiomyosarcoma | MFH | |
|---|---|---|---|
| Number of patients | 18 | 15 | 14 |
| Sex, n (%) | |||
| Male | 4 (22%) | 7 (47%) | 8 (57%) |
| Female | 14 (78%) | 8 (53%) | 6 (43%) |
| Time from diagnosis to enrollment, months |
|||
| Median | 22 | 20.5 | 21 |
| Range | 4-120 | 1-336 | 9-192 |
| Sites of metastases, n | |||
| Lung | 11 | 9 | 10 |
| Liver | 6 | 1 | 1 |
| Extremity/bone | 6 | 4 | 5 |
| Abdominal | 7 | 8 | 5 |
| Prior treatments, n (%) | |||
| CTX 1 regimen | 5 (28%) | 4 (27%) | 6 (43%) |
| CTX 2 regimens | 5 (28%) | 4 (27%) | 0 (0%) |
| CTX 3 regimens | 3 (17%) | 2 (13%) | 3 (21%) |
| XRT | 7 (39%) | 5 (33%) | 10 (71%) |
MFH, malignant fibrous histiocytoma; CTX, chemotherapy; XRT, radiotherapy.
One enrolled patient with fibrosarcoma was not included.
Safety
All patients received at least one dose of sunitinib malate and therefore are included in our toxicity evaluation. Patients were enrolled on study for a median of 8 weeks, and a total of 50% required reduction of sunitinib malate dose. Twenty-one patients (44%) were dose reduced to the 37.5 mg level. Two patients (4%) were dose reduced to the 25 mg level. The primary reason for dose reduction was the occurrence of drug-related adverse events.
The most frequent adverse events are listed in Table 2. The majority of events were grade 1 or 2, with the most common symptoms being fatigue/asthenia and other gastrointestinal complaints (diarrhea, dysguesia, and dyspepsia). Other symptoms seen in at least one-third of the patients were thrombocytopenia and mucositis. Five patients had grade 3 neutropenia, 3 patients had grade 3 thrombocytopenia, 4 patients had grade 3 fatigue/asthenia, and grade 3 vomiting and dyspnea were seen in one patient each. One patient with grade 4 reduction in LVEF systolic function and one patient with grade 4 hemorrhage were observed.
Table 2.
Hematologic and non-hematologic adverse events related to sunitinib (N=48)
| Grade 1/2 | Grade 3 | Grade 4 | All | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | |
| Anorexia | 8 | 17 | 0 | 0 | 0 | 0 | 8 | 17 |
| Fatigue/asthenia | 24 | 50 | 4 | 8 | 0 | 0 | 28 | 58 |
| Diarrhea | 20 | 42 | 0 | 0 | 0 | 0 | 20 | 42 |
| Mucositis | 17 | 35 | 0 | 0 | 0 | 0 | 17 | 35 |
| Vomiting | 11 | 23 | 1 | 2 | 0 | 0 | 12 | 25 |
| Dysguesia | 18 | 38 | 0 | 0 | 0 | 0 | 18 | 38 |
| Dyspepsia | 17 | 35 | 0 | 0 | 0 | 0 | 17 | 35 |
| Thrombocytopenia | 16 | 33 | 3 | 6 | 0 | 0 | 19 | 40 |
| Neutropenia | 9 | 19 | 5 | 10 | 0 | 0 | 14 | 29 |
| Hypertension | 7 | 15 | 0 | 0 | 0 | 0 | 7 | 15 |
| Left ventricular systolic dysfunction |
0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 |
| Dyspnea | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 2 |
| Hemorrhage/bleeding | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 |
| Rash | 13 | 27 | 0 | 0 | 0 | 0 | 13 | 27 |
Efficacy
Only one PR was achieved in the leiomyosarcoma group, and the patient remained on therapy for 56 weeks. The patient presented with pulmonary metastasis from a previously resected high-grade lung primary STS and was enrolled on trial after failing to respond to an adriamycin-based regimen (2 cycles) and to docetaxel-gemcitabine (3 cycles). He was removed from study primarily due to continued toxicities (fatigue, anorexia) that had already required a previous protocol dose reduction (50 mg to 37.5 mg). His restaging scan at the time of removal showed signs of tumor growth that did not meet criteria for progressive disease. SD (assessed at 6 weeks) was achieved in 82% of liposarcoma patients, 92% of leiomyosarcoma patients, and 54% of MHF patients. Given these findings, our ORR was only 2% (Table 3). Four patients were not assessable for response.
Table 3.
Response by RECIST criteria and median progression-free and overall survival results
| Response by RECIST | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | Partial Response | Stable Disease | Progressive Disease | ||||
|
|
|||||||
| N | No. | % | No. | % | No. | % | |
| Liposarcoma | 17 | 0 | 0 | 14 | 82 | 3 | 18 |
| Leiomyosarcoma | 13 | 1 | 8 | 12 | 92 | 0 | 0 |
| MFH | 13 | 0 | 0 | 7 | 54 | 6 | 46 |
| Total | 43 | 1 | 2 | 33 | 77 | 9 | 21 |
| Progression-Free and Overall Survival | |||||
|---|---|---|---|---|---|
|
| |||||
| N | Progression-Free Survival, months | Overall Survival, months | |||
|
|
|||||
| N | Exponential, Median (95% CI) |
Kaplan-Meier, Median (95% CI) |
Exponential, Median (95% CI) |
Kaplan-Meier, Median (95% CI) |
|
| Liposarcoma | 18 | 3.4 (2.2-5.8) | 3.9 (2.8-4.4) | 16.9 (10.1-33.8) | 18.6 (5.2-27.7) |
| Leiomyosarcoma | 15 | 3.7 (2.4-6.7) | 4.2 (2.0-8.3) | 9.2 (5.7-17.3) | 10.1 (4.1-11.9) |
| MFH | 14 | 4.6 (2.8-8.6) | 2.5 (1.4-5.5) | 15.3 (9.0-31.9) | 13.6 (3.1-……) |
For RECIST results, 1 liposarcoma, 2 leiomyosarcoma, and 1 MFH patient were not assessable for response. In addition, 1 enrolled patient with fibrosarcoma was not included. For survival results, 1 enrolled patient with fibrosarcoma was not included.
Table 3 also shows the PFS and OS estimated with both the K-M and the exponential method, and the corresponding survival curves are depicted in Figures 1 and 2. In all, six survival curves were examined; the exponential distribution assumption was not rejected. The median PFS times (months) for liposarcoma, leiomyosarcoma, and MFH were 3.4, 3.7, and 4.6, respectively, and did not reach statistical significance (Figure 1). The median OS times (months) for liposarcoma, leiomyosarcoma, and MFH were 16.9, 9.2, and 15.3, respectively, and again did not reach statistical significance (Figure 2). The 3- and 6-month PFS for all subgroups were 57.5% and 25.5%, respectively. The 3-month progression-free rates (PFR) in the untreated and pretreated (chemotherapy) patients with liposarcoma, leiomyosarcoma, and MFH (as defined by the EORTC) were 75% and 69.2%, 60% and 62.5%, and 25% and 44.4%, respectively.
Figure 1.
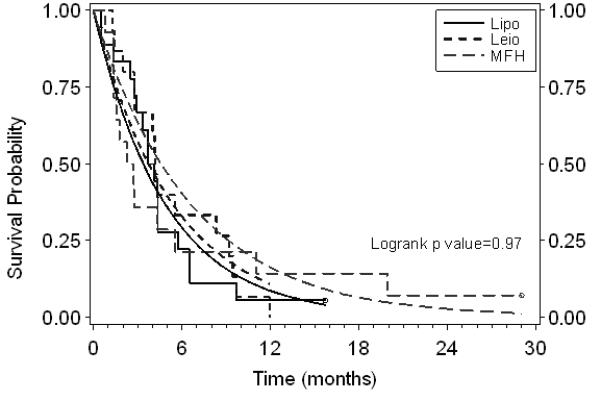
Progression-free survival by subgroup. Step functions are the Kaplan-Meier fits. Smooth curves are the exponential model fits. Censored patients are shown with open circles on Kaplan-Meier curves. :Lipo, liposarcoma; Leio, leiomyosarcoma; MFH, malignant fibrous histiocytoma.
Figure 2.
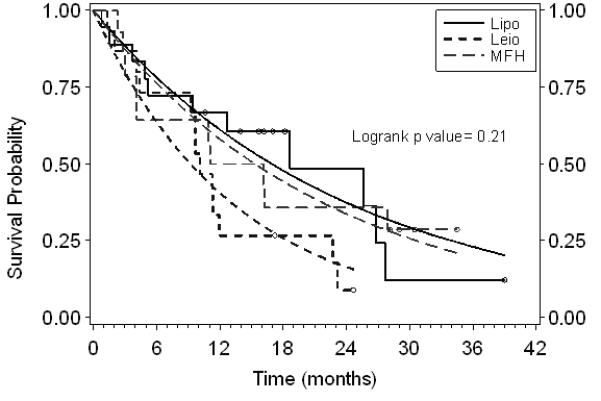
Overall survival by subgroup. Step functions are the Kaplan-Meier fits. Smooth curves are the exponential model fits. Censored patients are shown with open circles on Kaplan-Meier curves.
DISCUSSION
In this phase II study, we report toxicities similar to those described in a previous sunitinib malate study.11 Unfortunately, we failed to show a desirable ORR with sunitinib malate in three of the most frequent histologic subtypes of STS. Although this could reflect lack of the drug’s efficacy, this could also be a reflection of the population studied, that is, mostly heavily pretreated patients (68% of the patients had previously received at least one line of chemotherapy and 35% had received at least two lines), many with potentially indolent disease (the majority of patients were enrolled 20-22 months after their initial diagnosis). Furthermore, recent evidence suggests that ORR may not be the best surrogate endpoint to assess TKI drug activity in other malignancies, where, despite low ORRs, notable antitumor benefit has been reported.16
Specifically in regard to STS, van Glabbeke and the EORTC Soft Tissue and Bone Sarcoma Group have advocated replacing ORR with the “progression-free rate (PFR),” defined as “the absence of progression at a fixed point,” as a better efficacy endpoint for future phase II trials, and they have established thresholds for when a drug should be considered active in STS using the proposed PFR. The recommendation is that, in second-line or pretreated patients, a 3-month PFR ≥ 40% should suggest activity; conversely, a PFR ≤ 20% should suggest the opposite.17 Additionally, Verweij and others have suggested several other changes to be considered in future STS trials, including subdividing the patients by histology18 when drug activity is being assessed.
This change in approach, however, is not devoid of obstacles, particularly due to the relative paucity of STS patients in general and more so when patients are divided by histologies, which in turn could restrict the trial design to only a few of the most frequent histologies or to a minimum sample size so that the trial can be completed in a reasonable and/or timely manner. The consequences of the latter, of course, are well recognized, especially when endpoints like PFS are utilized. Nevertheless, subdividing trials by histologies seems necessary as drug activity in one type is no guarantee of activity in the others, especially when specific targeted therapies are studied.
A potential solution to this issue is to select a method to analyze survival that better reflects the results that would be obtained with desired larger trials. Since exponential distribution estimations have a smaller expected variance than K-M estimations, fitting PFS and OS data to an exponential model is valuable, as sample size calculations for time-to-event data may be reduced. This occurs in part because the K-M medians are obtained at the time of the first failure where the KM curve drops below 0.5, which could be well below 50% given the censoring pattern. The exponential estimates, however, are obtained right at the median. Moreover, they smooth out the step junction nature of the K-M curves. Thus, we believe that exponential estimates better reflect what would be obtained from larger trials. Additionally, in our study, the efficiency of the K-M fit to the exponential fit for estimating the median was 0.48, which means that it would take a sample twice as large with the K-M method (1/0.48 = 2.1) to obtain an estimate as precise as with the exponential method.
The exponential fit for the MFH subgroup provides a higher estimate of the median PFS than does the K-M fit (Figure 1). The difference is due to the fact that, although over half of the patients progressed rather early, two of the patients had long times to progression (> 18 months). Thus, the K-M median survival estimate fails to capture the overall PFS experience of all MFH patients compared to the other subgroups, as the exponential fit does.
Based on the EORTC PFR-established parameters for drug activity, our results suggest that sunitinib malate, despite the negligible ORR observed, may be active against leiomyosarcomas and liposarcomas and potentially also against MFH, where the median PFS observed (exponential method) was the highest. However, because progression of disease was not strictly documented, using RECIST, with two measurements prior to study enrollment, the application of the EORTC parameters is limited, as the pre-study tumor growth trajectory is unknown (potential for enrollment of very slow growing tumors).
A possible explanation for the response and relatively higher PFS seen in patients with leiomyosarcoma is the potential role that VEGF levels play in tumor angiogenesis and thus pathogenesis (studies have demonstrated higher degrees of VEGF expression in leiomyosarcoma than in leiomyomas),19 and the importance of VEGF expression in STS has been highlighted by two studies demonstrating association between immunohistochemically determined VEGF expression and tumor grade even though no association between VEGF expression and disease-free survival and OS was found.20, 21 However, another study that used quantitative enzyme-linked immunosorbent assays found that VEGF levels above the median were independently associated with poor prognosis with worse OS and metastases-free survival.22 Some of the consequences of high VEGF levels that lead to poor prognosis include accelerated cell growth, higher potency to disseminate, and chemoresistance. Increased rates of PDGF expression (also a target for sunitinib malate) have been found in leiomyosarcoma, liposarcoma, and MFH, but clear association with grade of tumor and prognosis has not been determined.23
Recently, two studies examining different multi-targeted TKIs (sorafenib and pazopanib) and one study that used sunitinib malate for non-GIST STS have been published.24-26 A comparison of these studies to ours is provided in Table 4. One PR in the leiomyosarcoma group was noted in our study, as well as in the sorafenib and pazopanib study, but not in the other sunitinib malate study. One patient with liposarcoma in the other sunitinib malate study had a durable response, with SD for 28 weeks. No such response was noted in our study group.
Table 4.
Comparison of phase II studies with tyrosine kinase inhibitors in soft tissue sarcoma
| Study/Histology | Number | ORR, % |
PFS, months |
OS, months |
|---|---|---|---|---|
| Sorafenib (Maki et al24) | 147 | 4.9 | 3.2 | 14.3 |
| Leiomyosarcoma | 37 | 2.7 | 3.2 | 22.4 |
| Synovial sarcoma | 12 | 12.5 | 2.5 | 10.3 |
| MFH/HGUPS | 12 | 0 | 2.4 | 12.2 |
| Pazopanib (Sleijfer et al23) | 142 | 6.3 | (not given) | (not given) |
| Adipocyte sarcoma | 19 | 0 | 2.6 | 6.5 |
| Leiomyosarcoma | 42 | 2.4 | 3.0 | 11.6 |
| Synovial sarcoma | 38 | 13.2 | 5.3 | 10.2 |
| Sunitinib (George et al25) | ||||
| Multiple | 48 | 2.0 | 1.8 | (not given) |
| Sunitinib (Mahmood et al, present study) |
47 | 2.1 | 3.8 | 13.6 |
| Liposarcoma | 18 | 0 | 3.9 | 18.6 |
| Leiomyosarcoma | 15 | 6.7 | 4.2 | 9.2 |
| MFH | 14 | 0 | 2.5 | 13.6 |
ORR, objective response rate.
The median PFS results for patients with leiomyosarcoma in the sorafenib, pazopanib, and our sunitinib malate study were 3.2, 3.0, and 3.7 months. OS for patients with leiomyosarcoma was 22.4 months in the sorafenib study compared with 11.6 and 9.2 months, respectively, in the pazopanib and in our sunitinib malate study. The median PFS for patients with adipocyte/liposarcoma in the pazopanib study was 2.6 months, but 3.4 months in our sunitinib malate study. The median PFS for patients with MFH in the sorafenib study was 2.4 months and 4.6 months in our study. Given the low number of patients and biases of cross-trial comparisons, definitive efficacy determinations cannot be stated; however, trends toward improved PFS can be seen with sunitinib malate in the leiomyosarcoma and liposarcoma groups.
Although this study was negative with failure to achieve goal ORR, patterns of response have emerged in our study and in others that may make evaluation of TKIs in the phase III setting possible. For instance, when retrospectively calculating 3-month PFR estimates in our study, rates above 40% were achieved in both liposarcoma and leiomyosarcoma, suggesting active drug. With the caveats that a minority of patients with potentially indolent or low grade disease may have been included and that small numbers are analyzed, a 3-month PFR >40% suggests activity for sunitinib malate at least in liposarcomas and leiomyosarcomas. Additionally, activity in leiomyosarcoma was seen in most trials, and the fact that responses and stable disease were found in heavily treated populations is encouraging. Finally, a “pause” containing dosing schedule for targeted therapies (4 weeks on and 2 weeks off sunitinib dosing schedule) has been recently questioned, and evidence now suggests that a continuous dosing schedule has the potential to be more effective.27 Together, these findings demonstrate the need to tailor specific molecularly targeted treatments to particularly susceptible subtypes of STS, which is a promising development in a previously similarly treated group of diseases.
Impact statement.
The treatment of advanced soft tissue sarcomas requires major improvements, and novel targeted therapies can potentially achieve such a goal. Clear parameters, based on survival, for the selection of “active” agents in soft tissue sarcoma have been previously proposed. In this study, we evaluate the activity of sunitinib malate in soft tissue sarcomas and utilize such parameters to conclude on the activity of the drug.
ACKNOWLEDGMENTS
We acknowledge study support and provision of study drugs from Pzifer, Inc. We thank Dr. Gang Han for conducting the Hollander-Proschan tests for exponentiality, Xiuhua Zhao for producing the figures, and Rasa Hamilton for editorial assistance.
Footnotes
CONFLICTS OF INTEREST C. Garrett served on the March 2008 Pfizer Advisory Board.
REFERENCES
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. Mosby; St. Louis, MO: 2001. [Google Scholar]
- 2.Cruz AB, Jr, Thames EA, Jr, Aust JB, Metter G, Ramirez G, Fletcher WS, Altman SJ, Frelick RW, Hill GJ., 2nd Combination chemotherapy for soft-tissue sarcomas: a phase III study. J Surg Oncol. 1979;11:313–23. doi: 10.1002/jso.2930110406. [DOI] [PubMed] [Google Scholar]
- 3.Edmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S, Parkinson DR. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–75. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 4.Santoro A, Tursz T, Mouridsen H, Verweij J, Steward W, Somers R, Buesa J, Casali P, Spooner D, Rankin E, Kirkpatrick A, Van Glabbeke M, van Oosterom A. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–45. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Younus J, Stys-Norman D, Haynes AE, Blackstein M, Members of the Sarcoma Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev. 2008;34:339–47. doi: 10.1016/j.ctrv.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Toma S, Palumbo R, Sogno G, Venturino A, Santi L. Doxorubicin (or epidoxorubicin) combined with ifosfamide in the treatment of adult advanced soft tissue sarcomas. Ann Oncol. 1992;3(Suppl 2):S119–S123. doi: 10.1093/annonc/3.suppl_2.s119. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 8.Tamborini E, Bonadiman L, Greco A, Gronchi A, Riva C, Bertulli R, Casali PG, Pierotti MA, Pilotti S. Expression of ligand-activated KIT and platelet-derived growth factor receptor beta tyrosine kinase receptors in synovial sarcoma. Clin Cancer Res. 2004;10:938–43. doi: 10.1158/1078-0432.ccr-03-0059. [DOI] [PubMed] [Google Scholar]
- 9.Hayes AJ, Mostyn-Jones A, Koban MU, A’Hern R, Burton P, Thomas JM. Serum vascular endothelial growth factor as a tumour marker in soft tissue sarcoma. Br J Surg. 2004;91:242–7. doi: 10.1002/bjs.4398. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 11.Kollmannsberger C, Soulieres D, Wong R, Scalera A, Gaspo R, Bjarnason G. Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. 2007;1(2 Suppl):S41–S54. doi: 10.5489/cuaj.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Miller RG. What price Kaplan-Meier? Biometrics. 1983;39:1077–81. [PubMed] [Google Scholar]
- 14.Meier P, Karrison T, Chappell R, Xie H. The price of Kaplan-Meier. J Am Stat Assoc. 2004;99:890–6. [Google Scholar]
- 15.Hollander M, Proschan F. Testing to determine the underlying distribution using randomly censored data. Biometrics. 1979;35:393–401. [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Van Glabbeke M, Van Glabbeke M, Verweij J, Judson I, Nielsen OS, EORTC Soft Tissue and Bone Sarcoma Group Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543–9. doi: 10.1016/s0959-8049(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 18.Verweij J. Soft tissue sarcoma trials: one size no longer fits all. J Clin Oncol. 2009;27:3085–7. doi: 10.1200/JCO.2009.21.8180. [DOI] [PubMed] [Google Scholar]
- 19.Hong T, Shimada Y, Uchida S, Itami A, Li Z, Ding Y, Kaganoi J, Komoto I, Sakurai T, Imamura M. Expression of angiogenic factors and apoptotic factors in leiomyosarcoma and leiomyoma. Int J Mol Med. 2001;8:141–8. doi: 10.3892/ijmm.8.2.141. [DOI] [PubMed] [Google Scholar]
- 20.Chao C, Al-Saleem T, Brooks JJ, Rogatko A, Kraybill WG, Eisenberg B. Vascular endothelial growth factor and soft tissue sarcomas: tumor expression correlates with grade. Ann Surg Oncol. 2001;8:260–7. doi: 10.1007/s10434-001-0260-9. [DOI] [PubMed] [Google Scholar]
- 21.Pakos EE, Goussia AC, Tsekeris PG, Papachristou DJ, Stefanou D, Agnantis NJ. Expression of vascular endothelial growth factor and its receptor, KDR/Flk-1, in soft tissue sarcomas. Anticancer Res. 2005;25:3591–6. [PubMed] [Google Scholar]
- 22.Yudoh K, Kanamori M, Ohmori K, Yasuda T, Aoki M, Kimura T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br J Cancer. 2001;84:1610–5. doi: 10.1054/bjoc.2001.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palman C, Bowen-Pope DF, Brooks JJ. Platelet-derived growth factor receptor (beta-subunit) immunoreactivity in soft tissue tumors. Lab Invest. 1992;66:108–15. [PubMed] [Google Scholar]
- 24.Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, Collin F, Pandite L, Marreaud S, De Brauwer A, van Glabbeke M, Verweij J, Blay JY. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–32. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 25.Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–40. doi: 10.1200/JCO.2008.20.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, Harmon DC, Bhuchar G, O’Mara MM, D’Adamo DR, Morgan J, Schwartz GK, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27:3154–60. doi: 10.1200/JCO.2008.20.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–96. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]


