Abstract
Background
Whether mitral valve repair (MVRep) during coronary artery bypass grafting (CABG) improves survival in patients with ischemic mitral regurgitation (MR) remains unknown.
Methods and Results
Patients with ejection fraction ≤ 35% and coronary artery disease amenable to CABG were randomized at 99 sites worldwide to medical therapy (MED) with or without CABG. The decision to treat the mitral valve during CABG was left to the surgeon. The primary endpoint was mortality. Of 1212 randomized patients, 435 (36%) had none/trace, 554 (46%) mild, 181 (15%) moderate, and 39 (3%) severe MR. In the medical arm, 70 deaths (32%) occurred in patients with none/trace, 114 (44%) with mild and 58 (50%) in moderate-severe MR. In patients with moderate-severe MR, there were 29 deaths (53%) among 55 patients randomized to CABG who did not receive mitral surgery (HR vs. MED 1.20, 95% CI 0.77–1.87) and 21 deaths (43%) among 49 patients who received mitral surgery (HR vs. MED 0.62, 95% CI 0.35–1.08). After adjustment for baseline prognostic variables, the HR for CABG with mitral surgery vs. CABG alone was 0.41 (95%CI 0.22–0.77; p=0.006).
Conclusions
While these observational data suggest that adding MVRep to CABG in patients with LV dysfunction and moderate-severe MR may improve survival compared with CABG alone or MED alone, a prospective randomized trial would be necessary to confirm the validity of these observations.
Keywords: cardiomyopathy, coronary disease, mitral valve, surgery, trials
Chronic ischemic mitral regurgitation (MR) is associated with heart failure and increased mortality.1–3 The severity of ischemic MR adversely affects survival after percutaneous or surgical myocardial revascularization.4–6 The optimal treatment strategy for ischemic MR remains controversial. European practice guidelines recommend mitral valve repair (MVRep) in patients with severe, or even moderate, ischemic MR and an ejection fraction (EF) >30% who are undergoing coronary artery bypass grafting (CABG).7 However, retrospective analyses using propensity score matching showed no survival benefit of adding MVRep to CABG.8–10 The need to add MVRep in patients with an indication for CABG becomes even less clear when MR is less severe and left ventricular (LV) dysfunction is more severe. Few data exist comparing surgical and medical management of patients with significant ischemic MR.10 To address this major knowledge gap in our understanding of ischemic MR, we analyzed the impact of MR on survival in the recently reported Surgical Treatment for Ischemic Heart Failure (STICH) trial.11
This report focuses on the 1212 STICH Hypothesis 1 patients randomized to CABG (N=610) or medical therapy (MED) (N=602). Among patients undergoing CABG, the decision to repair or not repair the mitral insufficiency was not randomized. However, as every patient had to be eligible for MED alone, the MED cohort provides a unique control group whose subsequent survival on MED alone can be compared with CABG patients with similar magnitudes of MR for whom a MVRep was an option but not required.
In this report we evaluate the prognostic influence of baseline MR severity in patients enrolled in STICH; compare survival of patients with varying degrees of MR severity by treatment assignment to MED or CABG; and compare survival among patients with moderate-severe MR who did or did not receive MVRep at the time of CABG versus those assigned to MED.
Methods
The design, protocol, and primary outcome of the STICH trial have been reported.11, 12 This multicenter non-blinded study randomized 1212 patients at 99 medical centers in 22 countries over 4.8 years.13 Eligible patients had coronary artery disease amenable to CABG and LVEF ≤ 35%. Major exclusion criteria included significant (>50%) left main disease and/or Canadian Cardiovascular Society Class 3 or 4 angina, recent acute myocardial infarction, hemodynamic instability, planned percutaneous revascularization, or aortic valvular surgery. Patients were randomly (1:1) assigned to intensive MED alone or to CABG in addition to MED.
Patients with all grades of MR could be randomized to MED with or without CABG. In patients assigned to CABG, the decision to perform adjunctive mitral surgery was left to the clinical judgment of the surgeon. The 50% chance of a MED assignment created a cohort for whom a definite need for CABG with MVRep was not apparent at the time of randomization.
The baseline case report form permitted the baseline MR grade to be characterized by the site study investigators as either “none or trace”, “mild”, “moderate”, “severe”, “or not assessed”. The STICH trial cardiovascular imaging core laboratories were staffed by investigators blinded to the randomized treatment. However, the core laboratory assessments of MR were not available to the study surgeon at the time of randomization and could not have influenced the MVRep decision in patients randomized to CABG. Therefore, we elected to use the site-reported assessment of MR severity to group patients for analysis.
All STICH surgeons were certified by the STICH Surgical Committee as having had performed at least 25 CABGs on patients with an EF <40% with operative mortality below five percent. Medical therapy was optimized under the guidance of the lead cardiologist at each site. Patients were followed up at 30 days (or at discharge, whichever occurred earlier), at four month intervals during the first year, and every six months thereafter.
Statistical Analysis
Baseline patient characteristics and details of the CABG surgery and perioperative course were summarized using the median and quartiles for continuous variables and frequencies and percentages for categorical variables. Group comparisons of continuous and ordinal variables were performed using non-parametric procedures (Wilcoxon rank-sum test for two-group comparisons and Kruskal-Wallis analysis of variance for three-group comparisons). Categorical factors were compared using the chi-square test or Fisher’s Exact Test. Cumulative mortality rates were calculated using the Kaplan-Meier method.14 All death or censoring times were measured from the time of randomization. Significance of mortality differences between patient groups was assessed using the Cox regression model.15 The Cox model provided relative risks, expressed as hazard ratios with associated 95% confidence intervals.
Mortality comparisons of the randomized treatment arms were performed according to the intention-to-treat principle. Because mitral valve surgery could be added only in those patients assigned to CABG who actually underwent the treatment assigned, the per protocol analysis of mortality in the group of patients with moderate to severe MR excluded patients who did not receive their assigned treatment within a year following randomization. All other per protocol analyses are presented in the Online Supplement.
A propensity analysis among patients with moderate or severe MR treated with CABG performed using logistic multiple regression identified baseline factors associated with subsequent performance of mitral valve surgery. The long-term mortality of the patients with moderate or severe MR undergoing CABG with versus without mitral valve surgery was compared using the Cox model, adjusting for key baseline factors to minimize the possible effects of confounding. The adjustment variables included enrollment region, age, LVEF, number of diseased vessels, New York Heart Association heart failure class, diabetes, and six-minute walk distance. Patients unable to perform the walk test were assigned a walk distance of zero.
Results
Study population
Between July 24, 2002 and May 5, 2007, 1212 patients were enrolled in the STICH H1 Trial. MR was not assessed in 3 patients. Tables 1 and 2 summarize baseline characteristics of the 1,209 patients with severity of MR reported as none or trace in 435 (36%), mild in 554 (46%), moderate in 181 (15%), and severe in 39 (3%). Increasing MR grade was associated with larger LV end-systolic volume index (ESVI), lower LVEF, higher NYHA class, shorter six-minute walk distance and higher risk at randomization score (calculated based on a model previously developed using the Duke Cardiovascular Database Registry13). (Table 1). Although enrollment was not stratified on MR severity, baseline characteristics of medically and surgically treated patients were well balanced at each level of MR (Table 2).
Table 1.
Baseline Characteristics of 1209 patients by Mitral Regurgitation Severity.
| Characteristic | None or Trace MR (n=435) |
Mild MR (n=554) |
Moderate-Severe MR (n=220) |
P Value |
|---|---|---|---|---|
| Age, median (25th, 75th), yrs. | 59 (54, 66) | 60 (54, 68) | 60 (54, 68) | 0·287 |
| Male, no. (%) | 394 (91) | 479 (86) | 188 (85) | 0·076 |
| Previous MI, no. (%) | 330 (76) | 434 (78) | 167 (76) | 0·598 |
| Hyperlipidemia, no. (%) | 278 (64) | 322 (58) | 127 (58) | 0·126 |
| Hypertension, no. (%) | 277 (64) | 331 (60) | 118 (54) | 0·046 |
| Diabetes, no. (%) | 187 (43) | 202 (37) | 87 (40) | 0·114 |
| Chronic renal disease, no. (%) | 35 (8) | 38 (7) | 21 (10) | 0·442 |
| Previous stroke, no. (%) | 34 (8) | 40 (7) | 18 (8) | 0·883 |
| Previous PCI, no. (%) | 49 (11) | 66 (12) | 41 (19) | 0·019 |
| Previous CABG, no. (%) | 11 (3) | 13 (2) | 11 (5) | 0·118 |
| Current CCS angina class, no. (%) | 0·061 | |||
| 0 | 174 (40) | 181 (33) | 84 (38) | |
| 1 | 69 (16) | 85 (15) | 33 (15) | |
| 2 | 172 (40) | 266 (48) | 87 (40) | |
| 3 | 17 (4) | 18 (3) | 13 (6) | |
| 4 | 3 (1) | 4 (1) | 3 (1) | |
| Highest NYHA class in last 3 months, no. (%) | <0.001 | |||
| I | 29 (7) | 30 (5) | 10 (5) | |
| II | 182 (42) | 191 (35) | 63 (29) | |
| III | 175 (40) | 261 (47) | 103 (47) | |
| IV | 49 (11) | 72 (13) | 44 (20) | |
| Region, no. (%) | <0·001 | |||
| Europe | 210 (48) | 344 (62) | 119 (54) | |
| US and Canada | 93 (21) | 111 (20) | 37 (17) | |
| Other | 132 (30) | 99 (18) | 64 (29) | |
| Risk at randomization* | 8 (3, 16) | 11 (5, 19) | 17 (10, 24) | <0·001 |
| CAD distribution, no. of vessels stenosed (75% criterion), no. (%) | 0·534 | |||
| ≤1 | 111 (26) | 143 (26) | 52 (24) | |
| 2 | 178 (41) | 195 (35) | 88 (40) | |
| 3 | 146 (34) | 215 (39) | 80 (36) | |
| Left main (≥ 50% stenosis), no. (%) | 7 (2) | 17 (3) | 8 (4) | 0·218 |
| Proximal LAD (≥75% stenosis), no. (%) | 318 (73) | 372 (67) | 133 (61) | 0·004 |
| Duke CAD index, median (25th, 75th), 0–100 | 65 (39, 77) | 65 (39, 77) | 59 (39, 77) | 0·474 |
| LV ejection fraction, median (25th, 75th), % | 29 (23, 35) | 27 (21, 33) | 25 (20, 32) | <0·001 |
| ESVI, median (25th, 75th), mL/m2 | 72 (57, 94) | 80 (62, 101) | 89 (65, 122) | <0·001 |
| Six-minute walk test | ||||
| Able to perform, no. (%) | 365 (85) | 488 (88) | 188 (86) | 0·260 |
| Distance walked, median (25th, 75th), m | 350 (281, 414) | 329 (250, 407) | 335 (255, 400) | 0·044 |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; ESVI, end-systolic volume index; LAD, left anterior descending; MED, medical therapy; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
The risk at randomization was estimated based on a model previously developed using data from the Duke Cardiovascular Database Registry13)
Table 2.
Baseline Characteristics of 1209 Patients by Mitral Regurgitation Severity and Randomized Treatment Assignment.
| Characteristic | None or Trace MR | Mild MR | Moderate-Severe MR | |||
|---|---|---|---|---|---|---|
| MED (n=222) |
CABG (n=213) |
MED (n=261) |
CABG (n=293) |
MED (n=116) |
CABG (n=104) |
|
| Age, median (25th, 75th), yrs. | 59 (53, 66) | 59 (54, 66) | 59 (54, 67) | 61 (54, 68) | 58 (53, 67) | 61 (55, 69) |
| Male, no. (%) | 202 (91) | 192 (90) | 226 (87) | 253 (86) | 96 (83) | 92 (88) |
| Previous MI, no. (%) | 175 (79) | 155 (73) | 207 (79) | 227 (77) | 87 (75) | 80 (77) |
| Hyperlipidemia, no. (%) | 142 (64) | 134 (64) | 161 (62) | 161 (55) | 64 (55) | 63 (61) |
| Hypertension, no. (%) | 143 (64) | 154 (63) | 160 (61) | 171 (58) | 65 (56) | 53 (51) |
| Diabetes, no. (%) | 92 (41) | 95 (45) | 96 (37) | 106 (36) | 48 (41) | 39 (38) |
| Chronic renal disease, no. (%) | 15 (7) | 20 (9) | 18 (7) | 20 (7) | 12 (10) | 9 (9) |
| Previous stroke, no. (%) | 12 (5) | 22 (10) | 20 (8) | 20 (7) | 9 (8) | 9 (9) |
| Previous PCI, no. (%) | 22 (10) | 27 (13) | 32 (12) | 34 (12) | 20 (17) | 21 (20) |
| Previous CABG, no. (%) | 4 (2) | 7 (3) | 5 (2) | 8 (3) | 4 (3) | 7 (7) |
| Current CCS angina class, No. (%) | ||||||
| 0 | 89 (40) | 85 (40) | 89 (34) | 92 (31) | 44 (38) | 40 (38) |
| 1 | 40 (18) | 29 (14) | 27 (10) | 58 (20) | 24 (21) | 9 (9) |
| 2 | 83 (37) | 89 (42) | 136 (52) | 130 (44) | 41 (35) | 46 (44) |
| 3 | 10 (5) | 7 (3) | 6 (2) | 12 (4) | 7 (6) | 6 (6) |
| 4 | 0 (0) | 3 (1) | 3 (1) | 1 (<1) | 0 (0) | 3 (3) |
| Highest NYHA class in last 3 months, no. (%) | ||||||
| I | 17 (8) | 12 (6) | 16 (6) | 14 (5) | 6 (5) | 4 (4) |
| II | 96 (43) | 86 (40) | 80 (31) | 111 (38) | 33 (28) | 30 (29) |
| III | 90 (41) | 85 (40) | 128 (49) | 133 (45) | 52 (45) | 51 (49) |
| IV | 19 (9) | 30 (14) | 37 (14) | 35 (12) | 25 (22) | 19 (18) |
| Treatment distribution by region, no. (%) | ||||||
| Europe | 114 (51) | 96 (45) | 164 (63) | 180 (61) | 62 (53) | 57 (55) |
| US and Canada | 48 (22) | 45 (21) | 49 (19) | 62 (21) | 18 (16) | 19 (18) |
| Other | 60 (27) | 72 (34) | 48 (18) | 51 (17) | 36 (31) | 28 (27) |
| Risk at randomization, median (25th, 75th) | 8 (3, 15) | 8 (3, 16) | 11 (5, 19) | 11 (6, 19) | 16 (10, 24) | 18 (11, 23) |
| CAD distribution, no. of vessels stenosed (≥ 75%), No. (%) | ||||||
| 1 | 54 (24) | 57 (27) | 75 (29) | 68 (23) | 29 (25) | 23 (22) |
| 2 | 96 (43) | 82 (38) | 86 (33) | 110 (38) | 47 (41) | 41 (39) |
| 3 | 72 (32) | 74 (35) | 101 (39) | 114 (39) | 40 (34) | 40 (39) |
| Left main (≥ 50% stenosis), no. (%) | 4 (2) | 3 (1) | 6 (2) | 11 (4) | 4 (3) | 4 (4) |
| Proximal LAD (≥75% stenosis), no. (%) | 165 (74) | 153 (72) | 177 (68) | 195 (67) | 70 (60) | 63 (61) |
| Duke CAD index, median (25th, 75th), 0–100 | 65 (39, 77) | 52 (39, 77) | 65 (39, 77) | 65 (39, 77) | 52 (39, 77) | 65 (39, 77) |
| LV ejection fraction, median (25th, 75th), % | 30 (23, 35) | 27 (22, 34) | 27 (21, 33) | 27 (22, 32) | 25 (20, 32) | 25 (20, 31) |
| ESVI, median (25th, 75th), mL/m2 | 73 (58, 91) | 71 (56, 100) | 80 (58,105) | 81 (65, 99) | 90 (66,123) | 86 (65,122) |
| Six-minute walk test | ||||||
| Able to perform, no. (%) | 195 (89) | 170 (80) | 233 (89) | 255(87) | 105 (91) | 83 (81) |
| Distance walked, median (25th, 75th), m | 350 (287, 410) | 351 (280, 416) | 324 (245, 410) | 340 (259, 402) | 345 (255, 400) | 333 (255, 399) |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; ESVI, end-systolic volume index; LAD, left anterior descending; LV, left ventricular; MED, medical therapy; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Among 104 patients assigned to CABG with site-reported moderate-severe MR, 91 underwent CABG and 49 received an adjunctive concomitant mitral valve procedure (48 repairs and one bioprosthesis implantation). Patients who received mitral valve surgery had significantly lower LVEF, larger LV ESVI, and a longer distance on the 6-minute walk test (Table 3). The extent of coronary artery disease was similar in both groups. Diabetes was more prevalent in those receiving CABG alone. The estimated risk at randomization was similar in both groups. Propensity modeling showed lower LVEF (p<0.001) and enrollment in Europe (p<0.001) to relate most strongly to receiving MVRep with CABG.
Table 3.
Baseline Characteristics of 91 patients with moderate-severe mitral regurgitation Randomized to CABG who Received CABG with versus without Mitral Valve Repair.
| Characteristic | No MV Repair (n=42) |
MV Repair (n=49) |
P Value |
|---|---|---|---|
| Age, median (25th, 75th), yrs. | 64 (56, 70) | 60 (54, 68) | 0.276 |
| Male, no. (%) | 37 (88) | 44 (90) | 1.000 |
| Previous MI, no. (%) | 31 (74) | 40 (82) | 0.369 |
| Hyperlipidemia, no. (%) | 19 (45) | 35 (73) | 0·008 |
| Hypertension, no. (%) | 21 (50) | 29 (59) | 0.380 |
| Diabetes, no. (%) | 23 (55) | 13 (27) | 0.006 |
| Chronic renal disease, no. (%) | 2 (5) | 5 (10) | 0.445 |
| Previous stroke, no. (%) | 3 (7) | 5 (10) | 0.721 |
| Previous PCI, no. (%) | 4 (10) | 14 (29) | 0.023 |
| Previous CABG, no. (%) | 2 (5) | 2 (4) | 1.000 |
| Current CCS angina class, no. (%) | 0.119 | ||
| 0 | 13 (31) | 23 (47) | |
| 1 | 3 (7) | 5 (10) | |
| 2 | 23 (55) | 17 (35) | |
| 3 | 1 (2) | 4 (8) | |
| 4 | 2 (5) | 0 (0) | |
| Highest NYHA class in last 3 months, no. (%) | 0.527 | ||
| I | 2 (5) | 2 (4) | |
| II | 14 (33) | 13 (27) | |
| III | 19 (45) | 25 (51) | |
| IV | 7 (17) | 9 (18) | |
| Region, no. (%) | <0.001 | ||
| Europe | 13 (31) | 35 (71) | |
| US and Canada | 9 (21) | 8 (16) | |
| Other | 20 (48) | 6 (12) | |
| Treatment distribution by region, no. (%) | <0.001 | ||
| Europe | 13 (27) | 35 (73) | |
| US and Canada | 9 (53) | 8 (47) | |
| Other | 20 (77) | 6 (23) | |
| Risk at randomization | 19 (15, 24) | 17 (9, 23) | 0.282 |
| CAD distribution, no. of vessels stenosed (75% criterion), No. (%) | 0.909 | ||
| 1 | 9 (21) | 12 (24) | |
| 2 | 17 (41) | 18 (37) | |
| 3 | 16 (38) | 19 (39) | |
| Left main (≥50% stenosis), no. (%) | 2 (5) | 1 (2) | 0.593 |
| Proximal LAD (≥75% stenosis), no. (%) | 26 (62) | 29 (59) | 0.791 |
| Duke CAD index, median (25th, 75th), 0–100 | 59 (43, 77) | 52 (37, 77) | 0.697 |
| LV ejection fraction, median (25th, 75th), % | 30 (25, 35) | 22 (19, 28) | <0.001 |
| ESVI, median (25th, 75th), mL/m2 | 75 (54, 109) | 97 (69, 135) | 0.008 |
| Six-minute walk test | |||
| Able to perform, no. (%) | 36 (88) | 39 (80) | 0.298 |
| Distance walked, median (25th, 75th), m | 289 (218, 385) | 353 (306, 400) | 0.042 |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; ESVI, end-systolic volume index; LAD, left anterior descending; LV, left ventricular; MI, myocardial infarction; MV, mitral valve; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Operative procedure
MR grade did not influence coronary revascularization conduct. A median of three grafts was done per patient, and 90% of patients received at least one arterial graft (Table 4). The proportion of patients with MVRep increased with MR grade. Table 5 provides operative details of the 91 patients with site-reported moderate-severe MR, who either received CABG alone or a concomitant MVRep. Of the 42 patients who underwent isolated CABG, 17 (40%) surgeries were performed without cardiopulmonary bypass. Cross clamp time, cardiopulmonary bypass time, and the duration of procedure were all longer in patients who had a concomitant MVRep compared with CABG only patients.
Table 4.
Operative Conduct and perioperative events by baseline mitral regurgitation severity of 555 patients randomized to CABG and treated surgically
| Surgical Data | None or Trace MR (N=198) |
Mild MR (N=266) |
Moderate-Severe MR (N=91) |
P Value |
|---|---|---|---|---|
| Number of distal anastomoses | 3 (2, 3) | 3 (2, 3) | 3 (2, 4) | 0.019 |
| Number of distal anastomoses, no. (%) | 0.019 | |||
| 0–1 | 25 (13) | 35 (13) | 10 (11) | |
| 2–3 | 133 (67) | 167 (63) | 49 (54) | |
| ≥4 | 40 (20) | 63 (24) | 32 (35) | |
| Arterial conduits ≥1, no. (%) | 184 (93) | 241 (91) | 80 (88) | 0.366 |
| Types of procedures on mitral valve, no. (%) | <0.001 | |||
| None | 196 (99) | 254 (95) | 42 (46) | |
| Repair | 2 (1) | 12 (5) | 48 (53) | |
| Bioprosthesis | 0 | 0 | 1 (1) | |
| Off-pump, no. (%) | 45 (23) | 54 (20) | 17 (19) | 0.695 |
| Cardioplegia, no. (%) | 0.309 | |||
| None | 55 (28) | 71 (27) | 24 (26) | |
| Crystalloid | 43 (22) | 66 (25) | 13 (14) | |
| Blood | 93 (47) | 119 (45) | 53 (58) | |
| Both | 6 (3) | 8 (3) | 1 (1) | |
| Time on aorta cross-clamp, median (25th, 75th), min | 48 (35, 64) | 51 (34, 70) | 79 (56, 110) | <0.0001 |
| Time on bypass pump, median (25th, 75th), min | 81 (63, 105) | 88 (67, 116) | 123 (95, 161) | <0.0001 |
| Total time in OR, median (25th, 75th), hrs. | 4.5 (3.8, 5.8) | 4.3 (3.5, 5,5) | 5.5 (4.4, 6.3) | <0.0001 |
| Total intubation time, median (25th, 75th), hrs. | 17 (11, 22) | 15 (11, 22) | 21 (14, 24) | <0.0001 |
| Total time in CCU/ICU, median (25th, 75th), hrs. | 49 (40, 92) | 47 (38, 90) | 91 (54, 155) | <0.0001 |
| Length of hospital stay after surgery, median (25th, 75th), days | 8 (7, 11) | 9 (7, 13) | 9 (7, 14) | 0.024 |
| Hospital stay longer than 30 days after surgery, no. (%) | 6 (3) | 14 (5) | 6 (7) | 0.331 |
| Inotropes for low CO, no. (%) | 67 (34) | 102 (38) | 47 (52) | 0.015 |
| Intraaortic balloon pump, no. (%) | 25 (13) | 40 (15) | 24 (26) | 0.010 |
| Death within 30 days after surgery, no. (%) | 6 (3) | 13 (5) | 7 (8) | 0.214 |
Abbreviations: CCU, cardiac care unit; CO, cardiac output; ICU, intensive care unit; MR, mitral regurgitation; OR, operating room.
Table 5.
Operative Conduct and perioperative events by operation received in 91 patients with moderate-severe MR who received CABG with versus without Mitral Valve Repair.
| Surgical Data | CABG Only (N=42) |
CABG+MV Repair (N=49) |
P Value |
|---|---|---|---|
| Number of distal anastomoses, median (25th, 75th) | 3 (2, 4) | 3 (3, 4) | 0.540 |
| Number of distal anastomoses, no. (%) | |||
| 1 | 6 (14) | 4 (8) | 0.540 |
| 2 | 7 (17) | 7 (14) | |
| 3 | 14 (33) | 21 (43) | |
| 4 | 11 (26) | 10 (20) | |
| 5 | 4 (10) | 7 (14) | |
| Arterial conduits ≥1, no. (%) | 36 (86) | 44 (90) | 0.552 |
| Types of procedures on mitral valve, no. (%) | |||
| Repair | 0 | 48 (98) | |
| Bioprosthesis | 0 | 1 (2) | |
| Off-pump, no. (%) | 17 (40) | 0 | |
| Cardioplegia, no. (%) | <0.001 | ||
| None | 23 (55) | 1 (2) | |
| Crystalloid | 4 (10) | 9 (18) | |
| Blood | 15 (36) | 38 (78) | |
| Both | 0 | 1 (2) | |
| Time on aorta cross-clamp, median (25th, 75th), min | 55 (47, 77) | 94 (69, 118) | <0.001 |
| Time on bypass pump, median (25th, 75th), min | 102 (84, 118) | 140 (110, 177) | 0.002 |
| Total time in OR, median (25th, 75th), hrs.) | 4.9 (4.0, 5.9) | 5.8 (4.8, 6.4) | 0.025 |
| Total intubation time, median (25th, 75th), hrs. | 19.5 (12.8, 22.8) | 22.4 (16.3, 31.0) | 0.014 |
| Total time in CCU/ICU, median (25th, 75th), hrs. | 67.0 (44.2, 109.8) | 102.7 (68.1, 188.8) | 0·001 |
| Length of hospital stay after surgery, median (25th, 75th), days | 8.0 (7.0, 11.0) | 11.0 (8.0, 17.0) | 0.011 |
| Hospital stay longer than 30 days after surgery, no. (%) | 1 (2) | 5 (10) | 0.214 |
| Inotropes for low CO, no. (%) | 14 (33) | 33 (67) | 0.001 |
| Intraaortic balloon pump, no. (%) | 8 (19) | 16 (33) | 0.142 |
| Mediastinitis, no. (%) | 2 (5) | 1 (2) | 0.593 |
| Other infection, no. (%)* | 0 | 10 (20) | 0.002 |
| Death within 30 days after surgery, no. (%) | 6 (14.3) | 1 (2.0) | 0.046 |
Refers to all other types of major postoperative infections (except mediastinitis), such as pneumonia, pyelonephritis, septicemia, and infections at the vein-harvest site.
Abbreviations: CABG, coronary artery bypass grafting; CCU, cardiac care unit; CO, cardiac output; ICU, intensive care unit; MV, mitral valve; OR, operating room.
Postoperative course
Patients with moderate-severe MR spent more time in the intensive care unit, and required more hemodynamic support with inotropes (34% vs. 38% vs. 52%; p=0.015, none or trace vs. mild vs. moderate-severe MR, respectively) and intraaortic balloon pumping (13% vs. 15% vs. 26%; p=0.010). Perioperative mortality tended to increase with higher MR grade (3% vs. 5% vs. 8%; p=0.214) (Table 4).
Patients with moderate-severe MR who had a MVRep required more intra-aortic balloon and inotrope use to support cardiac output and spent more time in the intensive care unit (median 103 vs. 67 hours; P=0.001). Median postoperative hospital stay was three days longer after MVRep. However, six (14%) CABG-only versus one (2%) CABG+MVRep (p=0.046) deaths occurred during the first 30 postoperative days (Table 5).
Adherence to guideline-based use of medication was high in all treatment cohorts throughout the study period (Supplemental Tables 3 and 4).
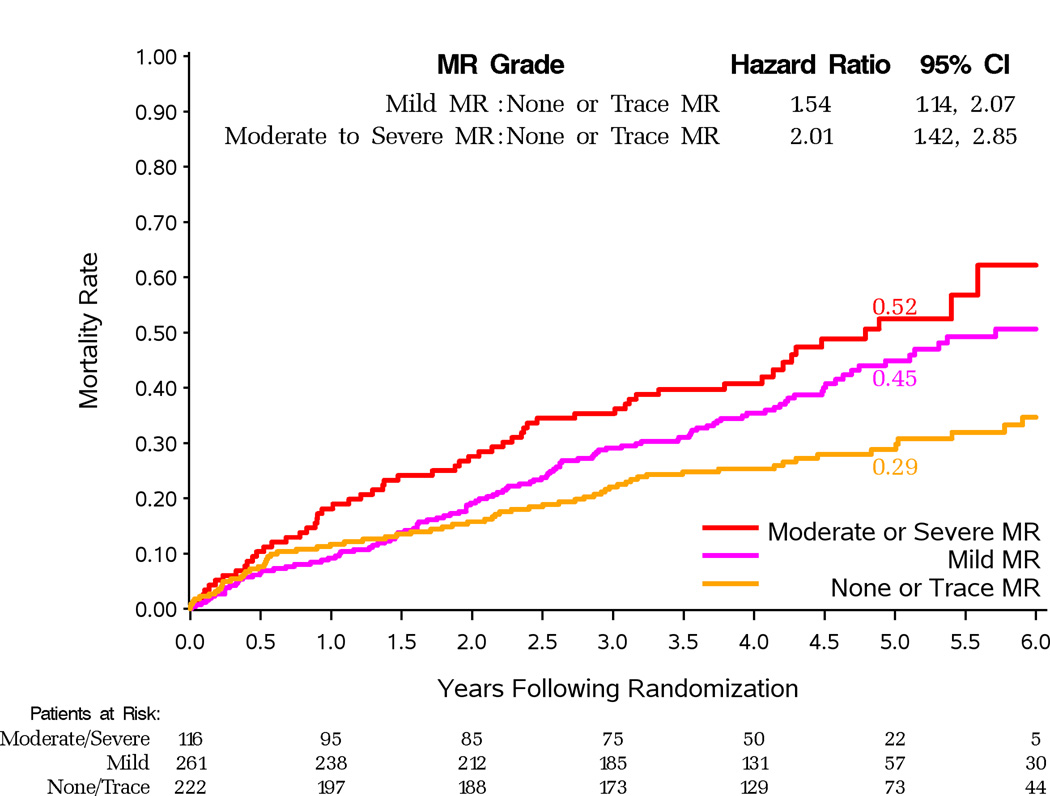
Survival
Follow-up for death was complete at a median of 56 months in the 1,209 patients with baseline MR grade reported. In the 599 patients randomized to MED alone, mortality was strongly related to MR severity at baseline (Figure 1). There were 70 deaths (32%) in 222 patients with none or trace MR, 114 (44%) in 261 with mild MR (HR vs. no MR 1.54, 95%CI 1.14–2.07) and 58 (50%) in 116 patients with moderate-severe MR (HR vs. no MR 2.01, 95%CI 1.42–2.85).
Figure 1.
Kaplan-Meier estimates of death from any cause among patients assigned to MED. Separate curves for patients with site-reported none or trace, mild, and moderate-severe MR are presented.
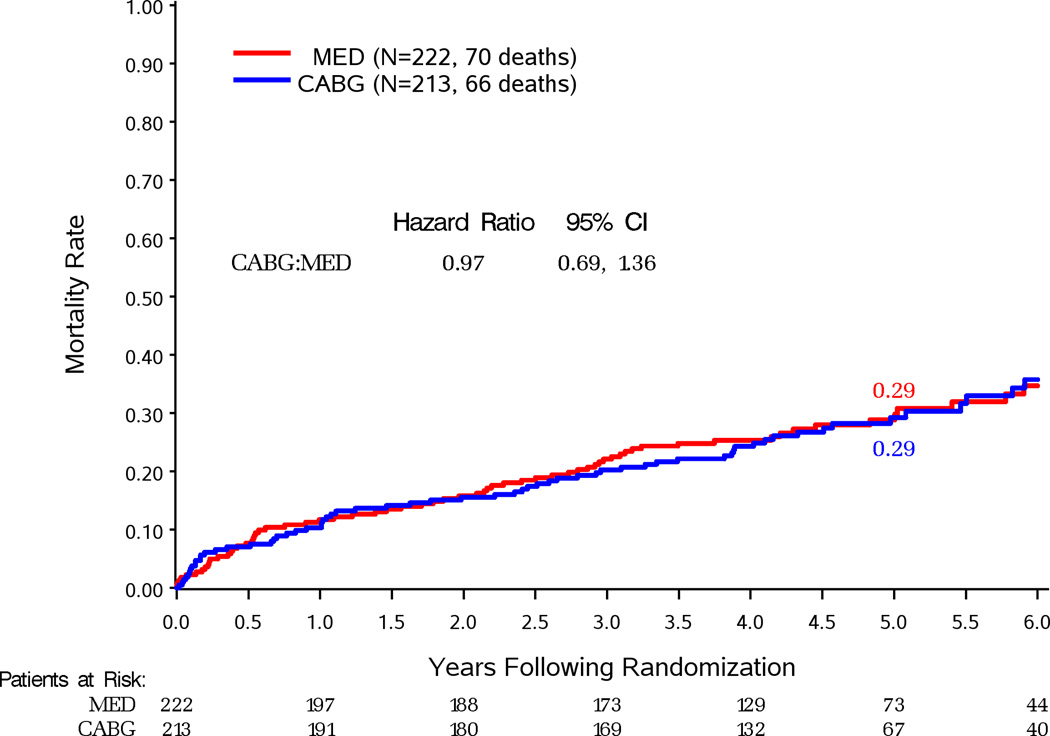
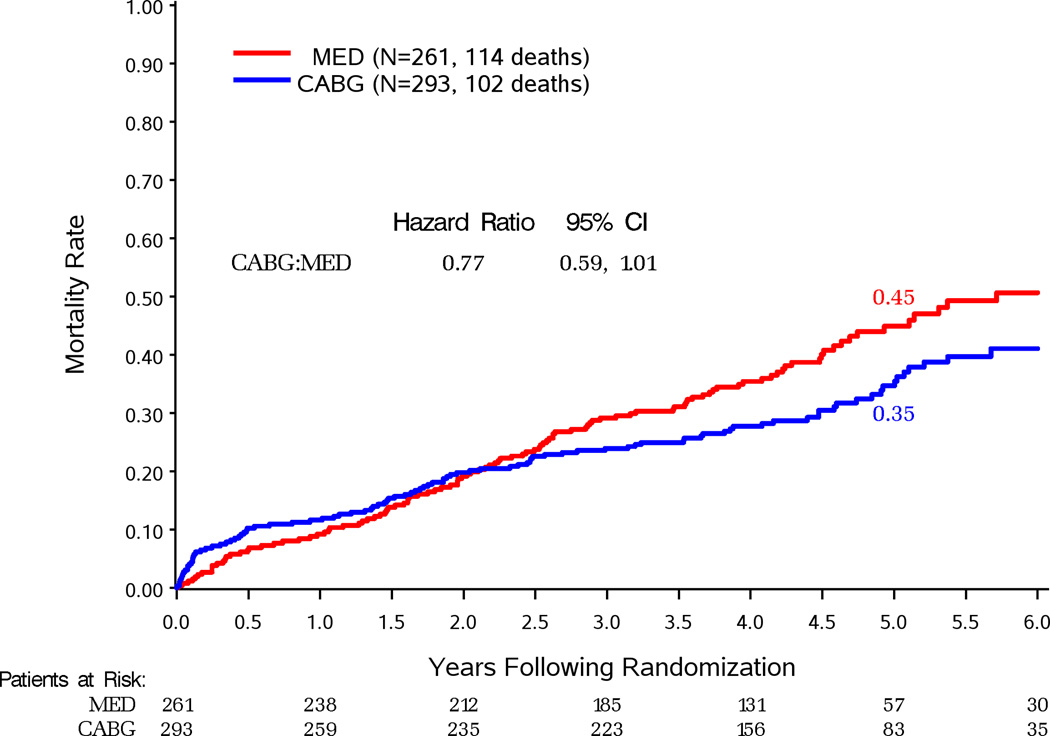
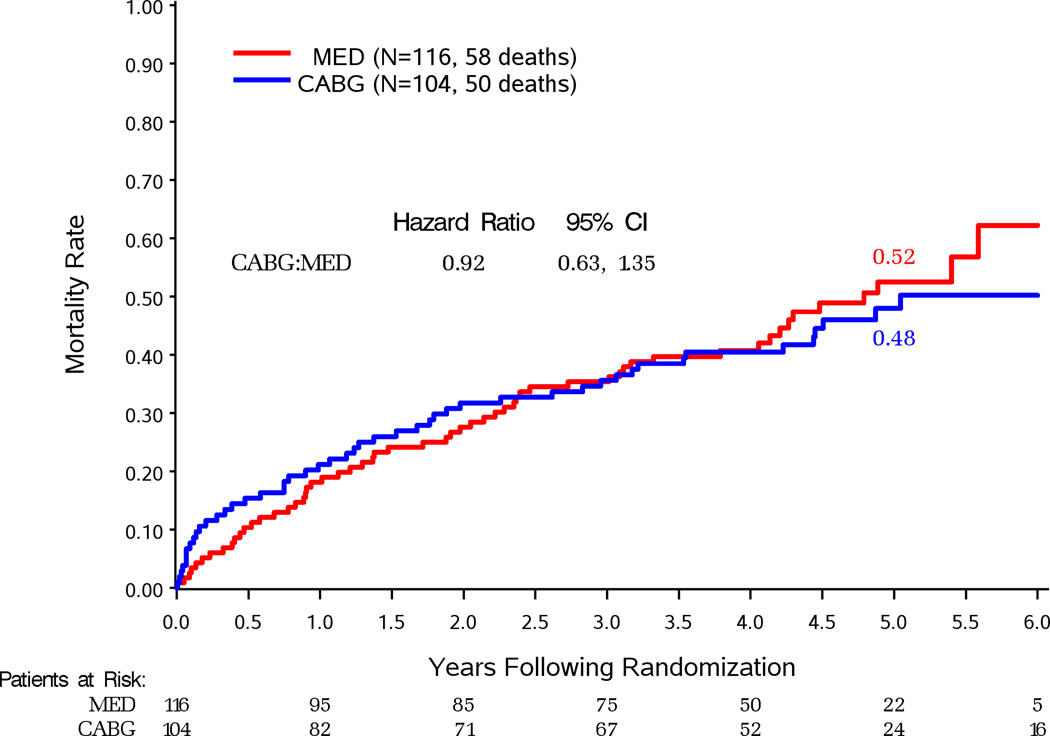
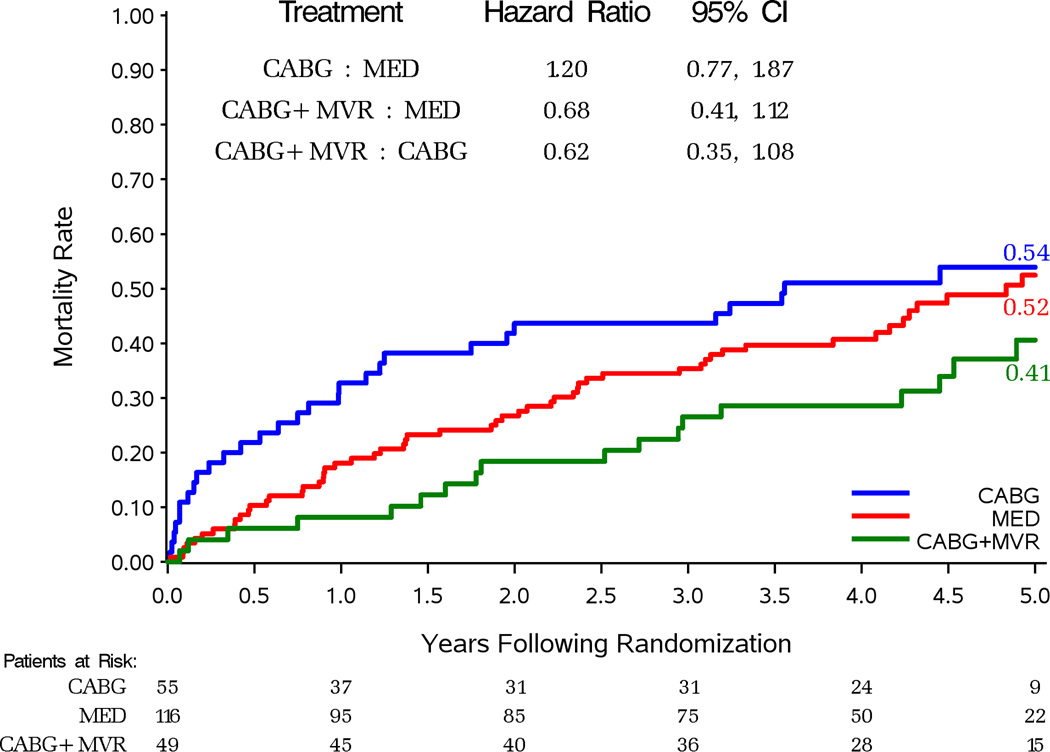
Figure 2 compares survival by randomized treatment assignment in patients grouped by their severity of MR. Death occurred in 66 (31%) of 213 patients with none or trace MR assigned to CABG (HR vs. MED 0.97, 95%CI 0.69–1.36) (Figure 2A). Death occurred in 102 (35%) of 293 patients with mild MR assigned to CABG (HR vs. MED 0.77, 95%CI 0.59–1.01; P=0.060) (Figure 2B). This difference was significant in per-protocol analysis of this subgroup (Supplemental Figure 2B). Death occurred in 50 (48%) of 104 patients with moderate-severe MR assigned to CABG (HR vs. MED 0.92, 95%CI 0.63–1.35) (Figure 2C). There were 29 (53%) deaths in the 55 CABG-assigned patients who did not receive a mitral procedure (HR vs. MED 1.20, 95%CI 0.77–1.87) and 21 (43%) deaths in 49 patients who received mitral surgery added to CABG (HR vs. MED 0.68, 95%CI 0.41–1.12) (Figure 3). There was a trend toward improved survival with performance of MVRep in addition to CABG when compared to the remaining CABG assigned patients (HR with CABG+MVRep vs. CABG only 0.62, 95%CI 0.35–1.08). Adjustment for other baseline prognostic variables further reduced the HR for CABG with MVRep compared with the remaining CABG arm patients (HR 0.41, 95%CI 0.22–0.77; p=0.006).
Figure 2.
Kaplan-Meier estimates of death from any cause in patients assigned to MED or MED and CABG with site-reported none or trace MR at baseline (A), mild MR at baseline (B), moderate-severe MR at baseline (C).
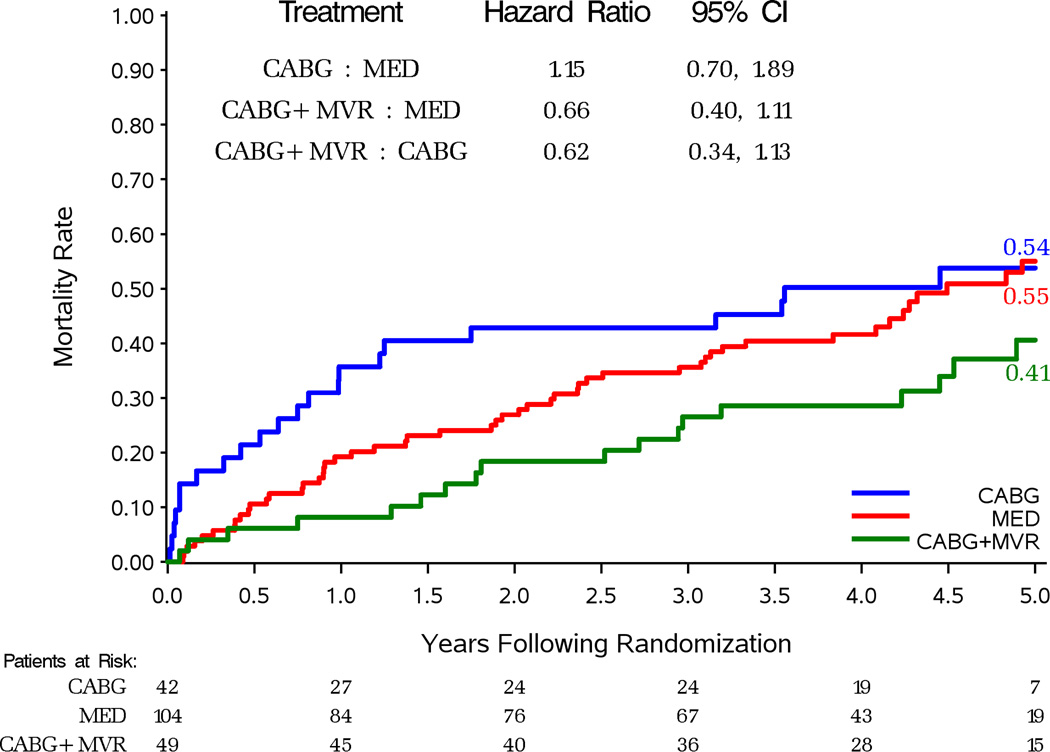
Figure 3.
Kaplan-Meier estimates of death from any cause in patients with moderate-severe MR at baseline: assigned to MED or MED and CABG who received or not mitral valve procedures (A), assigned and treated with MED or MED with CABG alone or MED with CABG and mitral valve procedure (B).
A per-protocol analysis of mortality in patients with moderate-severe MR excluded the 12 patients (10%) assigned to MED who received CABG within a year of randomization and the 13 patients (13%) assigned to CABG who did not undergo surgery. Out of 104 patients with moderate/severe MR assigned to MED and treated medically 53 died (51%). This compares to 22 deaths (52%) observed in 42 patients assigned to CABG and treated with CABG alone (HR vs. MED 1.15 95%CI 0.70–1.89). Performing MVRep at the time of CABG was associated with 21 deaths (43%) (HR vs. MED 0.66, 95%CI 0.40–1.11). A trend toward improved survival from adding MVRep to CABG (HR with CABG + MVRep vs. CABG only 0.62, 95%CI 0.34–1.13) only became significant after adjustment for other strongly prognostic baseline variables (HR 0.42, 95% CI 0.21–0.88; P=0.021).
Discussion
The STICH trial results are consistent with observational studies demonstrating that even mild MR is associated with decreased survival in medically-treated patients with ischemic cardiomyopathy.2, 3, 16 We report outcomes of an intraoperative decision to perform or not perform mitral valve surgery among ischemic cardiomyopathy patients randomized to a surgical versus medical strategy of care. Trichon at al.10 also reported an observational comparison of surgical versus medical therapy in prospectively defined ischemic MR cohorts using data from the Duke Databank for Cardiovascular Diseases. After propensity adjustment survival benefit was demonstrated when CABG was added to medical treatment in patients with 2+ or greater MR. However, no further survival advantage was observed from adding MVRep to CABG. Our results are concordant with those of Trichon’s for patients with mild MR. Other observational studies also show CABG to be adequate treatment for this group of patients.17
Other reports found that repair of ischemic MR at the time of CABG did not significantly increase perioperative mortality. Mortality was reported to be 4% in both groups by Diodato et al,8 3.5% vs. 1.8% by Goland et al,18 or 4.1% vs. 1.8% by Fattouch et al.19 In the report by Mihaljevic et al., hospital mortality was twice as high in the CABG-only group compared to the CABG with MVRep (7.4% vs. 3.7%, p=NS).9 Still, no long-term survival benefit of performing MVRep was demonstrated.
Our results differ from many reports that suggest adding MVRep to CABG increases early risk without adding long-term survival benefit.20–22 STICH patients with a MVRep added to CABG had a more complicated early postoperative period. The duration of intensive care unit stay was nearly doubled and more balloon pumps and inotropes were used postoperatively. Postoperative infections were common and 10% of CABG patients with an added MVRep were still in hospital 30 days postoperatively. Despite greater morbidity from adding MVRep to CABG, 6 of the 7 perioperative deaths in surgically-treated moderate-severe MR patients occurred in those treated with CABG-only. This suggests that there may also be harm from failure to address moderate to severe MR in patients undergoing CABG. Additional observations in larger numbers of patients undergoing CABG with moderate-severe MR are needed to confirm whether or not failure to repair moderate to severe MR in patients undergoing CABG decreases long-term survival.
Compared with other reports of operative treatment of moderate-severe ischemic MR, STICH patients had lower EF, less angina and more heart failure and were selected due to clinical equipoise regarding the need for CABG. While STICH data suggest that MVRep should be considered among STICH-like patients with moderate–severe MR undergoing CABG, our data do not support using the presence of moderate-severe MR as the primary indication for CABG. Additionally, the high 5 year mortality whatever the therapy indicates that in these patients alternative approaches including heart transplantation and left ventricular assist devices should also be considered.
Limitations
Our report is limited by the small number of patients in the moderate-severe MR cohort, and lack of a secondary randomization to MVRep. However, our study reports outcomes observed in a very well defined and prospectively followed group of patients in whom the decision to receive surgical treatment was randomized. Many confounding factors could influence the operative decision to perform or not perform MVRep. We cannot exclude the possibility, that surgeons were more reluctant to perform mitral procedure in less healthy patients. A tendency for STICH surgeons to perform CABG alone in higher risk patients and to add a mitral procedure to CABG in less risky patients might diminish survival in the CABG only cohort and enhance survival in the CABG+MVRep group with an intermediate outcome in those randomized to MED. Indeed we noted more diabetes among the patients in whom surgeons decided not to treat the mitral valve. On the other hand the risk at randomization index was similar in both groups. The patients subjected to MVRep had larger left ventricles on average, and lower ejection fractions. It appears that the main factor related to surgeon’s decision was the region of the world where the patient was enrolled. Moreover, statistical adjustment for baseline characteristics of STICH patients actually accentuated the survival advantage of CABG+MVRep over CABG alone. Still there are factors that could not be adjusted for in multivariable modeling. Information on surgical intraoperative decision making relative to MVRep was not collected and the potential for confounding based on operative conduct exists. While 40% of CABG only procedures were performed off pump, suggesting preoperative decisions against mitral valve surgery, intraoperative events cannot be excluded as a source of bias against CABG without MVRep.
Bearing in mind all of these limitations, we believe that these STICH patients are a uniquely valuable prospectively defined observational cohort typical of patients for whom some cardiac surgeons would and other cardiac surgeons would not repair the mitral valve. Moreover, despite not randomizing the MVRep decision, the two surgical groups in patients with moderate or severe baseline MR have similar numbers of patients with quite comparable baseline characteristics, risk at randomization index, and quality of revascularization. Our report provides the only prospectively defined survival comparison of adding MVRep to CABG in patients who were all defined as eligible for CABG but half of whom did not receive CABG by random assignment. The only other randomized comparison on this topic in the literature deals with patients with clear indications for CABG and minimal LV dysfunction.19 An ongoing Cardiothoracic Surgical Trials Network (CTSN) trial is now randomizing patients with moderate MR and a primary indication for CABG to mitral valve repair versus no repair. This trial is powered for a primary endpoint of reduction in LV ESVI, not mortality.23
Our data support the need for a randomized trial of medical vs. surgical therapy involving CABG with mitral valve repair in patients with low ejection fraction due to ischemic cardiomyopathy and at least moderate MR that would address survival as a primary endpoint. Our data also provide valuable survival information needed for estimating the cohort size necessary for a definitive trial. Executing such a trial is likely to be very challenging, however. A trial in which patients with moderate/severe MR before CABG are randomized to undergo or not MVRep has a high likelihood of being affected by a sizable number of intraoperative crossovers. Randomizing patients with a significant degree of MR to medical therapy may be even more difficult as evidenced by the small percentage of patients with moderate/severe MR that were included in the STICH trial. Thus our results may remain for a long time as the only comparison that has a well-defined medical cohort as a comparator group. Use of clinical site-reported MR severity rather than that reported by the STICH Echocardiography core laboratory may be viewed as a limitation or a strength of this report. The core lab result was a single time point reading not available to the operating surgeon. MR often varies with exercise, blood volume status, and afterload.24, 25 Many cardiac surgeons insist that an operative decision for MR should not be made based on a single echocardiography reading, particularly only an intraoperative one.26 The influence of the preoperative grading of MR on subsequent five-year survival in both medical and surgical randomized cohorts demonstrates site-reported MR severity to be strongly related to survival in medically and surgically treated patients.
Based on these observational data from the STICH trial, adding mitral valve repair to CABG in patients with LV dysfunction and moderate/severe MR may improve survival compared with CABG alone or MED alone. However, because of possible confounding by indication, a prospective randomized trial would be justified to confirm the validity of these observations.
Supplementary Material
Chronic ischemic mitral regurgitation (MR) is associated with heart failure and increased mortality. The optimal treatment strategy for ischemic MR remains controversial. European practice guidelines recommend mitral valve repair (MVRep) in patients with severe, or even moderate, ischemic MR and an ejection fraction (EF) <30% who are undergoing coronary artery bypass grafting (CABG), even though retrospective analyses using propensity score matching showed no survival benefit of adding MVRep to CABG. The need to add MVRep in patients with an indication for CABG becomes even less clear when left ventricular (LV) dysfunction is more severe. The STICH trial randomized 1212 patients with severe LV dysfunction (EF<35%) and coronary artery disease amenable to CABG to intensive medical therapy alone or in association with CABG. The decision to repair the mitral valve was left to the operating surgeon. The survival in the medically treated cohort depended strongly on MR grade at baseline with mortality hazard being increased twice in patients with moderate/severe MR in comparison to patients with no MR. In patients with mild MR CABG was associated with improved survival. In patients with moderate/severe MR adding mitral valve repair to CABG tended to improve survival compared with CABG alone or medical therapy alone. Unfortunately, the decision to repair the valve was not randomized and therefore, even though risk-adjustment actually accentuated the difference of survival in favor of adding MVRep, a randomized trial is required to confirm our findings.
Acknowledgements
The authors acknowledge the support of Vanessa Moore and Krysti Byrd in preparation of the manuscript.
Funding Sources: The trial was sponsored by the National Heart, Lung and Blood Institute (NHLBI). Additional support was provided by Abbott Laboratories, which had no role in the conduct or reporting of the trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: PAG is a consultant at Abbott Vascular Structural Heart, and BS is a consultant for Thoratec, St Jude, Cormatrx, and Abbot Laboratories. EJV has received grant funding from Abbott Vascular Structural Heart.
References
- 1.Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, Roger VL. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 2.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 3.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and Ventricular Enlargement Investigators. Circulation. 1997;96:827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 4.Di Mauro M, Di Giammarco G, Vitolla G, Contini M, Iaco AL, Bivona A, Weltert L, Calafiore AM. Impact of no-to-moderate mitral regurgitation on late results after isolated coronary artery bypass grafting in patients with ischemic cardiomyopathy. Ann Thorac Surg. 2006;81:2128–2134. doi: 10.1016/j.athoracsur.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Fattouch K, Sampognaro R, Speziale G, Salardino M, Novo G, Caruso M, Novo S, Ruvolo G. Impact of moderate ischemic mitral regurgitation after isolated coronary artery bypass grafting. Ann Thorac Surg. 2010;90:1187–94. doi: 10.1016/j.athoracsur.2010.03.103. [DOI] [PubMed] [Google Scholar]
- 6.Pastorius CA, Henry TD, Harris KM. Long-term outcomes of patients with mitral regurgitation undergoing percutaneous coronary intervention. Am J Cardiol. 2007;100:1218–1223. doi: 10.1016/j.amjcard.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 7.Wijns W, Kolh P, Danchin N, Di MC, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa UM, Taggart D, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Kolh P, Alfieri O, Dunning J, Elia S, Kappetein P, Lockowandt U, Sarris G, Vouhe P, Kearney P, von SL, Agewall S, Aladashvili A, Alexopoulos D, Antunes MJ, Atalar E, Brutel de la RA, Doganov A, Eha J, Fajadet J, Ferreira R, Garot J, Halcox J, Hasin Y, Janssens S, Kervinen K, Laufer G, Legrand V, Nashef SA, Neumann FJ, Niemela K, Nihoyannopoulos P, Noc M, Piek JJ, Pirk J, Rozenman Y, Sabate M, Starc R, Thielmann M, Wheatley DJ, Windecker S, Zembala M. Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 8.Diodato MD, Moon MR, Pasque MK, Barner HB, Moazami N, Lawton JS, Bailey MS, Guthrie TJ, Meyers BF, Damiano RJ., Jr Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78:794–799. doi: 10.1016/j.athoracsur.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Mihaljevic T, Lam BK, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM, Blackstone EH, Lytle BW. Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol. 2007;49:2191–2201. doi: 10.1016/j.jacc.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Trichon BH, Glower DD, Shaw LK, Cabell CH, Anstrom KJ, Felker GM, O'Connor CM. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation. 2003;108(Suppl 1):II103–II110. doi: 10.1161/01.cir.0000087656.10829.df. [DOI] [PubMed] [Google Scholar]
- 11.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, Yii M, Prabhakaran D, Szwed H, Ferrazzi P, Petrie MC, O'Connor CM, Panchavinnin P, She L, Bonow RO, Rankin GR, Jones RH, Rouleau JL. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velazquez EJ, Lee KL, O'Connor CM, Oh JK, Bonow RO, Pohost GM, Feldman AM, Mark DB, Panza JA, Sopko G, Rouleau JL, Jones RH. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones RH, White H, Velazquez EJ, Shaw LK, Pietrobon R, Panza JA, Bonow RO, Sopko G, O'Connor CM, Rouleau JL. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol. 2010;56:490–498. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 16.Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–543. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 17.Tolis GA, Jr, Korkolis DP, Kopf GS, Elefteriades JA. Revascularization alone (without mitral valve repair) suffices in patients with advanced ischemic cardiomyopathy and mild-to-moderate mitral regurgitation. Ann Thorac Surg. 2002;74:1476–1480. doi: 10.1016/s0003-4975(02)03927-9. [DOI] [PubMed] [Google Scholar]
- 18.Wong DR, Agnihotri AK, Hung JW, Vlahakes GJ, Akins CW, Hilgenberg AD, Madsen JC, MacGillivray TE, Picard MH, Torchiana DF. Long-term survival after surgical revascularization for moderate ischemic mitral regurgitation. Ann Thorac Surg. 2005;80:570–577. doi: 10.1016/j.athoracsur.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Goland S, Czer LS, Siegel RJ, DeRobertis MA, Mirocha J, Zivari K, Kass RM, Raissi S, Fontana G, Cheng W, Trento A. Coronary revascularization alone or with mitral valve repair: outcomes in patients with moderate ischemic mitral regurgitation. Tex Heart Inst J. 2009;36:416–424. [PMC free article] [PubMed] [Google Scholar]
- 20.Kang DH, Kim MJ, Kang SJ, Song JM, Song H, Hong MK, Choi KJ, Song JK, Lee JW. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation. 2006;114:I499–I503. doi: 10.1161/CIRCULATIONAHA.105.000398. [DOI] [PubMed] [Google Scholar]
- 21.Schurr P, Boeken U, Limathe J, Akhyari P, Feindt P, Lichtenberg A. Impact of mitral valve repair in patients with mitral regurgitation undergoing coronary artery bypass grafting. Acta Cardiol. 2010;65:441–447. doi: 10.2143/AC.65.4.2053903. [DOI] [PubMed] [Google Scholar]
- 22.Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E, Calvaruso D, Ruvolo G. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278–285. doi: 10.1016/j.jtcvs.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 23. http:,clinicaltrials.gov/ct2/show/NCT00806988.7-10-2011.
- 24.Levine RA. Dynamic mitral regurgitation--more than meets the eye. N Engl J Med. 2004;351:1681–1684. doi: 10.1056/NEJMe048165. [DOI] [PubMed] [Google Scholar]
- 25.Lancellotti P, Gerard PL, Pierard LA. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. Eur Heart J. 2005;26:1528–1532. doi: 10.1093/eurheartj/ehi189. [DOI] [PubMed] [Google Scholar]
- 26.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–758. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.