Abstract
A general method for the Pd-catalyzed coupling of methanol with (hetero)aryl halides is described. The reactions proceed under mild conditions with a wide range of aryl and heteroaryl halides to give methyl aryl ethers in high yield.
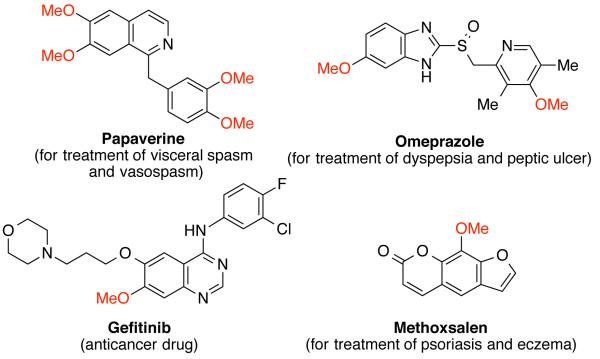
Methyl aryl ethers, including anisoles and methoxy-substituted heteroarenes, are structural components of natural products,1 pharmaceuticals,2,3 and agrochemicals.4 Figure 1 shows some examples of approved drug molecules containing methyl aryl ethers.2 The replacement of methoxy groups with trideuteriomethoxy groups in drug molecules can alter the metabolic activity of drugs and thus enhance their pharmaceutical potency.5 Therefore, the development of techniques for the intermoleculer construction of C-O bonds to synthesize methyl aryl ethers is of particular importance.
Figure 1.
Selected pharmaceutical molecules containing the methyl aryl ether motifs.
To date, various synthetic methods have been discovered for the preparation of methyl aryl ethers. These include nucleophilic aromatic substitution of aromatic halides with alkali metal methoxides,6 the Williamson7 and Mitsunobu8 ether syntheses, as well as the Brönsted/Lewis acid-mediated condensation between methanol and phenols.9 These traditional methods often require harsh reaction conditions and/or the use of toxic methylating agents (MeI, Me2SO4), and sometimes have limited substrate scope. Recently, the multistep synthesis of methyl aryl ethers via Pd-catalyzed arylation of hydroxide followed by the methylation of the resulting phenols has been reported.10 Methoxy-substituted silanes [Si(OMe)3H,11 Si(OMe)412] are also utilized as the methanol surrogates in Pd-catalyzed cross-coupling with aryl halides11 and in copper-catalyzed decarboxylative arylation with aromatic carboxylic acids.12 However, these methods are limited to electron-deficient and/or ortho-substituted (e.g., NO2, Me, OMe) aryl coupling partners.10b,11,12
The cross-coupling between methanol and aryl halides represents a direct, convenient, and atom-economical approach to synthesize methyl aryl ethers. We have previously reported two examples of Pd-catalyzed coupling of methanol with electron-deficient aryl halides.13 In 2012, Beller14 and Peruncheralathan5c reported more general Pd-catalyzed coupling of methanol and methanol-d4 with aryl halides to access a broader range of anisoles and methoxypyridines and their trideuteriomethoxy derivatives, by using sterically-demanding BippyPhos-based phosphine ligand and tBuXPhos ligand, respectively. In addition, copper-catalyzed coupling of methanol with aryl iodides also serves as a convenient method to prepare anisoles.15 Despite these advances, the substrate scope is generally limited to aryl halides and halopyridines. Moreover, a relatively high temperature (≥ 70 °C) is usually required. Herein, we report an improved, general method for the synthesis of a broader range of methyl aryl ethers and their trideuteriomethyl derivatives under mild reaction conditions enabled by the use of a bulky biarylphosphine ligand and a palladacycle precatalyst.
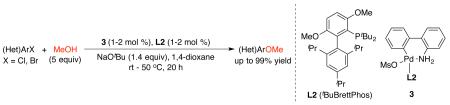
We recently reported an efficient arylation of primary and secondary alcohols to synthesize a variety of alkyl aryl ethers, using a Pd catalyst based on the bulky biarylphosphine ligand RockPhos (Table 1, L1).16,17 L1 could promote the productive reductive elimination of the proposed (L1)Pd(aryl)(alkoxy) intermediate relative to the undesirable, competing β-hydride elimination of the alkoxy group, thus facilitating the formation of alkyl aryl ethers rather than the arene side-products.16 Thus, we began our studies by examining the reaction of 4-chloroanisole with 5 equivalents of methanol in 1,4-dioxane at 100 °C, using a strong base, KOtBu, and a Pd catalyst based on Pd2dba3 (2 mol % Pd) and L1 (4 mol %) (Table 1, entry 1). Under the reaction conditions, the coupling reaction proceeded smoothly to give the desired product, 1,4-dimethoxybenzene (1), in excellent yield along with the minimal formation of anisole side-product (2). Similarly, the use of the structurally related BrettPhos-based ligands, tBuBrettPhos (L2)17 and AdBrettPhos (L3),18 also promoted the reaction to give 1 in excellent yields (Table 1, entries 2 and 3). In contrast, when the smaller ligands, BrettPhos (L4) and tBuXPhos (L5), were employed, the ratio of 1 to 2 dropped significantly (Table 1, entries 4 and 5), whereas the use of conformationally more rigid ligand Me4tBuXPhos (L6) resulted in a very low conversion (Table 1, entry 6). As L2 is available on kilogram scale, we selected it for subsequent studies.
Table 1.
Ligand Screen for the Pd-Catalyzed Arylation of MeOHa

| entry | ligand | temp (°C) |
conv (%)b |
yield of 1 (%)b |
yield of 2 (%)b |
|---|---|---|---|---|---|
| 1 | L1 | 100 | 100 | 93 | 7 |
| 2 | L2 | 100 | 100 | 92 | 7 |
| 3 | L3 | 100 | 100 | 93 | 7 |
| 4 | L4 | 100 | 53 | 13 | 40 |
| 5 | L5 | 100 | 100 | 73 | 27 |
| 6 | L6 | 100 | 27 | 20 | 7 |
| 7 | L2 | 80 | 100 | 96 | 4 |
| 8c | L2 | 80 | 94 | 85 | 7 |
| 9d | L2 | 80 | 100 | 93 | 7 |
| 10d | L2 | 50 | 100 | 94 | 6 |
| 11d | L2 | rt | 50 | 48 | 2 |
| 12e | L2 | rt | 100 | 95 | 5 |
Reaction conditions: 4-Chloroanisole (0.25 mmol), MeOH (1.25 mmol), NaOtBu (0.35 mmol), Pd2dba3 (1 mol %), ligand (4 mol %), 1,4-dioxane (0.5 mL, 0.50 M), 20 h.
Determined by GC.
Pd2dba3 (0.5 mol %), L2 (2 mol %), 24 h.
3 (1 mol %), L2 (1 mol %).
3 (2 mol %),L2 (2 mol %).
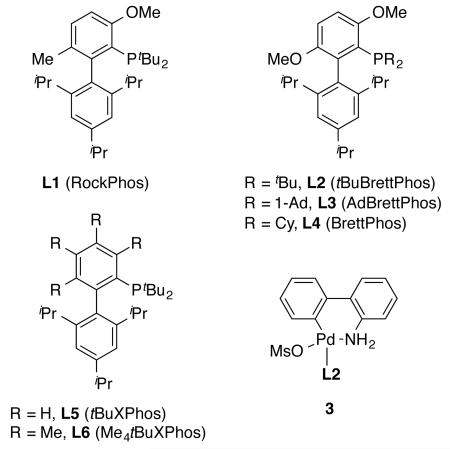
In light of the recent success of the use of aminobiphenyl palladacycle precatalysts to promote cross-coupling reactions under mild conditions,19 we utilized the precatalyst 3,19b which contains L2 pre-ligated to the Pd center, in the coupling reaction of methanol. While the Pd catalyst based on Pd2dba3/L2 still efficiently catalyzed the reaction at 80 °C (Table 1, entry 7), incomplete conversion of substrate was seen when the loading of Pd2dba3 was decreased to 0.5 mol % (1 mol % Pd) (Table 1, entry 8). In contrast, 1 mol % precatalyst 3 was sufficient to catalyze efficiently the reaction to afford 1 in excellent yield under otherwise identical conditions and even at 50 °C (Table 1, entries 9 and 10). At a higher loading of 3 (2 mol %), the reaction proceeded smoothly at room temperature (Table 1, entries 11 and 12).
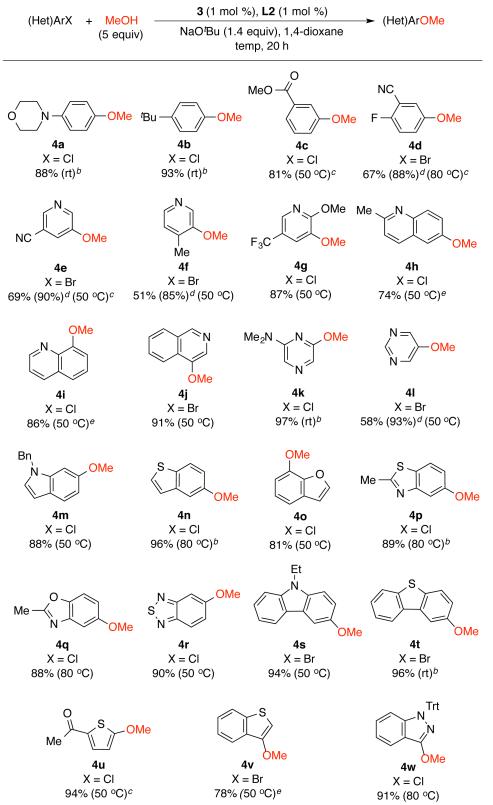
Next, we explored the scope of the Pd-catalyzed synthesis of methyl aryl ethers based on the use of precatalyst 3 (1-2 mol %) at 50 °C or at ambient temperature (Scheme 1). Both electron-rich and -deficient aryl halides could be coupled with methanol to afford anisoles in high yields (4a-d). Additionally, a wide range of heteroaryl halides were shown to be suitable coupling partners, including pyridines (4e-4g), (iso)quinolines (4h-4j), pyrazines (4k), pyrimidines (4l), indoles (4m), benzothiophenes (4n), benzofurans (4o), benzothiazoles (4p), benzoxazoles (4q), benzo-2,1,3-thiadiazoles (4r), carbazoles (4s), and dibenzothiophenes (4t). Significantly, the Pd-catalyzed coupling of methanol with five-membered heteroaryl halides was also demonstrated (4u-4w). Sterically hindered substrates were well-tolerated under the reaction conditions (4f, 4g, 4i, 4j), while the use of a weaker base, Cs2CO3, in place of NaOtBu, is necessary when the coupling partners contain base-sensitive functional groups (4c, 4d, 4e, 4u). For all substrates, we observed an excellent selectivity for the formation of methyl aryl ethers over the reduced products.20
Scheme 1.
Pd-catalyzed arylation of methanola
a Reaction conditions: (Het)ArX (1 mmol), MeOH (5 mmol), NaOtBu (1.4 mmol), 3 (1 mol %), L2 (1 mol %), 1,4-dioxane (2 mL, 0.50 M); isolated yields, average of two runs. b 3 (2 mol %), L2 (2 mol %). c Cs2CO3 (1.5 equiv). d 1H NMR yield of crude product. e An inseparable mixture of ether product and heteroarene (HetArH, 2-4%) was isolated.
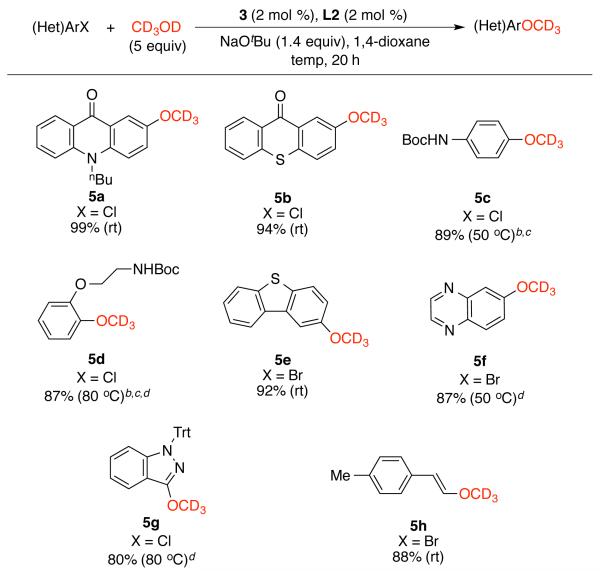
The reaction protocol based on precatalyst 3 also allowed for the coupling of methanol-d4 with a variety of (hetero)aryl halides under mild conditions, forming the corresponding trideuteriomethyl aryl ethers in good yields (Scheme 2, 5a-5g). A trideuteriomethyl vinyl ether could be prepared as well (5h).
Scheme 2.
Pd-catalyzed arylation of methanol-d4a
aReaction conditions: (Het)ArX (1 mmol), CD3OD (5 mmol), NaOtBu (1.4 mmol), 3 (2 mol %), L2 (2 mol %), 1,4-dioxane (2 mL, 0.50 M); isolated yields, average of two runs. b Cs2CO3 (1.5 equiv). c After the reaction, the reaction mixture was further heated with H2O (100 equiv) for 20 min at the reported temperature to form RNHBoc. d 3 (1 mol %) and L2 (1 mol %).
The homologue of methanol, ethanol, has seldom been studied in the Pd-catalyzed C-O coupling reactions.21 By utilizing the reaction protocol described above (Scheme 1), we found that the coupling of ethanol with heteroaryl halides proceeded to afford the ethoxy aryl ethers in good yields at ambient conditions (Scheme 3).
Scheme 3.
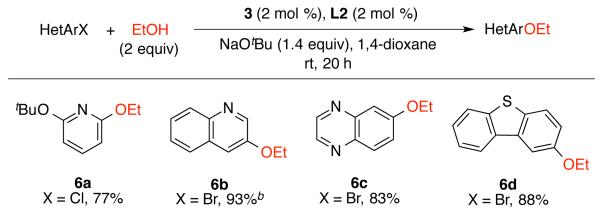
Pd-catalyzed arylation of ethanola
a Reaction conditions: (Het)ArX (1 mmol), EtOH (2 mmol), NaOtBu (1.4 mmol), 3 (2 mol %), L2 (2 mol %), 1,4-dioxane (2 mL, 0.50 M); isolated yield, average of two runs. bEtOH (5 equiv).
In conclusion, we have developed a method for the palladium-catalyzed arylation of methanol and methanol-d4 under mild conditions, with excellent selectivity for the formation of a variety of methyl- and deuteriomethyl aryl ethers. We also preliminarily demonstrated the palladium-catalyzed arylation of ethanol at room temperature. We expect these methods to be widely applicable to the synthesis of biologically active molecules containing the methoxy and deuteriomethoxy groups.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (GM58160) for financial support of this project. We are grateful to Drs J. Robb DeBergh (M.I.T.) and Nathan Jui (M.I.T.), and James Colombe (M.I.T.), for help with preparation of this manuscript. We acknowledge Nicholas C. Bruno (M.I.T.) for providing the Pd precatalyst 3 used in this study, and Dr. Naoyuki Hoshiya (M.I.T.) for the preparation of L2. C.W.C. thanks the Croucher Foundation (Hong Kong) for a postdoctoral fellowship. The Bruker 400 MHz NMR spectrometer used in this work was supported by the National Science Foundation (Grants CHE-9808061 and DBI-9729592). M.I.T. has patents on some of the ligands and precatalysts used in this work from which S.L.B. receives royalty payments.
Footnotes
Supporting Information Available. Experimental procedures along with experimental and spectroscopic data for all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Zhu J. Synlett. 1997:133–144. [Google Scholar]; (b) Manzan ACCM, Toniolo FS, Bredow E, Povh NP. J. Agric. Food Chem. 2003;51:6802–6807. doi: 10.1021/jf030161x. [DOI] [PubMed] [Google Scholar]; (c) Renouard S, Lopez T, Hendrawati O, Dupre P, Doussot J, Falguieres A, Ferroud C, Hagege D, Lamblin F, Laine E, Hano C. J. Agric. Food Chem. 2011;59:8101–8107. doi: 10.1021/jf201410p. [DOI] [PubMed] [Google Scholar]; (d) Ovenden SPB, Nielson JL, Liptrot CH, Willis RH, Tapiolas DM, Wright AD, Motti CA. J. Nat. Prod. 2011;74:1335–1338. doi: 10.1021/np200041v. [DOI] [PubMed] [Google Scholar]
- (2).The approved drugs containing methyl aryl ether motifs could be searched in the DrugBank [accessed on May 24, 2013]; http://www.drugbank.ca/
- (3).Vitaku E, Ilardi EA, Njarđarson JT. [accessed on May 24, 2013];Top 200 Pharmaceutical Products by US Retail Sales in 2011. http://cbc.arizona.edu/njardarson/group/top-pharmaceuticals-poster/
- (4). [accessed on May 24, 2013]; http://www.alanwood.net/pesticides/
- (5).(a) Sanderson K. Nature. 2009;458:269. doi: 10.1038/458269a. [DOI] [PubMed] [Google Scholar]; (b) Yarnell AT. Chem. Eng. News. 2009;87:36–39. [Google Scholar]; (c) Dash P, Janni M, Peruncheralathan S. Eur. J. Org. Chem. 2012:4914–4917. [Google Scholar]
- (6).For examples, see: Bunnett JF, Zahler RE. Chem. Rev. 1951;49:273–412. Chauvière G, Viodé C, Périé J. J. Heterocycl. Chem. 2000;37:119–126. Zilberman J. Org. Process Res. Dev. 2003;7:303–305.
- (7).For examples, see: Juršić B. Tetrahedron. 1988;44:6677–6680. Fuhrmann E, Talbiersky J. Org. Process Res. Dev. 2005;9:206–211. Massah AR, Mosharafian M, Momeni AR, Aliyan H, Naghash HJ. Synth. Commun. 2007;37:1807–1815.
- (8).For examples, see: Gentles RG, Wodka D, Park DC, Vasudevan A. J. Comb. Chem. 2002;4:442–456. doi: 10.1021/cc010090j.
- (9).For examples, see: Sowa FJ, Hennion GF, Nieuwland JA. J. Am. Chem. Soc. 1935;57:709–711. Simons JH, Passino HJ. J. Am. Chem. Soc. 1940;62:1624. Oku T, Arita Y, Ikariya T. Adv. Synth. Catal. 2005;347:1553–1557.
- (10).(a) Anderson KW, Ikawa T, Tundel RE, Buchwald SL. J. Am. Chem. Soc. 2006;128:10694–10695. doi: 10.1021/ja0639719. [DOI] [PubMed] [Google Scholar]; (b) Chen G, Chan ASC, Kwong FK. Tetrahedron Lett. 2007;48:473–476. [Google Scholar]
- (11).Milton EJ, Fuentes JA, Clarke ML. Org. Biomol. Chem. 2009;7:2645–2648. doi: 10.1039/b907784g. [DOI] [PubMed] [Google Scholar]
- (12).Bhadra S, Dzik WI, Goossen LJ. J. Am. Chem. Soc. 2012;134:9938–9941. doi: 10.1021/ja304539j. [DOI] [PubMed] [Google Scholar]
- (13).(a) Palucki M, Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1997;119:3395–3396. [Google Scholar]; (b) Torraca KE, Huang X, Parrish CA, Buchwald SL. J. Am. Chem. Soc. 2001;123:10770–10771. doi: 10.1021/ja016863p. [DOI] [PubMed] [Google Scholar]
- (14).Gowrisankar S, Neumann H, Beller M. Chem. Eur. J. 2012;18:2498–2502. doi: 10.1002/chem.201103347. [DOI] [PubMed] [Google Scholar]
- (15).(a) Wolter M, Nordmann G, Job GE, Buchwald SL. Org. Lett. 2002;4:973–976. doi: 10.1021/ol025548k. [DOI] [PubMed] [Google Scholar]; (b) Zhang H, Ma D, Cao W. Synlett. 2007:243–246. [Google Scholar]; (c) Altman RA, Shafir A, Choi A, Lichtor PA, Buchwald SL. J. Org. Chem. 2008;73:284–286. doi: 10.1021/jo702024p. [DOI] [PubMed] [Google Scholar]; (d) Naidu AB, Sekar G. Tetrahedron Lett. 2008;49:3147–3151. [Google Scholar]; (e) Niu J, Zhou H, Li Z, Xu J, Hu S. J. Org. Chem. 2008;73:7814–7817. doi: 10.1021/jo801002c. [DOI] [PubMed] [Google Scholar]; (f) Satyanarayana P, Maheswaran H, Kantam ML, Bull SB. Chem. Soc. Jpn. 2011;84:788–790. [Google Scholar]
- (16).Wu X, Fors BP, Buchwald SL. Angew. Chem. Int. Ed. 2011;50:9943–9947. doi: 10.1002/anie.201104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).For the synthesis of RockPhos (L1) and tBuBrettPhos (L2), see: Hoshiya N, Buchwald SL. Adv. Synth. Catal. 2012;354:2031–2037. doi: 10.1002/adsc.201200398.
- (18).For the synthesis of AdBrettPhos (L3), see: Su M, Buchwald SL. Angew. Chem. Int. Ed. 2012;51:4710–4713. doi: 10.1002/anie.201201244.
- (19).(a) Bruno NC, Tudge MT, Buchwald SL. Chem. Sci. 2013;4:916–920. doi: 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bruno NC, Buchwald SL. Org. Lett. 2013;15:2876–2879. doi: 10.1021/ol401208t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yang Y, Oldenhuis NJ, Buchwald SL. Angew. Chem. Int. Ed. 2012;52:615–619. doi: 10.1002/anie.201207750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).In the coupling reactions with most of the (hetero)aryl halides, no or only a trace of (hetero)arene side-products ((Het)ArH) were detected by GC-MS analysis. See Supporting Information for details.
- (21).To our knowledge, there is only one example of the Pd-catalyzed coupling of ethanol with aryl halide.13b
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.