Abstract
Bacterial vaginosis (BV), a common condition in women, is associated with increased shedding of HIV in the female genital tract. While the Lactobacillus species that comprise a healthy vaginal microbiota produce lactic acid, the bacteria common in BV produce high concentrations of short chain fatty acids (SCFAs) and succinic acid. Macrophages are abundant in the lower genital tract mucosa and are thought to play an important role in HIV infection. In this study, we investigated whether SCFAs and succinic acid impacted HIV expression in monocyte-derived macrophages. Monocytes differentiated with either granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage colony-stimulating factor (M-CSF) were infected with either HIVBal or an HIV-luciferase reporter virus and treated with SCFAs, succinic acid, or lactic acid. Butyric acid suppressed HIV expression while succinic acid significantly increased expression in macrophages differentiated with either GM-CSF or M-CSF. Acetic, propionic, and lactic acids had no effect on HIV expression. Only succinic acid resulted in a significant increase in interleukin-8 production by infected macrophages. Our results suggest that succinic acid present in increased concentrations in the genital tract of women with BV plays a pro-inflammatory role and increases HIV expression. This could be one factor contributing to increased virus shedding seen in women with BV.
Key words: bacterial vaginosis, HIV, inflammation, macrophage, short chain fatty acids, succinic acid
Introduction
The bacterial microbiota in the lower genital tract of many women is composed predominately of Lactobacillus. The most common species of genital lactobacilli are L. crispatus, L. iners, L. jensenii, and L. gasseri.1–4 Bacterial vaginosis (BV), in contrast, is a condition in which lactobacilli are relatively reduced in number and replaced by variable amounts of other genera of bacteria including Gardnerella, Prevotella, Atopobium, Megasphaera, Sneathia, Mobiluncus, Mycoplasma, and many others.1,5,6 BV is common in women of reproductive age in the developed world, and it is more prevalent in women that are sexually active. It is striking that BV can be present in up to 55% of reproductive aged women in some parts of Africa.7
The bacteria present in the female lower genital tract influence HIV-1 heterosexual transmission. Studies of virus shedding show that decreased levels of lactobacilli are associated with higher levels of HIV-1 RNA in the genital tract,2,3 and BV is also associated with higher levels of HIV-1 RNA.2–4,8 Additionally, Cohen et al.9 recently reported that women with BV were three times more likely to transmit HIV-1 to their male partners. Lingappa et al.10 recently found that BV was associated with a higher viral set point in male partners that seroconverted, suggesting the possibility that the size of the initial virus inoculum impacted set point.
Anaerobic bacteria in the female genital tract of women with BV produce fermentation products including succinic acid and short chain fatty acids (SCFAs) such as butyric, acetic, and propionic acids.11–15 This is in contrast to women with healthy microbiota consisting mostly of lactobacilli, with which SCFAs and succinic acid are low, but lactic acid is increased. Succinic acid concentration can be increased to more than 20 mM in vaginal fluid of women with BV compared to an average of 0.02 mM in women with predominantly Lactobacillus species colonizing their genital tract,12 and succinic acid has been shown along with SCFAs to have immunomodulatory effects.16–19 We recently found that SCFAs induce the production of pro-inflammatory cytokines by monocytes.20
Macrophages are abundant in mucosal sites and play an important role in HIV pathogenesis.21,22 Macrophages are thought to not only be among the first cells infected by HIV23 but also help to transmit virus particles to CD4+ T cells.24,25 Macrophages are also thought to harbor virus throughout the course of the infection26 and to maintain a latent reservoir for HIV,22,27–30 thereby playing a major role in HIV dissemination. Interestingly, macrophages in the female genital tract have been shown to not only be susceptible to HIV infection, but to also express virus at high levels once infected.31 Given the importance of macrophages in HIV-1 transmission, the increased concentrations of bacterial fermentation products in BV, and the increased HIV-1 shedding in the genital fluid of women with BV, we evaluated how SCFAs and succinic acid impacted HIV expression in monocyte-derived macrophages.
Materials and Methods
Virus production
HIVBal was obtained from the AIDS Reference and Reagent Program (ARRRP, www.aidsreagent.org, donated by Dr. Suzanne Gartner, Dr. Mikulas Popovic, and Dr. Robert Gallo, Division of AIDS, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) and propagated in phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells using standard procedures.
Luciferase-expressing reporter virus stocks were generated by co-transfecting Hek293 T cells using lipofectamine (Life Technologies, Grand Island, NY) with envelope-negative HIV plasmid pNL 4-3 (E2; ARRRP) expressing luciferase together with a plasmid expressing VSV-G.32
Infection of macrophages with HIVBal
To measure the effects of SCFAs on p24 levels, we created monocyte-derived macrophages (MDMs) as described previously.33 Briefly, peripheral blood mononuclear cells (PBMCs) were collected from healthy donors by density-gradient centrifugation. Informed consent was obtained from donors, and all studies were approved by the Institutional Review Board of Rush University. Monocytes were isolated by plastic adherence, placed in flasks at 2×106 cells/mL, and left to differentiate for 7 days in complete medium (RPMI-1640, supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine, and 50 μg/mL gentamicin) plus 20% autologous human serum and 100 ng/mL of M-CSF (Life Technologies). M-CSF was replenished on day 3. After 7 days of culture, MDMs were infected with HIVBal (1.5 ng p24 per culture, which corresponds to ∼1.8×107 HIV particles) for 4 h. Cells were then washed twice with RPMI and cultured for 14 days with 20 mM of acetic or butyric acids. Levels of p24 core antigen were detected by enzyme-linked immunosorbent assay (ELISA; SIAC, Fredrick, MD) 1, 9, 12, and 14 days after infection.
Infection of macrophages with a single-round virus
A modification of the method described by Hanley et al.34 was used to measure the effect of bacterial fermentation products on HIV expression by virus capable of only a single round of infection. Briefly, CD14+ monocytes were isolated from the PBMCs of healthy donors using anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA). Cells were cultured for 7 days in 48-well plates at 100,000 cells/well in complete medium, plus 10% autologous human serum and 100 ng/mL of either granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage colony-stimulating factor (M-CSF; Life Technologies) to generate either M1 or M2-like macrophages, respectively.35 Cells were then infected with a VSV-G-pseudotyped HIV-luciferase reporter virus (13 ng p24 per culture which corresponds to ∼1.6×108 HIV particles) for 4 h at 37°C. Wells were then washed five times with phosphate-buffered saline to remove unbound virus and cells were cultured for another 24 h in complete medium without fetal bovine serum and supplemented with 10% autologous human serum. Macrophages were then treated with 20 mM of succinic, lactic, acetic, or butyric acids (pH=7), or with 100 ng/mL of the TLR2 ligand Pam2CSK4 (Life Technologies) or 10 ng/mL tumor necrosis factor (TNF)α (eBioscience, San Diego, CA) for 42 h. Cells were then lysed with passive lysis buffer (Promega, Madison, WI). Luciferase levels were measured using luciferase substrate (Promega) and a luminometer. SCFAs, succinic acids, and lactic acids were purchased from Sigma (St. Louis, MO).
Cytokine measurement
Cell free supernatants were taken from HIV-infected macrophages treated for 42 h with either SCFAs, succinic acid, lactic acid, Pam2CSK4, or TNFα as indicated above and interleukin (IL)-8 levels were measured by ELISA (BD Biosciences, San Jose, CA).
Statistical analysis
Results were analyzed by Kruskal-Wallis test (nonparametric ANOVA), one-way analysis of variance (ANOVA), or two-way ANOVA; p-values <0.05 were considered significant.
Results
SCFAs do not increase p24 levels in cultures of HIVBal -infected macrophages
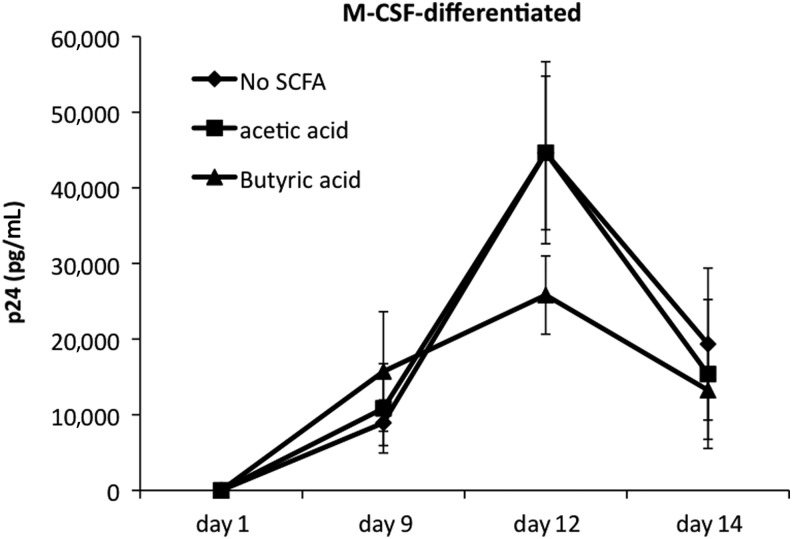
SCFAs levels can be as high as 20 mM in BV,12,15 and we previously showed that at 20 mM, both acetic and butyric acids induced production of pro-inflammatory cytokines by peripheral blood monocytes.20 Lower levels of SCFAs did not induce cytokine production. Here, we investigated the hypothesis that 20 mM SCFAs would also induce increased HIV expression in macrophages. We infected M-CSF–differentiated macrophages with HIVBal and then cultured the cells with 20 mM butyric or acetic acids for 14 days. In the absence of SCFAs, the levels of p24 increased over the first 12 days in HIVBal-infected macrophages (Fig. 1). In some donors, we observed a reduction in p24 levels, though this change was not significant. Unexpectedly, butyric acid–treated macrophages produced lower levels of p24 12 days postinfection compared to untreated macrophages, although this difference was not statistically significant (p>0.05, Fig. 1). Exposing infected macrophages to 20 mM acetic acid did not result in a significant difference in p24 levels in comparison to untreated cells over the 14-day culture period. Addition of SCFAs at 20 mM did not affect cell survival (data not shown).
FIG. 1.
Effects of short chain fatty acids (SCFAs) on HIVBal expression in macrophages. Monocyte-derived macrophages (MDMs) were differentiated in the presence of macrophage colony-stimulating factor (M-CSF; 100 ng/mL), infected with HIVBal and then cultured with 20 mM acetic or butyric acid for 14 days. Values shown are mean p24 concentrations in culture supernatants from quintuplicate cultures±standard deviation (SD). At each time point there was not a significant difference in p24 between SCFAs and control cultures (Kruskal-Wallis test, p-value >0.05). The experiment shown is representative of three independent experiments using cells from two different donors.
Bacterial fermentation products affect HIV expression in macrophages in a one-round virus infection system
In the cultures with HIVBal described above, multiple rounds of infection likely occurred over the 14-day period, and therefore the SCFAs could have affected virus infection, virus expression, or both. Additional experiments were subsequently performed with a pseudotyped luciferase-expressing HIV capable of only one round of infection in order to assess the effects of SCFAs only on the expression of virus. We also wanted to expand on our previous observations by testing the effects of additional fermentation products in the pseudotyped system. Furthermore, we obtained macrophages differentiated in the presence of GM-CSF or M-CSF because they have been reported to have distinct functional phenotypes.35
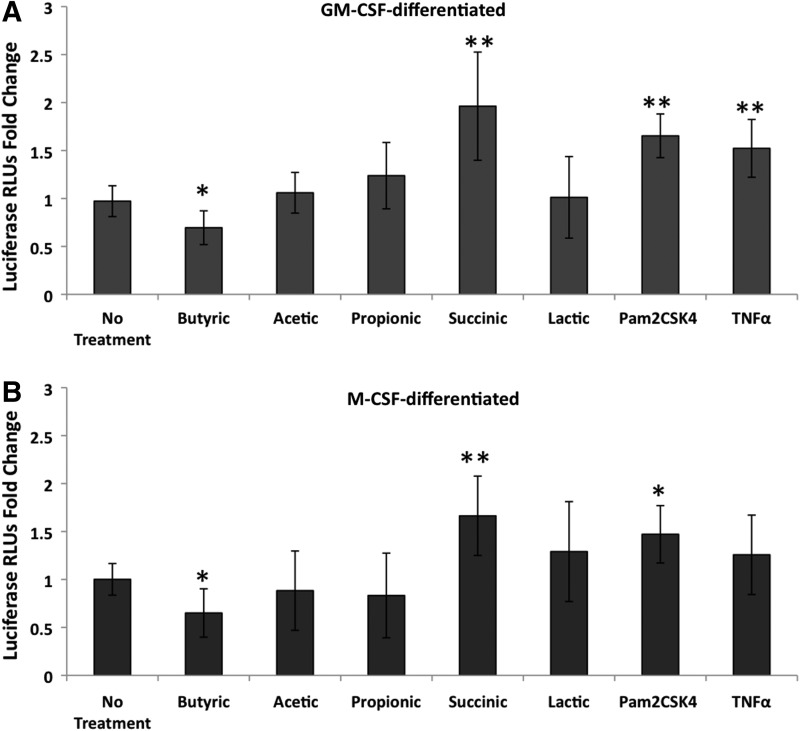
Similar to what has been reported previously,34 Pam2CSK4 (TLR2 ligand) significantly increased HIV expression as measured by luciferase (Fig. 2). TNFα also increased HIV expression, although this was significant in only the GM-CSF–differentiated cells. Consistent with the lower p24 levels found in experiments using virus capable of multiple rounds of infection, butyric acid decreased HIV expression in both M-CSF– and GM-CSF–differentiated macrophages infected with the pseudotyped virus (Fig. 2A, B).
FIG. 2.
Succinic acid up-regulates HIV expression. MDMs differentiated with (A) granulocyte-macrophage colony-stimulating factor (GM-CSF) or (B) M-CSF were infected with a luciferase-expressing HIV and 24 h later incubated with either 20 mM of SCFAs or succinic acid, 100 ng/mL of the TLR2 ligand Pam2CSK4, or 10 ng/mL TNFα for 48 h. Cell extracts were assayed for luciferase activity. Mean fold changes compared to untreated cells±SD from all three donors are shown. RLUs, relative light units. **p-values<0.001 and *p-values <0.01 were determined by Tukey-Kramer posttest following two-way analysis of variance, comparing untreated cells to treated cells. The results shown are from three independent experiments from three different donors with the exception of lactic acid, which was tested in two independent experiments using two different donors.
Of all the bacterial fermentation products tested, only succinic acid at 20 mM significantly increased HIV expression in infected macrophages. The increase was 2-fold in GM-CSF–differentiated macrophages (Fig. 2A) and 1.6-fold in M-CSF–differentiated macrophages (Fig. 2B) averaged across three experiments. In some experiments, 2 mM succinic acid was tested in parallel and also significantly increased HIV expression, while 2 mM butyric acid had no significant effect on HIV expression (data not shown). Acetic, propionic, and lactic acids at 20 mM did not significantly affect HIV expression in infected macrophages (Fig. 2).
Succinic acid induces IL-8 production
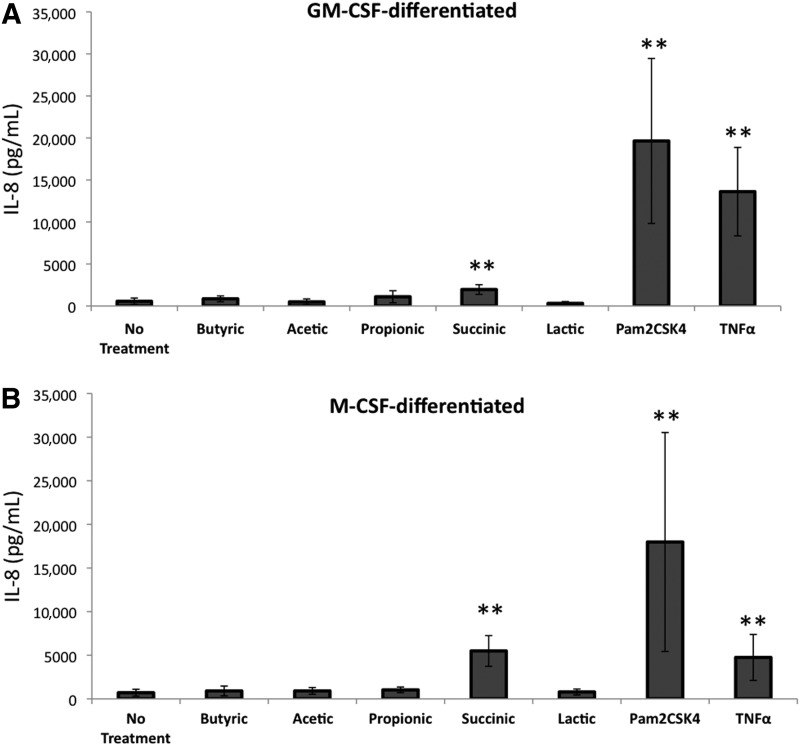
BV and other vaginal infections create an inflammatory milieu that potentially increases HIV-1 expression and facilitates HIV-1 infection.2–4,9,36–40 IL-8 is a pro-inflammatory cytokine that is reported to be increased in some studies of BV, recruits neutrophils and macrophages, and enhances HIV-1 replication in macrophages, T cells, and cervical explant tissue.41,42 To assess how the bacterial fermentation products tested above impacted IL-8 production by infected macrophages, we measured IL-8 in the supernatants of the SCFA-treated HIV pseudotyped-infected macrophages. In GM-CSF–differentiated macrophages, succinic acid increased IL-8 production by virus-infected cells 3.5-fold compared to controls (Fig. 3A). The ability of succinic acid to induce IL-8 production was even more robust in M-CSF–differentiated macrophages in which IL-8 levels were 7.8-fold higher relative to untreated cells (Fig. 3B). TNFα and Pam2CSK4 also up-regulated IL-8 production. In contrast, none of the SCFAs or lactic acid significantly increased IL-8 production by infected macrophages (Fig. 3).
FIG. 3.
Succinic acid induces interleukin (IL)-8 production in HIV-infected macrophages. MDMs derived in the presence of (A) GM-CSF or (B) M-CSF were infected with a luciferase-expressing HIV and treated as in Fig. 2. IL-8 levels in the supernatant were measured by ELISA. **p-values <0.001 were determined by two-way analysis of variance (ANOVA) comparing untreated cells to treated cells. The results shown are means of two independent experiments using two different donors.
Discussion
BV is the primary reason for women presenting to STD clinics in the United States. It is estimated to be present in 29% of U.S. women between the ages of 14 and 49 years, and the incidence is even higher in women in sub-Saharan Africa.43,44 It has been well established that BV, as well as other genital tract infections such as Trichomonas vaginalis, increase susceptibility to HIV-1 infection and increase the risk of transmitting the virus.3,9,36,39 SCFAs including butyric, acetic, and propionic acids are produced in higher concentrations in the female genital tract of women with BV. SCFAs have been studied extensively in the gut and have been shown to impact the immune system in a variety of ways, although their effects in the female genital tract are unclear. SCFAs have been shown by our group to increase the production of pro-inflammatory cytokines by monocytes.15,20 Moreover, some SCFAs have been shown to increase latent HIV expression by inhibiting histone deactylase activity.45 Therefore, we hypothesized that SCFAs could induce HIV expression in vitro and potentially contribute to the higher HIV-1 shedding seen in women with BV. We found, however, that butyric acid did not increase p24 levels in macrophages infected with HIVBal and significantly reduced luciferase expression by macrophages infected with an HIV-reporter virus in vitro, while acetic acid had no significant effect (Fig. 1). Butyric acid has anti-inflammatory properties in certain settings,16,19 and we found that neither acetic nor butyric acids resulted in an increase in IL-8 production by HIV-infected macrophages (Fig. 3). The inability of these SCFAs to increase HIV expression in macrophages may be mechanistically related to their lack of induction of pro-inflammatory cytokine production by these cells, although this needs further study.
We also studied the effects of succinic acid on HIV expression. Succinic acid, found at high concentrations in BV, is similar in structure to SCFAs and has also been shown to have immunomodulatory effects.12,18,46 Our results show that succinic acid significantly increased HIV-1 expression in macrophages differentiated with either GM-CSF or M-CSF. Succinic acid is produced in variable amounts by different bacteria that are increased in BV. Al-Mushrif et al.12 found that Prevotella and Mobiluncus produce on average twice as muchsuccinic acid as Gardnerella vaginalis. Since the specific composition of bacterial species in BV varies widely among individuals,1,5,6,11 this could explain the varying levels of succinic acid present in women with BV.12 Further study is needed to determine if women with BV who have the highest levels of succinic acid also have higher levels of HIV-1 shedding.
We studied the effects of these bacterial fermentation products on HIV-1 expression in macrophages because macrophages are found in abundance in mucosal sites, including the vagina and ectocervix, and are thought to remain infected throughout the course of HIV-1 infection.47 Understanding factors that influence HIV-1 expression in macrophages is therefore pivotal in determining viral pathogenesis. It has been demonstrated that vaginal macrophages express monocyte-like cell surface markers and, unlike intestinal macrophages, are permissive to HIV-1 entry.31
Macrophages can also behave differently depending on their exposure to either GM-CSF or M-CSF during their development.35,48 It has been proposed that macrophages can develop into pro-inflammatory M1 macrophages or anti-inflammatory M2 macrophages depending on their exposure to GM-CSF or M-CSF, respectively.35,48 Other researchers have noted differences between M1- and M2-like macrophages in the way they interact with HIV. For example, it has been shown that HIV-1 infection of macrophages induces their differentiation into a pro-inflammatory (M1-like) phenotype.49,50 Also, a recent study showed that HIV-1 proteins (Tat, gp120, and especially Nef ) preferentially activated M2-like macrophages but then drove the cell toward a more pro-inflammatory (M1-like) phenotype.51 We found that macrophages differentiated in the presence of GM-CSF expressed substantially more luciferase than those differentiated in the presence of M-CSF, although there was some variability across donors (data not shown). This suggests that HIV-1 might take advantage of the specific cellular environment created when macrophages are differentiated in the presence of GM-CSF. More studies are needed to determine why GM-CSF differentiated macrophages express more virus when they are exposed to succinic acid.
Finally, we found that succinic acid—but not acetic, butyric, or propionic acids—increased the production of IL-8 by HIV-infected macrophages. Pro-inflammatory cytokines such as IL-8 are increased in the vaginal fluids of women with BV, especially in those co-infected with Trichomonas vaginalis.37,52 These inflammatory mediators have been suggested to play a variety of roles that make the genital tract more susceptible to HIV-1 infection. It has been postulated that they could be involved in recruiting CD4+ cells, suppressing innate antimicrobial responses, or disrupting the epithelial barrier.40 Additionally, these cytokines can activate the long-terminal repeat promoter region of HIV-1 through activation of the nuclear factor (NF)-κB pathway.53
Because of the importance of IL-8 in HIV infection, the production of IL-8 by succinic acid represents one way by which this microbial fermentation product may impact HIV dissemination. It will be important to assess the impact of these fermentation products on other pro-inflammatory cytokines such as interferon γ. Clearly, the effects of SCFAs on NF-κB, cytokine production, and HIV expression need to be more carefully examined.
The increase of viral gene expression and IL-8 production induced by succinic acid could be a contributing factor to the increased HIV-1 shedding observed in HIV-infected women with BV, thereby enhancing female-to-male transmission. Our findings suggest one possible mechanism by which the microbiota of the female genital tract may impact HIV dissemination.
Abbreviations
- BV
bacterial vaginosis
- ELISA
enzyme-linked immunosorbent assay
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL
interleukin
- M-CSF
macrophage colony-stimulating factor
- MDM
monocyte-derived macrophage
- NF
nuclear factor
- PBMC
peripheral blood mononuclear cell
- PHA
phytohemagglutinin
- SCFA
short chain fatty acid
- TNF
tumor necrosis factor
Acknowledgments
HIVBal was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH; HIV-1Ba-L from Dr. Suzanne Gartner, Dr. Mikulas Popovic, and Dr. Robert Gallo. This work was supported by the NIH (grant number P01 AI08297) and the Dean's Fellowship at Rush University Medical Center (to LSG and LK).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ravel J. Gajer P. Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sha BE. Zariffard MR. Wang QJ, et al. Female genital-tract HIV load correlates inversely with lactobacillus species but positively with bacterial vaginosis and mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 3.Coleman JS. Hitti J. Bukusi EA, et al. Infectious correlates of HIV-1 shedding in the female upper and lower genital tracts. AIDS. 2007;21:755–759. doi: 10.1097/QAD.0b013e328012b838. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell C. Balkus JE. Fredricks DN, et al. Interaction between lactobacilli, bacterial vaginosis-associated bacteria and HIV-1 RNA and DNA genital shedding in US and Kenyan women. AIDS Res Hum Retroviruses. 2013;29:13–19. doi: 10.1089/aid.2012.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillier SL. Krohn MA. Rabe LK, et al. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16(Suppl 4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 6.Hill GB. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169(2 Pt 2):450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 7.Gray RH. Wawer MJ. Sewankambo N, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:1780. doi: 10.1016/s0140-6736(05)63612-4. [DOI] [PubMed] [Google Scholar]
- 8.Cu-Uvin S. Hogan JW. Caliendo AM, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–896. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 9.Cohen CR. Lingappa JR. Baeten JM, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9:e1001251. doi: 10.1371/journal.pmed.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lingappa J. Thomas KK. Hughes JP, et al. Partner characteristics predicting HIV-1 setpoint in sexually acquired HIV-1 among African seroconverters. AIDS Res Hum Retroviruses. 2013;29:164–171. doi: 10.1089/aid.2012.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gajer P. Brotman RM. Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Mushrif S. Eley A. Jones BM. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J Med Microbiol. 2000;49:1023–30. doi: 10.1099/0022-1317-49-11-1023. [DOI] [PubMed] [Google Scholar]
- 13.Ison CA. Easmon CS. Dawson SG, et al. Non-volatile fatty acids in the diagnosis of non-specific vaginitis. J Clin Pathol. 1983;36:1367–1370. doi: 10.1136/jcp.36.12.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudry AN. Travers PJ. Yuenger J, et al. Analysis of vaginal acetic acid in patients undergoing treatment for bacterial vaginosis. J Clin Microbiol. 2004;42:5170–5175. doi: 10.1128/JCM.42.11.5170-5175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirmonsef P. Gilbert D. Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65:190–5. doi: 10.1111/j.1600-0897.2010.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavaglieri CR. Nishiyama A. Fernandes LC, et al. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003;73:1683–1690. doi: 10.1016/s0024-3205(03)00490-9. [DOI] [PubMed] [Google Scholar]
- 17.Maslowski KM. Vieira AT. Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotstein OD. Nasmith PE. Grinstein S. pH-dependent impairment of the neutrophil respiratory burst by the bacteroides byproduct succinate. Clin Invest Med. 1988;11:259–265. [PubMed] [Google Scholar]
- 19.Segain JP. Raingeard de la Bletiere D. Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirmonsef P. Zariffard MR. Gilbert D, et al. Short-chain fatty acids induce pro-inflammatory cytokine production alone and in combination with toll-like receptor ligands. Am J Reprod Immunol. 2012;67:391–400. doi: 10.1111/j.1600-0897.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghiglione Y. Turk G. Nef performance in macrophages: the master orchestrator of viral persistence and spread. Curr HIV Res. 2011;9:505–513. doi: 10.2174/157016211798842080. [DOI] [PubMed] [Google Scholar]
- 22.Smith PD. Meng G. Salazar-Gonzalez JF, et al. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leuk Biol. 2003;74:642–9. doi: 10.1189/jlb.0503219. [DOI] [PubMed] [Google Scholar]
- 23.Philpott SM. HIV-1 coreceptor usage, transmission, and disease progression. Curr HIV Res. 2003;1:217–27. doi: 10.2174/1570162033485357. [DOI] [PubMed] [Google Scholar]
- 24.Groot F. Welsch S. Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 25.Gousset K. Ablan SD. Coren LV, et al. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuitemaker H. Kootstra NA. de Goede RE, et al. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahl SM. Orenstein JM. Immune stimulation and HIV-1 viral replication. J Leuk Biol. 1997;62:67–71. doi: 10.1002/jlb.62.1.67. [DOI] [PubMed] [Google Scholar]
- 28.Lambotte O. Taoufik Y. de Goer MG, et al. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Zhu T. HIV-1 in peripheral blood monocytes: an underrated viral source. J Antimicrob Chemother. 2002;50:309–311. doi: 10.1093/jac/dkf143. [DOI] [PubMed] [Google Scholar]
- 30.Sharova N. Swingler C. Sharkey M, et al. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005;24:2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen R. Richter HE. Clements RH, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji X. Olinger GG. Aris S, et al. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J Gen Virol. 2005;86(Pt 9):2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]
- 33.Cheney KM. McKnight A. Interferon-alpha mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication. PLoS One. 2010;5:e13521. doi: 10.1371/journal.pone.0013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley TM. Viglianti GA. Nuclear receptor signaling inhibits HIV-1 replication in macrophages through multiple trans-repression mechanisms. J Virol. 2011;85:10834–10850. doi: 10.1128/JVI.00789-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A. Sica A. Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Taha TE. Hoover DR. Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: Association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Cauci S. Culhane JF. Modulation of vaginal immune response among pregnant women with bacterial vaginosis by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, and yeast. Am J Obstet Gynecol. 2007;196:133. doi: 10.1016/j.ajog.2006.08.033. .e1–7. [DOI] [PubMed] [Google Scholar]
- 38.Donders GG. Bosmans E. Dekeersmaecker A, et al. Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol. 2000;182:872–878. doi: 10.1016/s0002-9378(00)70338-3. [DOI] [PubMed] [Google Scholar]
- 39.Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S17–21. [PubMed] [Google Scholar]
- 40.Thurman AR. Doncel GF. Innate immunity and inflammatory response to trichomonas vaginalis and bacterial vaginosis: relationship to HIV acquisition. Am J Reprod Immunol. 2011;65:89–98. doi: 10.1111/j.1600-0897.2010.00902.x. [DOI] [PubMed] [Google Scholar]
- 41.Narimatsu R. Wolday D. Patterson BK. IL-8 increases transmission of HIV type 1 in cervical explant tissue. AIDS Res Hum Retroviruses. 2005;21:228–233. doi: 10.1089/aid.2005.21.228. [DOI] [PubMed] [Google Scholar]
- 42.Smythies LE. Maheshwari A. Clements R, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leuk Biol. 2006;80:492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 43.Allsworth JE. Peipert JF. Prevalence of bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 2007;109:114–120. doi: 10.1097/01.AOG.0000247627.84791.91. [DOI] [PubMed] [Google Scholar]
- 44.van De Wijgert JH. Mason PR. Gwanzura L, et al. Intravaginal practices, vaginal flora disturbances, and acquisition of sexually transmitted diseases in Zimbabwean women. J Infect Dis. 2000;181:587–594. doi: 10.1086/315227. [DOI] [PubMed] [Google Scholar]
- 45.Kantor B. Ma H. Webster-Cyriaque J, et al. Epigenetic activation of unintegrated HIV-1 genomes by gut-associated short chain fatty acids and its implications for HIV infection. Proc Natl Acad Sci USA. 2009;106:18786–18791. doi: 10.1073/pnas.0905859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donders GG. Vereecken A. Bosmans E, et al. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG. 2002;109:34–43. doi: 10.1111/j.1471-0528.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 47.Shen R. Richter HE. Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65:261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verreck FA. de Boer T. Langenberg DM, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown JN. Kohler JJ. Coberley CR, et al. HIV-1 activates macrophages independent of toll-like receptors. PLoS One. 2008;3:e3664. doi: 10.1371/journal.pone.0003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porcheray F. Samah B. Leone C, et al. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006;349:112–120. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 51.Chihara T. Hashimoto M. Osman A, et al. HIV-1 proteins preferentially activate anti-inflammatory M2-type macrophages. J Immunol. 2012;188:3620–3627. doi: 10.4049/jimmunol.1101593. [DOI] [PubMed] [Google Scholar]
- 52.Simhan HN. Anderson BL. Krohn MA, et al. Host immune consequences of asymptomatic trichomonas vaginalis infection in pregnancy. Am J Obstet Gynecol. 2007;196:59. doi: 10.1016/j.ajog.2006.08.035. .e1–5. [DOI] [PubMed] [Google Scholar]
- 53.Osborn L. Kunkel S. Nabel GJ. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci USA. 1989;86:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]