Abstract
It is expected that use of adult multipotential mesenchymal stem cells (MSCs) for bone tissue engineering (TE) will lead to improvement of TE products. Prior to clinical application, biocompatibility of bone TE products need to be tested in vitro and in vivo. In orthopedic research, sheep are a well-accepted model due to similarities with humans and are assumed to be predictive of human outcomes. In this study we uncover differences between human and ovine bone marrow–derived MSCs (BMSCs) and adipose tissue–derived MSCs (ADSCs) in response to osteogenic media. Osteogenic differentiation of BMSCs and ADSCs was monitored by alkaline phosphatase (ALP) activity and calcium deposition. Mineralization of ovine BMSC was achieved in medium containing NaH2PO4 as a source of phosphate ions (Pi), but not in medium containing β-glycerophosphate (β-GP), which is most often used. In a detailed study we found no induction of ALP activity in ovine BMSCs and ADSCs upon osteogenic stimulation, which makes β-GP an unsuitable source of phosphate ions for ovine cells. Moreover, mineralization of human ADSCs was more efficient in osteogenic medium containing NaH2PO4. These results indicate major differences between ovine and human MSCs and suggest that standard in vitro osteogenic differentiation techniques may not be suitable for all types of cells used in cell-based therapies. Since mineralization is a widely accepted marker of the osteogenic differentiation and maturation of cells in culture, it may lead to potentially misleading results and should be taken into account at the stage of planning and interpreting preclinical observations performed in animal models. We also present a cell culture protocol for ovine ADSCs, which do not express ALP activity and do not mineralize under routine pro-osteogenic conditions in vitro. We plan to apply it in preclinical experiments of bone tissue–engineered products performed in an ovine model.
Key words: cell culture, stem cells, tissue engineering
Introduction
Bone regeneration requires a multidisciplinary approach. This includes the use of appropriate scaffolds and the selection of an optimal cell source and type of animal model for preclinical studies. Mesenchymal stem cells (MSCs) are of particular interest in tissue engineering (TE) due to their ability to differentiate into mesenchymal tissues, such as bone, muscle, cartilage, and fat.1,2 Because of these unique properties MSCs are attractive candidates for cell-based tissue repair techniques and are expected to give rise to mature and functional osteoblasts, resulting in improved and enhanced biological properties of bone implants.3 Bone marrow1 and adipose tissue4 are the two most frequently investigated sources of MSCs and have been considered suitable for the generation of tissue engineered bone in mouse, rat, dog, goat, pig, and sheep.5 The clinical applicability of MSCs depends on our ability to predictably direct these multipotent cells to differentiate into the desired phenotypes. In bone TE particular emphasis is placed on guidance of cell capacity towards bone formation both in vivo and in vitro. Since clinical application of cell-based TE products should be preceded by experimental implantation into animal models, culture and differentiation potency verification of cells derived from animal tissues is an indispensable element of the preclinical evaluation. Differentiation potency of human and animal MSCs has been extensively studied using mainly well-established combinations of different induction factors.6 Classically, osteogenic differentiation of human MSCs1,7 requires incubation in medium supplemented with fetal bovine serum (FBS), ascorbic acid, dexamethasone, and β-glycerophosphate (β-GP). Dexamethasone is a synthetic glucocorticoid, a potent stimulator of in vitro osteogenesis. It induces the expression of the runt-related transcription factor 2 (Runx2), Osterix (Osx), and bone matrix proteins.8 Ascorbic acid 2-phosphate, a long-acting derivative of vitamin C, plays an important role as a cofactor for the hydroxylation of proline and lysine residues in collagens, increasing synthesis of collagen type I, the main component of extracellular matrix,9 and activity of alkaline phosphatase (ALP) in osteogenic cells.10 The presence of both calcium and phosphate ions is essential for matrix mineralization. The addition of organic β-GP to osteogenic medium remains routinely used in differentiation assays with MSC cultures.11,12 A release of phosphate ions (Pi) from β-GP is probably due to the activity of ALP.13 ALP is among the first genes expressed in the process of calcification, therefore it is frequently used as an osteogenic differentiation marker.14 However, MSCs from different tissues differ in their response to differentiation stimuli, growth abilities, phenotypic characteristics, or global gene expression pattern.15–17 Moreover, such differences were also shown for MSCs derived from different species18 and between mouse strains.19 Considering this heterogeneity, the differentiation protocol that is valid for MSCs from one tissue source or species may not be valid for others.
In the present study we demonstrated that the mineralization of animal MSCs in culture may by significantly different compared with human cells, which in turn may substantially affect conclusions coming from animal models. In bone TE, the sheep model is widely accepted20 and this is why we focused on ovine cell performance in culture. There is some evidence of successful differentiation and consequently mineralization of ovine MSC in standard osteogenic medium containing β-GP.21–23 However, in our experimental system, based on Heather sheep, we noticed in culture of ovine bone marrow–derived MSCs (BMSCs) as well as adipose tissue–derived MSCs mineralization is not convincing or even not present at all. Therefore, prior to in vivo experiments, we decided to resolve the problem; that is, identify the reasons for the observed phenomenon and to ensure osteoblastic phenotype acquisition and maturation by ovine BMSCs and ADSCs. Therefore, we performed systematic studies on the in vitro mineralization of ovine and human cells. We hypothesized that the observed disruption of mineralization may result from insufficient availability of phosphate ions due to the lack of ALP activity in the ovine cell cultures. The main aim of this study was to provide cell culture conditions in which the osteogenic capacity of differentiated ovine BMSCs and ADSCs could be demonstrated by successful mineralization. We believe that the correctness of TE observations in animal models requires significant attention be paid to the problems demonstrated in our study.
Materials and Methods
Subjects
The study was approved by the local ethics committee, and written consent was obtained from all individuals included in the study. The biological material used in this study would have been otherwise discarded during standard surgical procedures.
Human BMSCs, ADSCs, and fibroblasts
Bone marrow aspirates as well as pieces of periarticular connective tissues were collected during total hip replacement. The age of the patients ranged from 49 to 78 years. Adipose tissue samples were obtained during cosmetic liposuction.
Ovine BMSCs and ADSCs
Bone marrow aspirates from the proximal humerus and subcutaneous adipose tissue were obtained from nine 2-year-old Heather sheep, in total.
Isolation and culture of cells
Unless otherwise indicated, all reagents and materials used in this work were obtained from Sigma-Aldrich (St. Louis, MO).
BMSCs
Human and ovine bone marrow cells were fractionated by Histopaque density centrifugation and the mononuclear cell fraction was seeded into culture flasks at a density of 5×105 cells/cm2 in culture medium composed of Dulbecco's modified Eagle's medium (DMEM), 20% FBS (human [h]BMSCs) or 10% FBS (ovine [o]BMSCs), 1% antibiotic-antimycotic (all components from Gibco, Invitrogen, Carlsbad, CA) and maintained at 37°C in a humidified atmosphere and 5% CO2. Cells were allowed to adhere for 48 h at 37°C and 5% CO2, after which cells were washed with phosphate-buffered saline (PBS) to remove nonadherent cells. Cells became confluent in 10 to 20 days. The cells from passages 3–5 were used for the subsequent assays.
ADSCs
Twenty-five-milliliter aliquots of fat were washed three times with equal volume PBS and incubated in 50% volume 400 U/ml collagenase for 60 min at 37°C shaking at 250 rpm, followed by centrifugation at 400 g for 10 min to separate the oil and remaining fat lobules from the stromal vascular fraction (SVF). The pelleted SVF was resuspended in DMEM containing 10% FBS and 1% antibiotic-antimycotic and filtered using a 100-μm filter mesh (BD Bioscience). Cells were plated in 75-cm2 culture flasks at a density of 5×106/cm2 with DMEM containing 10% FBS and 1% antibiotic-antimycotic. After 24 h, the medium was changed to remove nonadherent cells. The adhered cells were expanded for 5–7 days, then trypsinized and counted. Cells (106 cells/mL) were cryopreserved in Bambanker (Lymphotec, Japan).
Ovine adipose tissue was washed with a solution of PBS and 1% antibiotic-antimycotic (Gibco), and minced into small fragments with a blade. The fragments were further processed similar to human lipoaspirates.
Human fibroblasts
The pieces of connective tissue were cut and subjected to collagenase digestion for 30 min at 37°C. After removal of the collagenase, pieces were rinsed with PBS and explanted to culture flasks in DMEM containing 10% FBS and 1% antibiotic-antimycotic. Within 6–7 days, cells migrated from the fragmented tissue. Pieces of tissue were then removed, and the cells were further cultured to confluence.
Phenotypic analysis
Cells were analyzed for expression of mesenchymal surface antigens by flow cytometry using FACS Calibur instrument (Becton Dickinson, San Jose, USA). The following fluorochrome-conjugated monoclonal antibodies were used for human cells: CD166-PE (R&D Systems, #FAB6561P), CD105-PE (BD, #560839), CD73-PE (BD, #550257), CD90-APC (BD, #559869), CD45-FITC (BD, #555482), CD34-APC (BD, #555824), CD31-PE (BD, #555446), and CD29-APC (Novus Biologicals, #110-81703). Sheep cells were stained with CD45-PE (Lifespan Biosciences, #C44436), CD44-FITC (Lifespan Biosciences, #C43382), CD31-FITC (Lifespan Biosciences, #C43279), and CD29-APC (Novus Biologicals, #110-81703). Cells stained with isotype control antibodies (all from R&D Systems) were included in all experiments. Cells were detached from culture flasks by 5–10 min incubation with Accutase solution, resuspended in culture medium and washed in PBS with 1% bovine serum albumin (BSA). Afterwards, (1–2)×105 cells were resuspended in PBS 1% BSA and stained with a relevant antibody for 30 min at 4°C. The final antibody concentration was 10 μg/mL, as advised by most manufacturers. After staining, cells were washed twice in PBS with 1% BSA and fixed in 1% PBS-buffered paraformaldehyde. Cells were stored in 4°C until analysis, which was performed within 48 h from staining. Results were analyzed with FCS Express (De Novo Software). The percentage of antigen-expressing cells was determined by Overton Subtraction method, with isotype control stained cells used as background signal.
Osteogenic differentiation
Approximately 2×103/cm2 hBMSCs (passage 2–3) and 103/cm2 oBMSCs, hADSCs, and oADSCs were plated in each well of a flat-bottom 24-well plate in 1 mL of culture medium. Osteogenic differentiation and mineralization was initiated 24 h after plating by replacing culture medium with DMEM supplemented with 10% FBS (20% for hBMSCs), 100 nM dexamethasone, 50 μg/mL ascorbic acid-2-phosphate. For mineralization, 10 mM β-GP or 3 mM NaH2PO4 was added to osteogenic medium.
Free phosphate assay
Supernatants were collected 0 and 24 h after medium change at day 21 of experiment. The release of Pi from β-GP was monitored using a Phosphate Colorimetric Assay Kit (BioVision, Mountain View, CA). The amount of free phosphate released was measured at A620nm (BMG Labtech) and compared with a standard curve.
DNA content
We added 150 μL of cell lysis buffer (0.1% Triton X-100 in 10 mM Tris-HCl, pH 7.4) to each well of 24-well plates, which were then homogenized for 4 h at 4°C and frozen. DNA content was determined by the Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen) according to manufacturer's guideline. Briefly, 50 μL of PicoGreen reagent was added to 50 μL of each cell lysate and incubated in the dark at room temperature for 5 min. The fluorescence was read at an excitation/emission of 485–538 nm on the plate reader (BMG Labtech) and compared with a standard curve.
ALP activity
The release of p-nitrophenol from p-nitrophenol phosphate, indicating osteogenic differentiation, was used to determine the specific activity of ALP. Briefly, 130 μL of P-nitrophenol phosphate solution was added to 20 μL of cell lysate (prepared as before) and incubated at room temperature for 30 min. The reaction was stopped by adding 100 μL of 3 M NaOH. The absorbance was measured at 405 nm (BMG Labtech) and compared with a standard curve. ALP levels were normalized to DNA levels from the same well.
Alizarin Red S
Alizarin Red S (40 mM) was prepared in dH2O, and the pH was adjusted to 4.1–4.3 using 0.5% (v/v) ammonium hydroxide. At day 28 of osteogenic differentiation, the cells were washed in PBS followed by fixation in 10% buffered formalin for 20 min. The monolayers were then washed twice with excess dH2O prior to addition of 1 mL of 40 mM Alizarin Red S per well. The plates were incubated at room temperature for 20 min with gentle shaking. After aspiration of the unincorporated dye, the wells were washed four times with 2 mL of dH2O while shaking for 5 min. Stained monolayers were visualized by phase microscopy using an inverted microscope (Nikon). For quantification of staining, 500 μL of 10% (v/v) cetylpyridinium chloride was added to each well, and the plate was incubated at room temperature for 30 min with shaking. The dye was then removed and 100-μL aliquots were transferred to a 96-well plate prior to reading at 560 nm (BMG Labtech).
Statistical analysis
All statistical analyses in this study were performed using GraphPad InStat (La Jolla, CA) using Kruskal-Wallis test and post hoc analysis.
Results
Immuno-phenotype of human and ovine BMSCs and ADSCs
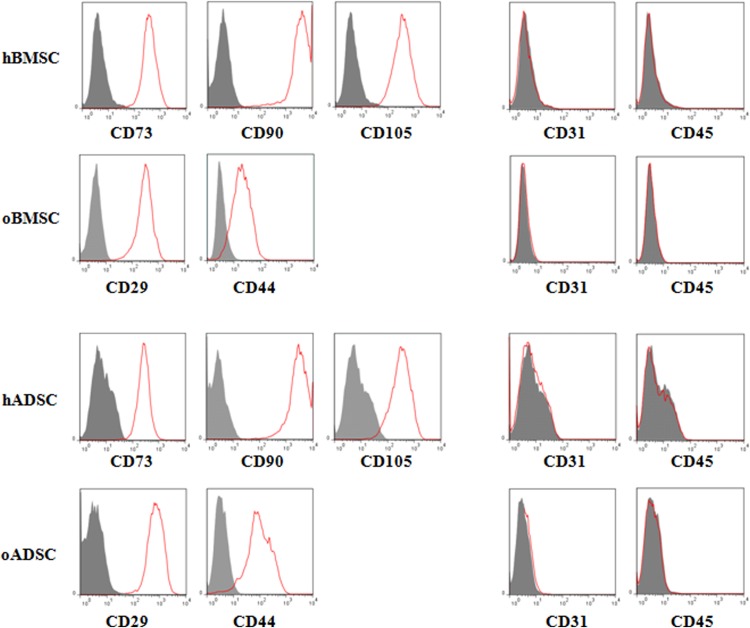
Fluorescence-activated cell sorting (FACS) analysis of surface antigens expression on human cells was performed according to criteria set by International Society for Cellular Therapy.24 Over 95% of hBMSCs and hADSCs expressed CD73, CD90, and CD105 (Fig. 1). Ovine cells were positive for CD29 and CD44, identified as markers associated with oBMSCs and oADSCs.25 Additionally all analyzed cells did not express hematopoietic (CD45) and endothelial (CD31) markers.
FIG. 1.
Immuno-phenotype of human (h) and ovine (o) BMSC and ADSC. Immunolabeling with antibodies against the indicated antigens was performed on cell suspension followed by single color flow cytometry. Representative histograms are demonstrated. The BMSCs or ADSCs of human or ovine origin are represented by the red line. The respective isotype control is shown in gray. All populations did not react with the hematopoietic markers CD31 and CD45. BMSC, bone marrow–derived mesenchymal stem cell; ADSC, adipose tissue–derived mesenchymal stem cell.
Phosphate ions source modulates mineralization
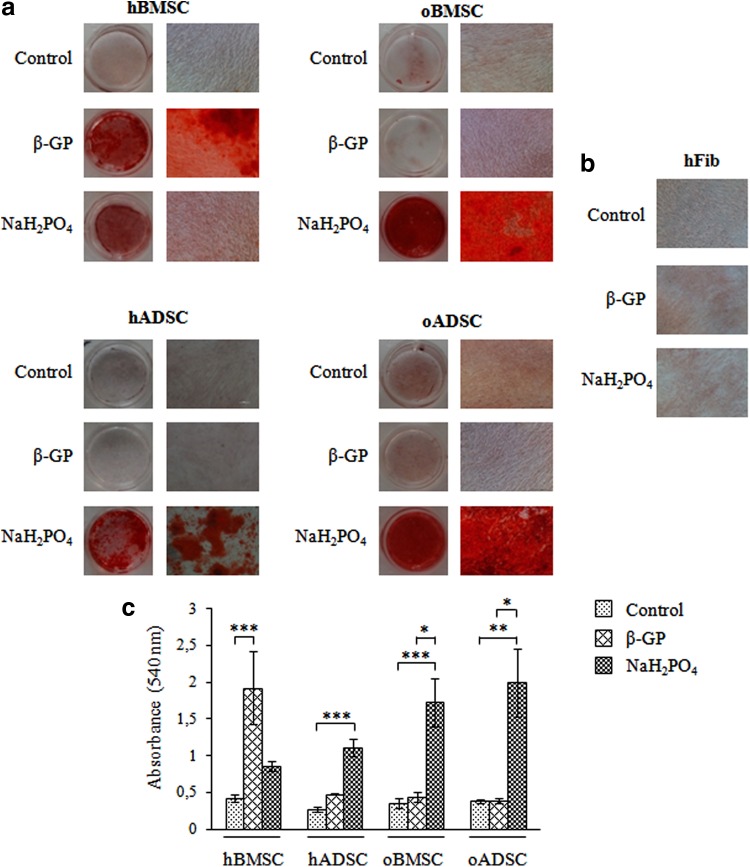
The effect of phosphate ions source on mineralization of human and ovine BMSC and ADSC was evaluated by Alizarin Red S staining. Cells were cultured in control or in osteogenic medium containing dexamethasone, ascorbic acid-2-phosphate and β-GP or NaH2PO4 as a source of phosphate ions. At day 28 in the presence of culture medium (Control), no mineralization of ovine or human BMSCs and ADSCs was observed by Alizarin Red S staining (Fig. 2a). As expected, mineralization of hBMSCs was detected at day 28 in medium containing β-GP but surprisingly not in osteogenic medium containing NaH2PO4 as source of phosphate ions. In contrast to hBMSCs, mineralization of oBMSCs and oADSCs was only occurred in medium containing NaH2PO4. Interestingly, abundant mineralization of hADSCs at day 28 was observed in medium supplemented with NaH2PO4. Prolonged (up to 42 days) incubation of hADSCs in medium with β-GP and hBMSCs in medium containing NaH2PO4 led to moderate extracellular matrix mineralization (data not shown). Mineralized nodules were not detected in the cultures of fibroblasts performed in osteogenic media (Fig. 2b). A quantitative analysis of Alizarin Red S staining, by extraction of the calcified mineral from the stained monolayer at low pH, is shown in Figure 2c.
FIG. 2.
Mineral deposition analyzed by Alizarin Red S staining of representative human (h) and ovine (o) BMSCs, ADSCs (a), and human fibroblasts (hFib) (b) after 4 weeks of culture in control and osteogenic medium containing β-glycerophosphate (β-GP) or NaH2PO. Single experiment shown is representative of four to seven independent ones. Histograms represent corresponding staining quantification and represent the mean values±SD (c). *p<0.05; **p<0.001, ***p<0.001.
ALP activity
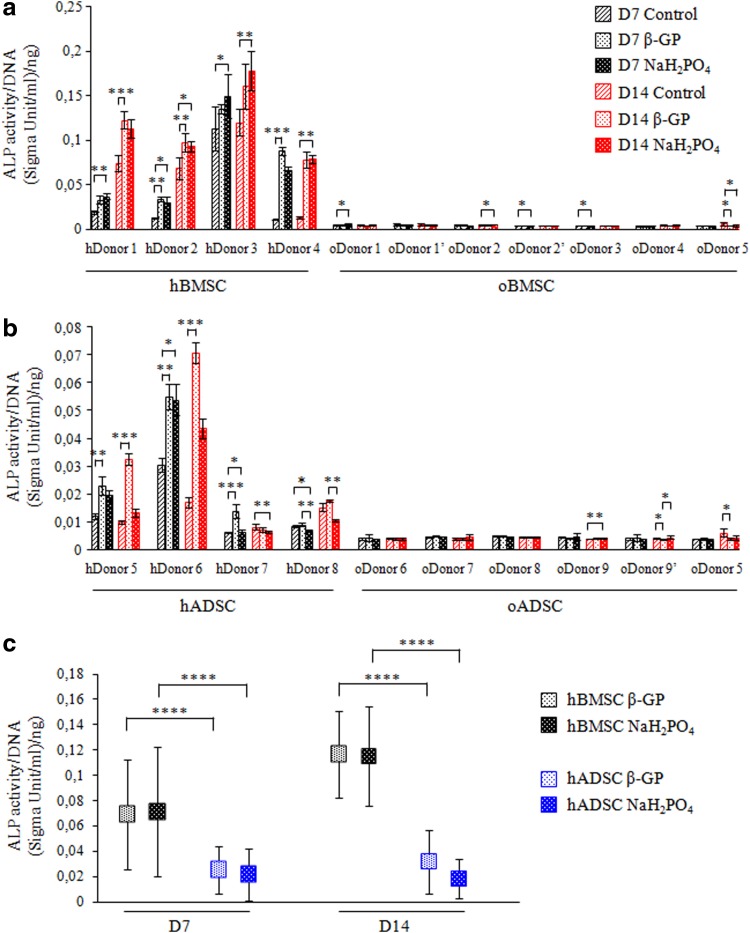
Changes in ALP activity, an assay widely used for osteogenic cultures, were analyzed to determine whether insufficient ALP activity could cause lack of mineralization of oBMSCs and oADSCs. hBMSCs from three donors (with the exception of donor 3) showed significant up-regulation of ALP activity when cultured with β-GP or NaH2PO4 at day 7 and day 14 when compared to their respective controls (Fig. 3a). Donor 3–derived hBMSCs showed high basal ALP activity under control conditions (which was not significantly affected by exposure to osteogenic medium). Moderate increase in ALP activity was observed in hADSCs upon stimulation with osteogenic media (Fig. 3b). Interestingly, at day 14 the level of ALP enzymatic activity induced by β-GP was greater than that induced by NaH2PO4 in cells derived from donors 5 and 6. The ALP activity observed in ovine cells derived from bone marrow and adipose tissue cells was found to be very low under all conditions. Comparison of mean ALP activity of hBMSCs and hADSCs upon stimulation with osteogenic medium showed significantly lower activity of this enzyme in hADSCs at analyzed time-points (Fig. 3c).
FIG. 3.
ALP activity of human and ovine BMSCs (a) and ADSCs (b) shown for each donor separately or as mean value from all combined hBMSCs or hADSCs (c). Donors marked with a prime symbol (′) indicates cells derived from the same donor but used in different round of experiments. Cells were cultured in control or osteogenic medium containing β-GP or NaH2PO4. ALP activity was measured by colorimetric assay at day 7 (D7) and 14 (D14). Each measurement was normalized for total DNA, as assayed by the Picogreen method. Values shown are means±SD. *p<0.05; **p<0.001, ***p<0.001, ****p<0.0001.
Release of phosphate ions from β-GP
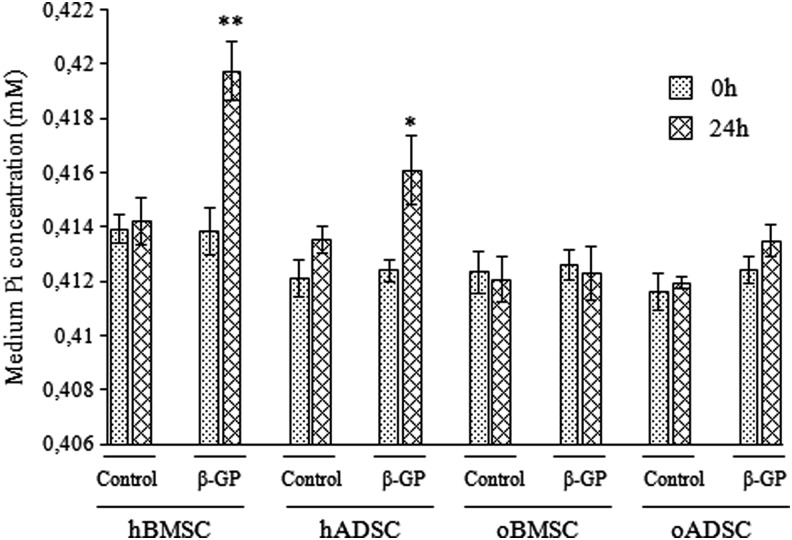
Culture supernatants were collected and assayed for presence of free phosphate ions (Pi). Release of Pi from β-GP is catalyzed by ALP activity. In all supernatants collected from control cultures, changes in Pi levels between time-points 0 and 24 h were not significant (Fig. 4). In culture supernatant containing β-GP, collected 24 h after medium change from hBMSCs and hADSCs, significant increases in Pi level were detected (p<0.01 and p<0.05 respectively). No Pi increase was detected in supernatants containing β-GP collected from ovine cell cultures.
FIG. 4.
The release of free phosphate (Pi) from β-GP in human and ovine BMSC and ADSC cultures. After 21 days of culture supernatants were collected at 0 and 24 hours after medium change. The release of Pi was monitored using a colorimetric assay. Values shown are means±SD. * p<0.05; ** p<0.01.
Discussion
In recent years MSCs have attained significant attention in cell-based strategies of regenerative medicine. In particular, bone tissue engineered products containing BMSCs, and lately ADSCs, are being widely considered for clinical application. Basic investigations focused on MSC-biomaterial interaction, MSC expansion and differentiation, as well as the optimization of MSC-based TE products are performed in culture systems in vitro. This mode of observation allows using human biological material. However, due to the standards, in vivo validation of bone tissue engineered products is required. Preclinical studies are performed on several model organisms, mainly rabbits, rats, dogs, sheep, and pigs.26 Among them sheep remain the most often recommended large animal experimental model for examination of bone substitutes.27 Since there is no immunodeficient large animal model for biocompatibility studies, in case of cell containing TE products, species-compatible implants are needed for in vivo observations. For such purpose, in parallel to the examinations of human MSCs, we investigated oBMSCs as well as oADSCs. We have confirmed stemness of the isolated cells taken for observation (Fig. 1). However, our numerous attempts to differentiate oBMSCs and oADSCs did not result in successful mineralization (Fig. 2a), which is widely regarded as a sign of osteoblastic phenotype. Jaiswal et al.7 have shown that higher initial seeding density of BMSCs significantly increased mineral deposition. Therefore, we speculated that at early stages of experiments, the lack of mineralization of ovine cells could be attributed to low seeding density (2000–3000 cells/cm2). We checked several high seeding densities and found that cultures with more than 3000 cells/cm2 peeled off the culture surface, preventing analysis at further time-points. Thus, we used the highest possible cell density for experiments, and we showed that the lack of mineralization of oBMSCs and oADSCs in standard osteogenic medium containing β-GP results from absence of ALP activity (Fig. 3a, 3b). The importance of ALP activity in mineralization is widely known. Its enzymatic activity is crucial for hydrolysis of β-GP,28 which is used in osteogenic medium as a source of organic phosphate known to support mineralization in vitro.29 The addition of 10 mM β-GP to osteogenic medium has remained routinely used by a wide range of groups performing differentiation assays with MSC cultures1,7,29–31 of different origin including sheep.21–23,32 It was shown that β-GP degraded to Pi (phosphate ions) in a few hours33 and that almost 80% of β-GP was hydrolyzed by chick bone cells within 24 h.11 Such results were confirmed by us for human but not for ovine cells. In the absence of ALP activity in ovine cells and in consequence lack of free phosphate ions, as shown by Pi measurement in culture (Fig. 4), mineralization of extracellular matrix was halted. Unlike human osteogenic cells, in which ALP activity is routinely considered, there is little know about ALP activity in oMSCs upon stimulation with osteogenic medium. To date there is no evidence of absence of ALP activity in ovine cells in osteogenic medium. Rentsch et al.22 only mentioned that they were not able to detect ALP activity in oBMSC culture using various methods, without showing the results. Contradictory data was presented by Reichert and colleagues23 in which ALP activity was measured at days 14 and 28 and showed typical for human rise–fall pattern. Several laboratories demonstrated that BMSCs from different species produce higher ALP activity when cultured in 10 nM dexamethasone compared with 100 nM.34 We tested whether it might have been the case in our study and found no difference in ALP activity in regards to dexamethasone concentration (data not shown).
In order to confirm that the absence of Pi causes the lack of mineralization in the β-GP–containing medium, we replaced it with inorganic source of Pi. Two alternative and uncommon sources of phosphate ions used as a component of osteogenic medium were reported (i.e., NaH2PO4 or KH2PO4).35,36 Following the protocol published by Mrugala et al.,35 we used 3 mM concentration of NaH2PO4. Since Chung and colleagues11 postulated that medium supplementation with β-GP or Pi should not exceed 2 mM, we tested if Pi supplementation, which we applied, could lead to nonphysiological mineral deposition. To examine this, we cultured human fibroblasts in both types of osteogenic media and confirmed the absence of mineral deposition (Fig. 2b). In contrast, upon addition of NaH2PO4 to osteogenic medium we achieved abundant mineralization of ovine cells extracellular matrix (Fig. 2a). Among the published observations showing mineralization in oBMSC culture, Mrugala et al.,35 Zannettino et al.,25 and McCarty et al.36 used osteogenic media containing an inorganic phosphate source. However, Reichert23 reported positive Alizarin red S staining in oBMSC cultures in the presence of β-GP. Among those publications, only Reichert23 confirmed ALP activity in the culture; Rentsch et al.22 was not able to detect it, while the others did not address this issue. In our opinion, differences in ALP activity between donors derived from different sheep strains is due to vast heterogeneity among these animals since the sheep population is large and partly subdivided as a result of geographic factors.
Besides obvious differences between human and ovine cells we also observed differences between BMSCs and ADSCs of human origin. In contrast to hADSCs, hBMSCs cultured in osteogenic medium containing NaH2PO4 did not have detectable mineral deposition, which might be a consequence of insufficient supply of NaH2PO4. Surprisingly, we did not detect mineralization of human ADSCs at day 28 in standard osteogenic medium (β-GP). It might be due to a lower rate of mineral formation with β-GP than that of Pi (NaH2PO4) since β-GP undergoes hydrolysis to liberate Pi, which is a time-dependent process and may take longer than in cultures supplemented directly with Pi. Signs of mineral deposition at later time points (day 35, data not shown) support this hypothesis. Additionally, limited mineralization efficiency of ADSCs in β-GP–containing media is accompanied by low ALP activity, which confirms the tendency that we revealed in the culture of ovine cells.
In conclusion, we demonstrate significant differences between human and ovine MSC mineralization capacity in osteogenic culture conditions, and we identify the reasons due to interspecies variations. Since mineralization is a widely accepted marker of the osteogenic differentiation and maturation of cells in culture, it may lead to potentially misleading results, and this phenomenon should be taken into account at the stage of planning and interpreting preclinical observations performed in animal models. We plan to use inorganic source of phosphorus ions (i.e., NaH2PO4 instead of the routinely used β-GP [organic source of P ions]) in oADSC culture in experiments testing bone tissue engineered products in the ovine model.
Abbreviations
- ADSC
adipose tissue–derived MSC
- ALP
alkaline phosphatase
- β-GP
β-glycerophosphate
- BMSC
bone marrow–derived MSC
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- h
human
- MSC
mesenchymal stem cell
- o
ovine
- PBS
phosphate-buffered saline
- Pi
phosphate ions
- Runx2
runt-related transcription factor 2
- SVF
stromal vascular fraction
- TE
tissue engineering
Acknowledgment
This work was supported by the European Regional Development Fund within the Innovative Economy Operational Programme in the frame of project BIO-IMPLANT (Grant No. POIG.01.01.02-00-022/09).
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger MF. Mackay AM. Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Gong Z. Calkins G. Cheng EC, et al. Influence of culture medium on smooth muscle cell differentiation from human bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2009;15:319–330. doi: 10.1089/ten.tea.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruder SP. Kraus KH. Goldberg VM, et al. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA. Zhu M. Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Reichert JC. Saifzadeh S. Wullschleger ME, et al. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials. 2009;30:2149–2163. doi: 10.1016/j.biomaterials.2008.12.050. [DOI] [PubMed] [Google Scholar]
- 6.Vater C. Kasten P. Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Jaiswal N. Haynesworth SE. Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 8.Igarashi M. Kamiya N. Hasegawa M, et al. Inductive effects of dexamethasone on the gene expression of Cbfa1, Osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J Mol Histol. 2004;35:3–10. doi: 10.1023/b:hijo.0000020883.33256.fe. [DOI] [PubMed] [Google Scholar]
- 9.Choi KM. Seo YK. Yoon HH, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105:586–594. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- 10.Hitomi K. Torii Y. Tsukagoshi N. Increase in the activity of alkaline phosphatase by L-ascorbic acid 2-phosphate in a human osteoblast cell line, HuO-3N1. J Nutr Sci Vitaminol (Tokyo) 1992;38:535–544. doi: 10.3177/jnsv.38.535. [DOI] [PubMed] [Google Scholar]
- 11.Chung CH. Golub EE. Forbes E, et al. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. 1992;51:305–311. doi: 10.1007/BF00334492. [DOI] [PubMed] [Google Scholar]
- 12.Tenenbaum HC. Role of organic phosphate in mineralization of bone invitro. J Dent Res. 1981;60:1586–1589. doi: 10.1177/0022034581060003S0801. [DOI] [PubMed] [Google Scholar]
- 13.Fortuna R. Anderson HC. Carty RP, et al. Enzymatic characterization of the matrix vesicle alkaline phosphatase isolated from bovine fetal epiphyseal cartilage. Calcif Tissue Int. 1980;30:217–225. doi: 10.1007/BF02408631. [DOI] [PubMed] [Google Scholar]
- 14.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2:81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 15.Baksh D. Yao R. Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 16.Rebelatto CK. Aguiar AM. Moretao MP, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 17.Wagner W. Wein F. Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Levi B. Nelson ER. Brown K, et al. Differences in osteogenic differentiation of adipose-derived stromal cells from murine, canine, and human sources in vitro and in vivo. Plast Reconstr Surg. 2011;128:373–386. doi: 10.1097/PRS.0b013e31821e6e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peister A. Mellad JA. Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 20.den Boer FC. Patka P. Bakker FC, et al. New segmental long bone defect model in sheep: quantitative analysis of healing with dual energy x-ray absorptiometry. J Orthop Res. 1999;17:654–660. doi: 10.1002/jor.1100170506. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes NP. Srivastava JK. Smith RF, et al. Heterogeneity in proliferative potential of ovine mesenchymal stem cell colonies. J Mater Sci Mater Med. 2004;15:397–402. doi: 10.1023/b:jmsm.0000021109.21807.f0. [DOI] [PubMed] [Google Scholar]
- 22.Rentsch C. Hess R. Rentsch B, et al. Ovine bone marrow mesenchymal stem cells: isolation and characterization of the cells and their osteogenic differentiation potential on embroidered and surface-modified polycaprolactone-co-lactide scaffolds. In Vitro Cell Dev Biol Anim. 2010;46:624–634. doi: 10.1007/s11626-010-9316-0. [DOI] [PubMed] [Google Scholar]
- 23.Reichert JC. Woodruff MA. Friis T, et al. Ovine bone- and marrow-derived progenitor cells and their potential for scaffold-based bone tissue engineering applications in vitro and in vivo. J Tissue Eng Regen Med. 2010;4:565–576. doi: 10.1002/term.276. [DOI] [PubMed] [Google Scholar]
- 24.Dominici M. Le Blanc K. Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 25.Zannettino AC. Paton S. Itescu S, et al. Comparative assessment of the osteoconductive properties of different biomaterials in vivo seeded with human or ovine mesenchymal stem/stromal cells. Tissue Eng Part A. 2010;16:3579–3587. doi: 10.1089/ten.TEA.2010.0153. [DOI] [PubMed] [Google Scholar]
- 26.Pearce AI. Richards RG. Milz S, et al. Animal models for implant biomaterial research in bone: A review. Eur Cells Mater. 2007;13:1–10. doi: 10.22203/ecm.v013a01. [DOI] [PubMed] [Google Scholar]
- 27.Turner AS. Experiences with sheep surgery: strengths and as an animal model for shoulder shortcomings. J Shoulder Elbow Surg. 2007;16:158s–163s. doi: 10.1016/j.jse.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Robison R. The possible significance of hexosephosphoric esters in ossification. Biochem J. 1923;17:286–293. doi: 10.1042/bj0170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peter SJ. Liang CR. Kim DJ, et al. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem. 1998;71:55–62. doi: 10.1002/(sici)1097-4644(19981001)71:1<55::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Jaiswal RK. Jaiswal N. Bruder SP, et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 31.Halleux C. Sottile V. Gasser JA, et al. Multi-lineage potential of human mesenchymal stem cells following clonal expansion. J Musculoskelet Neuronal Interact. 2001;2:71–76. [PubMed] [Google Scholar]
- 32.Eslaminejad MB. Falahi F. Nazarian H, et al. Differentiation potential and culture requirements of mesenchymal stem cells from ovine bone marrow for tissue regeneration applications. Iranian J Vet Surg. 2007;2:53–65. [Google Scholar]
- 33.Bellows CG. Heersche JN. Aubin JE. Inorganic phosphate added exogenously or released from beta-glycerophosphate initiates mineralization of osteoid nodules in vitro. Bone Miner. 1992;17:15–29. doi: 10.1016/0169-6009(92)90707-k. [DOI] [PubMed] [Google Scholar]
- 34.Hoemann CD. El-Gabalawy H. McKee MD. In vitro osteogenesis assays: influence of the primary cell source on alkaline phosphatase activity and mineralization. Pathol Biol (Paris) 2009;57:318–323. doi: 10.1016/j.patbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Mrugala D. Bony C. Neves N, et al. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67:288–295. doi: 10.1136/ard.2007.076620. [DOI] [PubMed] [Google Scholar]
- 36.McCarty RC. Gronthos S. Zannettino AC, et al. Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J Cell Physiol. 2009;219:324–333. doi: 10.1002/jcp.21670. [DOI] [PubMed] [Google Scholar]