Abstract
Currently, adult mesenchymal stem cells (MSCs) are being evaluated for a wide variety of therapeutic approaches. It has been suggested that MSCs possess regenerative properties when implanted or injected into damaged tissue. However, the efficacy of MSCs in several of the proposed treatments is still controversial. To further explore the therapeutic potential of these cells, it is necessary to trace the fate of individual donor or manipulated cells in the host organism. Recent studies from our lab showed that human placental alkaline phosphatase (hPLAP) is a marker with great potential for cell tracking. However, a potential concern related to this marker is its enzymatic activity, which might alter cell behavior and differentiation by hydrolyzing substrates in the extracellular space and thereby changing the cellular microenvironment. Therefore, the aim of this study was to characterize bone marrow MSCs (BMSCs) derived from hPLAP-transgenic inbred F344 rats (hPLAP-tg) in comparison to wild type (wt) BMSCs. Here, we show that BMSCs from wt and hPLAP-tg donors are indistinguishable in terms of cell morphology, viability, adhesion, immune phenotype, and proliferation as well as in their differentiation capacity over six passages. The expression of the hPLAP marker enzyme was not impaired by extensive in vitro cultivation, osteogenic, adipogenic, or chondrogenic differentiation, or seeding onto two- or three-dimensional biomaterials. Thus, our study underscores the utility of genetically labeled BMSCs isolated from hPLAP-tg donors for long-term tracking of the fate of transplanted MSCs in regenerative therapies.
Keywords: model organisms for research, stem cells, tissue engineering
Introduction
In the past decade, adult pluripotent mesenchymal stem cells (MSC) have increasingly been applied in the field of regenerative medicine and tissue engineering. The known potential of MSCs to differentiate into several distinct cell types1 makes them an attractive source for regeneration of tissues such as bone, cartilage, tendon, or myocardium.2 Although recent studies have clearly demonstrated the potential for MSC-based therapies to ameliorate or cure diseases of various tissues or even organs,3–5 basic aspects of the underlying mechanisms are still unclear. At present, we know little about the fate and function of applied cells. The percentage of MSCs that survive after systemic or local application, either in solution or in a supportive biomatrix, is an often unknown factor in many of the earlier studies. In addition, disease pathophysiology could be altered through many factors. The pluripotent differentiation capacity, cytokine secretion, immune modulation, or direct cell–cell interactions with damaged tissues are some of the most relevant factors to be considered. The success of future clinical applications of MSCs clearly depends on a thorough understanding of the fate and function of applied cells. Therefore, robust methods for cell tracking are required.
Currently, a variety of cell labeling methodologies are available for tracking purposes. The transfection of MSCs with a marker is one possibility—a challenging method when primary cells are used. Fluorescent markers like CM-Dil and CFSE have lethality levels reaching from 45% to 90%. Other tracking dyes like Hoechst 33342 are not suitable for long-term studies. 4′,6-Diamidino-2-phenylindole as a marker can lead to false-positive interpretations of results after the dye is released from dead cells.6 Even markers with excellent detection properties like PKH26 lose fluorescence intensity with subsequent cell divisions, limiting their use in long-term studies.6 Thus, the permanent integration of a marker gene into the genome of transgenic animals is a more convenient option for long-term cell tracking because a steady expression of the marker in the progeny of a specific cell type is obtained. Thereby, the main requirement for efficient long-term tracking of stem cells in the course of the application in tissue engineering and regenerative medicine (TERM) could be achieved.
Recent studies from our lab showed that human placental alkaline phosphatase (hPLAP) is a marker with great potential for cell tracking.7–9 Since the hPLAP enzyme is heat-stabile, labeled cells or tissues consisting of labeled cells can be processed by paraffin and plastic embedding procedures for histological analysis without interfering with the labeling of these cells. Endogenous alkaline phosphatases can be completely inactivated by heat exposure, allowing background-free detection of labeled cells in histological sections with excellent morphological preservation and circumventing the problems associated with autofluorescence of specific tissues when fluorescent cell tracking markers are used.10
A potential concern associated with hPLAP as a genetic marker is its enzymatic activity as an alkaline phosphatase. hPLAP is mainly expressed in the cell membrane,7,9 and by hydrolyzing substrates in the extracellular space, hPLAP could change the cellular microenvironment. Transgenic inbred Fischer 344 rats (hPLAP-tg) ubiquitously expressing the hPLAP enzyme under the control of the ROSA βgeo 26 promoter are indistinguishable from wild type (wt) F344 rats.9,11 Therefore, it is unlikely that the transgene has any major cellular effects. However, it is currently unknown whether the cellular presence of the genetic marker hPLAP may influence the behavior and differentiation of hPLAP-labeled MSCs.
In the present study, we sought to characterize further bone marrow MSCs (BMSCs) derived from hPLAP-tg F344 rats. We hypothesized that BMSCs isolated from hPLAP-tg rats provide answers to many open questions in regenerative medicine due to their excellent cell tracking properties. In order to provide a complete characterization of these cells, their cell viability and proliferation, pluripotent differentiation capacity, and behavior in two-dimensional as well as in three-dimensional culture models were assessed in comparison with BMSCs isolated from wt F344 rats.
Materials and Methods
Animals
Hemizygous R26-hPLAP-tg F344 rats were mated with wt F344 rats, and the resulting offspring was genotyped for hPLAP expression by histochemical staining using tail blood drops as previously described.11 Female 4- to 5-week-old wt and hPLAP-tg rats were used for all BMSC isolations.
Isolation and culture of BMSCs
BMSCs were isolated by adapting a protocol described by Farrell et al.12 A combination of collagenase digestion and Ficoll gradient centrifugation was introduced to the latter protocol. The animals were killed by carbon dioxide asphyxia immediately before tissue collection. After defleshing, femurs and tibias were cut at both epiphyses and incubated in sterile modified Eagle's medium with Earle's Salts and L-glutamine (MEM; PAA Laboratories GmbH, Pasching, Austria) containing 2.5 mg/mL collagenase type II (Gibco™, Invitrogen, Carlsbad, CA) for 2 h at 37°C, 5% CO2, and 3% O2. Subsequently, the bone marrow was flushed out with 10 mL of complete MEM supplemented with 10% heat-inactivated fetal calf serum (FCS; PAA Laboratories GmbH) and 1% penicillin/streptomycin (PAA Laboratories GmbH). Cells were sedimented and resuspended in fresh complete culture medium. Afterwards, the mononuclear cell fraction was collected by density gradient centrifugation using Ficoll-Paque™ (500 g, 30 min; GE Healthcare Ltd., Little Chalfont, United Kingdom), washed and finally resuspended in complete MEM. Subsequently, the cell suspension was plated in T75 cell culture flasks (PAA Laboratories GmbH). After 24 h in culture, the culture medium was replaced to remove nonadherent cells. Upon reaching 80% confluency, cells were passaged and replated. BMSCs were subcultured up to passage (P)6. During culture, the medium was changed every third day and the cells were maintained at 37°C, 5% CO2, and 3% O2 (hereafter referred to as standard culture conditions).
BMSC characterization
Colony-forming unit assay
The colony-forming unit (CFU-f) assay was performed on hPLAP-tg and wt BMSCs in all obtained passages (P1 to P6) according to established protocols.13 In brief, cultures of 1000 cells/60 cm2 were seeded in triplicates for each genotype and maintained under standard culture conditions for 10 and 14 days. Subsequently, the cells were washed two times with Dulbecco's phosphate-buffered saline (DPBS; pH 7.4; PAA Laboratories GmbH) and stained with 0.5% crystal violet solution in methanol for 10 min at room temperature (RT). Stained cultures were washed with DPBS and subsequently photographed. Colonies were counted using the ImageJ image analysis software (National Institutes of Health, Bethesda, MD).
Flow cytometry analysis
BMSCs harvested from wt and hPLAP-tg animals were cultured for up to six passages. After every passage (P2 to P6), the cells were enzymatically detached using trypsin/EDTA (PAA Laboratories GmbH) and examined by flow cytometry (FCM). In brief, cells were resuspended in DPBS, supplemented with 2% FCS, and counted. Cells (1×106) were used for staining with the following combination of monoclonal antibodies: antimouse/rat CD90.1-eFluor® 450 (clone HIS51; eBioscience, Santa Clara, CA), antimouse/rat CD29-APC (clone eBioHMb1-1, eBioscience), antihuman/rat CD34-PE (clone ICO115; Santa Cruz Biotechnology, Santa Cruz, CA), and antirat CD45-PE (clone OX-1; BioLegend, San Diego, CA). Appropriate isotype controls were included. In addition, live and dead cells were distinguished by using a Live/Dead Near-IR kit (Invitrogen) according to the manufacturer's recommendations. Only viable cells were included in the analyses. FCM analyses were performed on a FACSCanto II (BD Biosciences, Franklin Lakes, NJ) collecting at least 10,000 events.
Cell viability and proliferation
For assessment of viability and proliferation capability, wt and hPLAP-tg BMSCs from P2 to P6 were seeded at a density of 1×104 cells/well in 24-well tissue culture plates (TPP; Techno Plastic Products AG, Trasadingen, Switzerland). The cells were cultured under standard culture conditions for up to 7 days. Latex rubber (Velos-Perforex, Sevenoaks, United Kingdom) was used as a positive control for induction of cell death. Cell viability was determined by means of a standard MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay according to the manufacturer's instructions (CellTiter 96; Promega, Fitchburg, WI) and modified using previously described protocols.14,15 Briefly, the cell monolayer was treated with 200 μL/well of MTS reagent solution (5:1 ratio in serum-free MEM without phenol red) and incubated for 3 h at 37°C in a humidified environment containing 5% CO2 and 3% O2. One hundred microliters of medium from each well was transferred into a 96-well plate, and the absorbance at 490 nm was determined in a plate reader (EnSpire; Perkin Elmer, Waltham, MA). All samples were tested in triplicate in three independent assays.
Cell proliferation was evaluated by DNA quantification using the PicoGreen assay (Molecular Probes, Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Before performing the assay, the cell monolayer was carefully washed with DPBS and then lysed by osmotic and thermal shock. The obtained supernatant was used for DNA analysis. Fluorescence was measured (485 nm excitation and 528 nm emission) in a microplate reader (EnSpire; Perkin Elmer) and the DNA amounts were calculated according to a standard curve. The samples were tested in triplicate in three independent assays.
In addition, the cell viability was assessed by live-dead staining using calcein-AM (Sigma-Aldrich, St. Louis, MO) and propidium iodide (Sigma-Aldrich). After 1, 3, and 7 days, monolayer cultures were carefully washed with DPBS and subsequently stained using a DPBS solution containing 2 μM calcein-AM and 1.5 μM propidium iodide. After 30 min of incubation at 37°C, cultures were observed under a fluorescence microscope (Axioskop 2 plus; Zeiss, Oberkochen, Germany).
Differentiation capacity of BMSCs
BMSCs at P2 to P6 were evaluated in vitro for adipogenic, osteogenic, and chondrogenic differentiation according to standard procedures16,17 described in detail in the Supplementary Data. All experiments were performed in triplicate, and the results were reported in comparison to untreated control cells.
Seeding of hPLAP-tg cells onto different biomaterials
PCL membranes
For the production of PCL membranes, a solvent casting methodology was used.18,19 In brief, 20% (w/v) solution from PCL (Tone™ PCL-787; Union Carbide Chemicals and Plastics Division, Piscataway, NJ) in chloroform was prepared and 2 mL was poured into 100-mm glass petri dishes, resulting in 105±30-μm thick films as determined by scanning electron microscopy. The polymeric membranes were allowed to air-dry overnight at RT and were incubated in a vacuum at 35°C until a constant weight was reached. Each membrane was cut into 3 by 3 cm pieces and subsequently fitted into an empty insert (CellCrown™ 24; Scaffdex, Tampere, Finland). The inserts containing the membranes were sterilized by ethylene oxide for further cell culture use.
Fibrin hydrogels
The fibrin hydrogels were prepared at RT by mixing the same volumes of fibrinogen 67–106 mg/mL, dissolved in 3000 kIU/mL aprotinine, and thrombin (500 IU/mL), dissolved in 40 μmol/mL CaCl2 (all components obtained from Tisseel, Baxter, Vienna, Austria).20–22 The clots were formed inside a sterile plastic mold of 1 cm in diameter and allowed to polymerize at 37°C for 30 min.22 All steps were performed under sterile conditions and immediately before the cell seeding experiments.
PCL scaffolds
Individual scaffolds were cut out of a commercially available 50 mm×50 mm×1.25 mm PCL mesh with 70% porosity (Osteopore™, The Alpha, Singapore), using an 8-mm punch biopsy device. Thus, the final scaffolds had a spherical shape with a diameter of 8 mm. They were subsequently sterilized by ethylene oxide for further cell culture experiments.
For cell seeding experiments, 1×104 wt or hPLAP-tg BMSCs in 1 mL of culture medium were seeded onto each PCL membrane, PCL scaffold, and fibrin hydrogel using 24-well plates.22,23 After 1, 3, and 7 days the seeded biomaterials were collected and subsequently analyzed for hPLAP expression of the cells and SEM observations.
hPLAP expression
For the evaluation of hPLAP expression, histochemical staining was performed. In brief, the cells were fixed in ice-cold acetone–methanol mixture (30:70) for 5 min. Subsequently, the cells were washed two times with PBS and endogenous heat-labile alkaline phosphatases were inactivated by incubation in TMN substrate buffer (0.1 M Tris-HCl, pH 9.5 containing 0.1 M NaCl and 5 mM MgCl2) at 60°C for 30 min. Substrate buffer was discarded and staining for heat-stable PLAP was performed by using fresh TMN buffer containing 0.175 mg/mL of 5-bromo-4-chloro-3-indolyl phosphate (BCIP; Sigma-Aldrich) and 0.45 mg/mL of nitrotetrazolium blue chloride (NBT; Sigma-Aldrich).8 The staining was performed at RT for at least 3 h. Samples were washed two times with PBS and observed under an inverted light microscope (Axiovert 25; Zeiss) or stereomicroscope (Stemi SV6; Zeiss). Each sample was stained at least in triplicate. Wt BMSCs served as negative control in all cases.
Scanning electron microscopy
Scanning electron microscopy (SEM) was performed to evaluate the morphology of cells growing on the membranes, gels, and scaffolds. The samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4, 2% sucrose) for 30 min and subsequently dehydrated by increasing concentrations of ethanol. Incubation in hexamethyldisilazane was performed three times for 10 min each. Finally, the specimens were allowed to air-dry and sputter-coated with gold before SEM observation (Philips XL20, Eindhoven, The Netherlands), using operating conditions of 15 kV and SE detection.
Statistical analysis
All obtained values are reported as mean±standard deviation. The statistical analysis was performed with OriginPro 8.0 (Microcal1 software; OriginLab Corp, Northampton, MA). Normal distribution of the data was analyzed by Shapiro-Wilk test. Differences between wt and hPLAP-tg BMSCs were determined using Student's t test for two independent samples and one-way ANOVA when more than two groups were compared; p values<0.05 were considered significant.
Results and Discussion
The main intention behind the cellular system developed in this work was the possibility of cell tracking after therapeutic application of MSC. TERM strategies are mainly based on the combination of stem cells, growth factors, and biomaterials to create a bioconstruct to be injectable or implantable into the body with the final aim to regenerate injured tissues or organs. Thus, the hPLAP-tg cells need to fulfill two important features to be a suitable cell tracking system for TERM applications: to maintain continuous and stable expression of the hPLAP enzyme and to have the capability of normal growth and proliferation after seeding onto commonly used biomaterials.
Cell growth, morphology, and colony formation
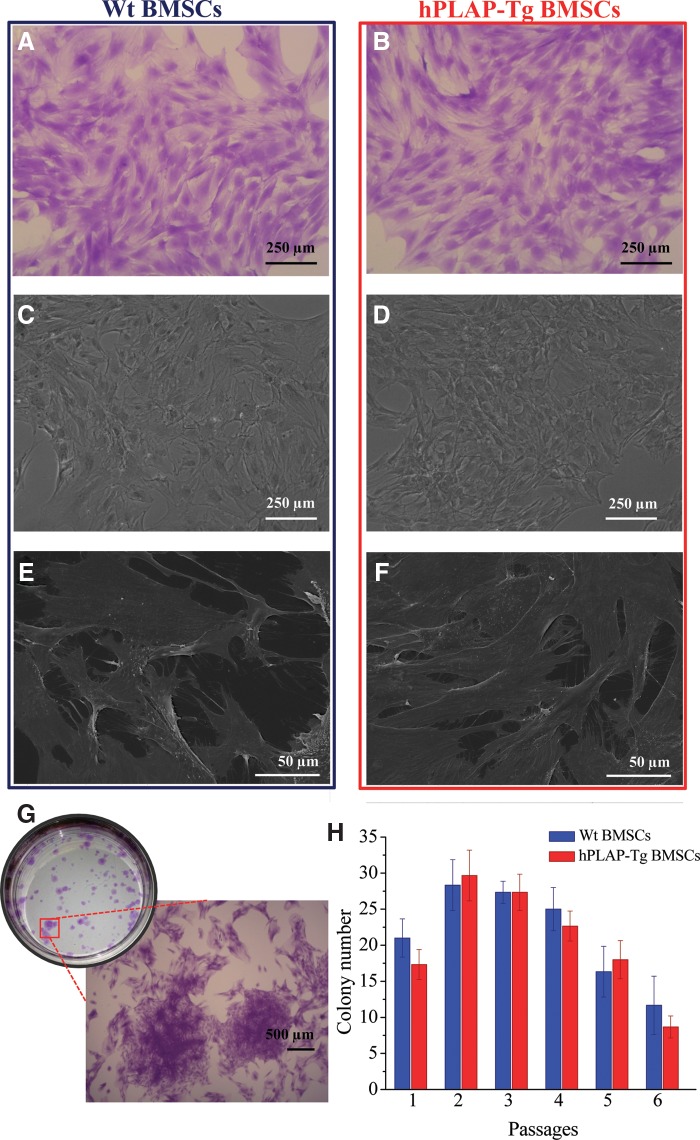
hPLAP-tg BMSCs were successfully isolated from tg donors by adapting previously described standard protocols.12 Isolated hPLAP-tg cells were expanded up to P6. hPLAP-tg cells had the same morphology as wt cells exemplarily shown for P2 (Fig. 1A–D). In addition, scanning electron micrographs of the cells after 7 days of culture showed good adherence to the culture plastic surface for both tg and wt cells (Fig. 1E, F). Very close cell–cell contacts and a high number of filopodia could be observed in both cases. No morphological differences were observed from P0 to P6 between hPLAP-tg and wt BMSCs.
FIG. 1.
Morphological characteristics and colony-forming capacity of hPLAP-tg BMSCs in comparison with identically isolated and expanded wt BMSCs. Light microscopy: (A, B) crystal violet staining, (C, D) phase contrast. (E, F) Scanning electron microscopy. (G, H) Colony-forming capacity of in vitro expanded wt and hPLAP-tg BMSCs. (G) Crystal violet stained plate of CFU-f assay performed for tg cells in passage (P)2. Inset shows morphological details of the colonies at higher magnification. (H) Effect of subsequent passaging of wt and hPLAP-tg cells on the CFU-f capacity. Values are mean±standard deviation (n=3 each). hPLAP, human placental alkaline phosphatase; BMSCs, bone marrow mesenchymal stem cells, tg, transgenic; wt, wild type; CFU-f, colony-forming unit.
The capability of hPLAP-tg BMSCs to generate colonies after plating at low densities was evaluated by means of CFU-f assay (Fig. 1G). In fact, no significant differences in the colony number could be observed when comparing tg and wt BMCSs at each of the six passages evaluated (p>0.05 for all passages; Fig. 1H). Wt and hPLAP-tg cells retained their ability to generate single cell–derived colonies up to P6. However, from P4 on, the colony-forming ability decreased in both groups with increasing passages, a finding which is in accordance with earlier reports.24–26
Cell viability and proliferation upon in vitro cultivation
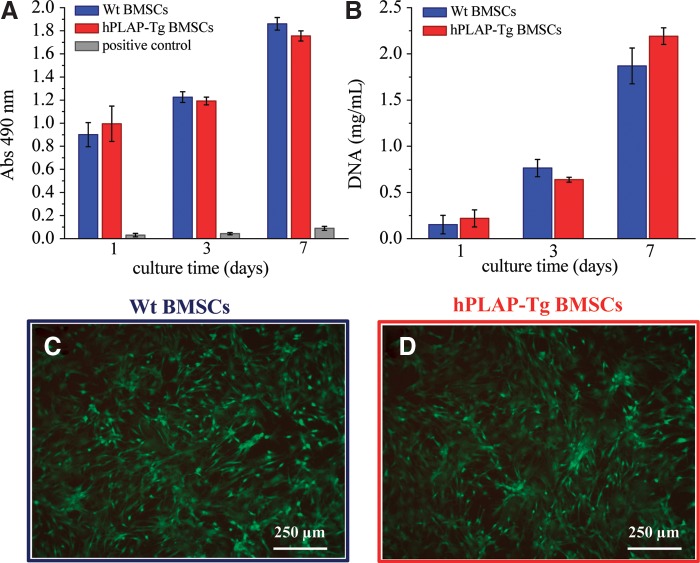
The viability and proliferative capacity of hPLAP-tg and wt BMSCs was evaluated after each passage and for up to 7 days of culture. Viability and proliferation rate of hPLAP-tg BMSCs were similar to wt controls after each passage of in vitro cultivation (exemplarily shown for P3 in Fig. 2A, B). The proliferation rate increased with increasing passage number for all cells (tg and wt). In addition, the viability of the hPLAP-tg cells was also confirmed by calcein AM-PI staining (Fig. 2C, D).
FIG. 2.
Cell viability and proliferation of hPLAP-tg BMSCs in culture for up to 7 days compared to wt BMSCs. (A) Cell viability assessed by MTS assay. For positive control, BMSCs were treated with latex rubber to induce cell death. (B) Cell proliferation assessed by DNA quantification. (C, D) Calcein-AM/propidium iodide live/dead staining of wt and hPLAP-tg BMSCs after 7 days of culture, P3. MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Abs, absorbance.
Immunophenotypic characterization of hPLAP-tg BMSCs
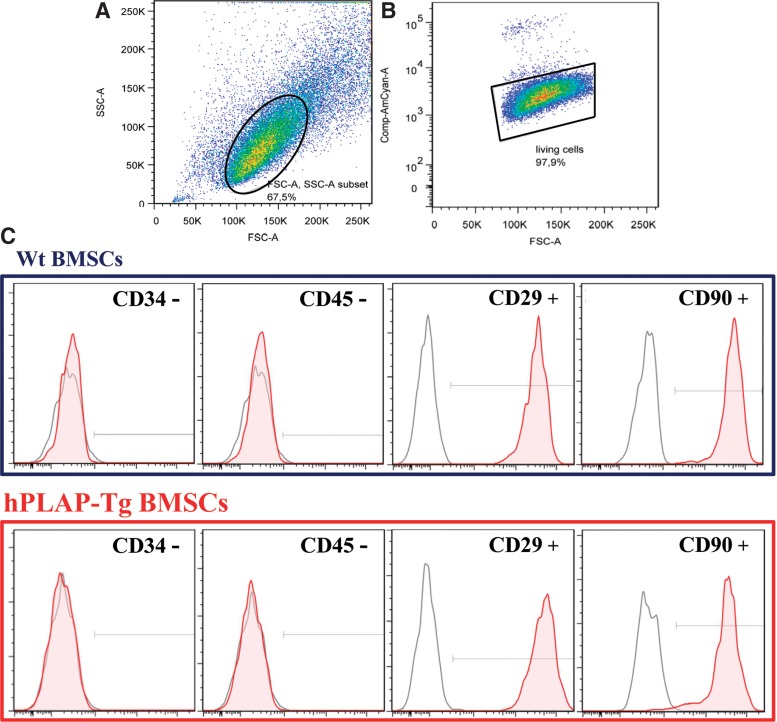
To determine whether isolated BMSCs from hPLAP-tg rats expressed typical stem cell surface markers but not hematopoietic markers, flow cytometric analysis was performed for CD34, CD45, CD29, and CD90 (Fig. 3). hPLAP-tg BMSCs from three different donors and at P2 to P6 were positive for the adhesion marker CD29 (>99.8% positive) and the rat stem cell antigen CD90 (>94.3% positive) exemplarily shown for P4 (Fig. 3C and data not shown). In addition, these cells were negative for typical hematopoietic lineage markers like CD34 (<1% positive) and CD45 (<1.2% positive). No differences were observed when comparing tg with wt cells related to the expression of the analyzed surface markers. Hence, isolated hPLAP-tg and wt cells expressed characteristic MSC cell surface markers.
FIG. 3.
Flow cytometric analysis of isolated and expanded wt and hPLAP-tg BMSCs. The cells were cultured for up to six passages and stained after every passage (from P2 to P6) for stem cell (CD29 and CD90) and hematopoietic markers (CD34 and CD45). (A) Density plot for FSC versus SSC and (B) percentage of cell viability of hPLAP-tg BMSCs at P4. (C) Expression of stem cell surface markers on hPLAP-tg and wt BMSCs at P4. Cells stained with the corresponding antibody are indicated by filled red histograms, negative isotype controls are indicated by open gray histograms.
Osteogenic, chondrogenic, and adipogenic differentiation capacity of hPLAP-tg BMSCs
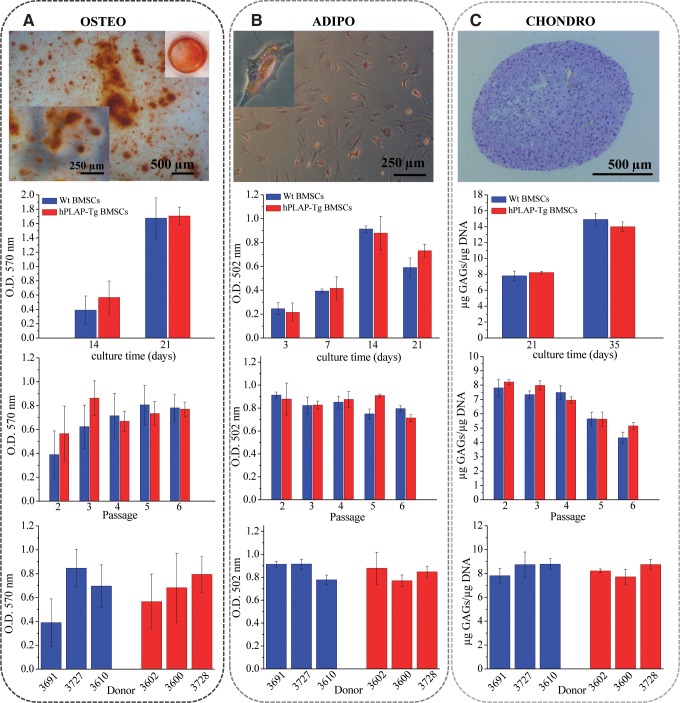
To examine the differentiation capacities of the isolated MSCs, hPLAP-tg and wt BMSCs from all passages (P2 to P6) were induced to differentiate into osteogenic, adipogenic, and chondrogenic lineages. Osteogenesis was induced by culturing the cells in dexamethasone containing culture medium up to 21 days.16 Alizarin red staining showed no significant differences (p>0.05) in osteogenic differentiation when comparing hPLAP-tg with wt BMSCs (Fig. 4A). Mineralization increased profoundly between 14 and 21 days of osteogenic stimulation for wt (p=0.003) and hPLAP-tg cells (p=0.0016; Fig. 4A). Moreover, their osteogenic differentiation potential was not impaired by cell passaging up to P6 and independent of the donor (middle and lower panels in Fig. 4A).
FIG. 4.
Multiple lineage differentiation of wt and hPLAP-Tg BMSCs. (A) Osteogenic differentiation of wt and hPLAP-tg BMSCs (P2) cultured in osteogenic media for up to 21 days as indicated by alizarin red staining. (B) Adipogenic differentiation of wt and hPLAP-Tg BMSCs (P2) cultured in adipogenic media for up to 21 days as indicated by oil red O staining. (C) Chondrogenic differentiation of wt and hPLAP-tg BMSCs (P2) pellets cultured in chondrogenic media for up to 35 days as indicated by toluidine blue staining. Bar graphs show quantification of alizarin red–positive cultures (A), oil red O–positive cultures (B), and GAGs quantification (C) depending on culture time, passage (14 days of culture) and donor (cells in P2 after 14 days of culture).
Adipogenic differentiation was induced by insulin, dexamethasone, and isobutyl-methylxanthine.16 After 3 days of stimulation the first lipid droplet accumulations could be observed microscopically. The upper panel in Figure 4B shows oil red O staining of tg BMSCs after 7 days of stimulation where abundant oil red O-stained lipid droplets are visible. Lipid accumulation increased from 3 to 14 days of adipogenic differentiation and was equal in tg and wt cells regardless of passage number or donor (Fig. 4B), indicating a robust adipogenic differentiation potential of wt and tg BMSCs during culturing.
To assess the chondrogenic capacity of the hPLAP-tg BMSCs, a pellet culture system was used.27 The cell pellets were stimulated with standard chondrogenic medium in presence of transforming growth factor-β1 up to 35 days.27,28 Figure 4C shows the toluidine blue staining of the hPLAP-tg cell pellet after 35 days of chondrogenic differentiation. A homogenously distributed matrix throughout the pellet can be observed where embedded cells appeared spherical and morphologically similar to chondrocytes. Glycosaminoglycan (GAG) measurements in papain-digested pellets showed increased production of proteoglycans in both tg and wt cells after 35 days of culture (Fig. 4C, upper graph). However, the GAG production decreased significantly with increasing cell passages (p=0.0011 for wt and p=0.00004 for tg cells; Fig. 4C, middle graph), indicating a diminished capability of the cells towards chondrogenic differentiation after prolonged in vitro culture. Interestingly, this effect was much more pronounced during chondrogenic differentiation than in adipogenesis or osteogenesis. In analogy to our findings, it was reported before by other authors that BMSCs lose their multidifferentiation capacity after extensive in vitro passaging.25,26,29,30 Nevertheless, there was no significant difference in the differentiation potential of wt and hPLAP-tg BMSC (p>0.05).
Maintenance of the hPLAP expression
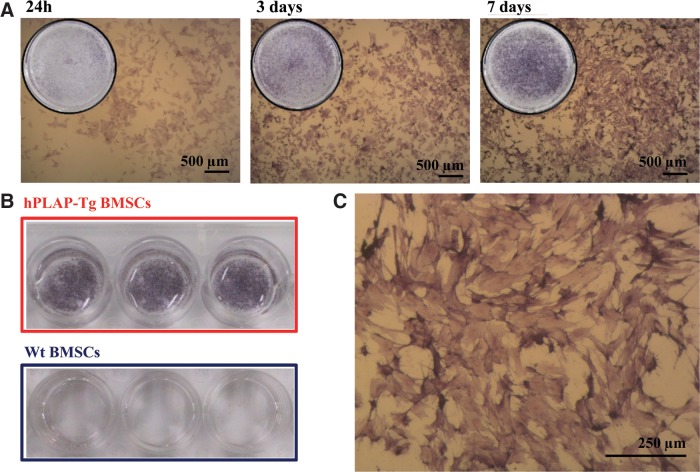
hPLAP-tg BMSCs ought to carry on their marker expression during in vitro expansion, differentiation, and after their seeding onto diverse biomaterials. Therefore, the maintenance of hPLAP expression was carefully evaluated. The results of the histochemical staining for hPLAP after in vitro culture of tg and wt BMSCs up to 7 days is shown in Figure 5. All adherent hPLAP-tg BMSCs showed clear hPLAP staining at all time points (Fig. 5A, C), whereas staining was absent in wt cells (Fig. 5B). In addition, osteogenic, chondrogenic, and adipogenic differentiation procedures did not impair hPLAP expression in hPLAP-tg cells (Fig. 6).
FIG. 5.
hPLAP expression of BMSCs isolated from wt and hPLAP-tg rats. (A) Histochemical hPLAP staining using NBT/BCIP after heat inactivation of hPLAP-tg BMSCs cultured for 24 h, 3 days, and 7 days after seeding. (B) Negative expression of the enzyme in wt BMSCs isolated and culture under identical conditions. (C) Higher magnification of hPLAP-tg BMSCs after 7 days of culture. NBT, nitrotetrazolium blue chloride; BCIP, 5-bromo-4-chloro-3-indolyl phosphate.
FIG. 6.
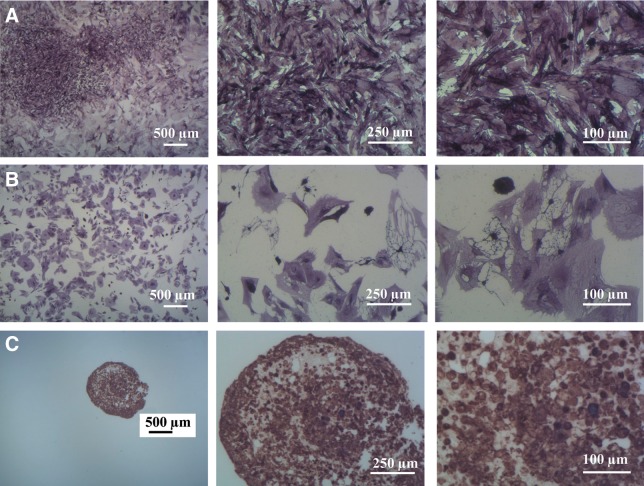
Stable expression of the hPLAP enzyme by hPLAP-tg BMSCs after 21 days of induced multidifferentiation in vitro. (A) Osteogenic differentiated hPLAP-tg cells, (B) adipogenic differentiated hPLAP-tg cells, and (C) chondrogenic differentiated hPLAP-tg cell pellet. Histochemical hPLAP staining was carried out using NBT/BCIP after heat inactivation.
Cell seeding on two- and three-dimensional scaffolds for tissue engineering applications
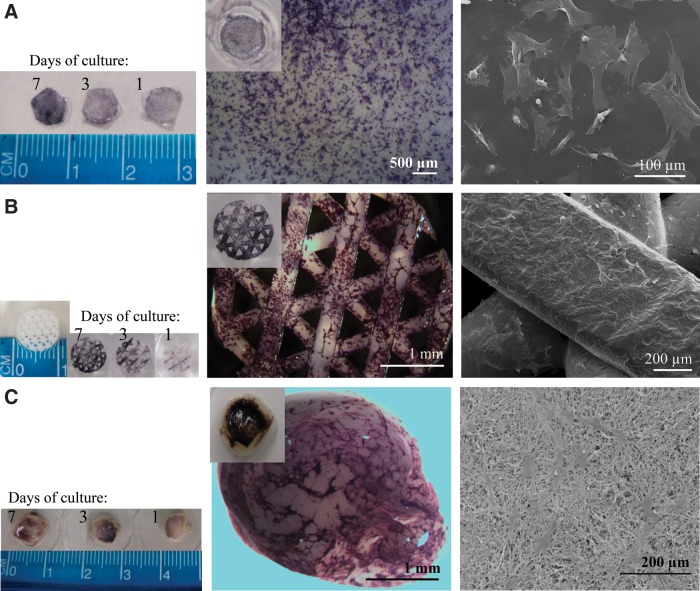
For TERM applications, hPLAP-tg BMSCs should be able to adhere and proliferate onto different biomaterials' surfaces in the same manner as wt BMSCs, and should maintain hPLAP expression. Polymeric-based membranes and scaffolds as well as fibrin gels seeded with hPLAP-tg cells were stained for hPLAP at 1, 3, and 7 days after seeding (Fig. 7A–C, left and middle). The hPLAP expression was very robust and reproducible in all cases, and a good cell adhesion to the material surface could be observed. In addition, SEM micrographs (Fig. 7, right) revealed good adhesion of the hPLAP-tg cells to the different biomaterials. No differences in morphology, adhesion, or proliferation were observed between tg and wt control cells (data not shown).
FIG. 7.
Maintenance of the expression of the hPLAP enzyme by hPLAP-tg BMSCs after seeding on different biomaterials. (A) PCL membrane, (B) PCL scaffold, and (C) fibrin hydrogel. Left and middle panels show histochemical staining for hPLAP enzymatic activity of hPLAP-tg BMSCs. Right panels show morphological details of the seeded cells as observed by SEM (after 3 days of culture).
Conclusions
The successful development of TERM applications using MSCs is highly dependent on our capability to accurately track the applied cells. We need to have a full understanding of the cells' fate and function after they are injected or implanted into a patient. Therefore, improvements in our ability to reliably and reproducibly track labeled MSCs in a long-term fashion will certainly facilitate the successful application of MSCs in tomorrow's clinical practice. In the current study we successfully isolated and characterized genetically labeled BMSCs from hPLAP-tg donor rats and demonstrated their multidifferentiation capacity. hPLAP-tg BMSCs stably expressed the hPLAP marker enzyme after in vitro culture for up to six passages and after seeding on different biomaterials of interest. The behavior, viability, and differentiation capacity of BMSCs isolated from wt and hPLAP-tg F344 rats were indistinguishable, suggesting that hPLAP is a neutral genetic marker for BMSCs. In combination with our recently introduced concept of marker tolerance,7 hPLAP labeling of MSCs allows the long-term in vivo study of labeled cells in the complete absence of immune-mediated rejection in immunocompetent hosts. A limitation of hPLAP as a cell tracking marker in vivo is that it is a histological marker for end-point analyses and thus not suitable for longitudinal follow-up studies in individual organisms. However, histology is still considered the gold standard for cell tracking.
Supplementary Material
Abbreviations
- BCIP
5-bromo-4-chloro-3-indolyl phosphate
- BMSC
bone marrow MSC
- CFU-f
colony-forming unit
- FCM
flow cytometry
- FCS
fetal calf serum
- GAG
glycosaminoglycan
- hPLAP
human placental alkaline phosphatase
- MEM
modified Eagle's medium
- MSC
mesenchymal stem cell
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- NBT
nitrotetrazolium blue chloride
- P
passage
- RT
room temperature
- SEM
scanning electron microscopy
- TERM
tissue engineering and regenerative medicine
- tg
transgenic
- TPP
tissue culture plate
- wt
wild type
Acknowledgments
The authors would like to thank Fritz Seidl, M.A. Interpreting and Translating, for English linguistic corrections. This work was supported by a grant from the Austrian Science Fund (FWF P 21903-B13) to R.G.E.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Pittenger MF. Mackay AM. Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Rastegar F. Shenaq D. Huang J, et al. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells. 2010;2:67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cowan CM. Shi YY. Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 4.Gnecchi M. Danieli P. Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol. 2012;57:48–55. doi: 10.1016/j.vph.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Koga H. Muneta T. Ju Y-J, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25:689–696. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 6.Yan L. Han Y. He Y, et al. Cell tracing techniques in stem cell transplantation. Stem Cell Rev. 2007;3:265–269. doi: 10.1007/s12015-007-9004-y. [DOI] [PubMed] [Google Scholar]
- 7.Odörfer KI. Unger NJ. Weber K, et al. Marker tolerant, immunocompetent animals as a new tool for regenerative medicine and long-term cell tracking. BMC Biotechnol. 2007;7:30. doi: 10.1186/1472-6750-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odörfer KI. Egerbacher M. Unger NJ, et al. Hematopoietic bone marrow cells participate in endothelial, but not epithelial or mesenchymal cell renewal in adult rats. J Cell Mol Med. 2011;15:2232–2244. doi: 10.1111/j.1582-4934.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger NJ. Odoerfer KI. Weber K, et al. Utility of human placental alkaline phosphatase as a genetic marker for cell tracking in bone and cartilage. Histochem Cell Biol. 2007;127:669–674. doi: 10.1007/s00418-007-0286-6. [DOI] [PubMed] [Google Scholar]
- 10.Jackson KA. Snyder DS. Goodell MA. Skeletal muscle fiber-specific green autofluorescence: Potential for stem cell engraftment artifacts. Stem Cells. 2004;22:180–187. doi: 10.1634/stemcells.22-2-180. [DOI] [PubMed] [Google Scholar]
- 11.Kisseberth WC. Brettingen NT. Lohse JK, et al. Ubiquitous expression of marker transgenes in mice and rats. Dev Biol. 1999;214:128–138. doi: 10.1006/dbio.1999.9417. [DOI] [PubMed] [Google Scholar]
- 12.Farrell E. O'Brien FJ. Doyle P, et al. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12:459–468. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- 13.Pochampally R. Colony forming unit assays for MSCs. In: Prockop DJ, editor; Phinney DG, editor; Bunnell BA, editor. Mesenchymal Stem Cells: Methods and Protocols. Humana Press Springer Science; Totowa, NJ: 2008. pp. 83–91. [DOI] [PubMed] [Google Scholar]
- 14.Balmayor ER. Feichtinger GA. Azevedo HS, et al. Starch-poly-e-caprolactone microparticles reduce the needed amount of BMP-2. Clin Orthop Relat Res. 2009;467:3138–3148. doi: 10.1007/s11999-009-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgado AJ. Coutinho OP. Reis RL. Novel starch-based scaffolds for bone tissue engineering: cytotoxicity, cell culture, and protein expression. Tissue Eng. 2004;10:465–474. doi: 10.1089/107632704323061825. [DOI] [PubMed] [Google Scholar]
- 16.da Silva Meirelles L. Covas DT. Phenotypic analysis and differentiation of murine mesenchymal stem cells. In: Vemuri M, editor. Mesenchymal Stem Cells Assays and Applications, Methods in Molecular Biology. Springer: LLC; 2011. pp. 331–350. [DOI] [PubMed] [Google Scholar]
- 17.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045–1056. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu SZ. Wang XH. Guo G, et al. Preparation and properties of nano-hydroxyapatite/PCL-PEG-PCL composite membranes for tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2011;97B:74–83. doi: 10.1002/jbm.b.31788. [DOI] [PubMed] [Google Scholar]
- 19.Tiaw KS. Teoh SH. Chen R, et al. Processing methods of ultrathin poly(caprolactone) films for tissue engineering applications. Biomacromolecules. 2007;8:807–816. doi: 10.1021/bm060832a. [DOI] [PubMed] [Google Scholar]
- 20.Ho ST. Cool SM. Hui JH, et al. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2010;31:38–47. doi: 10.1016/j.biomaterials.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 21.O'Cearbhaill ED. Murphy M. Barry F, et al. Behavior of human mesenchymal stem cells in fibrin-based vascular tissue engineering constructs. Ann Biomed Eng. 2010;38:649–657. doi: 10.1007/s10439-010-9912-x. [DOI] [PubMed] [Google Scholar]
- 22.Nürnberger S. Wolbank S. Peterbauer-Scherb A, et al. Properties and potential alternative applications of fibrin glue. In: Byern J, editor; Grunwald I, editor. Biological Adhesive Systems. Springer; Vienna: 2010. pp. 237–259. [Google Scholar]
- 23.Zhu H. Schulz J. Schliephake H. Human bone marrow stroma stem cell distribution in calcium carbonate scaffolds using two different seeding methods. Clin Oral Implants Res. 2010;21:182–188. doi: 10.1111/j.1600-0501.2009.01816.x. [DOI] [PubMed] [Google Scholar]
- 24.Çelebi B. Elçin AE. Elçin YM. Proteome analysis of rat bone marrow mesenchymal stem cell differentiation. J Proteome Res. 2010;9:5217–5227. doi: 10.1021/pr100506u. [DOI] [PubMed] [Google Scholar]
- 25.Lo Surdo J. Bauer SR. Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:877–889. doi: 10.1089/ten.tec.2011.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkel JM. Rites of (stem cell) passage. J Proteome Res. 2009;8:2137. doi: 10.1021/pr900198a. [DOI] [PubMed] [Google Scholar]
- 27.Hildner F. Peterbauer A. Wolbank S, et al. FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-beta in human adipose derived stem cells. J Biomed Mater Res A. 2010;94:978–987. doi: 10.1002/jbm.a.32761. [DOI] [PubMed] [Google Scholar]
- 28.Handorf AM. Li WJ. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One. 2011;6:e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mareschi K. Ferrero I. Rustichelli D, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 30.Zhou S. Greenberger JS. Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.