Abstract
The use of lytic viruses to preferentially infect and eliminate cancer cells while sparing normal cells is a promising experimental therapeutic approach for treating cancer. However, the efficacy of oncolytic virotherapy is often limited by two innate immunity pathways, the protein kinase PKR and the 2′-5′-oligoadenylate (OAS)/RNase L systems, which are widely present in many but not all tumor cell types. Previously, we reported that the anticancer drug, sunitinib, an inhibitor of VEGF-R and PDGF-R, has off-target effects against both PKR and RNase L. Here we show that combining sunitinib treatments with infection by an oncolytic virus, vesicular stomatitis virus (VSV), led to the elimination of prostate, breast, and kidney malignant tumors in mice. In contrast, either virus or sunitinib alone slowed tumor progression but did not eliminate tumors. In prostate tumors excised from treated mice, sunitinib decreased levels of the phosphorylated form of translation initiation factor, eIF2-α, a substrate of PKR, by 10-fold while increasing median viral titers by 23-fold. The sunitinib/VSV regimen caused complete and sustained tumor regression in both immunodeficient and immunocompetent animals. Results indicate that transient inhibition of innate immunity with sunitinib enhances oncolytic virotherapy allowing the recovery of tumor-bearing animals.
Introduction
There is a critical need for innovative and novel approaches for the treatment of advanced metastatic cancer. Oncolytic viruses (OVs) are therapeutically used microbes, either naturally occurring or genetic engineered, that preferentially infect and replicate in cancer cells.1,2 The goal of OV research is the elimination of malignant tumors without serious toxicity. Oncolytic virotherapy for cancer was initially conceived based on the observation of transient remission of cancer patients during viral infections.3 OVs target tumor cells precisely because the same genetic alterations that allow malignant tumor cells to proliferate and survive also promote growth of lytic viruses. In some instances, genetic and epigenetic changes in cancer cells facilitate viral replication by suppressing the interferon (IFN) antiviral response.4,5 However, because the IFN pathway is multifaceted and complex, cancer cells are unlikely to be completely deficient in innate immunity.
To maximize therapeutic modalities that are either synergistic or sequential in their activities, OV are sometimes used in combination with more traditional anticancer agents, in particular with chemotherapy drugs, but also with angiogenesis inhibitors.3 OVs selectively eliminate cancer cells sparing noncancerous tissues and thus have a significant advantage compared to chemotherapy agents. Importantly, drug-resistant cancer cells and cancer stem cells retain their susceptibility to oncolytic viruses,6,7 and oncolytic viruses are also effective in hypoxic environments characteristic of solid tumors.8 Oncolytic viruses can be used locally or systemically to eliminate both primary tumors and metastases. Currently, at least ten different types of replication competent viruses have been investigated as oncolytic viruses, many of which are in clinical trials,3 including for use against prostate, breast, and kidney cancer2,9,10,11,12—the types of cancer investigated in this study. Although the first generation of oncolytic viruses involved viruses that are pathogenic for humans, later studies used relatively nonpathogenic human or animal viruses.3 In this study, we used the rhabdovirus, vesicular stomatitis virus (VSV), a nonsegmented negative RNA stranded virus that is pathogenic for horses, cattle, and swine. In contrast, VSV infections are usually asymptomatic in humans, although mild flu-like symptoms have been reported.13,14 Natural infections with VSV are extremely rare in humans and therefore so are preexisting antibodies against VSV. VSV is considered an attractive oncolytic virus for use in humans because it has a broad tissue tropism (therefore it will infect all types of tumor cells). However, while VSV infects and kills many types of tumor cells, viral growth is attenuated in normal cell types.13 Mutant VSVs genetically engineered to be deficient in host shut-off activity, thereby enhancing IFN induction in response to infection, have been proposed for oncolytic virotherapy.13,15,16
Despite their early promise, oncolytic virotherapy has not reached its potential in the clinic due principally to the host immune response. One reason is because preexisting immunity or immunity as a result of repeated administration of an oncolytic virus reduces the effectiveness of virotherapy. Accordingly, immuno-suppressants have been used to transiently inhibit antiviral immunity during oncolytic virus regimens.17 However, cellular immune responses have been shown in some studies to be beneficial for virotherapy of cancer by enhancing tumor antigen presentation.18,19
IFNs provide the frontline innate defense against viral infections in mammalian cells. But while the IFN system is normally beneficial to the host, in the case of oncolytic virotherapy, the opposite is true because the IFN system restricts the spread of the oncolytic virus within tumors. Two principal pathways for viral resistance are the 2′,5′-oligoadenylate synthetase (OAS)-RNase L system that degrades viral RNA and the RNA-dependent protein kinase (PKR), which inhibits viral protein synthesis.20 In the OAS-RNase L pathway, type I IFNs produced in response to viral infections induce transcription of the OAS genes. OAS-1, -2, and -3 are activated by viral double-stranded RNA, resulting in the production of 2-5A [pppA(2′p5′A)n] that binds inactive RNase L monomers causing dimerization and activation of RNase L.21 Cleavage of viral and cellular ssRNAs by RNase L inhibits viral replication. Also, type I IFNs induce expression of the serine/threonine protein kinase, PKR. Viral double-stranded activates PKR, causing it to dimerize and phosphorylate the α-subunit of eukaryotic initiation factor 2, eIF2α.20 Phosphorylation of eIF2α inhibits the guanine nucleotide exchange factor (eIF2B), thereby trapping eIF2α in an inactive state thus inhibiting protein synthesis. Once a virus infects a tumor cell, these intracellular host defenses are often activated limiting viral replication. Many, but not all, tumor cell types harbor functional PKR and OAS/RNase L systems, including the tumor cells used in this study. These results suggested to us that an inhibitor of RNase L and PKR would improve the efficacy of oncolytic virotherapy. In this regard, we recently reported that the antiangiogenesis drug, sunitinib (Sutent), is a potent in vivo inhibitor of both RNase L and PKR.22 Sunitinib is a kinase inhibitor of VEGF-R and PDGF-R used clinically to suppress renal cancer by blocking angiogenesis.23 In addition, however, sunitinib impairs antiviral innate immune responses by blocking the activities of both RNase L and PKR in cultured cells as well as in mice.22 Inhibition of RNase L and PKR is likely mediated through interactions of sunitinib with highly homologous protein kinase-like and protein kinase domains, respectively, in these proteins.22 Sunitinib inhibits RNase L dimerization and activation, while it also prevents activation of PKR. Because sunitinib is a proviral drug, we explored the possibility that sunitinib used in combination with an oncolytic virus would result in enhanced viral replication in tumor tissues. We observed that the sunitinib/oncolytic virus combination inhibited eIF2α phosphorylation while increasing viral replication in tumors. Furthermore, prostate, breast, and kidney tumors were eliminated in mice treated with both sunitinib and virus.
Results
Inhibition of antiviral enzymes by sunitinib in tumor cells
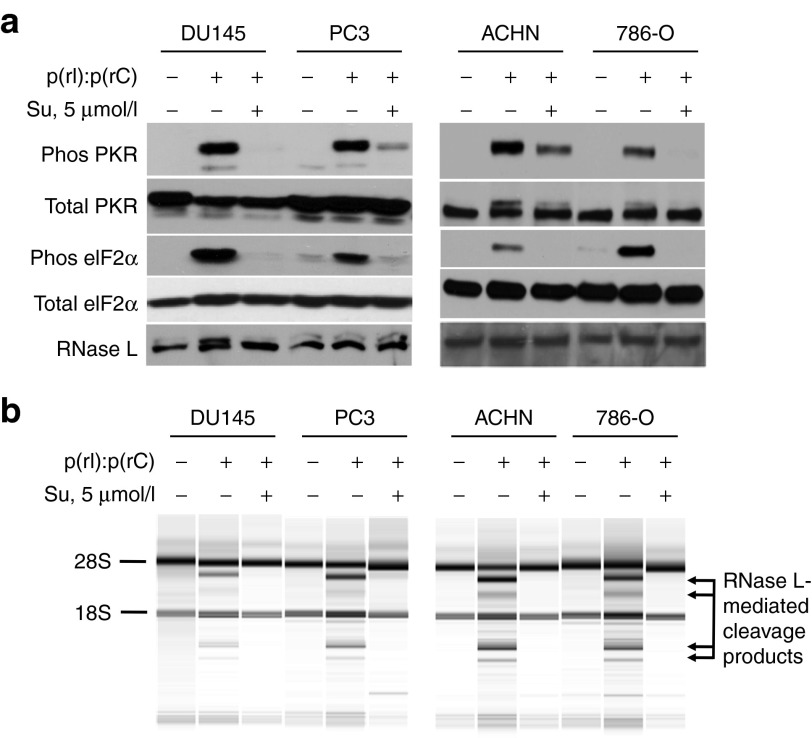
Previously we reported inhibition of the antiviral enzymes PKR and RNase L by sunitinib in mouse embryonic fibroblasts and in the human ovarian carcinoma cell line, Hey1b.22 To extend these finds to other types of human cancer cells, we used the prostate cancer cell lines DU145 and PC3 and the renal cell carcinoma cell lines ACHN and 786-O. Cells were treated with the synthetic double-stranded RNA, poly(rI):poly(rC), a virus surrogate that activates PKR and OASs (enzymes that produce the activator of RNase L, 2-5A, from ATP). In the absence of sunitinib, poly(rI):poly(rC) activated PKR, as measured by its autophosphorylation and by phosphorylation of its substrate, eIF2α (Figure 1a). In contrast, sunitinib pretreatment prevented or greatly reduced both PKR autophosphorylation and eIF2α phosphorylation in response to poly(rI):poly(rC) treatment in all four cancer cell lines. RNase L is present in all of these tumor cell lines as determined by immunoblotting (Figure 1a, bottom panel). To assay RNase L activity, rRNA was examined for characteristic and discrete cleavage products resulting from RNase L-mediated degradation of rRNA in intact ribosomes.24 Whereas RNase L degraded rRNA in poly(rI):poly(rC)-treated cells, there were no cleavage products detected in cells that were pretreated with sunitinib before transfection with poly(rI):poly(rC) (Figure 1b). We obtained similar results in the murine breast cancer cell line, 4T1, in which sunitinib inhibited activation of both PKR and RNase L (data not shown). Therefore, all of these cancer cell types have a functional PKR and OAS-RNase L system and sunitinib treatment effectively blocked both pathways.
Figure 1.
Sunitinib inhibits antiviral enzymes PKR and RNase L in prostate and kidney cancer cells. (a) Prostate cancer cells PC3 and DU145 and human kidney cancer cell lines ACHN and 786-0 cells (as indicated) were incubated with or without sunitinib (5 µmol/l) for 2 hours and then mock transfected or transfected with poly(rI):poly(rC) [p(rI):p(rC)] (2 µg/ml) for 3 hours. Proteins in cell extracts were separated by SDS/PAGE, transferred to membranes, and probed with antibodies to different proteins (as indicated). (b) RNase L activity in the cells was determined by isolating and separating total RNA on RNA chips using an Agilent Bioanalyzer. Positions of 28S rRNA, 18S rRNA, and RNase L-mediated cleavage products of rRNA are indicated. Su, sunitinib.
Sunitinib and VSV used in combination selectively targets cancer cells
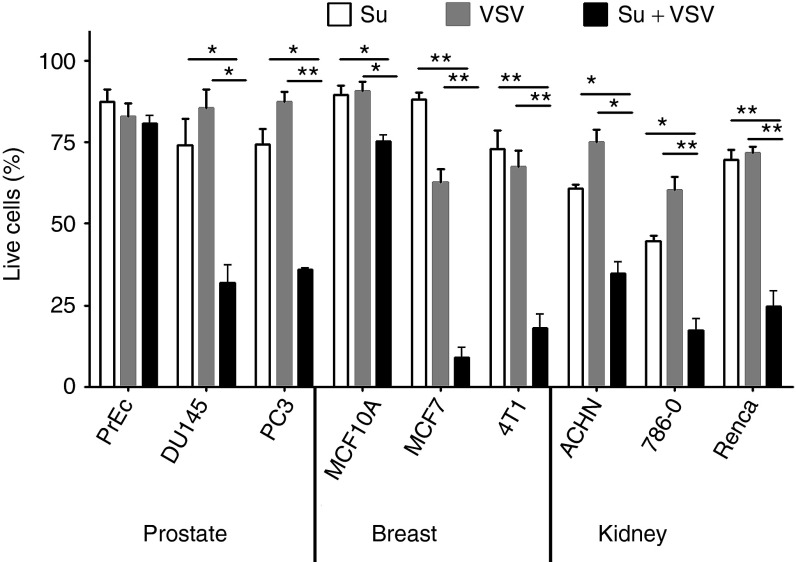
The effect of sunitinib and wild-type (WT) VSV alone and combination on cell survival was determined in nontumorigenic and cancer cell types. Cells were incubated in the absence or presence of sunitinib (5 µmol/l) for 2 hours followed by VSV infection for 8 hours at a multiplicity of infection of 1 and cell viability was determined by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assays (Materials and Methods). Normal prostate epithelial cells were resistant to single and combined treatments of sunitinib and VSV (Figure 2). By contrast, the combination of sunitinib and VSV caused about 66% loss of viable prostate cancer cells (PC3, DU145), while each agent alone resulted in up to about a 25% loss of viable cells. Similarly, the nontumorigenic human breast epithelial cell line MCF10 was relatively resistant to the anticellular effects of sunitinib plus VSV infection (25% loss of viable cells), whereas human breast adenocarinoma cells MCF7 and mouse breast cancer cell line 4T1 were highly susceptible to the combination of sunitinib plus VSV (91% and 82% loss of viable cells, respectively). Also, sunitinib plus VSV treatments of renal cell carcinoma (RCC) cell lines ACHN, 786-O, and Renca caused 65%, 83%, and 75% loss of viable cells, respectively. In the same RCC cell lines, sunitinib or VSV alone had significantly reduced anticellular effects. The malignant glioma cell lines, U87, U251, and SNB19, were also highly susceptible to the anticellular effects of sunitinib combined with VSV (data not shown).
Figure 2.
Sunitinib treatment combined with VSV infection leads to loss of viable cancer cells, whereas nontumorigenic cells are resistant. Cells were incubated 10 hours with 5 µmol/l of sunitinib, or infected with VSV, or were treated with 5 µmol/l of sunitinib for 2 hours followed by VSV infection for 8 hours (Su + VSV). Loss of viable cells was determined by MTS assays and results presented as percent of control (untreated cells). Data are from an average of six separate infections ± standard deviation. *P < 0.05; **P < 0.01. MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; Su, sunitinib; VSV, vesicular stomatitis virus.
Elimination of prostate tumors in mice with sunitinib/VSV combined therapy
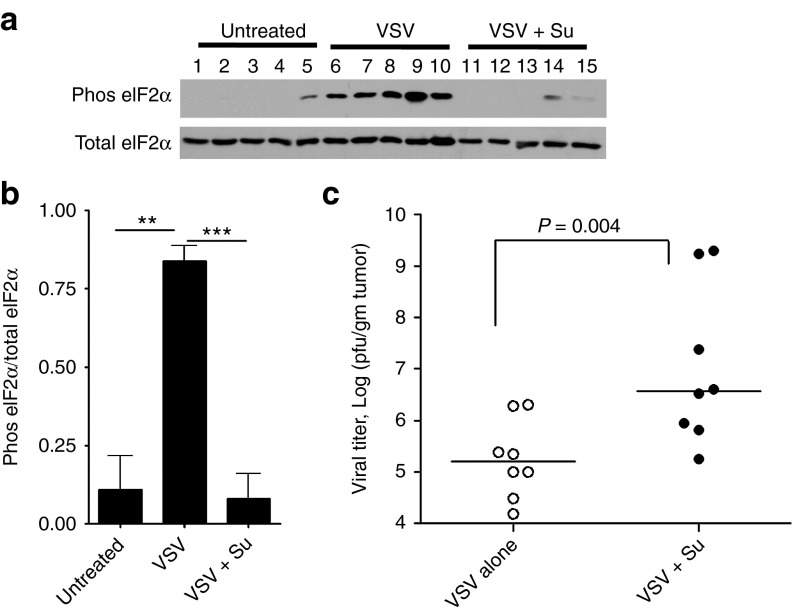
To evaluate suppression of antiviral innate immunity by sunitinib in vivo, eIF2α phosphorylation and viral titers were determined in PC3 tumors grown subcutaneously in nude mice (Figure 3). Tumors were excised 4 days after intratumoral injection with WT VSV in mice that received by oral gavage either saline or sunitinib daily beginning 2 days before infection (Figure 3). A western blot with proteins from individual tumor extracts showed a lack of phosphorylation of eIF2α, a substrate of PKR, in four of five tumors, and very low levels in one tumor, from untreated mice (Figure 3a, lanes 1–5). However, eIF2α was strongly phosphorylated in response to viral infection in five of five tumors (Figure 3a, lanes 6–10). In contrast, oral sunitinib treatments blocked or greatly reduced eIF2α phosphorylation in five of five tumors (Figure 3a, lanes 11–16). Quantitation of the western blots showed that sunitinib treatments reduced eIF2α phosphorylation in virus-infected mice to basal levels (Figure 3b). Viral titers were determined in the tumors extracts by performing viral plaque assays on indicator cells. The combination therapy of sunitnib with VSV enhanced median viral yields in the tumors by 23-fold (Figure 3c). These results are consistent with an inhibition of PKR activation by sunitinib resulting in enhanced viral replication.
Figure 3.
Sunitinib inhibits eIF2α phosphorylation in response to viral infection of prostate tumors while increasing viral replication. Nude mice with s.c. PC3 prostate tumors were used. (a) Protein in tumors harvested 4 days postinfection were separated by SDS/PAGE, transferred, and probed with antibody to phosphorylated (Phos) eIF2α or Total eIF2α (as indicated). Lanes 1–5, untreated mice; lanes 6–10, mice received saline orally and tumors were infected with VSV; and lanes 11–15, mice received sunitinib orally and tumors were infected with VSV. (b) Quantitation of results in panel A determined by imageJ software. Data from two separate experiments are combined. **P < 0.01, ***P < 0.005. (c) Viral titers in extracts of tumors from mice determined by plaque assays. Data analysis was performed in GraphPadPrizm using Mann–Whitney test for statistical significance. Su, sunitinib; VSV, vesicular stomatitis virus.
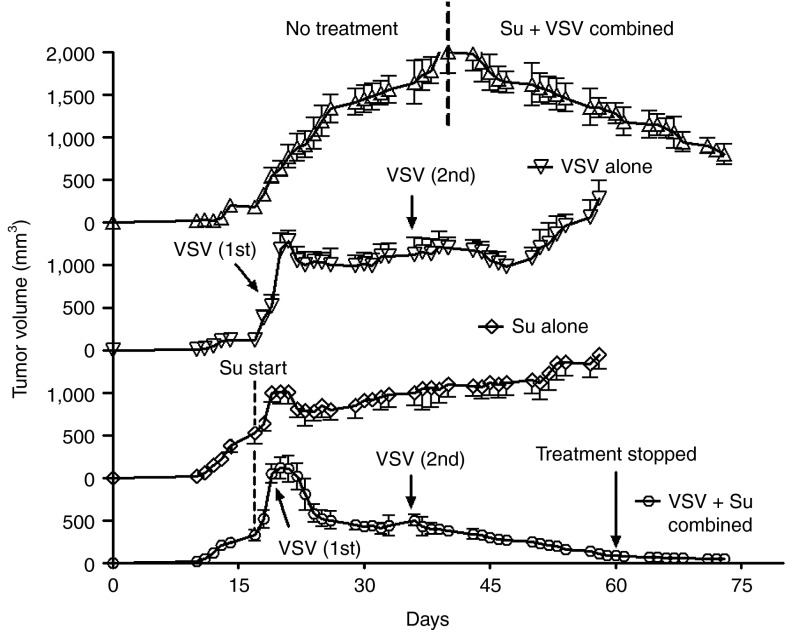
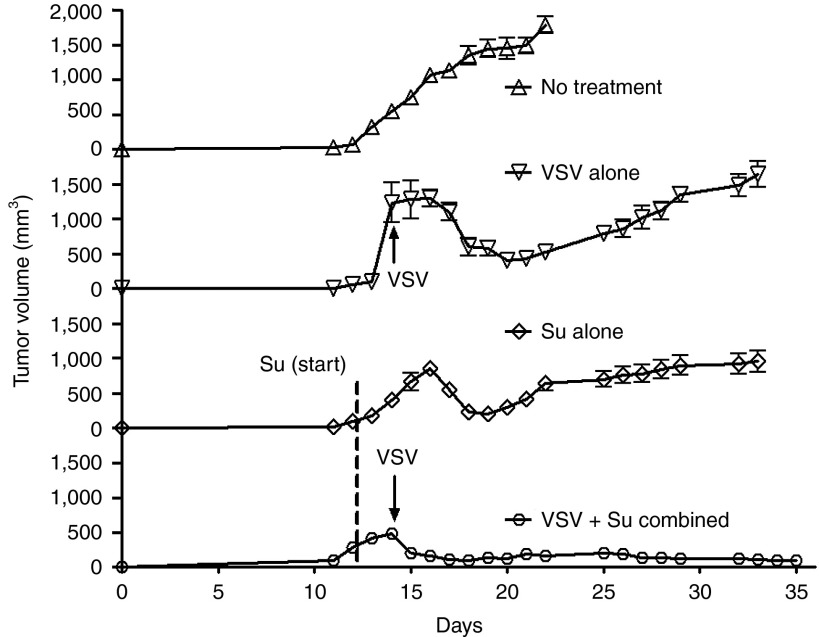
To determine the effects of sunitinib and VSV on prostate tumor growth, PC3 cells were implanted subcutaneously in nude mice. Once PC3 tumor sizes reached 6–8 mm in diameter (12 days), mice were separated into four groups: no treatment, sunitinib alone (oral gavage beginning on day 16), VSV infection alone by intratumoral injections on days 18 and 35, and combined sunitinib treatment with VSV infection (Figure 4). Sunitinib or VSV monotherapy had partial effects against PC3 tumors, reducing tumor growth rates without causing complete regression of the tumors. In the case of VSV infections alone, tumors resumed logarithmic growth at about 15 days after the second infection. In contrast, there was a dramatic and persistent antitumor effect of the combined therapy with VSV and sunitinib. In the presence of continued treatments with sunitinib, tumor sizes steadily declined following the second injection of VSV until tumors were no longer observed. Sunitinib treatment ceased on day 60, yet tumors did not reappear after 2 additional weeks (Figure 4, bottom plot, see arrow labeled “treatment stopped”). In the untreated mice, when tumor growth approached the size requiring euthanasia, the mice received treatments with sunitinib orally beginning on day 40, and the tumors were inoculated with VSV on day 41. The combined treatment of sunitinib and VSV also caused regression of these large tumors (Figure 4, top plot to the right of the vertical dashed line).
Figure 4.
Sunitinib enhances the oncolytic efficacy of VSV against PC3 prostate tumors in nude mice. Oral gavage with sunitinib began on day 16 postimplantation of PC3 cells. Intratumoral injections of VSV were done on days 19 and 36 (arrows) and day 41 (in upper plot) postimplantation. Tumor sizes were measured using a digital caliper and data were analyzed in GraphPadPrizm. Su, sunitinib; VSV, vesicular stomatitis virus.
Effect of sunitinib plus VSV against syngeneic 4T1 breast cancer in immunocompetent animals
To extend the sunitinib/VSV strategy to immunocompetent mice, the mouse 4T1 breast cancer cell line was implanted subcutaneously into Balb/cJ mice (Figure 5). A single VSV infection or oral sunitinib treatments retarded tumor progression. Remarkably, however, a single intratumoral inoculation of VSV reversed tumor growth in sunitinib-treated animals causing complete tumor regression in 5 days (Figure 5, bottom plot). In contrast, tumor growth in the untreated animals was rapid requiring euthanasia at 22 days postimplantation. Results show that sunitinib and VSV combined treatments are effective against an aggressive type of breast cancer even in the presence of an intact adaptive immune system.
Figure 5.
Sunitinib enhances the oncolytic efficacy VSV against 4T1 breast tumors in immunocompetent mice. Mouse mammary tumor cells 4T1 were implanted subcutaneously in BALB/c mice. Daily orally sunitinib treatments began on day 12 postimplantation (dashed line). Intratumoral injection with VSV was done on day 14 (arrows). Tumor sizes were measured using a digital caliper and data were analyzed in GraphPadPrizm. Su, sunitinib; VSV, vesicular stomatitis virus.
Effectiveness of an attenuated mutant VSV in the presence of sunitinib against RCC
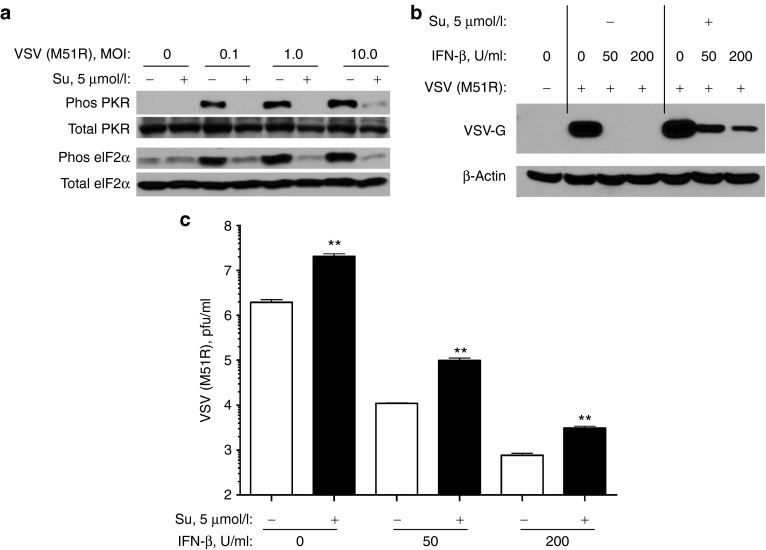
To extend the sunitinib/VSV strategy, we employed an RCC cell line (ACHN) and a mutant VSV (M51R) defective in host shut-off allowing robust viral induction of IFN.16 The VSV mutant, VSV (M51R), triggers a higher IFN response than WT VSV and may have an improved safety profile.13,15,16 To determine the effect of sunitinib on replication of VSV (M51R), cultured ACHN cells were treated with sunitinib (5 µmol/l) for 2 hours before VSV (M51R) infection for 24 hours. VSV (M51R) induced phosphorylation of PKR and eIF2α in untreated cells, but not in sunitinib treated cells (Figure 6a).
Figure 6.
Sunitinib inhibits phosphorylation of PKR and eIF2α in RCC ACHN cells infected with VSV (M51R) while increasing viral titers. (a) ACHN cells were incubated with or without sunitinib (5 µmol/l) for 2 hours and then mock infected or infected with VSV (M51R) at different multiplicity of infection (as indicated). The western blot was probed with antibodies as indicated. (b) VSV G protein and β-actin detection were by western blotting. (c) Viral titers by plaque assays were determined from ACHN cells incubated in the absence or presence of IFN-β (as indicated) for 3 hours followed by incubation in the absence or presence of sunitinib (5 µmol/l) for 2 hours (as indicated), and then infected with 1 multiplicity of infection VSV (M51R) for 13 hours in the continued presence of sunitinib where indicated. Data analysis was performed in GraphPadPrizm. **P < 0.01. IFN, interferon; MOI, multiplicity of infection; Su, sunitinib; VSV, vesicular stomatitis virus.
To measure the impact of sunitinib on viral growth, ACHN cells were preincubated in the absence or presence of IFN-β followed by further incubation in the absence or presence of 5 µmol/l sunitinib. The cells were then infected with VSV (M51R) for 13 hours. Virus growth was monitored by western blot assay for VSV G protein and by measuring viral titers in plaque assays (Figure 6b,c, respectively). IFN treatment alone at 50 and 200 µ/ml resulted in an absence of detectable levels of VSV G protein and about two and three log10 units of inhibition of viral growth. Sunitinib treatment, however, enhanced virus replication by 6.8-, 8.9-, and 4.0-fold in cells preincubated with 0, 50, and 200 µ/ml of IFN-β, respectively (Figure 6c). In addition, VSV G protein was detected in IFN and sunitinib treated ACHN cells (Figure 6b). These results indicate that sunitinib enhances growth of VSV (M51R) even in tumor cells that have already been exposed to IFN, although not to levels obtained in the absence of prior treatment with IFN.
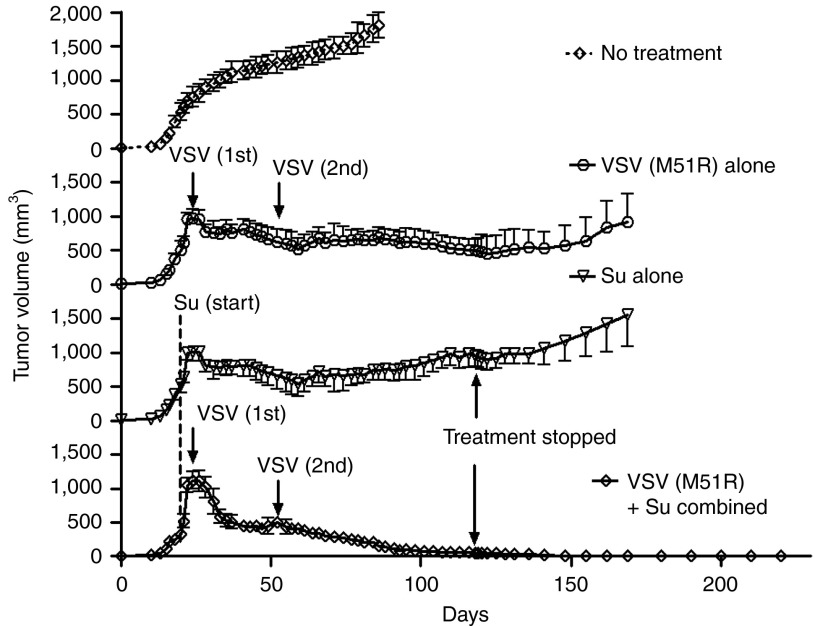
To determine the effect of VSV (M51R) in the presence or absence of sunitinib on tumor growth, ACHN cells were implanted subcutaneously into nude mice. Once ACHN tumor sizes reached 6–8 mm in diameter, mice were separated into four groups: no treatment; sunitinib alone (by oral gavage daily beginning on day 23), VSV (M51R) infection alone injected intratumoral on days 25 and 52, and combined sunitinib treatments with VSV (M51R) infections. Sunitinib alone or VSV (M51R) alone had partial effects against ACHN tumors, reducing tumor growth rates but not causing tumors to completely regress. In contrast, sunitinib treatments beginning 2 days before VSV (M51R) inoculation caused complete regression of the ACHN tumors (Figure 7). The mice were considered cured because the tumors did not reappear, even 12 weeks after sunitinib treatments ceased.
Figure 7.
Elimination of RCC ACHN tumors in nude mice by combined treatments with sunitinib and attenuated mutant VSV (M51R). ACHN were implanted subcutaneously in nude mice (Methods). Oral sunitinib treatments began on day 20 postimplantation. Intratumoral injections of VSV (M51R) were done on days 25 and 52 postimplantation (arrows). Tumor sizes were measured using a digital caliper and data were analyzed in GraphPadPrizm. Su, sunitinib; VSV, vesicular stomatitis virus.
Discussion
Inhibitor of innate immunity enhances oncolytic virotherapy
Our results show that whereas monotherapy with sunitinib or oncolytic virus retard malignant tumor growth, neither caused complete or sustained tumor regression. In contrast, orally delivered sunitinib combined with intratumoral injections of VSV eliminated three different types of solid tumors in mice. Sunitinib is an ATP competitive inhibitor of VEGF-R and PDGF-R used clinically to treat RCC,23,25 gastrointestinal stromal tumor,26 and neuroendocrine pancreatic tumors.27 Previously, we reported that sunitinib also impairs innate immunity by inhibiting the antiviral enzymes PKR and RNase L in cells and in mice.22 Our findings support a prior report in which intraperitoneal injections of sunitinib in C57/bl6 mice was used in combination with intravenously delivered reovirus or VSV against B16 melanoma expressing VEGF165.28 The rationale of that study was to transiently inhibit VEGF signaling with sunitinib, cease treatment, and then allow a “VEGF burst” leading to viral infection and lysis of the endothelial cells thus enhancing viral spread from blood vessels into tumor tissues. In our studies, it was not necessary to withdraw orally delivered sunitinib treatment to achieve a potent antitumor effect. In addition, we provide a different, additional mechanism by showing that sunitinib impairs host antiviral enzymes in tumor cells, presumably allowing for greater spread of virus throughout tumor tissues. Because PKR and the OAS/RNase L system inhibit many types of viruses, we predict that sunitinib will enhance lysis of cancer cells by other types of oncolytic viruses. Sunitinib treatment combined with VSV infections was effective against a wide range of tumor cell lines grown in culture. Tumor cells are often deficient in the IFN antiviral response at a variety of different levels.29 In contrast, nontumorigenic cell types, prostate epithelial cells and MCF10, were resistant to the combination of sunitinib and VSV. Therefore, we suggest that while normal cells retain the ability to respond to IFN through alternative antiviral pathways (even when PKR and RNase L are inhibited by sunitinib) tumor cells rapidly succumb to viral infection.
VSV preferentially targets and destroys tumor vasculature,30 whereas sunitinib inhibits angiogenesis by blocking VEGF-R activity in tumors.23 Therefore, combination of sunitinib and VSV might be expected to have a synergistic effect against tumor vasculature involving effects on different host factors. In addition, an oncolytic vaccinia virus expressing granulocyte-macrophage colony stimulating factor (JX-594) followed by sorafenib, another VEGF-R inhibitor, showed combined efficacy against hepatocellular carcinoma in preclinical studies and in patients.31 Interestingly, the same study included a case report of a metastatic RCC patient treated with JX-594 followed by sunitinib who had a complete whole body tumor response lasting more than 4 years.
Effects of sunitinib on the host innate immune response
We chose to study PC3 cells as a model for prostate cancer because they are normally resistant to VSV32 due to the presence of functional OAS/RNase L and PKR systems.32,33 PC3 cells originated from advanced, metastatic (bone) prostate cancer and are castration resistant.34,35 The remarkable effectiveness of the sunitinib/VSV combination strategy against PC3 tumors was apparent from the observation that tumor regression occurred even in advanced tumors. Also, when treating smaller tumors, complete regression of the tumors was obtained and tumors did not reappear after sunitinib treatment ceased. Our hypothesis that antiviral innate immunity is impaired by sunitinib was supported by demonstrating enhanced viral replication in PC3 tumors from sunitinib-treated animals compared with untreated animals. Furthermore, intratumoral eIF2α phosphorylation induced by VSV infection was inhibited by orally delivered sunitinib. Phosphorylation of eIF2α by PKR blocks protein synthesis in virus infected cells by preventing recycling of eIF2α-GDP to eIF2α-GTP.20 While sunitinib is known to inhibit PKR, it is possible that it also inhibits other eIF2α kinases, such as the endoplasmic reticulum stress kinase, PERK.36,37
We show that sunitinib is also an inhibitor of the antiviral enzyme RNase L (Figure 1).22 Relevant to the possible uses of sunitinib/oncolytic virus against prostate cancer, genetic deficiency in RNase L occurs in some cases of hereditary prostate cancer,38 although the majority of prostate cancer cases are WT for RNase L.39 Nevertheless, genetic deficiencies in RNase L are predicted to increase, and not decrease, the antitumor activity of oncolytic viruses because such mutations reduce innate immune responses to viruses. Therefore, mutations in the RNase L gene should not be an impediment to sunitinib/oncolytic virus therapy. RNase L is related to the kinase-nuclease IRE1 that functions in the unfolded protein response to endoplasmic reticulum stress.40 Inhibition of IRE1 with a small molecule greatly enhanced the oncolytic activity of a rhabdovirus.41 Sunitinib was reported to inhibit human IRE1,42 therefore it is possible that sunitinib-mediated inhibition of IRE1 also contributes to its antitumor activity when used in conjunction with VSV. In addition, whereas we show here that VSV infection activates PKR, we were unable to demonstrate RNase L activation by VSV by monitoring rRNA cleavage (data not shown). Therefore, PKR may be the principal molecular target of sunitinib in terms of its ability to suppress innate immunity against VSV, at least with the tumor cell types used in these studies. A predominant role for PKR in the inhibition of VSV infections is consistent a prior study showing that PKR−/− mice are exquisitely sensitive to WT VSV.15,43
While sunitinib blocks the direct antiviral effects of PKR and RNase L, it is possible that sunitinib also affects other viral sensors that trigger innate immune responses. Previously, we reported that viral induction of IFN-β in mice was modestly reduced (by as much as 50%) by sunitinib.22 Because RNase L contributes to viral induction of IFN-β these effects could be due to inhibition of RNase L by sunitinib, or to other viral sensors.44 However, consistent with a lack of viremia, only low levels (<25 units per ml) of systemic IFN were detected in blood of nude mice infected intratumoral with WT VSV in the presence or absence of sunitinib as determined by elisa (data not shown).
Adaptive immune responses (production of neutralizing antibody and cell mediated immunity) are barriers to effective oncolytic virotherapy.2 However, sunitinib combined with VSV infection was effective even in immunocompetent mice, perhaps by preventing viral spread early in infection (Figure 5 and ref. 28). Remarkably, a single inoculation of VSV eliminated 4T1 tumors in BALB/cJ immunocompetent mice within several days. In contrast, either VSV or suninitib monotherapies failed to control tumor growth.
An attenuated VSV mutant combined with sunitinib eliminates RCC in mice
We used a mutant VSV deficient in the ability to inhibit innate immunity, but still able to infect and lyse cancer cells to treat renal cell carcinoma (ACHN) tumors in mice.15 The mutation is in the gene encoding the matrix protein which counters innate immunity by impairing host transcription and nuclear-to-cytoplasm transport of RNA.15,16,45 As a result, IFN expression in response to infection is increased by certain M gene mutants of VSV, including the mutant used here, VSV (M51R).16 Mutant VSV (M51R) is highly attenuated for growth in normal mice, but nevertheless has potent antitumor activity. However, neither WT VSV nor mutant VSV (M51R) showed apparent toxicity in any of our mouse experiments. In addition, suntinib treatment followed by intravenous injections of VSV in mice to treat B16-VEGF melanoma tumors was previously performed.28 Survival was greatly enhanced in the mice treated with both sunitinib and VSV compared with the monotherapies. Those findings indicate that sunitinib treatment following intravenous injection of VSV does not lead to serious adverse effects in mice. In addition, we were unable to detect viremia in nude mice infected intratumorally with WT VSV in the presence or absence of sunitinib suggesting viral spread is limited beyond the tumor tissues (data not shown). Also, Balb/cJ and nude mice were injected with WT VSV (106 plaque-forming unit (pfu) by the ip route) plus oral sunitinib treatments with no apparent toxicity as determined by animal weight and general health (data not shown).
Sunitinib/VSV (M51R) therapy was highly effective against ACHN tumors in mice. Our cell culture studies showed that VSV (M51R) replicated to 7-fold higher titers in sunitinib-treated compared with untreated ACHN cells. In addition, a preexisting IFN-induced antiviral state was partially inhibited by sunitinib treatment. Phosphorylation of both PKR and eIF2α were induced in ACHN cells by VSV (M51R) infections while sunitinib effectively blocked phosphorylation of both proteins. The ACHN tumors steadily increased in size in untreated animals, requiring euthanasia at 80 days postimplantation. We used two intratumoral inoculations of VSV (M51R), which had a potent antitumor effect, but eventually tumor growth resumed and the animals had to be euthanized after 180 days. Similar findings were obtained in the sunitinib monotherapy group of mice. In contrast, there was a remarkable and durable antitumor effect of suninitib combined with VSV (M51R). Tumors disappeared after 120 days and did not reappear even 90 days after cessation of sunitinib treatments.
A promising experimental approach for enhancing the efficacy of oncolytic virotherapy
Our studies show that transient suppression of innate immunity with orally delivered sunitinib correlates with enhanced efficacy of oncolytic virotherapy. The dose of sunitinib used in all of our studies, 20 mg/kg, was reported to result in serum concentrations equivalent to those of patients receiving sunitinib.46,47 However, it is not yet clear if VSV can be safely combined with sunitinib in cancer patients because mouse and human innate defenses may differ. In our studies, the sunitnib/VSV strategy cured mice of prostate, kidney, or breast tumors. We also show that sunitinib inhibits both PKR and RNase L in cell culture and that the phosphorylation of eIF2-α is inhibited in tumors implanted into mice. Our results suggest that in addition to suppressing angiogenesis, sunitinib enhances replication and spread of virus within tumors by inhibiting antiviral innate immunity mediated by PKR (and possibly also by RNase L) leading to tumor elimination. However, establishing direct involvement of PKR and/or RNase L in the antitumor mechanism of action of sunitinib combined with VSV, and determining why normal cells are not similarly affected are outstanding questions requiring further investigation.
Materials and Methods
Reagents, antibodies, and chemicals. Chemicals, unless stated otherwise, were analytical grade from Sigma-Aldrich (St Louis, MO). Sunitinib was obtained from Pfizer (New York, NY) through the Cleveland Clinic pharmacy. Poly(rI):poly(rC) was from Calbiochem, EMD Chemicals, (Gibbstown, NJ) and matrigel was from BD-Bioscience (San Jose, CA). Antibodies against total PKR, and phosphorylated PKR [p-PKR Antibody (Thr 451)] were from Santa Cruz Biotechnology (Santa Cruz, CA), and antibodies against total and phosphorylated eIF2α were from Cell Signaling (Danvers, MA). Monoclonal antibody against human RNase L was generated as we described.48 Mouse anti-VSV G antibody was obtained from Roche Applied sciences (catalogue #1667351). Human recombinant IFN-β [200–400 × 106 units (U)/mg] was a gift from Serono (Geneva, Switzerland).
Cell lines and cell culture. Prostate cancer cells PC3 and DU145, human breast adenocarcinoma cells MCF7, nontumorigenic human breast epithelial cell line MCF-10A, mouse mammary tumor cell 4T1 and human kidney cancer cell lines ACHN, Renca, and 786-0 were all from the American Type Culture Collection (Manassas, VA). Prostate epithelial cells cells were purchased from Lonza (Walkersville, MD). MCF-7 and ACHN were grown in complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). PC3, DU145, 786-O, Renca, and 4T1 were grown in RPMI medium supplemented with 10% FCS. Prostate epithelial cells were grown in PrEGM SingleQuots (Lonza) and MCF 10A was grown in DMEM supplemented with 10% FCS and 100 ng/ml cholera toxin.
Viruses. VSV Indiana strain was a kind gift from Amiya Banerjee (Cleveland Clinic, Cleveland, OH) and the attenuated mutant VSV (M51R) (Indiana strain) was a kind gift from Douglas S. Lyles (Wake Forest University, Winston Salem, NC). Viruses were propagated in BHK21 cells grown in DMEM supplemented with 10% FCS and titered as described.49,50
Transfection of cells with poly(rI):poly(rC). Cells were seeded at ~60% confluence and grown for 14–16 hours at 37 °C and transfected with 2 µg/106 cells in 1 ml media using lipofectamine 2000 (Life Technologies, Carlsbad, CA). After transfection, cells were incubated 2–4 hours and harvested for isolation of total RNA or protein. Total RNA was isolated using Trizol (Life Technologies) while the protein was isolated from a separate aliquot of cells using nonidet P-40 (NP40) lysis buffer (50 mmol/l Tris–HCl, pH 7.2, 0.15 mol/l NaCl, 1% Nonidet P-40, 2 mmol/l EDTA, and 5 mmol/l DTT) containing protease inhibitor cocktail (Roche, Nutley, NJ) and 200 µmol/l of sodium orthovanadate.
Western blot assays. Proteins from cells or tissues were isolated in NP40 lysis buffer as described22 and quantified using the Bradford method (Bio-Rad, Hercules, CA). Equal amounts of protein (25–50 µg) were separated by SDS-PAGE and transferred on to Immun-Blot PVDF membrane (Bio-Rad) for protein blotting. The blots were incubated overnight in primary antibodies and developed using HRP conjugated secondary antibodies (Cell Signaling).
Analysis of RNase L-mediated cleavage of rRNA. Total RNA (200 ng) from cells either transfected with poly(rI):poly(rC) or infected with viruses at a multiplicity of infection of 1 with and without a 1–2 hour pretreatment with sunitinib were isolated using Trizol, separated and analyzed using RNA chips on an Agilent 2000 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Animals. BALB/cJ and NCR athymic nude mice of age 6–8 weeks weighing 20–30 grams were purchased from the Case Western Reserve University transgenic animal core facility and housed in ventilated cages under pathogen-free conditions on a 12 hour light/dark schedule. Viral infections were done in a biosafety level 2 (BSL2) animal laboratory. The animals were handled in accordance with the Institutional Animal Care and Use Committee of the Cleveland Clinic.
Sunitinib and VSV treatments of nude mice. Male mice were allowed to acclimate for at least 1 week before the start of the experiments. NCR athymic nude mice were injected subcutaneously in the right flank with 2 × 106 PC3 cells or 3 × 106 ACHN cells in 75 µl of complete culture media plus 75 µl of matrigel. Tumor sizes were measured using a digital caliper. Once tumors were established (about 6–8 mm in diameter) mice were divided into four experimental groups of five animals each: [100 µl saline 5 days/week (on week days) by oral gavage]; sunitinib [20 mg/kg (body weight) in 100 µl of saline 5 days/week (on week days) by oral gavage; intratumoral injection of VSV or VSV (M51R) (Indiana strain) 106 pfu/mice; sunitinib [20 mg/kg (body weight) in 100 µl of saline 5 days/week (on week days) by oral gavage]; and intratumoral injection of VSV or VSV (M51R) (Indiana strain) 106 pfu/mice as indicated (see figure legends). Tumor volumes were monitored using digital caliper measurements by the formula: (4π/3) × (w/2)2 × (l/2), where w = width and l = length and data analyzed using Wilcoxon signed rank test (P < 0.05) in GraphPadPrizm.
Sunitinib and VSV treatments of BALB/c mice. Female BALB/c mice, 6–8 weeks old were injected subcutaneously with 5 × 104 4T1 cells in phosphate buffered saline in the right flank. Once tumors were established (6–8 mm in diameter) mice were divided into four experimental groups [as described in the previous section except oral treatment with saline or sunitinib were done daily (7 days/week)].
Measuring viral titers in PC3 tumors. NCR athymic nude mice were injected subcutaneously in the right flank with 2 × 106 PC3 cells in matrigel plus complete media. Once tumors were established (6–8 mm in diameter) mice given either saline (100 µl) or sunitinib [20 mg/kg (body weight) in 100 µl of saline] orally daily for 2 days followed by intratumoral injection of WT VSV (106 pfu/mice). Saline or sunitinib treatments continued for 4 days. Mice were euthanized and tumors were excised and sectioned and blood was harvested. Tumor tissues were washed with phosphate buffered saline and homogenized using a Sample Grinding kit (GE Healthcare, Piscataway, NJ). The tissue homogenates were centrifuged at 500g for 15 minutes at 4 °C in a swinging bucket rotor to remove the debris. The supernatants were collected and sonicated for 20 seconds four times (20 micron pulse each) and used for plaque assays on the indicator cell line L929.22
Assaying eIF2α phosphorylation in PC3 tumors. Separate pieces of the same PC3 tumors (as described in the previous section) were extracted using Sample Grinding kit (GE Healthcare) and NP40 lysis buffer. Protein amounts were estimated using the Bradford method (Bio-Rad), and 25 µg of total protein was separated on SDS-PAGE, transferred onto membranes, and probed with either antibody against total or phosphorylated eIF2α as described earlier.
Effects of IFN-β and sunitinib on VSV (M51R) replication in ACHN cells. ACHN cells grown in 6-well plates were incubated with 0, 50, and 200 U/ml of IFN-β for 3 hours followed by further incubation with or without sunitinib (5 µmol/l)in fresh media for 2 hours. Cells were then infected with VSV (M51R) at a multiplicity of infection of 1 in DMEM medium without serum. After 1 hour, media containing virus was removed, cells were washed at room temperature with phosphate buffered saline, and fresh complete DMEM media with 10% fetal bovine serum with or without sunitinib (5 μmol/l) was added. Cells were incubated for 12 hours in a humidified CO2 incubator at 37 °C. The supernatants were collected for viral plaque assays performed on BHK21 cells. Plaques were counted and data analyzed using Student's t-test (P < 0.05) in GraphPadPrizm. Protein in cell extracts were separated by SDS-PAGE and transferred onto membranes, and probed with antiVSV G protein antibody and reprobed with anti-β-actin antibody.
Measuring phosphorylation of PKR and eIF2α in response to VSV (M51R) infection in ACHN cells. ACHN cells were plated at 60–70% confluency and then pretreated with sunitinib (5 µmol/l) for 2 hours and infected with VSV (M51R) at different multiplicity of infections in serum-free DMEM medium. After 1 hour, the supernatants were removed, cells were washed at room temperature with phosphate buffered saline, and fresh complete DMEM media with 10% fetal bovine serum with or without sunitinib (5 µmol/l) was added. After 24 hours incubation in a humidified CO2 incubator at 37 °C, cells were lysed in NP40 lysis buffer containing sodium orthovanadate and sodium fluoride. The activation of PKR and eIF2α was monitored using specific antibodies on western blots.
Acknowledgments
We thank Brian Rini (Cleveland Clinic) for assisting us in obtaining sunitinib and to him and James Finke (Cleveland Clinic) for valuable discussions and Douglas Lyles (Wake Forest University) for the vesicular stomatitis virus (M51R) mutant. These studies were supported by the Mal and Lea Bank Chair Fund, the Cleveland Clinic Global Innovation Fund, the Maltz Family Foundation, and National Institutes of Health/National Cancer Institute grant number CA044059 to R.H.S. The authors declared no conflict of interest.
References
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennier ST, Liu J, McFadden G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. Curr Pharm Biotechnol. 2012;13:1817–1833. doi: 10.2174/138920112800958850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echchgadda I, Chang TH, Sabbah A, Bakri I, Ikeno Y, Hubbard GB, et al. Oncolytic targeting of androgen-sensitive prostate tumor by the respiratory syncytial virus (RSV): consequences of deficient interferon-dependent antiviral defense. BMC Cancer. 2011;11:43. doi: 10.1186/1471-2407-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447–3453. doi: 10.1158/0008-5472.CAN-04-4316. [DOI] [PubMed] [Google Scholar]
- Cripe TP, Wang PY, Marcato P, Mahller YY, Lee PW. Targeting cancer-initiating cells with oncolytic viruses. Mol Ther. 2009;17:1677–1682. doi: 10.1038/mt.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahller YY, Williams JP, Baird WH, Mitton B, Grossheim J, Saeki Y, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS ONE. 2009;4:e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghi MK, Liu TC, Rabkin S, Martuza RL. Hypoxia enhances the replication of oncolytic herpes simplex virus. Mol Ther. 2009;17:51–56. doi: 10.1038/mt.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Stricker H, Peabody J, Pegg J, Paielli D, Movsas B, et al. Five-year follow-up of trial of replication-competent adenovirus-mediated suicide gene therapy for treatment of prostate cancer. Mol Ther. 2007;15:636–642. doi: 10.1038/sj.mt.6300068. [DOI] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lyles DS, Rupprecht CE.2007Rhabdoviridae, in Field's Virology5th edn., vol. 1Lippincott Williams and Wilkins: Philadelphia, PA.pp. 1364–1408. [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Ma B, Pang X, Wu TC, Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther. 2009;17:1365–1372. doi: 10.1038/mt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–4381. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar SA, Pan DA, Marcato P, Garant KA, Lee PW. Oncolytic virus-initiated protective immunity against prostate cancer. Mol Ther. 2011;19:797–804. doi: 10.1038/mt.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Jha BK, Silverman RH. New insights into the role of RNase L in innate immunity. J Interferon Cytokine Res. 2011;31:49–57. doi: 10.1089/jir.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha BK, Polyakova I, Kessler P, Dong B, Dickerman B, Sen GC, et al. Inhibition of RNase L and RNA-dependent protein kinase (PKR) by sunitinib impairs antiviral innate immunity. J Biol Chem. 2011;286:26319–26326. doi: 10.1074/jbc.M111.253443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer. Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- Silverman RH, Skehel JJ, James TC, Wreschner DH, Kerr IM. rRNA cleavage as an index of ppp(A2'p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J Virol. 1983;46:1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959–1968. doi: 10.1016/j.ejca.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H, et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest. 2010;120:1551–1560. doi: 10.1172/JCI41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach CJ, De Silva NS, Falls TJ, Aladl U, Evgin L, Paterson J, et al. Targeting tumor vasculature with an oncolytic virus. Mol Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Breitbach CJ, Moon A, Kim CW, Patt R, Kim MK, et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther. 2011;19:1170–1179. doi: 10.1038/mt.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey BL, Ahmed M, Puckett S, Lyles DS. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol. 2008;82:12104–12115. doi: 10.1128/JVI.01508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Condit RC, Vijaysri S, Jacobs B, Williams BR, Silverman RH. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J Virol. 2002;76:5251–5259. doi: 10.1128/JVI.76.10.5251-5259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Gravina GL, Marampon F, Piccolella M, Biordi L, Ficorella C, Motta M, et al. Antitumor effects of carnertinib in castration resistant prostate cancer models: a Comparative study with erlotinib. Prostate. 2011;71:1481–1491. doi: 10.1002/pros.21363. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpten J, Nupponen N, Isaacs S, Sood R, Robbins C, Xu J, et al. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet. 2002;30:181–184. doi: 10.1038/ng823. [DOI] [PubMed] [Google Scholar]
- Silverman RH. Implications for RNase L in prostate cancer biology. Biochemistry. 2003;42:1805–1812. doi: 10.1021/bi027147i. [DOI] [PubMed] [Google Scholar]
- Dong B, Niwa M, Walter P, Silverman RH. Basis for regulated RNA cleavage by functional analysis of RNase L and Ire1p. RNA. 2001;7:361–373. doi: 10.1017/s1355838201002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney DJ, Lefebvre C, Allan K, Brun J, Sanaei CA, Baird S, et al. Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered caspase-2 cell death. Cancer Cell. 2011;20:443–456. doi: 10.1016/j.ccr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Ali MM, Bagratuni T, Davenport EL, Nowak PR, Silva-Santisteban MC, Hardcastle A, et al. Structure of the Ire1 autophosphorylation complex and implications for the unfolded protein response. EMBO J. 2011;30:894–905. doi: 10.1038/emboj.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, et al. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity. 2000;13:129–141. doi: 10.1016/s1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria PA, Chakraborty P, Levay A, Barber GN, Ezelle HJ, Enninga J, et al. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol Cell. 2005;17:93–102. doi: 10.1016/j.molcel.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2012;130:1948–1959. doi: 10.1002/ijc.26219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre S, Raymond E, Boucher E, Douillard J, Lim HY, Kim JS, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- Dong B, Silverman RH. 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J Biol Chem. 1995;270:4133–4137. doi: 10.1074/jbc.270.8.4133. [DOI] [PubMed] [Google Scholar]
- Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BL, Rhodes RB, McKenzie M, Lyles DS. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]