Abstract
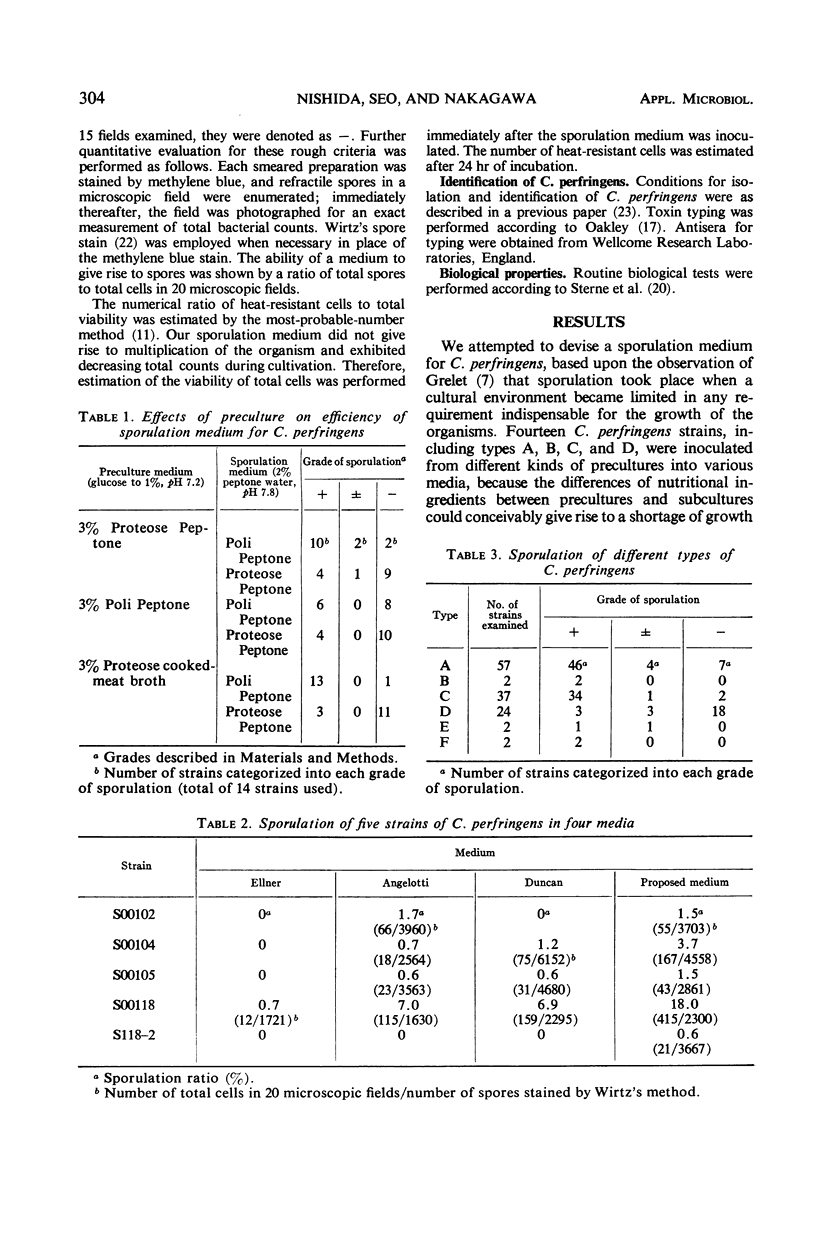
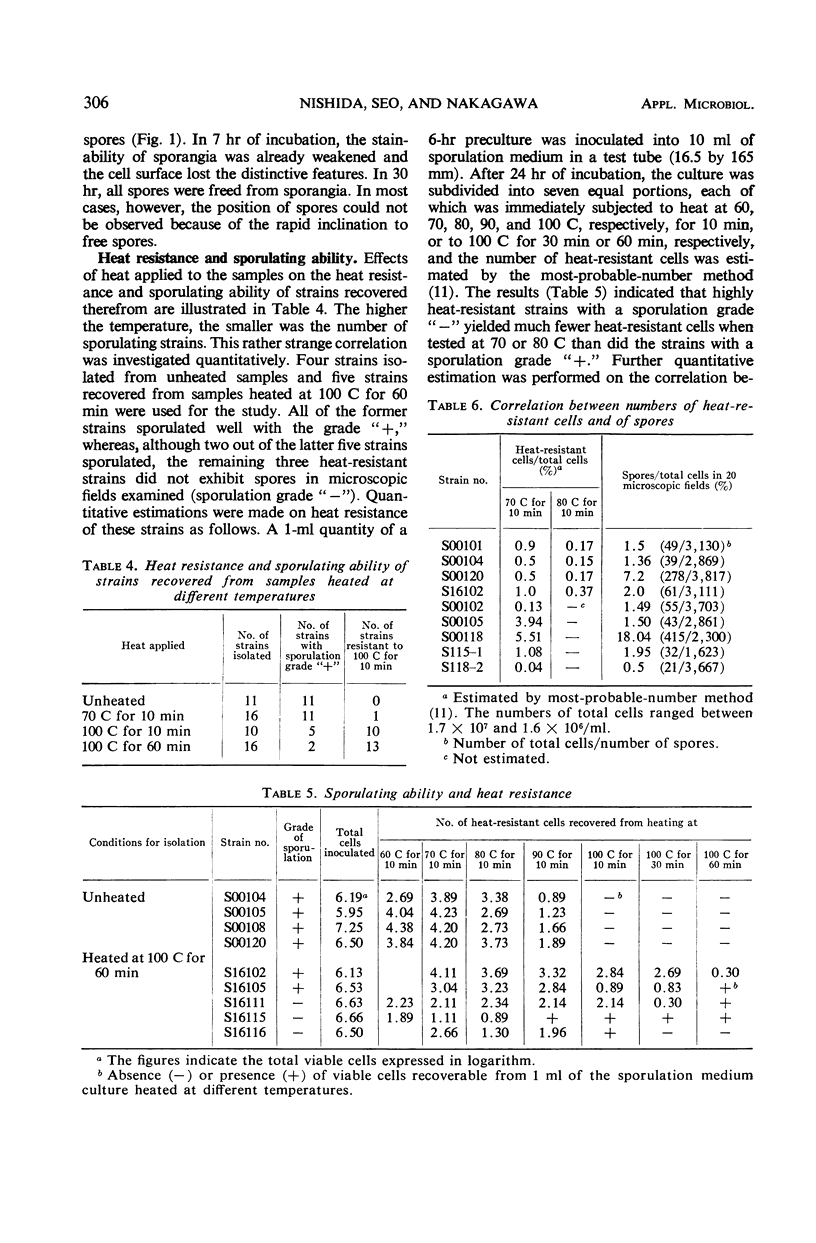
A sporulation medium for 134 Clostridium perfringens strains, including types A, B, C, D, E, and F, was devised according to Grelet's observation that sporulation occurred when cultural environment became limited in any nutritional requirement indispensable for the growth of the organism. Sporulation took place most prominently when 10% cooked-meat broth (pH 7.2) containing 3% Proteose Peptone and 1% glucose was used for the preculture and 2% Poli Peptone medium (pH 7.8) was used for the subculture medium. Sometimes, terminal spores could be observed. A correlation between sporulation and heat resistance was examined by use of C. perfringens strains isolated from samples heated at different temperatures. Almost all strains isolated from unheated samples and from those heated at lower temperatures gave rise to spores in our sporulation medium, but the spores were weakly heat-resistant, whereas strains isolated from samples heated at 100 C for 60 min were highly heat-resistant but sporulated poorly. A majority of these heat-resistant strains were non-gelatinolytic and definitely salicin-fermenting.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGELOTTI R., HALL H. E., FOTER M. J., LEWIS K. H. Quantitation of Clostridium perfringens in foods. Appl Microbiol. 1962 May;10:193–199. doi: 10.1128/am.10.3.193-199.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Davidoff-Abelson R. Altered sporulation and respiratory patterns in mutants of Bacillus subtilis induced by acridine orange. J Bacteriol. 1966 Jul;92(1):229–240. doi: 10.1128/jb.92.1.229-240.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson L. P., Zamenhof S. Studies on induction of mutations by heat in spores of Bacillus subtilis. Can J Microbiol. 1966 Feb;12(1):43–46. doi: 10.1139/m66-007. [DOI] [PubMed] [Google Scholar]
- Curran H. R., Evans F. R. The Importance of Enrichments in the Cultivation of Bacterial Spores Previously Exposed to Lethal Agencies. J Bacteriol. 1937 Aug;34(2):179–189. doi: 10.1128/jb.34.2.179-189.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLNER P. D. A medium promoting rapid quantitative sporulation in Clostridium perfringens. J Bacteriol. 1956 Apr;71(4):495–496. doi: 10.1128/jb.71.4.495-496.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom R. A., Strong D. H. Sporulation of Clostridium perfringens (welchii) in four laboratory media. J Appl Bacteriol. 1966 Aug;29(2):308–318. doi: 10.1111/j.1365-2672.1966.tb03481.x. [DOI] [PubMed] [Google Scholar]
- HALL H. E., ANGELOTTI R., LEWIS K. H., FOTER M. J. CHARACTERISTICS OF CLOSTRIDIUM PERFRINGENS STRAINS ASSOCIATED WITH FOOD AND FOOD-BORNE DISEASE. J Bacteriol. 1963 May;85:1094–1103. doi: 10.1128/jb.85.5.1094-1103.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBBS B. C., SMITH M. E., OAKLEY C. L., WARRACK G. H., CRUICKSHANK J. C. Clostridium welchii food poisoning. J Hyg (Lond) 1953 Mar;51(1):75–101. doi: 10.1017/s0022172400015515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C. T., TAMAI K., NISHIDA S. TAXONOMY OF CLOSTRIDIUM BIFERMENTANS AND CLOSTRIDIUM SORDELLII. 3. AGGLUTINABILITY OF HEAT-RESISTANT SUBSTRAINS OFCLOSTRIDIUM SORDELLII. J Bacteriol. 1965 Aug;90:391–394. doi: 10.1128/jb.90.2.391-394.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSSON I., SMITH L. D. Atypical strains of Clostridium perfringens from swine. Acta Pathol Microbiol Scand. 1962;55:342–348. doi: 10.1111/j.1699-0463.1962.tb04134.x. [DOI] [PubMed] [Google Scholar]
- NISIDA S., TAMAI K., YAMAGISHI T. TAXONOMY OF CLOSTRIDIUM BIFERMENTANS AND CLOSTRIDIUM SORDELLII. I. THEIR TOXIGENICITY, UREASE ACTIVITY, AND SPORULATING POTENCY. J Bacteriol. 1964 Dec;88:1641–1646. doi: 10.1128/jb.88.6.1641-1646.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson F. E. Factors which Influence the Growth of Heat-treated Bacteria: I. A Comparison of Four Agar Media. J Bacteriol. 1943 Apr;45(4):395–403. doi: 10.1128/jb.45.4.395-403.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OAKLEY C. L., WARRACK G. H. Routine typing of Clostridium welchii. J Hyg (Lond) 1953 Mar;51(1):102–107. doi: 10.1017/s0022172400015527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Schaeffer P., Ionesco H. Classification cytologique, par leur stade de blocage, des mutants de sporulation de Bacillus subtilis Marburg. Ann Inst Pasteur (Paris) 1966 Mar;110(3):305–315. [PubMed] [Google Scholar]
- TAMAI K., NISHIDA S. TAXONOMY OF CLOSTRIDIUM BIFERMENTANS AND CLOSTRIDIUM SORDELLII. II. TOXIGENIC AND SPORULATING POTENCIES IN SUBSTRAINS OF A CLOSTRIDIUM SORDELLII STRAIN. J Bacteriol. 1964 Dec;88:1647–1651. doi: 10.1128/jb.88.6.1647-1651.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGISHI T., ISHIDA S., NISHIDA S. ISOLATION OF TOXIGENIC STRAINS OF CLOSTRIDIUM PERFRINGENS FROM SOIL. J Bacteriol. 1964 Sep;88:646–652. doi: 10.1128/jb.88.3.646-652.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]




