Abstract
Purpose.
Thrombospondin-1 (THBS1) has been suggested as a corneal wound-healing modulator. Therefore, we compromised the integrity of the cornea to elucidate the role of THBS1.
Methods.
Full-thickness penetrating corneal incisions (1.5 mm) were created in wild type (WT, 129S2/SvPas) and THBS1-deficient mice (Thbs1−/−, 129S2/SvPas-Thbs1tm1Hyn/Thbs1tm1Hyn), and allowed to heal up to 1 month, while being monitored by slit-lamp and intravital corneal examinations. Corneas also were examined by transmission electron microscopy and indirect immunofluorescence. To determine how THBS1 was involved in the healing process, we examined THBS1 and α-smooth muscle actin (SMA), a marker of myofibroblasts and myoepithelial cells.
Results.
In WT mice by 1 month, corneas appeared transparent with a thin scar, and endothelium and Descemet's membrane (DM) were restored. In contrast, Thbs1−/− corneas exhibited chronic edema and persistent opacity after wounding. The DM and endothelium were not restored, and wound contraction was impaired. The THBS1 was localized in epithelial cells at early stages of the healing process, and in the stroma and endothelial cells during later stages. The SMA-positive epithelial cells and myofibroblasts were observed within the healing area at day 4, peaked at day 14, and disappeared at day 30. The SMA-positive cells were reduced greatly in Thbs1−/− mice.
Conclusions.
In the current study, we demonstrated that corneal restoration is strikingly compromised by a penetrating incision in Thbs1−/− mice. The wound results in persistent edema and wound gaping. This appears to be the result of the lack of endothelial migration and DM restoration. In addition, myofibroblast formation is compromised, resulting in the lack of wound contraction.
Keywords: thrombospondin-1, corneal wound healing, penetrating wound, endothelial restoration, corneal epithelium
Thrombospondin-1 is a large glycoprotein with multiple functions, including the ability to activate TGF-B. Mice lacking thrombospondin-1 exhibited multiple healing defects of the stroma and endothelium following penetrating wounds.
Introduction
Thrombospondin-1 (THBS1, also referred to as TSP-1) is a large (450 kDa) trimeric extracellular glycoprotein1,2 released by platelets, and epithelial and mesenchymal cells in response to many of the physiologic processes, including development, wound healing, angiogenesis, tumor cell migration, platelet aggregation, and cell adhesion.3–8 It is not known if THBS1 has a significant role in normal tissue homeostasis; however, it is present during development of many embryonic tissues, and its expression is increased dramatically during the wound-healing process.9–12 Absence of THBS1 leads to prolonged inflammation, delayed wound healing, and delayed scab loss.13,14
In the healthy cornea, THBS1 is localized in the basement membrane zone (BMZ), Descemet's membrane (DM), and corneal endothelium (CE), but not within the stroma of human, bovine,15 and rat corneas.16 In addition, Sekiyama et al.17 demonstrated that limbal and corneal epithelium express Thbs1 mRNA in human corneas. THBS1 and Thbs1 mRNA expression is increased immediately during corneal wound healing after injury16–20; however, the mechanisms of action and function remain unclear. Uno et al. suggest that epithelial defects in the cornea stimulate the expression of THBS1 in the wound area, resulting in the accelerated reepithelialization of the cornea and that lack of vitamin A reduced THBS1 expression.20,21 Recently, Matsuba et al.16 proposed that THBS1 might be involved in the transformation of the keratocytes into myofibroblasts during wound healing after a corneal keratectomy in rats. One of the potential roles of THBS1 is the activation of TGF-β. THBS1 has been demonstrated to be one of the most important activators of TGF-β1,6,22,23 which induces keratocyte proliferation, myofibroblast differentiation, and extracellular matrix (ECM) production.24–26 TGF-β1 is released and suspended within the ECM in a latent form, which is activated in response to injury.27 It also has been observed that adhesion and migration were impaired in vitro in Thbs1−/− mouse corneal endothelium,28 thus showing that THBS1 has a major role during endothelial wound healing as well.29
Since THBS1 is known to be expressed in remodeling corneal epithelium,20 corneal stroma,16 and corneal endothelium,28,29 and is an activator of latent TGF-β1,6,22,23 we hypothesized that THBS1 has an important role in corneal wound repair when the corneal barrier's integrity is compromised. We addressed this hypothesis by performing a full-thickness incision wound in the central cornea of adult THBS1-deficient mice (Thbs1−/−) and comparing them to wild type (WT) mice.
Materials and Methods
All studies were conducted in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. A full-thickness incision was performed with a 1.5-mm blade (Fine Science Tools, Foster City, CA) in the central cornea of 12- to 18-week-old male 129S2/SvPas (WT; The Jackson Laboratory, Bar Harbor, ME), and 12- to 18-week-old male and female 129S2/SvPas- Thbs1tm1Hyn/Thbs1tm1Hyn mice (Thbs1−/−; a kind gift from Jack Lawler, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA). THBS1-deficient mice were generated in D3 ES cells, which were derived from the 129S2/SvPas strain.13,30 The knockout allele is called Thbs1tm1Hyn. Contralateral eyes served as controls. Approximately 70 animals of WT and Thbs1−/− were examined, allowing for at least 3 corneas to be examined per condition per time point.
Full-Thickness Penetrating Incision
At 20 minutes before the procedure, one drop of 1% atropine sulfate ophthalmic solution (Bausch and Lomb, Inc., Rochester, NY) was instilled in the right eye. In a preliminary experiment without topical instillation of atropine, chronic iris incarceration into the corneal incision was observed. Under the microscope, a nasal-temporal orientated full-thickness penetrating incision (1.5 mm in length) was created in the center of the cornea with a surgical blade. Animals were monitored with a slit-lamp (Topcon Medical Systems, Inc., Oakland, NJ) everyday for a week and then weekly until the end of the experiment. Intravital corneal examination also was performed at days 14 and 30 using a Heidelberg Retina Tomograph III (HRT; Heidelberg Engineering, Heidelberg, Germany). At the appropriate time (1, 2, 4, 7, 14, and 30 days) animals were euthanized and corneas either were processed for indirect immunofluorescence (IF; frozen sections and whole mounts) or transmission electron microscopy (TEM).
IF Microscopy
For frozen sections, the eyes were enucleated, frozen in OCT, 6 μm sections were cut, and IF was performed.16 For whole mount, the corneas were enucleated, fixed in prechilled 100% methanol and dimethyl sulfoxide (4:1) for 2 hours at 20°C, and then stored in 100% methanol at 20°C until ready to use. The corneas were prepared for immunofluorescence as described by Pal-Ghosh et al.31 The lens, iris, and retina were removed, and the corneas were cut in half, perpendicular to the original line of incision. Two cuts were placed in each half of the cornea to allow the corneas to lie flat, and then the sections and whole mounts were incubated at 4°C overnight with the following primary antibodies: SMA-FITC (Sigma-Aldrich, St. Louis, MO) and THBS1 (Abcam, Cambridge, MA). Then, the secondary antibody, rhodamine-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was applied for a 1-hour incubation at room temperature (sections) or overnight at 4°C (whole mounts). Coverslips were mounted with mounting media containing 4′6-diamidino-2-phenylindole (DAPI, Vectashield; Vector Laboratories, Burlingame, CA), a marker of all cell nuclei. The sections were examined and documented with a fluorescence microscope (Nikon Eclipse E800; Nikon, Melville, NY) equipped with a digital camera (SPOT; Diagnostic Instruments, Sterling Heights, MI). Whole mounts were examined with a Leica TCS-SP5 laser confocal scanning microscope (LCSM; Leica Microsystems, Bannockburn, IL). Three-dimensional image projections were performed with LAS AF Lite software (Leica Microsystems). Negative controls, where the primary antibody was omitted, were run with all experiments. As an additional control, irrelevant antibodies of the same isotype were compared to ensure specificity.
Transmission Electron Microscopy
Corneas were fixed in half-strength Karnovsky's fixative and processed for TEM, as described previously.32 Briefly, fixed corneas were rinsed for 24 hours with cacodylate buffer 0.1 M and postfixed in 1% osmium tetroxide for 1 to 2 hours at room temperature. The tissue then was dehydrated in ascending concentrations of acetone, infiltrated with propylene oxide, and embedded in Epon plastic. Sections 60 to 90 angstroms (Å) thick were cut on an ultramicrotome (LKB ultramicrotome; LKB, Bromma, Sweden) and examined with an electron microscope (Philips 410 electron microscope; Philips Research Laboratories, Eindhoven, The Netherlands).
Results
Pathologic Manifestations of Lack of Thbs1
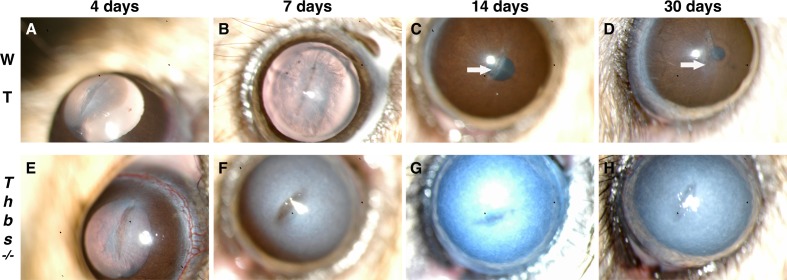
In WT corneas, the incision was sealed by wound-healing epithelium within 1 day. Incipient edema was observed during the first days around the incision, but corneas became transparent after 4 to 7 days (Figs. 1A, 1B). All Thbs1−/− corneas became chronically opaque from day 4 until the end of the experiment (Figs. 1E–H). In the WT mice, the stromal wound closed rapidly (Fig. 1B), healing with a thin scar (Figs. 1C, 1D). However, in Thbs1−/− corneas the incision gaped rather than sealing shut (Figs. 1E, 1F) and never appeared to close fully (Figs. 1G, 1H).
Figure 1.
Slit-lamp evaluation of WT (A–D) and Thbs1−/− (E–H) mouse corneas. In WT mice, stromal matrix contraction around the closed wound can be seen at 7 days (B). WT mice show transparent corneas with a thin reflective line of subepithelial haze in the incision area at 14 (C) and 30 (D) days. Scar indicated by white arrows. Lack of contractility with unclosed wounds can be observed at 4 (E) and 7 (F) days in Thbs1−/−. Persistent corneal edema with full opacity is observed from day 4 (E) until the end of the experiment (G, H).
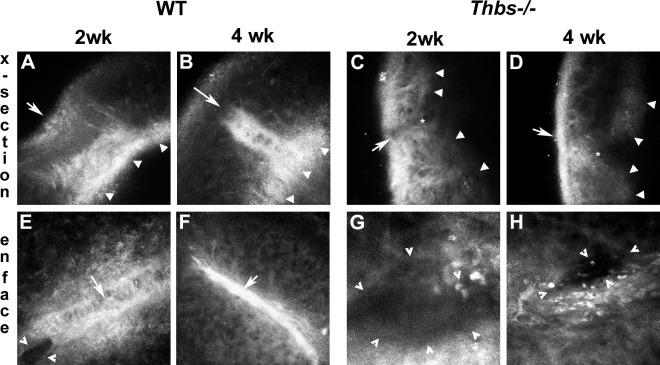
These findings were confirmed via intravital examination with the HRT. A highly reflective stromal tissue was observed under the epithelium of WT corneas by cross-section (Figs. 2A, 2B) and enface (Figs. 2E, 2F) scanning. At 2 weeks, WT corneas recovered almost the total thickness with a newly deposited stromal tissue (appears brightly) and a hyperplasic epithelium (Fig. 2A). However, both sides of the cleft remained separated (Fig. 2E). At 4 weeks, the incision was tightly contracted with a highly reflective matrix (Figs. 2B, 2F). Minimal reflective stromal tissue or stromal healing activity was found in Thbs1−/− corneas (Figs. 2C, 2D, 2G, 2H). In the endothelial layer, WT corneas showed high healing activity at 2 (Fig. 2A) and 4 (Fig. 2B) weeks after injury, as indicated by the high level of reflectance (arrowheads). No evidence of endothelial-healing activity was observed in Thbs1−/− corneas (Figs. 2C, 2D). The lack of endothelial repair in the Thbs1−/− corneas is consistent with the persistent edema seen in Figure 1, suggesting that THBS1 has an important role in endothelial migration during wound repair.
Figure 2.
HRT intravital evaluation of WT (A, B, E, F) and Thbs1−/− (C, D, G, H) mouse corneas 2 and 4 weeks after wounding showing cross-section (x-section) and enface images. A reflective fibrotic process can be observed in the WT stroma at 2 weeks (A, E) with the area of fibrosis appearing to contract at 4 weeks (B, F). Highly reflective endothelium indicating wound repair also can be observed at 2 and 4 weeks (A, B). In contrast, the absence of stromal or endothelial wound healing is evident in the Thbs1−/− corneas (C, D, G, H). White arrows (A–F) indicate the incision. Closed arrowheads (A–D) indicate extent of posterior cornea. Open arrowheads (E, G, H) indicate gape in stroma, demonstrating lack of wound closure. Asterisk (C, D) indicates tip of cleft in Thbs1−/− corneas. Also note highly pigmented cells in wound area of Thbs1−/− corneas (G, H). At least three corneas per time point were examined with similar results.
Localization of THBS1 After Incision
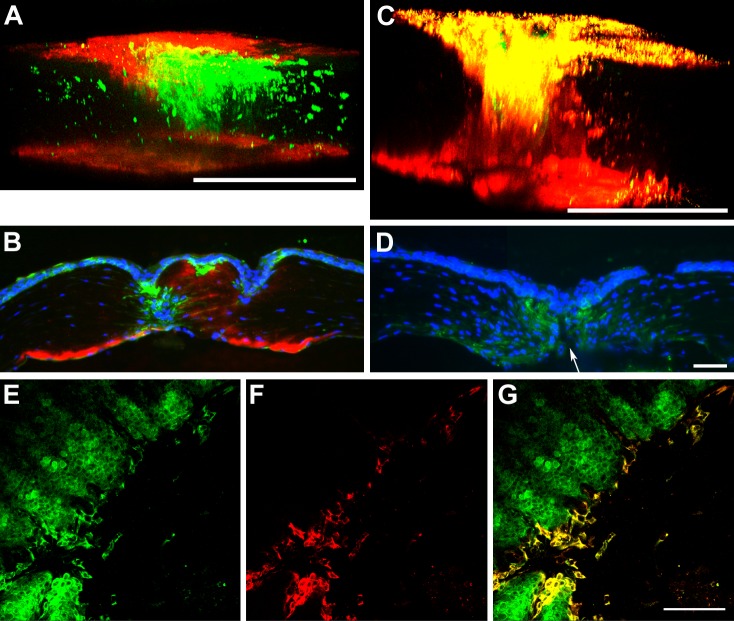
In corneal16,20 and nonocular1 models, THBS1 is deposited within 48 hours after injury. As seen in Figure 3, THBS1 was observed only in the endothelium of unwounded WT corneas (Fig. 3A), and then in the epithelium covering the incision 24 hours after wounding (Fig. 3B). At 48 hours after wounding, THBS1 expression was extended to surrounding epithelial cells and into the cleft created by the incision (Fig. 3D). As expected, no THBS1 was observed in the Thbs1−/− mouse corneas 24 and 48 hours after incision (Figs. 3C, 3E). Expression of THBS1 gradually increased by days 4 (Fig. 4A) and 7 (Fig. 4B), and was maximal at day 14 (Fig. 4C), where an interrupted column of THBS1 localization was visualized from the top of the epithelium to the endothelium. THBS1 levels were diminished by 30 days after injury (data not shown). Strikingly, THBS1 was elevated in the endothelium at these later time points (Figs. 4A–C). Localization of THBS1 in the epithelial cells was seen clearly in enface imaging (Figs. 4E–G). Presence of THBS1 in peripheral stroma, or epithelium of injured or unwounded corneas was not observed.
Figure 3.
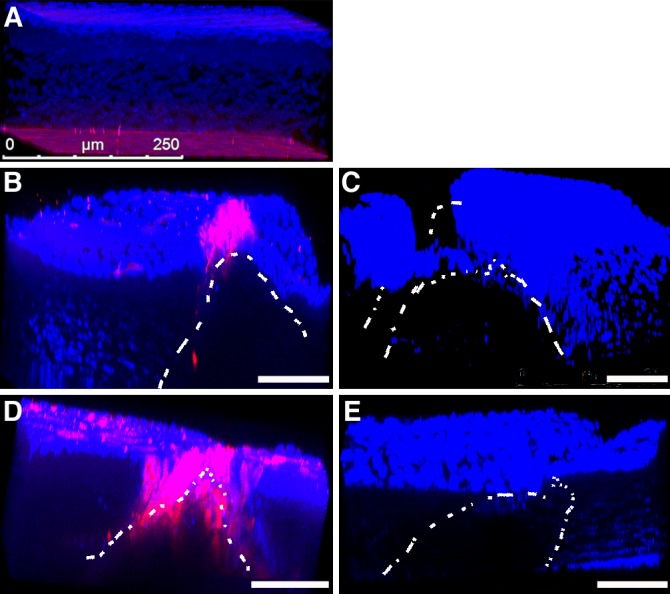
Three-dimensional projection of whole mount corneas immunolabeled with THBS1 (pink) in WT (A, B, D) and Thbs1−/− (C, E) mice, unwounded (A), and 24 (B, C) and 48 (D, E) hours after wounding. In unwounded cornea (A), THBS1 is present primarily in the endothelial cell layer. A narrow line of THBS1 is localized in the corneal epithelium of WT 24 hours after incision (B), which expanded to peripheral epithelium and stroma by 48 hours (D). As expected, no THBS1 was detected in Thbs1−/− mice (C, E). White lines show the borders of the incision. DAPI (blue) is a marker of all cell nuclei. Scale bars: 100 μm (B–E).
Figure 4.
Immunolocalization of SMA (green) and THBS1 (red) in WT (A–C, E–G) and Thbs1−/− (D) mice. (A, C) Three-dimensional projection of whole mount corneas at 4 (A) and 14 (C) days after injury. (B, D) Cross-sections at 7 days after incision in WT (B) and Thbs1−/− (D) mice. (E–G) Epithelial localization in whole mount corneas 4 days after wounding: (E) SMA, (F) THBS1, and (G) SMA/THBS1 overlay. Note the expression of THBS1 in the epithelium, upper stroma, and endothelium after wounding (A–C). Colocalization of THBS1 and SMA is evident at 14 days (C), and SMA-positive cells extend further from edge of wound than THBS1-positive cells (E–G). Also, note the gape (arrow) in the Thbs1−/− cornea (D) and reduced level of SMA localization compared to (B). DAPI (blue) is a marker of all cell nuclei. Scale bars: 250 μm (A, C), 50 μm (D, G).
Myofibroblast Differentiation Appears to Correlate With THBS1 Expression
Cytoplasmic colocalization of THBS1 and α-smooth muscle actin (SMA) was evident in the epithelial cells of WT mice 4 days after wounding (Figs. 4E–G). In the Thbs1−/− corneas, few, if any, SMA-positive epithelial cells were observed (Fig. 4D). The Thbs1−/− corneas appeared to lack the ability to contract, as the wound remained open longer and appeared to gape (Fig. 1). In WT mice, myofibroblasts were observed on days 4 (Fig. 4A) and 7 (Fig. 4B) in the anterior aspect of the incision. By 14 days (Fig. 4C), as with THBS1, the presence of myofibroblasts reached its maximum, and a column of SMA-positive cells was observed connecting the epithelium with the endothelium (Fig. 4C). By 30 days after incision, no myofibroblasts or SMA-positive epithelial cells were observed, and the wound was fully sealed by new ECM (data not shown). Greatly reduced numbers of myofibroblasts were observed in the Thbs1−/− corneas (Fig. 4D).
Corneal Endothelial Barrier Restoration Is Impaired Severely in Thbs1−/− Corneas
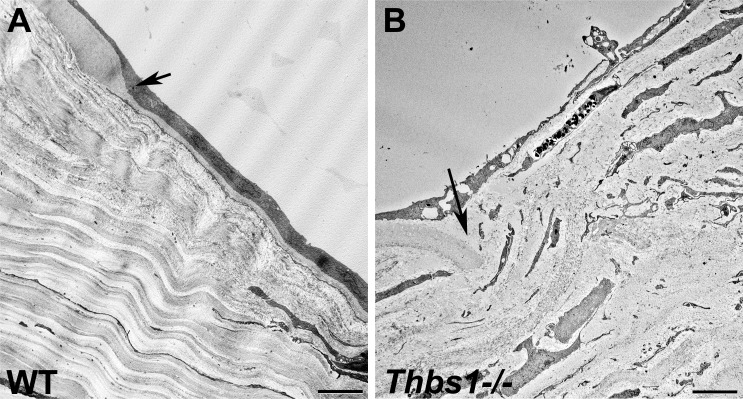
A proper endothelial function is essential to maintain the stroma with the appropriate hydration for keeping corneal transparency. Incipient edema in the corneas was observed around the incision in the WT mice before becoming transparent after 4 to 7 days (Figs. 1A, 1B). However, Thbs1−/− corneas remained chronically opaque until the end of the experiment (Figs. 1G, 1H). A reflective new tissue was observed at endothelial level in WT corneas, indicating a wound-healing process at 2 (Fig. 2A) and 4 (Fig. 2B) weeks, suggesting that the corneal endothelium is healing. This was confirmed in Figure 5 by TEM, where a new layer of endothelial cells was observed in the WT mice at day 15 after incision (Fig. 5A). Of interest, a new thin well-organized DM was found to be present between the stroma and the new corneal endothelial cells 2 weeks after injury (Fig. 5A). This incipient DM can be observed at the border of the wound area as a continuation of the unwounded DM. The lack of endothelial barrier in the Thbs1−/− was confirmed in vivo by slit-lamp and HRT examination (Figs. 2C, 2D). In the Thbs1−/−, attenuated corneal endothelial cells were apparent in the wound area; however, no new DM was observed (Fig. 5B). The original DM appeared to be broken and curved into the stroma without continuation (Fig. 5B).
Figure 5.
TEM micrographs of WT (A) and Thbs1−/− (B) mouse corneas 15 days after incision. In WT corneas (A), the endothelium has migrated to cover the wound area and the DM is partially restored. In contrast, only highly attenuated endothelial cells are seen in Thbs1−/− corneas (B) and no signs of DM regeneration were observed. Arrows indicate edge of cut DM. Scale bars: 5 μm.
Discussion
Previously, we demonstrated that THBS1 expression accompanied myofibroblast generation during the healing process after keratectomy in rats.16 THBS1 was deposited into the stroma following a wound where the BMZ was damaged16; however, when the BMZ was left intact, THBS1 was expressed only in the most superficial aspect of the stroma, corresponding to the BMZ.20 In concordance with Uno et al.,20 we also showed previously that THBS1 expression was maximal early after wounding and gradually decreased.16 These studies suggest that THBS1 has an important role in the healing process. In the current report, we made use of Thbs1−/− mice to examine further the role of THBS1, and we demonstrated that stromal, as well as endothelial healing, was severely impaired in mice lacking Thbs1. In the current model, a full-thickness corneal incision was performed. In WT mice at 24 hours, the expression of THBS1 was observed in the epithelium that covers the incision, but not in the stroma (Fig. 3B). Later, THBS1 expression extended gradually to the surrounding epithelial cells and down into the stroma around the wound, peaking at 14 days (Fig. 4C). At this time point, THBS1 was observed in an interrupted column pattern filling both sides of the incision from the epithelial surface to the endothelium (Fig. 4C); however, no THBS1 was observed in the unwounded peripheral stroma or epithelium (data not shown). One of the unexpected and most striking results of our investigation was that Thbs1−/− corneas rapidly became opaque after an incision wound and never appeared to heal. These results indicated that THBS1 is a necessary component of endothelial wound repair. This is supported further by the observation that THBS1 expression increased in the endothelial cell layer after wounding in the WT mice (Figs. 4A–C).
When the corneal integrity is compromised, epithelial cells quickly cover the damaged area by proliferation and migration.33 In the stroma, keratocytes within the wound area undergo apoptosis, leaving the stroma devoid of cells that must be replaced, and the keratocytes surrounding the wound area proliferate and become fibroblasts.34 These fibroblasts then migrate to fill the stromal wound area, with many of them differentiating into myofibroblasts, a cellular phenotype with contractile properties and ECM synthesis capabilities.24,35,36 Such a healing environment, with myofibroblasts and new ECM, produces an optical phenomenon called light backscattering or subepithelial haze,24,26 which usually disappears when the wound-healing process is done.24 In the current work, subepithelial haze (Figs. 1A, 1B), reflective stromal and endothelial tissue (Figs. 2A, 2B, 2E, 2F), myofibroblasts (Fig. 4), and new ECM were observed in the WT mice. However, all of these processes were strikingly absent or reduced in Thbs1−/− mice. Previously, we observed that the expression of THBS1 correlated with the appearance of myofibroblasts after a corneal keratectomy in rats,16 where THBS1 was localized in the wound area beginning at 1 day, and myofibroblasts were detected in the same area by day 4. A similar pattern was observed in the current work in mice. These data clearly suggested that keratocytes differentiate to become myofibroblasts in response to THBS1. Since it is known that THBS1 can activate TGF-β1, one possible mechanism of action would be the stimulation of keratocytes/fibroblasts by TGF-β1, which was activated by THBS1 in the wound area. One unclear aspect from our previous work16 was whether fibroblasts, myofibroblasts, or epithelial cells were responsible for THBS1 expression. Confocal images revealed intracellular expression of THBS1 by epithelial and endothelial cells. Interestingly, many of the epithelial cells expressing THBS1 also expressed SMA, suggesting that they were myoepithelial cells. Presence of myoepithelial cells is well known in other tissues,37,38 but is not well documented in the cornea. Hiscott et al. suggested a main role of THBS1 in the maintenance of the epithelial structure during wound repair.39 This was confirmed by our results in the Thbs1−/− mice.
In contrast to the epithelial cells, no myofibroblasts or keratocytes expressed intracellular THBS1. While this does not rule out the possibility that myofibroblasts, nerves, or inflammatory cells are producing THBS1, our data were consistent with the epithelial cells being a primary source. Interestingly, the corneal endothelium also appeared to be a source of THBS1. In our study, we extended our work to demonstrate that THBS1 is critical also for endothelial restoration, since Thbs1−/− mice develop chronic edema and persistent corneal opacification. Neither of these healing defects was observed in the WT mice. In the WT mice, THBS1 expression was observed to increase in the healing endothelium from day 4 (Fig. 4A) until the end of the experiment, reaching maximal intensity at 14 days (Fig. 4C). The new monolayer rests over a thin, but well-defined DM (Fig. 5A). Dramatically, all of these components are missing in the Thbs1−/− mouse corneas. It is well established that endothelial cell proliferation is lacking in humans40; however, if the endothelial barrier is damaged, a restorative process occurs by migration and/or hypertrophy of surrounding endothelial cells.41,42 In lung microvascular endothelia, THBS1 increases tyrosine phosphorylation of components in the cell-cell adherens junctions, inducing actin reorganization and focal adhesion disassembly.43,44 Migration, hypertrophy, and adhesion are impaired in vitro in Thbs1−/− mouse endothelial cells,28 suggesting a major role of THBS1 during the endothelial barrier repair,29 which is confirmed strongly by our in vivo results. In addition, previous studies have demonstrated that TGF-β1 stimulates endothelial cells to proliferate, migrate, and produce ECM.45–47 The THBS1 binds the TGF-β–LAP,11 leading to activation of endogenous TGF-β expressed by endothelial cells.48 It also has been suggested that TGF-β1 has a role in maintaining the endothelial cells in a nonproliferative state.49 Our results suggested that TGF-β1 and THBS1 have an important role in endothelial cell repair.
In our current study, we made the novel observation that penetrating wounds in Thbs1−/− corneas fail to heal, and exhibit persistent edema and prolonged wound gaping. We have demonstrated that lack of endothelial healing and wound contraction is the functional mechanism that appears to cause these defects. However, we have not uncovered the molecular mechanism of THBS1 in these processes. While THBS1 has been shown to have an important role in activation of TGF-β1, which certainly may have a role in these processes, THBS1 has been demonstrated to have multiple effects in the cornea. Haddadin et al. have demonstrated that Thbs1−/− corneas are thinner than WT by 6.5% and that intraocular pressure was reduced by 10%.50 This could make the cornea more susceptible to healing defects. In addition, THBS1 has an important role in maintaining immune privilege, in that THBS1 derived from antigen-presenting cells is necessary to suppress immune rejection.51 It is not clear if immune rejection has a role in our studies; however, THBS1 derived from immune cells certainly could be involved in the healing response. In addition to its role in immune suppression, lack of THBS1 also has been reported to result in ocular surface defects that mimic dry eye.52 Again, it is not clear if this impacts our studies, but it is possible that these ocular surface defects make the cornea susceptible to wound-healing defects. Finally, Sekiyama et al.17 suggest that THBS1 is bound or trapped by Bowman's layer in human corneas. Our data in mice and rats, which lack Bowman's layer, supported their findings in that we see minimal accumulation of THBS1 at the epithelial/stromal interface.16 In total, there appear to be multiple potential molecular mechanisms of THBS1 that may affect corneal wound repair.
We concluded that THBS1 has critical roles during corneal restoration when its integrity is compromised by a penetrating incision, including endothelial migration and stromal contraction. Based on our results and other published reports, THBS1 expression and persistence in the tissue seem to be related to the extent of the injury, ranging from a few hours in the BMZ20 of an epithelial wound to a few days in the upper aspect of the stroma of a superficial keratectomy16 to one month in all the corneal layers following a penetrating wound. THBS1 also appears to be expressed by the corneal epithelium and endothelium, and has a role in epithelial, stromal, and endothelial repair. These experiments raise many questions regarding the mechanisms of THBS1 in wound repair; however, these questions will be addressed in further work.
Acknowledgments
Supported by National Institutes of Health/National Eye Institute Grants EY005665 (JDZ), EY05665-25S1 (JDZ), and EY03790 (Core-JDZ).
Disclosure: J.T. Blanco-Mezquita, None; A.E.K. Hutcheon, None; J.D. Zieske, None
References
- 1. Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001; 107: 929– 934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008; 65: 672– 686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baenziger NL, Brodie GN, Majerus PW. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci U S A. 1971; 68: 240– 243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000; 12: 634– 640 [DOI] [PubMed] [Google Scholar]
- 5. Leung LL. Role of thrombospondin in platelet aggregation. J Clin Invest. 1984; 74: 1764– 1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy-Ullrich JE, Gurusiddappa S, Frazier WA, Hook M. Heparin-binding peptides from thrombospondins 1 and 2 contain focal adhesion-labilizing activity. J Biol Chem. 1993; 268: 26784– 26789 [PubMed] [Google Scholar]
- 7. Sheibani N, Frazier WA. Thrombospondin-1, PECAM-1, and regulation of angiogenesis. Histol Histopathol. 1999; 14: 285– 294 [DOI] [PubMed] [Google Scholar]
- 8. Taraboletti G, Roberts DD, Liotta LA. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol. 1987; 105: 2409– 2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990; 63: 21– 29 [PubMed] [Google Scholar]
- 10. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993; 122: 103– 111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy-Ullrich JE, Schultz-Cherry S, Hook M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992; 3: 181– 188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993; 328: 1828– 1835 [DOI] [PubMed] [Google Scholar]
- 13. Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002; 161: 831– 839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Streit M, Velasco P, Riccardi L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000; 19: 3272– 3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hiscott P, Seitz B, Schlotzer-Schrehardt U, Naumann GO. Immunolocalisation of thrombospondin 1 in human, bovine and rabbit cornea. Cell Tissue Res. 1997; 289: 307– 310 [DOI] [PubMed] [Google Scholar]
- 16. Matsuba M, Hutcheon AE, Zieske JD. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp Eye Res. 2011; 93: 534– 540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sekiyama E, Nakamura T, Cooper LJ, et al. Unique distribution of thrombospondin-1 in human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2006; 47: 1352– 1358 [DOI] [PubMed] [Google Scholar]
- 18. Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest Ophthalmol Vis Sci. 2002; 43: 2897– 2904 [PubMed] [Google Scholar]
- 19. Hiscott P, Armstrong D, Batterbury M, Kaye S. Repair in avascular tissues: fibrosis in the transparent structures of the eye and thrombospondin 1. Histol Histopathol. 1999; 14: 1309– 1320 [DOI] [PubMed] [Google Scholar]
- 20. Uno K, Hayashi H, Kuroki M, et al. Thrombospondin-1 accelerates wound healing of corneal epithelia. Biochem Biophys Res Commun. 2004; 315: 928– 934 [DOI] [PubMed] [Google Scholar]
- 21. Uno K, Kuroki M, Hayashi H, Uchida H, Kuroki M, Oshima K. Impairment of thrombospondin-1 expression during epithelial wound healing in corneas of vitamin A-deficient mice. Histol Histopathol. 2005; 20: 493– 499 [DOI] [PubMed] [Google Scholar]
- 22. Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem. 2004; 279: 38032– 38039 [DOI] [PubMed] [Google Scholar]
- 23. Bornstein P. Thrombospondins function as regulators of angiogenesis. J Cell Commun Signal. 2009; 3: 189– 200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999; 18: 311– 356 [DOI] [PubMed] [Google Scholar]
- 25. Jester JV, Barry-Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea. 1997; 16: 177– 187 [PubMed] [Google Scholar]
- 26. Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998; 17: 627– 639 [DOI] [PubMed] [Google Scholar]
- 27. Barcellos-Hoff MH. Latency and activation in the control of TGF-beta. J Mammary Gland Biol Neoplasia. 1996; 1: 353– 363 [PubMed] [Google Scholar]
- 28. Scheef EA, Huang Q, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of corneal endothelial cells from wild type and thrombospondin-1 deficient mice. Mol Vis. 2007; 13: 1483– 1495 [PubMed] [Google Scholar]
- 29. Munjal ID, Crawford DR, Blake DA, Sabet MD, Gordon SR. Thrombospondin: biosynthesis, distribution, and changes associated with wound repair in corneal endothelium. Eur J Cell Biol. 1990; 52: 252– 263 [PubMed] [Google Scholar]
- 30. Lawler J, Sunday M, Thibert V, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998; 101: 982– 992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA. A mouse model for the study of recurrent corneal epithelial erosions: alpha9beta1 integrin implicated in progression of the disease. Invest Ophthalmol Vis Sci. 2004; 45: 1775– 1788 [DOI] [PubMed] [Google Scholar]
- 32. Gipson IK, Kiorpes TC, Brennan SJ. Epithelial sheet movement: effects of tunicamycin on migration and glycoprotein synthesis. Dev Biol. 1984; 101: 212– 220 [DOI] [PubMed] [Google Scholar]
- 33. Zieske JD. Expression of cyclin-dependent kinase inhibitors during corneal wound repair. Prog Retin Eye Res. 2000; 19: 257– 270 [DOI] [PubMed] [Google Scholar]
- 34. Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001; 72: 33– 39 [DOI] [PubMed] [Google Scholar]
- 35. Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast transformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002; 100: 411– 433 [PMC free article] [PubMed] [Google Scholar]
- 36. Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001; 42: 2490– 2495 [PubMed] [Google Scholar]
- 37. Berman JJ. Neoplasms: Principles of Development and Diversity. Sudbury, MA: Jones and Bartlett Publishers; 2009. [Google Scholar]
- 38. Raubenheimer EJ. The myoepithelial cell: embryology, function, and proliferative aspects. Crit Rev Clin Lab Sci. 1987; 25: 161– 193 [DOI] [PubMed] [Google Scholar]
- 39. Hiscott P, Paraoan L, Choudhary A, Ordonez JL, Al-Khaier A, Armstrong DJ. Thrombospondin 1, thrombospondin 2 and the eye. Prog Retin Eye Res. 2006; 25: 1– 18 [DOI] [PubMed] [Google Scholar]
- 40. Bahn CF, Glassman RM, MacCallum DK, et al. Postnatal development of corneal endothelium. Invest Ophthalmol Vis Sci. 1986; 27: 44– 51 [PubMed] [Google Scholar]
- 41. Jacobi C, Zhivov A, Korbmacher J, et al. Evidence of endothelial cell migration after Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2011; 152: 537– 542 [DOI] [PubMed] [Google Scholar]
- 42. Watson SL, Abiad G, Coroneo MT. Spontaneous resolution of corneal oedema following Descemet's detachment. Clin Experiment Ophthalmol. 2006; 34: 797– 799 [DOI] [PubMed] [Google Scholar]
- 43. Goldblum SE, Young BA, Wang P, Murphy-Ullrich JE. Thrombospondin-1 induces tyrosine phosphorylation of adherens junction proteins and regulates an endothelial paracellular pathway. Mol Biol Cell. 1999; 10: 1537– 1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu A, Mosher DF, Murphy-Ullrich JE, Goldblum SE. The counteradhesive proteins, thrombospondin 1 and SPARC/osteonectin, open the tyrosine phosphorylation-responsive paracellular pathway in pulmonary vascular endothelia. Microvasc Res. 2009; 77: 13– 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grant MB, Khaw PT, Schultz GS, Adams JL, Shimizu RW. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992; 33: 3292– 3301 [PubMed] [Google Scholar]
- 46. Plouet J, Gospodarowicz D. Transforming growth factor beta-1 positively modulates the bioactivity of fibroblast growth factor on corneal endothelial cells. J Cell Physiol. 1989; 141: 392– 399 [DOI] [PubMed] [Google Scholar]
- 47. Usui T, Takase M, Kaji Y, et al. Extracellular matrix production regulation by TGF-beta in corneal endothelial cells. Invest Ophthalmol Vis Sci. 1998; 39: 1981– 1989 [PubMed] [Google Scholar]
- 48. Sumioka T, Ikeda K, Okada Y, Yamanaka O, Kitano A, Saika S. Inhibitory effect of blocking TGF-beta/Smad signal on injury-induced fibrosis of corneal endothelium. Mol Vis. 2008; 14: 2272– 2281 [PMC free article] [PubMed] [Google Scholar]
- 49. Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2. Invest Ophthalmol Vis Sci. 2002; 43: 2152– 2159 [PubMed] [Google Scholar]
- 50. Haddadin RI, Oh DJ, Kang MH, et al. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2012; 53: 6708– 6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zamiri P, Masli S, Kitaichi N, Taylor AW, Streilein JW. Thrombospondin has a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci. 2005; 46: 908– 919 [DOI] [PubMed] [Google Scholar]
- 52. Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjögren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009; 175: 1136– 1147 [DOI] [PMC free article] [PubMed] [Google Scholar]