Abstract
Purpose.
Neuroprotectin D1 (NPD1) is an anti-inflammatory and proresolving lipid mediator biosynthesized from the omega-3-polyunsaturated fatty acid docosahexaenoic acid (DHA). The purpose of this study is to test the therapeutic potential of NPD1 for the treatment of herpes simplex virus (HSV)–induced stromal keratitis (SK) using a mouse model.
Methods.
C57BL/6 mice were infected ocularly with HSV-1 strain RE. Infected animals were treated topically with methyl ester prodrug NPD1 (300 ng/eye, 5-μL drop). Development of SK lesions, infiltration of inflammatory cells into the cornea, and production of proinflammatory cytokines, chemokines, and angiogenic factors were compared to untreated animals using slit-lamp biomicroscopy, flow cytometry, ELISA, and quantitative PCR (qPCR).
Results.
Topical administration of NPD1 resulted in a significant reduction in the severity and incidence of SK, as well as the extent of corneal neovascularization in the NPD1-treated animals compared to their untreated counterparts. Infiltration of fewer neutrophils and pathogenic CD4+ T cells into the cornea, along with a lower number of cells that could be induced ex vivo to produce IFN-γ and IL-17, occurred with NPD1 treatment. Additionally, treatment with NPD1 diminished the production of proinflammatory cytokines, chemokines, and angiogenic factors, such as IL-6, CXCL1, CXCL-10, CCL-20, VEGF-A, MMP-2, and MMP-9 in the corneas of infected animals. Importantly, treatment with NPD1 increased the production of the anti-inflammatory cytokine, IL-10.
Conclusions.
Our novel findings demonstrate that NPD1 treatment could represent a valuable therapeutic approach to control SK lesions.
Keywords: NPD1, immunopathology, HSV-1, herpes stromal keratitis
In this report, we demonstrate the efficacy of topical therapy with neuroprotectin D1 against ocular disease caused by HSV.
Introduction
Herpes simplex virus (HSV) infection of the eye is the leading infectious cause of vision impairment in the developed countries.1 Frequent recurrent episodes may lead to a chronic inflammatory reaction in the corneal stroma that is characterized by neovascularization of the normally avascular cornea, which together with stromal opacification impedes vision and can result in blindness.2 Management of stromal keratitis (SK) lesions usually depends on a combination of antiviral and anti-inflammatory drugs, such as corticosteroids, with the latter often needed for lengthy periods, which can result in unwanted side effects.3–5 As such, there is an urgent need for alternative and more effective therapies to control SK. One approach would be to exploit the fact that the host itself produces numerous counter-inflammatory molecules to regulate inflammatory responses and accelerate their resolution.6 Neuroprotectin D1 (NPD1) represents one such group of molecules,6,7 but to our knowledge these have not yet been tested for their effects in chronic immunopathologic disorders caused by viral infections, which include SK.
NPD1 is an endogenously-produced lipid mediator that is derived from the omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA).6 NPD1 was shown to control acute inflammatory events and possess proresolution properties leading to tissue repair in a number of noninfectious inflammatory conditions.8–11 Activities reported for NPD1 include reduced neutrophil recruitment,12 increased phagocytosis of apoptotic neutrophils,13 impairment of T cell migration,14 reduced production of several proinflammatory cytokines and chemokines,15 reduced oxidative stress,16 and promotion of corneal wound healing.17 Since many of these activities are considered to contribute to tissue damage in SK,18 we surmised that treatment of ongoing SK lesions with NPD1 would represent a valuable means of controlling herpetic ocular disease.
In this report, we demonstrated the efficiency of topical therapy with NPD1 against ocular disease caused by HSV. The results demonstrated that NPD1 administration markedly reduced SK lesion severity and the extent of corneal neovascularization, and was linked to decreased influx of effector CD4+ T cells and neutrophils, and production of proinflammatory cytokines, chemokines, and proangiogenic molecules. Thus, NPD1 therapy may represent a novel approach to control HSV-induced ocular immunopathologic lesions, the most common infectious cause of blindness in humans.
Materials and Methods
Mice and Virus
Female C57BL/6 mice were purchased from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). The animals were housed in American Association of Laboratory Animal Care–approved facilities at the University of Tennessee, Knoxville, Tennessee. All investigations followed guidelines of the Institutional Animal Care and Use Committee, and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. HSV-l RE strain was used in all procedures. Virus was grown in Vero cell monolayers (American Type Culture Collection, Manassas, VA), titrated, and stored in aliquots at −80°C until used.
Corneal HSV Infection
Corneal infections of all mouse groups (6–10 weeks old) were conducted under a deep anesthesia (tribromoethanol). The mice were scarified lightly on their corneas with a 27-gauge needle and a 3-μl drop containing 2 × 104 plaque-forming units (PFU) of HSV-1 RE was applied to the eye.
NPD1 Administration
The methyl ester prodrug of NPD1 (RX-20001; Resolvyx, Bedford, MA) was applied topically to the cornea (300 ng/eye, 5-μL drop) twice daily starting from 1 day before infection until day10 after infection (pi), or starting at day 6 pi until day 12 pi. The vehicle control group received an equal volume of PBS. The dose of NPD1 was chosen based on our preliminary studies (data not shown).
Detection of Infectious Virus on HSV-Infected Corneas
Mouse corneas were swabbed with sterile swabs (Fisher HealthCare, Thermo Fisher Scientific, Waltham, MA) at 3, 6, and 8 days pi. The swabs then were placed in 0.5 mL RPMI 1640 and frozen at −80°C until used for assays. Samples were added to confluent Vero cells, incubated for 75 minutes at 37°C, and overlaid with 1% methylcellulose. The cultures were incubated for 72 hours, fixed with buffered formalin, stained with crystal violet, and viral cytopathic effect was examined.
Clinical Observations
The eyes were examined on different days after infection for the development of clinical lesions by slit-lamp biomicroscopy (Kawa Co., Nagoya, Japan), and the clinical severity of keratitis of individually scored mice was recorded by a blinded observer. The scoring system was as follows: 0, normal cornea; +0.5, slight corneal haze; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity, but iris visible; +4, opaque cornea and corneal ulcer; +5, corneal rupture and necrotizing stromal keratitis. The severity of angiogenesis was recorded as described previously.19 In reference to the angiogenic scoring system, the method relied on quantifying the degree of neovessel formation based on three primary parameters: the circumferential extent of neovessels (as the angiogenic response is not uniformly circumferential in all cases), the centripetal growth of the longest vessels in each quadrant of the circle, and the longest neovessel in each quadrant was identified and graded between 0 (no neovessel) and 4 (neovessel in the corneal center) in increments of 0.4 mm (radius of the cornea is 1.5 mm). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm toward the corneal center. The score of the four quadrants of the eye then were summed to derive the neovessel index (range, 0–16) for each eye at a given time point.
Immunohistochemical Staining
At the termination of the experiment (day 15 pi), eyes were removed from control and NPD1-treated mice, and snap-frozen in OCT compound (Miles Lab, Elkhart, IN). Sections 6 μ thick were cut, and stained with hematoxylin and eosin as described previously.20
Flow Cytometric Analysis
At day 15 pi, corneas were excised, pooled group-wise, and digested with liberase (Roche Diagnostics Corporation, Indianapolis, IN) for 45 minutes at 37°C in a humidified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a syringe plunger on a cell strainer and a single-cell suspension was made in complete RPMI 1640 medium. The single-cell suspensions obtained from corneal samples were stained for different cell surface molecules for fluorescence-activated cell sorting (FACS) analyses. All steps were performed at 4°C. Briefly, a total of 1 × 106 cells was blocked first with an unconjugated anti-CD32/CD16 mAb for 30 minutes in FACS buffer. After washing with FACS buffer, the cells were stained with respective fluorochrome-labeled Abs for 30 minutes. Finally, the cells were washed three times and resuspended in 1% paraformaldehyde. The stained samples were acquired with a FACS Calibur (BD Biosciences, San Jose, CA) and the data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
To determine the number of IFN-γ– and IL-17–producing T cells, intracellular cytokine staining was performed. In brief, corneal cells were stimulated with anti-CD3 (5 μg/mL) and anti-CD28 (1 μg/mL) for 5 hours in the presence of brefeldin A (5 μg/mL) in U-bottom 96-well plates. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD Pharmingen, San Diego, CA) in accordance with the manufacturer's recommendations. The Abs used were anti-CD4 APC, anti-IFN-γ PE, and IL-17 Percp cy5.5. The stained samples were acquired with a FACS Calibur and the data were analyzed using FlowJo software.
Protein Quantification of Corneal Lysates by ELISA
The corneas were pooled group-wise (5 corneas/sample/group) and homogenized using a tissue homogenizer (Pellet Pestle mortar; Kontes, Vineland, NJ). The concentrations of various cytokines, IL-6, IL-10, CXCL1 (KC), and VEGF were measured by sandwich ELISA kits from eBioscience (IL-6, IL-10; San Diego, CA) and R&D Systems (VEGF-A, CXCL1; Minneapolis, MN) as per the manufacturer's instructions.
Quantitative PCR (qPCR)
At 15 days after ocular infection, the corneas were isolated and five corneas were pooled per sample/group. The corneal cells were lysed and total mRNA was extracted using TRIzol LS reagent (Invitrogen, Carlsbad, CA). Total cDNA was made with 1 μg of RNA using oligo(dT) primer. Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) with iQ5 real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA). The expression levels of different molecules were normalized to β-actin using ΔCt calculation. Relative expression between control and experimental groups was calculated using the 2-ΔΔCt formula. The primers used for CXCL10 and CXCL-20 are as follows: CXCL-10, 5′-TGC TGG GTC TGA AGT GGG ACT-3′ (forward) and 5′- AAG CTT CCC TAT GGC CCT CA-3′ (reverse); and CCL-20, 5′-GCCTCTCGTACATACAGACGC-3′ (forward) and 5′- CCAGTTCTGCTTTGGATCAGC-3′ (reverse). The PCR primers used for MMP-2 and MMP-9 were described previously.21
TaqMan miRNA qPCR (TaqMan qPCR)
At 15 days after ocular infection, the corneas were isolated and five corneas were pooled per sample/group. The miRNA were extracted from HSV-infected mice corneas using the mirVana miRNA Isolation Kit (Ambion, Austin, TX) as described previously.22 These extracted miRNA were converted to cDNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). TaqMan MicroRNA Assay (Applied Biosystems) was used to quantify for miR-132 with real-time PCR detection system (Applied Biosystems). Data were normalized to the internal control small nucleolar RNA 202.22
Statistical Analysis
Statistical significance was determined by Student's t-test unless otherwise specified. A P value of <0.05 was regarded as a significant difference between groups: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA) was used for statistical analysis.
Results
Topical Treatment With NPD1 Reduces the Severity of HSV-1–Induced Corneal Immunopathology
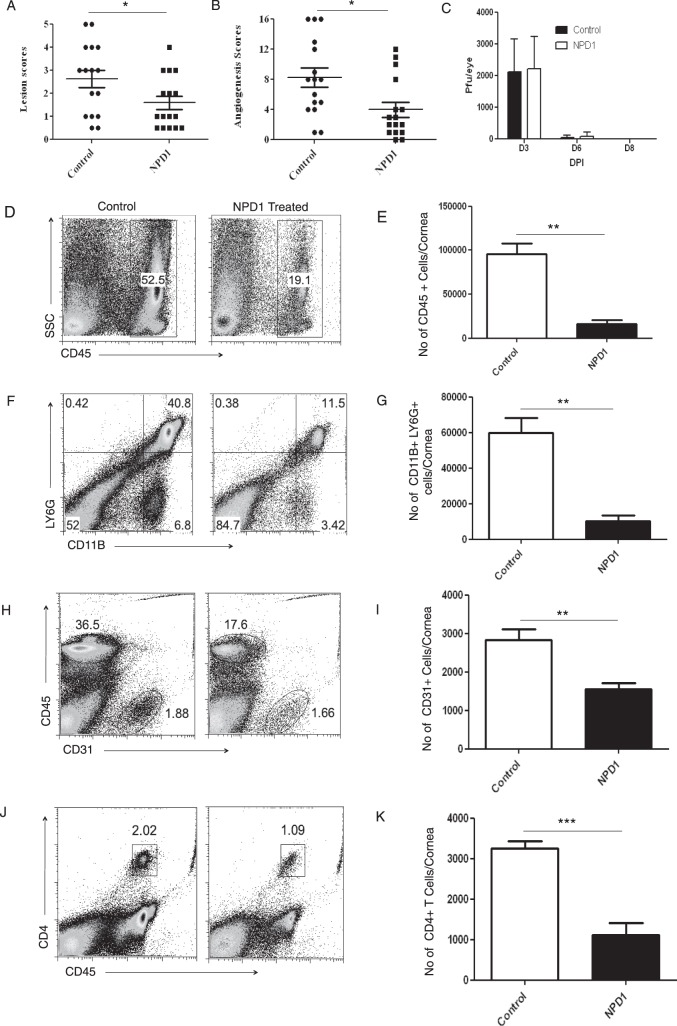
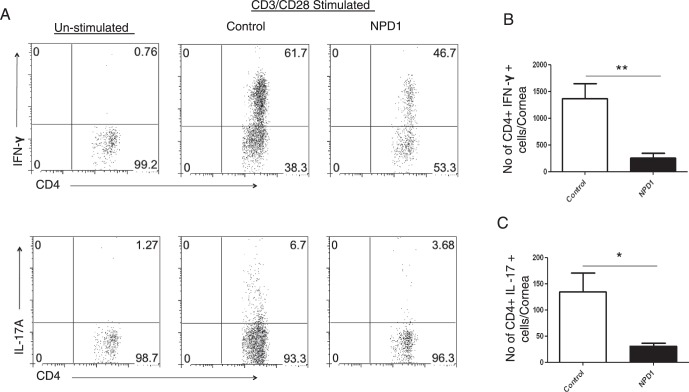
In the mouse model SK lesions become evident clinically approximately 1 week after HSV infection, at a time when the replicating virus no longer is detected in corneas.23 However, lesions continue to progress in severity.24 To investigate the effects of NPD1 treatment on SK pathogenesis, groups of animals were treated topically twice daily with the prodrug NPD1 or vehicle starting at 1 day before infection (prophylactic) or day 6 after infection (clinical phase). Animals were examined at intervals to record the severity of SK lesions, as well as the extent of corneal neovascularization. In animals where treatment was started at 1 day before infection, significant inhibition of SK lesions was observed in the NPD1-treated group compared to untreated controls (P < 0.05, Fig. 1A). Not only the frequency of lesions, but also their severity was reduced, with 25% of NPD1-treated animals showing a lesion score of ≥3 compared to 60% in control animals. Corneal neovascularization also was reduced significantly in the treated animals compared to controls (P < 0.05, Fig. 1B). Our data showed that prophylactic treatment with NPD1 starting at 1 day before infection did not affect virus load. Almost similar virus titers were observed in the NPD1-treated (starting at 1 day before infection) and control animals, and the virus was cleared completely from the eyes by day 8 after infection (Fig. 1C). At termination on day 15 pi, corneas were pooled, and isolated cells were identified and enumerated by flow cytometry. The numbers of total leukocytes (CD45+ cells), neutrophils (CD11b+Ly6G+ cells), CD31+(marker for endothelial cells), and CD4+ T cells were reduced by 83%, 82%, 45%, and 65%, respectively, in the NPD1-treated group compared to the control group, which was highly significant for all four cell populations (Figs. 1D–K). Importantly, when the frequency of subsets of CD4+ T cells producing IFN-γ or IL-17 was examined after stimulating with CD3/CD28, NPD1 treatment was shown to reduce markedly Th1 and Th17 populations by 81% and 76%, respectively (Figs. 2A–C).
Figure 1.
Effect of prophylactic treatment with NPD1 on SK severity and cellular infiltration. C57BL/6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from 1 day before infection until day 10 pi. The disease severity was examined on different days after infection. (A, B) SK lesion severity and angiogenesis scores at day 15 pi are shown. The level of significance was determined by Student's t-test (unpaired, n = 16–18 mice/group as indicated in the scatter plots). (C) At the indicated time points, eyes of HSV-infected mice were swabbed with a sterile swab and assayed for infectious virus by standard plaque assay. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM (n = 10 eyes). (D–K) The immune parameters were analyzed at the termination of the experiment (day 15 pi). Representative histograms show percentage of (D) leukocytes (CD45+), (F) neutrophils (CD11B+Ly6G+), (H) CD31+ cells, and (J) CD4+ T cells in the inflamed cornea of control and NPD1-treated animals at day 15 pi. Average numbers of (E) CD45+ cells, (G) CD11B+Ly6G+, (I) CD31+ cells, and (K) CD4+ T cells per cornea at indicated time point are shown. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM, n = 4 corneas per group. Experiments were repeated at least two times. *P ≤ 0.05. **P ≤ 0.01. ***P ≤ 0.001.
Figure 2.
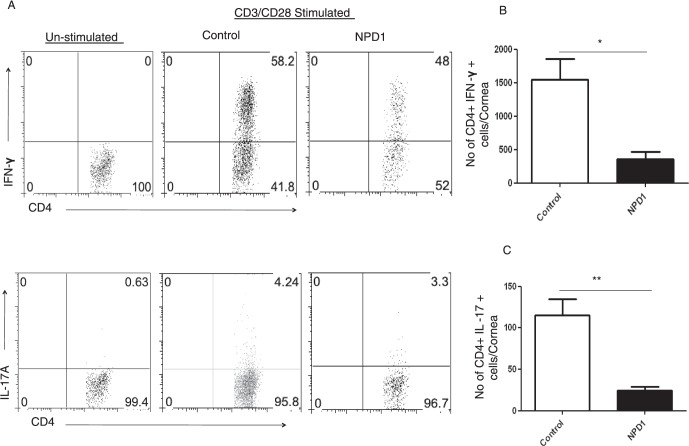
Prophylactic treatment with NPD1 reduces Th1 and Th17 cell responses in corneas of HSV-1–infected animals. C57BL/6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from 1 day before infection until day 10 pi. (A) Representative plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the corneas of control and NPD1-treated animals. Plots shown were gated on CD4+ T cells. (B, C) Average number of CD4 T cells producing (B) IFN-γ and (C) IL-17 in the cornea at day 15 are shown. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM, n = 4 corneas per group. Experiments were repeated at least two times *P ≤ 0.05. **P ≤ 0.01.
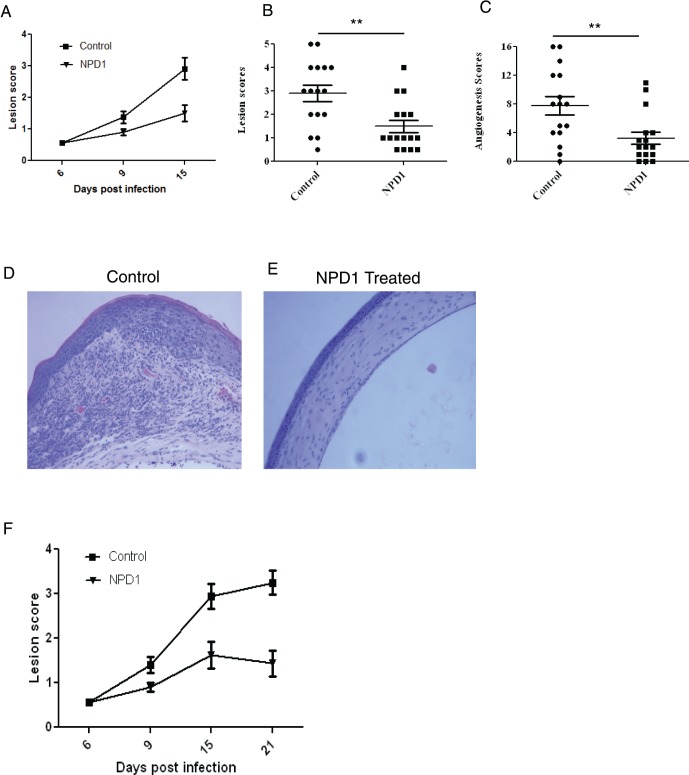
In the second series of experiments, NPD1 treatment was begun at day 6 pi, a time point when replicating virus is no longer detected on the HSV-infected corneas. This time point also corresponded to the beginning of the chronic stage of SK, with massive infiltration of pathogenic CD4+ T cells and neutrophils that orchestrate SK lesion development. The results demonstrated that, following topical administration of NPD1, significant inhibition of SK lesions was observed in the treated group compared to untreated controls (Figs. 3A–C). At the termination of experiment on day 15 pi, only 38% of corneas in the NPD1-treated group showed lesion score ≥ 2 compared to 81% in controls (Fig. 3B). In addition, lesions were more severe in untreated control mice, with a higher percentage of infected eyes exhibiting a lesion score of ≥3 than NPD1-treated mice. The extent of corneal neovascularization also was reduced significantly in the NPD1-treated animals compared to controls (Fig. 3C). Histopathologic analysis performed on the eyes of HSV-infected mice revealed that corneas from untreated mice were swollen and contained a massive infiltrate of inflammatory cells, which was essentially prevented in NPD1-treated mice (Figs. 3D–E). In some experiments, the disease progression was followed beyond day 15 pi until day 21 pi (treatment started at day 6 pi). Our results showed that there is no further progression in disease severity in the treated groups (Fig. 3F). These results demonstrated that NPD1 treatment delays the onset and prevents further progression of the disease.
Figure 3.
Topical administration of NPD1 started during clinical phase (day 6 pi) decreases SK severity. C57BL/6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from day 6 until day 12 pi. The disease severity was examined on different days after infection. (A–C) Disease progression (until day 15 pi), SK lesion severity, and angiogenesis at day 15 pi are shown. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM. Experiments were repeated at least three times, n = 16–18 mice/group as indicated in the scatter plots. (D, E) Mice were terminated at day 15 pi and eyes were processed for immunohistochemical analysis. Hematoxylin and eosin (H&E) staining was performed on 6-μm sections. The Figure shows the pictures of the sections taken at ×20 magnification. (F) Disease progression until day 21 pi. **P ≤ 0.01.
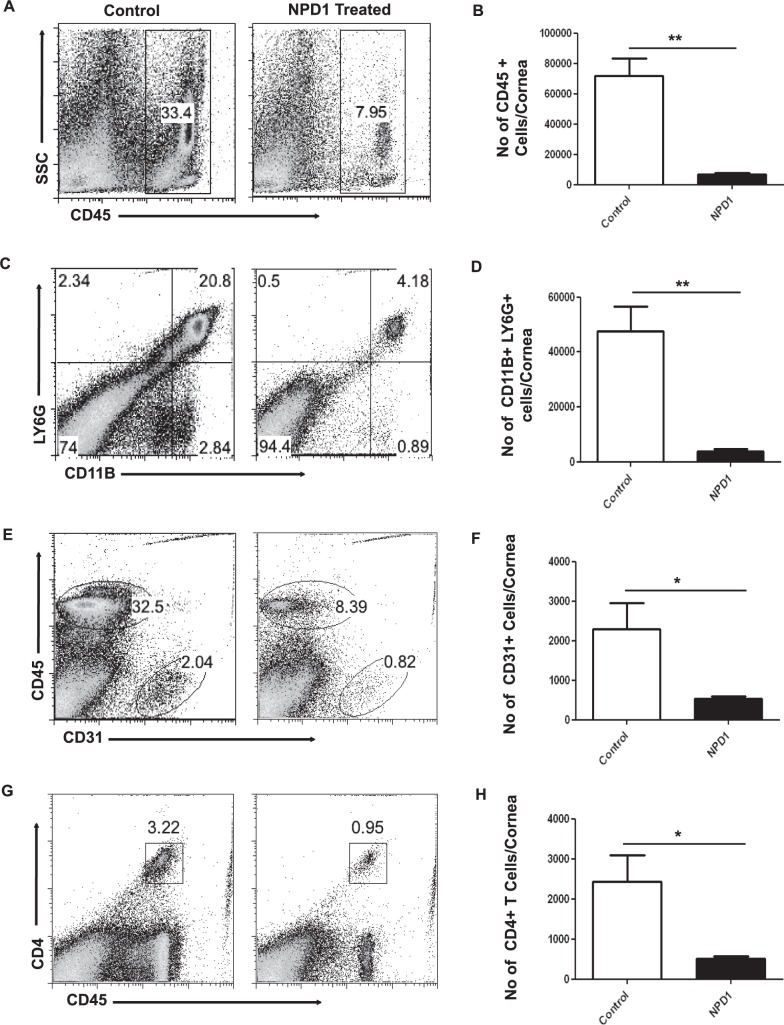
To determine further the effects of topical NPD1 therapy started at day 6 pi on the infiltration of immune cells, mice were killed at the termination of the experiment on day 15 pi. Corneas were pooled, and the isolated cells were enumerated and identified phenotypically by flow cytometry. The numbers of infiltrating leukocytes (CD45+ cells) including neutrophils (CD11B+Ly6G+ cells) in infected corneas were reduced by almost 90% in mice treated with NPD1 (Figs. 4A–D). In addition, CD31+ cells (a marker for blood vessel endothelial cells) were reduced markedly in the corneal samples obtained from NPD1-treated mice compared to control mice (Figs. 4E, 4F). The numbers of CD4+ T cells were reduced by greater than 75% in NPD1-treated mice compared to control mice (Figs. 4G, 4H). Importantly, when the numbers of CD4+ T cells producing IFN-γ or IL-17, respectively, were determined after stimulating with CD3/CD28, the NPD1-treated group showed significantly lower numbers of Th1 and Th17 cells in the cornea compared to untreated control mice (Figs. 5A–C). The numbers of Th1 and Th17 cells were reduced 4- to 5-fold as a result of NPD1 treatment.
Figure 4.
NPD1 treatment started during clinical phase (day 6 pi) reduces cellular infiltration in corneas of HSV-1–infected animals. C57BL/6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from day 6 until day 12 pi. The immune parameters were analyzed at the termination of the experiment (day 15 pi). Representative histograms show percentage of (A) leukocytes (CD45+), (C) neutrophils (CD11B+Ly6G+), (E) CD31+ cells, and (G) CD4+ T cells in the inflamed cornea of control and NPD1-treated animals at day 15 pi. Average number of (B) CD45+ cells, (D) CD11B+Ly6G+, (F) CD31+ cells, and (H) CD4+ T cells per cornea at indicated time point are shown. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM, n = 4 corneas per group. Experiments were repeated at least three times. *P ≤ 0.05. **P ≤ 0.01.
Figure 5.
Topical administration NPD1 started during clinical phase (day 6 pi) reduces Th1 and Th17 cell responses in corneas of HSV-1–infected animals. C57BL6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from day 6 until day 12 pi. (A) Representative plots show percentage of CD4+ T cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the corneas of control and NPD1-treated animals. Plots shown were gated on CD4+ T cells. (B, C) Average number of CD4 cells producing (B) IFN-γ and (C) IL-17 in the cornea at day 15 are shown. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM, n = 4 corneas per group. Experiments were repeated at least three times. *P ≤ 0.05. **P ≤ 0.01.
Effect of NPD1 Treatment on Cytokine and Chemokines Involved in SK Pathogenesis
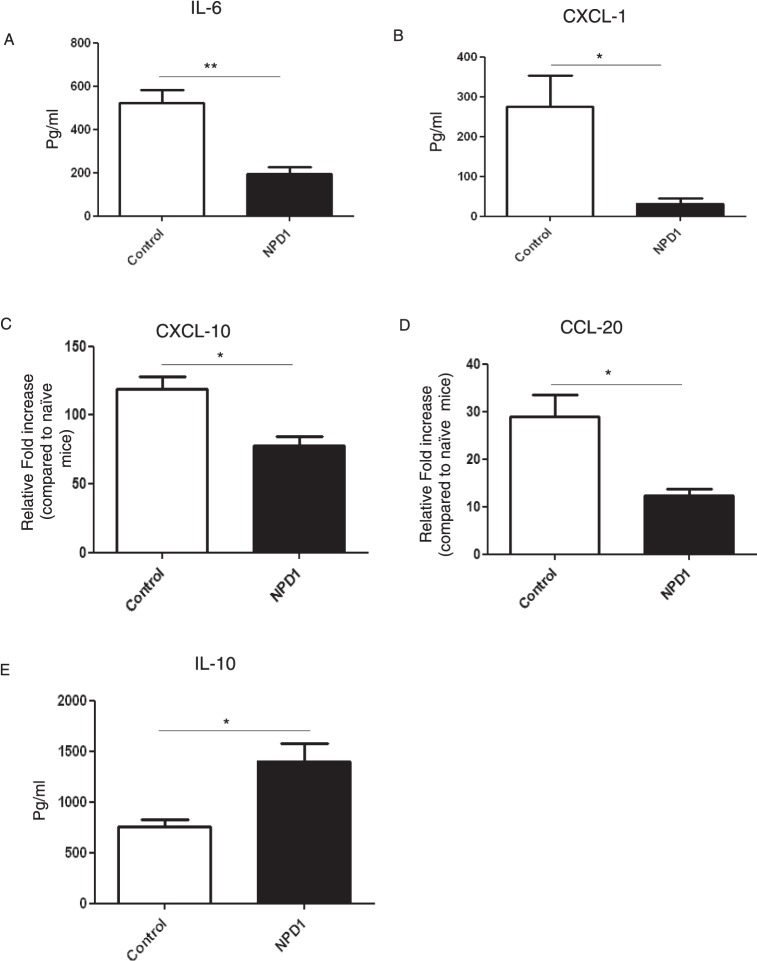
Pooled corneal extracts were collected on day 15 pi from control and from NPD1-treated animals (started at day 6 pi), and sample pools were analyzed for IL-6, CXCL1, CXCL-10, CCL-20, and IL-10 levels using ELISA or qPCR. The levels of IL-6, a key proinflammatory cytokine involved in SK immunopathology,25 were reduced by 4-fold in NPD1-treated mice compared to the control group (Fig. 6A). The levels of CXCL1, a prominent chemokine that has a major role in neutrophil recruitment, were reduced by 8-fold in the NPD1-treated group compared to untreated mice (Fig. 6B). Additionally, NPD1 treatment reduced the expression of the chemokines, CXCL-10 and CCL-20, which are involved in T cell trafficking (Figs. 6C, 6D). Notably, NPD1 administration increased the synthesis of the anti-inflammatory cytokine, IL-10, with levels approximately 2-fold higher in the corneal samples obtained from NPD1-treated mice compared to control animals (Fig. 6E).
Figure 6.
NPD1 treatment reduces the levels of cytokine and chemokines in corneas of HSV-1–infected animals. C57BL6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from day 6 until day 12. Mice were killed at day 15 pi, and levels of cytokines and chemokines were measured in three different pooled corneal samples, each consisting of five corneas/group in control and NPD1-treated animals by sandwich ELISA or QPCR. (A) IL-6 protein levels were quantified using sandwich ELISA. (B) CXCL1 levels were quantified using sandwich ELISA. (C, D) qPCR was used to measure the expression of (C) CXCL-10 and (D) CCL-20 in corneal samples obtained from control and NPD1-treated animals. (E) IL-10 protein levels were measured using sandwich ELISA. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM, n = 3 samples per group. *P ≤ 0.05. **P ≤ 0.01.
NPD1 Treatment Suppresses Proangiogenic Factors Involved in SK Pathogenesis
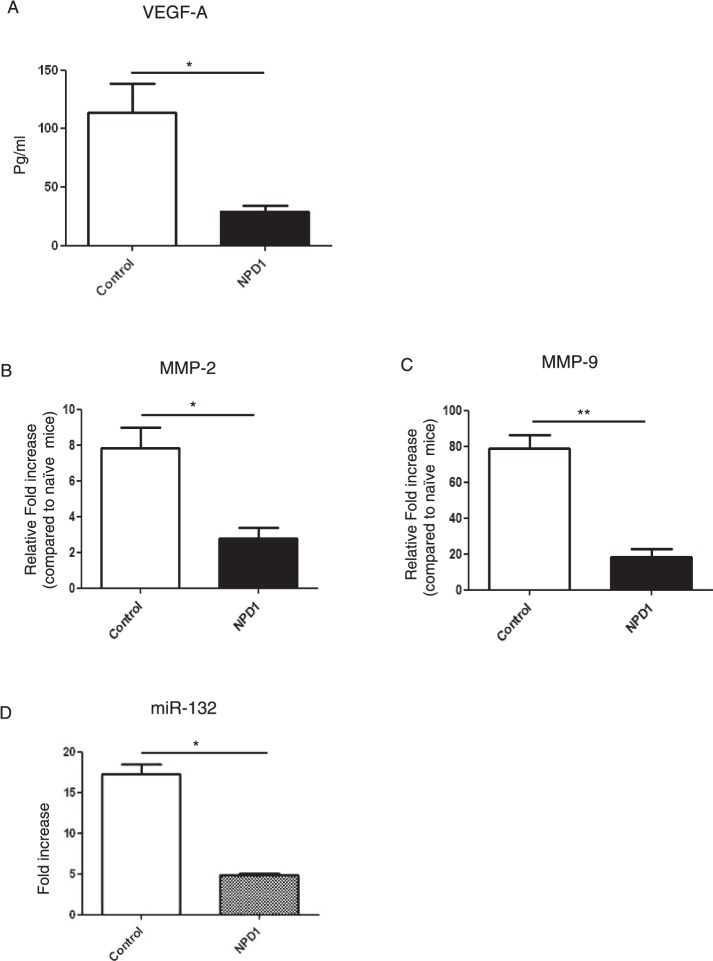
Consistent with a reduction in new vessel formation in corneas from NPD1-treated animals (started at day 6 pi), the VEGF-A levels were reduced by 4-fold (Fig. 7A). Besides VEGF-A, we also examined the expression of MMP-2 and MMP-9, molecules that also are associated with the neovascularization process in the eye.26 The qPCR data demonstrated that levels of MMP-2 were reduced by 3-fold and MMP-9 by 4-fold in corneal tissues obtained from NPD1-treated group compared to the control group (Figs. 7B, 7C). Notably, our data showed that NPD1 treatment significantly reduced the expression of miR-132, which is involved in corneal neovascularization.22 NPD1 treatment decreased the expression of miR-132 by almost 4-fold when compared to control (Fig. 7D).
Figure 7.
NPD1 treatment diminishes the levels of proangiogenic factors in corneas of HSV-1–infected animals. C57BL6 mice infected with 2 × 104 PFU of HSV-1 RE were given NPD1 topically twice daily starting from day 6 until day 12. Mice were killed at day 15 and (A) VEGF-A protein levels in three different pooled corneal samples, each consisting of five corneas/group in control and NPD1-treated animals, were quantified using quantikine sandwich ELISA kit. (B, C) qPCR was used to measure the expression of (B) MMP-2 and (C) MMP-9 in three different pooled corneal samples, each consisting of five corneas/group in control and NPD1-treated animals. (D) Mice were killed at day 15 and the expression levels of miR-132 were quantified using TaqMan qPCR in three different pooled corneal samples, each consisting of five corneas/group in control and NPD1-treated animals. The level of significance was determined by Student's t-test (unpaired). Error bars represent mean ± SEM, n = 3 samples per group. *P ≤ 0.05. **P ≤ 0.01.
Discussion
In our report, we used a novel approach to control SK lesions in a mouse model of SK using NPD1, which is biosynthesized from the omega-3 PUFA, DHA, and was shown to possess potent anti-inflammatory bioactions.6,7 Our results demonstrated that topical administration of the NPD1 prodrug diminished the severity of SK lesions by inhibiting several key events involved in SK pathogenesis. These included inhibitory effects on the trafficking of inflammatory cells, particularly neutrophils and effector CD4+ T cells, into the cornea, and inhibition of proinflammatory cytokines and chemokines. Importantly, there was an increase in the levels of IL-10, an anti-inflammatory cytokine associated with the resolution of SK lesions.27,28 NPD1 treatment also reduced corneal neovascularization, because of its inhibitory effects on the production of the proangiogenic factor, VEGF-A, and matrix metalloproteinases, MMP-2 and MMP-9, and miR-132 that are involved in corneal neovascularization.22,25,26 Thus, NPD1 therapy could represent a novel therapeutic option for the management of HSV-induced ocular inflammatory lesions.
HSV-induced stromal keratitis is an immunopathologic lesion in the corneal stroma that impairs vision and can result in blindness.3,29 Past studies in mice by our group and others have demonstrated that SK is orchestrated primarily by CD4+ T cells that are mainly of the Th1 subset with a supplementary role for Th17 cells later in the disease process.20,29 However, the actual damage to the eye and the ensuing vision impairment likely is caused by neutrophils.29,30 NPD1 treatment represents a rational approach for controlling SK lesions, since several studies have established that a key action of resolvins and protectins in accelerating resolution of an acute inflammatory event is through the prevention of neutrophil migration into inflamed tissues combined with an increased phagocytosis by macrophages of apoptotic neutrophils,8,13,15 knowing that the neutrophil is the primary cell type that dominates inflammatory reactions in SK.29,30
In our studies, we could show that the influx of neutrophils and T cells was reduced significantly after NPD1 treatment. Several present findings may explain the regulation of neutrophils and T cells in the HSV-infected cornea. Firstly, NPD1 attenuated CD4 T cell responses, which orchestrate the perpetuating immune response in SK.20,28 NPD1 reduced the expression of chemokines, such as CXCL-10 and CCL-20, which are involved in T cell migration.31 Furthermore, NPD1 treatment attenuated levels of the chemokine, CXCL1 (KC), which is a prominent regulator of neutrophil migration.32 Another critical finding in our study with NPD1 was the upregulation of IL-10, which also is considered a major regulator of immune responses and is known to attenuate SK pathogenesis.27 The production of IL-10 also could explain the efficacy of NPD1 treatment in the SK model. Another key event in regulating the severity of SK is the control of the proinflammatory cytokine IL-6.25 IL-6 not only contributes to the regulation of neutrophil migration into inflamed tissues, but also is required for expansion of Th17 cells.33 The source of IL-6 may be either leukocytic or could derive from the HSV-infected corneal cells.25
An important aspect of switching an active inflammatory event towards resolution is the efficient clearance of apoptotic neutrophils through the activation of resolution-phase macrophages.34 It is well documented that NPD1 promotes phagocytic removal of apoptotic neutrophils. In the current study, we did not specifically attempt to quantify subsets of macrophages as either of predominantly proinflammatory or proresolution phenotype, but we speculated that resolution-type macrophages could be the source of IL-10 in response to NPD1 exposure. We currently are investigating this possibility.
Corneal neovascularization is a key event in the pathogenesis of SK, with the lesion being limited in severity by procedures that inhibit the production of molecules involved in new blood vessel development.24 Our findings revealed that NPD1 inhibited the release of proangiogenic factors, such as VEGF-A, MMP-2, and MMP-9, which likely contributed to the marked reduction in corneal neovascularization in NPD1-treated mice. Moreover, the inhibition of neovascularization also may be attributed to down-regulation of miR-132 expression in corneal tissues of NPD1-treated mice. Recent findings from our group demonstrated that targeting miR-132 reduced the extent of corneal neovascularization following HSV-1 infection, suggesting a role for miR-132 in SK pathogenesis.22 In addition, NPD1 could be mediating antiangiogenesis through its inhibitory effects on proangiogenic cytokines, such as IL-6, which may act to cause VEGF production.25 Consistent with our observations, control of neovascularization by NPD1 has been demonstrated previously using the laser-induced choroidal neovascularization model,35 or in the retina, using the oxygen-induced retinopathy model.36
SK represents an important cause of vision loss in humans and current treatment modalities are not considered ideal. For example, prolonged use of corticosteroids has several side-effects that include cataract formation, increased intraocular pressure, and increased susceptibility to glaucoma, which affect vision.5 Since protectins, as well as some other groups of molecules derived from omega 3 fatty acids, such as the resolvins,37 are endogenous molecules responsible for resolving inflammatory reactions under normal circumstances, they are expected to have limited side effects and could represent a safe alternative. In our reported studies, topical application of prodrug NPD1 was found to be highly effective in reducing the severity of SK lesions. In conclusion, the use of NPD1 could represent a useful approach for the therapeutic management of virus-induced ocular inflammatory lesions.
Acknowledgments
The authors thank Tamara Veiga Parga, Fernanda Gimenez, Sid Bhela, and Leon Richardson for assistance during research and manuscript preparation.
Supported by Grant EY005093 from the National Eye Institute.
Disclosure: N.K. Rajasagi, None; P.B.J. Reddy, None; S. Mulik, None; P. Gjorstrup, Resolvyx Pharmaceuticals, Inc. (E, I, S); B.T. Rouse, None
References
- 1. Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001; 20: 1– 13 [DOI] [PubMed] [Google Scholar]
- 2. Deshpande S, Banerjee K, Biswas PS, Rouse BT. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev Mol Med. 2004; 6: 1– 14 [DOI] [PubMed] [Google Scholar]
- 3. Colin J. Ganciclovir ophthalmic gel, 0.15%: a valuable tool for treating ocular herpes. Clin Ophthalmol. 2007; 1: 441– 453 [PMC free article] [PubMed] [Google Scholar]
- 4. Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009; 54: 226– 234 [DOI] [PubMed] [Google Scholar]
- 5. McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002; 25: 33– 55 [DOI] [PubMed] [Google Scholar]
- 6. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008; 8: 349– 361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011; 111: 5922– 5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution pathways. Nature. 2007; 447: 869– 875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007; 178: 496– 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Periz A, Planaguma A, Gronert K, et al. Docosahexanenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006; 20: 2537– 2539 [DOI] [PubMed] [Google Scholar]
- 11. Duffield JS, Hong S, Vaidya V, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006; 177: 5902– 5911 [DOI] [PubMed] [Google Scholar]
- 12. Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006; 176: 1848– 1859 [DOI] [PubMed] [Google Scholar]
- 13. Serhan CN, Fredman G, Yang R, et al. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011; 18: 976– 987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ariel A, Li PL, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005; 280: 43079– 43086 [DOI] [PubMed] [Google Scholar]
- 15. Bannenberg GL, Chiang N, Ariel A, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005; 174: 4345– 4355 [DOI] [PubMed] [Google Scholar]
- 16. Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004; 101: 8491– 8496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M. Laniado Schwartzman MA. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005; 280: 15267– 15278 [DOI] [PubMed] [Google Scholar]
- 18. Biswas PS, Rouse BT. Early events in HSV keratitis–setting the stage for a blinding disease. Microbes Infect. 2005; 7: 799– 810 [DOI] [PubMed] [Google Scholar]
- 19. Kim B, Sarangi PP, Lee Y, Deshpande S, Lee S, Rouse BT. Depletion of MCP-1 increases development of herpetic stromal keratitis by innate immune modulation. J Leukocyte Biol. 2006; 80: 1405– 1415 [DOI] [PubMed] [Google Scholar]
- 20. Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992; 149: 3035– 3039 [PubMed] [Google Scholar]
- 21. Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus induced ocular inflammatory lesions with the lipid derived mediator Resolvin E1. J Immunol. 2011; 186: 1735– 1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulik S, Xu J, Reddy PB, et al. Role of microRNA-132 in angiogenesis after ocular infection with herpes simplex virus. Am J Pathol. 2012; 181: 525– 534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rajasagi NK, Suryawanshi A, Sehrawat S, et al. Galectin-1 reduces the severity of herpes simplex virus-induced ocular immunopathological lesions. J Immunol. 2012; 188: 4631– 4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gimenez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis–a focus on corneal neovascularization. Prog Retin Eye Res. 2012; 33: 1– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp Eye Res. 2006; 82: 46– 54 [DOI] [PubMed] [Google Scholar]
- 26. Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Invest. 2002; 110: 1105– 1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarangi PP, Sehrawat S, Suvas S, Rouse BT. IL-10 and natural regulatory T cells: two independent anti-inflammatory mechanisms in herpes simplex virus-induced ocular immunopathology. J Immunol. 2008; 180: 6297– 6306 [DOI] [PubMed] [Google Scholar]
- 28. Tumpey TM, Elner VM, Chen SH, Oakes JE, Lausch RN. Interleukin-10 treatment can suppress stromal keratitis induced by herpes simplex virus type 1. J Immunol. 1994; 153: 2258– 2265 [PubMed] [Google Scholar]
- 29. Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Prog Retin Eye Res. 2013; 32: 88– 101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997; 158: 1383– 139 [PubMed] [Google Scholar]
- 31. Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008; 9: 970– 980 [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008; 13: 2400– 2407 [DOI] [PubMed] [Google Scholar]
- 33. Steinman L. A brief history of Th17, the first major revision in the Th1/Th2 hypothesis of T-cell mediated tissue damage. Nat Med. 2007; 13: 139– 145 [DOI] [PubMed] [Google Scholar]
- 34. Bystrom J, Evans I, Newson J, et al. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 2008; 112: 4117– 4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheets KG, Zhou Y, Ertel MK, et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010; 16: 320– 329 [PMC free article] [PubMed] [Google Scholar]
- 36. Connor KM, Paul J, Giovanni S, et al. Increased dietary intake of ω-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 13: 2007; 868– 873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Navarro-Xavier RA, Newson J, Silveira VLF, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J Immunol. 2010; 184: 1516– 1525 [DOI] [PubMed] [Google Scholar]