Abstract
Background
Combat-related PTSD has been associated with reduced gray matter volume in regions of the prefrontal and temporal cortex, hippocampus, insula, and amygdala. However, the relationship between gray matter volume and specific deployment and post-deployment experiences has not been investigated. The aim of this study was to delineate how such experiences may contribute to structural brain changes for combat veterans.
Methods
Operation Iraqi Freedom/Operation Enduring Freedom veterans (N = 32) completed magnetic resonance imaging, the Deployment Risk and Resilience Inventory, Alcohol Use Disorders Identification Test, and Clinical Administered PTSD Scale. Voxel-wise Huber robust multiple regressions were used to quantify the relationship between gray matter volume and deployment experiences (combat experiences, military social support) and post-deployment symptoms (PTSD, alcohol use).
Results
There was an interaction between severity of combat experiences and military social support for orbitofrontal gyrus gray matter volume. Specifically, individuals with more orbitofrontal gyrus gray matter volume reported less combat experiences and higher unit support. Individuals with more severe PTSD symptoms showed reduced gray matter volume within a large temporal region (inferior temporal and parahippocampal gyrus).
Conclusions
The identified association between unit support and orbitofrontal gyrus volume supports two potential resilience mechanisms to be delineated with future longitudinal studies. First, individuals with larger orbitofrontal gyrus may engage in greater quality of social interactions and thus experience combat as less stressful. Second, individuals who experience greater unit support may preserve a larger orbitofrontal gyrus, serving to “protect” them from aversive consequences of combat.
Introduction
Recent wars and political conflicts have exposed United States military men and women to increased levels of combat trauma. These experiences often lead not only to posttraumatic stress disorder (PTSD) but also to other highly comorbid mental health conditions, such as alcohol use disorders or major depressive disorders. It is estimated that approximately 20% of military veterans develop PTSD [1], [2] while approximately 7–10% develop alcohol use disorders and 17% develop major depressive disorder [1], [3]. Previous research has established a relationship between severity and amount of traumatic experiences (e.g., combat or other previous trauma) with subsequent development of PTSD and other mental health symptoms [4]–[9]. However, recent research suggests that there may be other experiences that may influence the likelihood or severity of subsequent mental health disorders. With respect to combat-related PTSD, identified resiliency factors include quality of non-combat deployment experiences (i.e., unit support or cohesion), as well as family and social support, among others [4], [5], [9]–[12].
Magnetic resonance imaging (MRI) has been helpful in delineating how trauma experiences and PTSD may relate to structure of various brain regions. PTSD has repeatedly been associated with reduced volume of hippocampal regions (see recent meta-analyses, [13], [14]), which may relate to severity of combat experiences [15] and/or severity of PTSD symptoms [16]. Studies have also identified reduced amygdala volume for PTSD versus non-PTSD with and without trauma exposure [14], though findings with this region have not been as consistently reported. Functional activation of the insula cortex (and its role in monitoring internal bodily states) has been increasingly implicated in PTSD and other anxiety disorders [17] and recent studies have also associated PTSD with reduced gray matter volume in this region [18]–[20]. Studies have additionally identified decreased volume within the temporal cortex and several frontal cortical regions, including anterior cingulate, orbitofrontal, middle frontal, and inferior frontal regions, for PTSD compared to non-PTSD groups [14], [21]–[24].
While there has been a plethora of studies focused on identifying structural abnormalities related to trauma exposure or PTSD, there has been a relative lack of research examining the impact of other factors, such as comorbid symptoms or factors related to resiliency. Alcohol use disorders in particular have been associated with structural differences that overlap with those identified for PTSD – including reduced volume within cortical regions, hippocampus, amygdala, and insula, as well as striatal regions [25]–[28]. However, a recent meta-analysis suggests that at least the hippocampal volumetric differences in PTSD cannot be fully attributed to alcohol use disorders [29].
Several investigators [30]–[34] have proposed that combat trauma and PTSD do not occur in a vacuum and that other life experiences – either good or bad, recent or long past – influence neural development and post-traumatic behavioral responses. The current study was designed to investigate how risk and resiliency factors related to deployment experiences and post-deployment symptoms of Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) veterans relate to differences in gray matter volume. Specifically, we utilize MRI and voxel-based methods to investigate gray matter volumes related to the severity of combat experiences and deployment-related social support, as well as to post-deployment PTSD symptoms and level of alcohol use. In light of previous literature, we hypothesized that combat experiences and PTSD symptoms would relate to reduced hippocampal, amygdala, insula, and temporal and frontal cortical volumes. Further, we hypothesized that greater quality of deployment social support would be a protective factor, serving as a moderator to reduce these volumetric effects. Lastly, we hypothesized that level of current alcohol use would relate to further volumetric reductions in hippocampal, amygdalar, and cortical regions as well as to volumetric reductions in striatal regions. Greater understanding of how these risk and resiliency factors relate to structural brain differences could provide insight concerning the development of post-traumatic mental health symptoms and potential strategies for prevention and treatment.
Materials and Methods
Ethics Statement
All participants were treated in accordance with the Declaration of Helsinki and written informed consent was obtained at the initial study session after full explanation of study procedures. The University of California – San Diego (UCSD) Human Research Protections Program and the Veterans Affairs San Diego Healthcare System Research and Development Office approved the study and all procedures were completed at these institutions.
Participants
Thirty-two veterans (all male; mean age 29.19, SD = 6.65) who reported experiencing combat as part of OIF/OEF volunteered for the current study. Participants were excluded if they reported experiencing significant head trauma and/or moderate to severe symptoms associated with traumatic brain injury (>30 minutes loss of consciousness or >1 day posttraumatic amnesia), significant suicidal ideation or otherwise unstable psychiatric symptoms, diagnoses of bipolar disorder or schizophrenia, irremovable ferromagnetic bodily material, or medical conditions contraindicated for MRI scanning. Other Axis I disorders, such as major depressive disorder and alcohol and substance abuse and dependence, were not specifically excluded. Fifteen individuals met current diagnostic criteria for PTSD; 9 for major depressive disorder; 1 for alcohol dependence, 3 for alcohol abuse, and 3 for other substance dependence (amphetamine, cocaine, THC). Nine individuals reported current psychotropic medications. Of these individuals, seven reported prescriptions for antidepressants (SSRIs, Bupropion, Amitriptyline), two reported prescriptions for sedatives to help with sleep (Eszopiclone, Zolpidem), one reported using a benzodiazepine as needed (Lorazepam), one reported daily use of methylphenidate, and one reported a prescription for Valproic Acid for anger symptoms.
Participants completed an MRI scan as well as the Clinician-Administered PTSD Scale (CAPS [35]), Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA-II [36]), Patient Health Questionnaire (PHQ[37], Alcohol Use Disorders Identification Test (AUDIT [38]), and the Deployment Risk and Resiliency Inventory (DRRI [39]).
Behavioral Analyses
For deployment variables of interest, we focused on the subscales of “combat experiences” (higher score indicates greater exposure to combat experiences) and “social support” (higher scores indicate greater quality of support and encouragement from the military, unit leaders, and unit members). For post-deployment variables of interest, we focused on PTSD symptoms, as measured by the CAPS, and alcohol use symptoms, as measured by the AUDIT. We focused on these aspects of post-deployment mental health due to the high prevalence of PTSD and alcohol use disorders reported among OEF/OIF veterans. While major depressive disorder is also highly prevalent among this population, high overlap and correlations between PTSD (CAPS) and depressive symptom (PHQ) measures precluded including both within regression analyses.
PTSD diagnosis was determined using the CAPS while alcohol/substance abuse and dependence and major depressive disorder were diagnosed using the SSAGA-II. Total severity scores were calculated for the CAPS (total of frequency and intensity scores across all symptom clusters), AUDIT, PHQ, and DRRI combat experiences and deployment social support subscales. The relationship between each of these variables of interest was investigated using Spearman's correlation analyses. In addition, three Huber robust regression analyses [40] (conducted using R Statistical package [41]) were used to investigate interaction effects of combat experiences and deployment social support on (1) PTSD symptoms (CAPS score), (2) depressive symptoms (PHQ score), and (3) level of alcohol use (AUDIT score). One participant was excluded from the post-deployment analyses involving the PHQ due to not completing this self-report questionnaire (leaving N = 31). In addition, three individuals had significantly higher AUDIT scores (score>19, with all other participants having scores<10). Thus, post-deployment analyses involving this measure were computed with these outliers removed (leaving N = 29). Results were considered significant at p<0.05.
MRI Data Acquisition and Analyses
All scanning was conducted on a 3T GE (Milwaukee, WI) scanner at the UCSD Center for Functional MRI. High-resolution sagittal T1-weighted FSPGR anatomical images were acquired (TE = 2.976 ms, TR = 7.772 ms, flip angle 12°, slice thickness 1.0 mm, matrix 256×256×172).
The high-resolution FSPGR images were subjected to the FSL implementation [42] of voxel-based morphometry [43], [44]. The images were initially brain-extracted using AFNI's [45] 3dSkullStrip followed by manual editing to remove any remaining non-brain material. The resultant voxels were then segmented in to grey, white and cerebrospinal fluid components using FAST4 [46]. These were then warped to MNI152 standard space using the affine transform implemented in FLIRT [47], [48]. Further refinement of the alignment was accomplished using the non-linear transform implemented in FNIRT [49], [50]. The warped data was then averaged to create a study-specific template to which the native grey matter images were then non-linearly registered. The registered partial volume images were then multiplied by the Jacobian of the warp field [44]. This compensates for any expansion or contraction due to the non-linear part of the transformation (http://dbm.neuro.uni-jena.de/vbm/segmentation/modulation/) thus obviating the need to correct for total intracranial volume [51] and permitting inference on the local GM volume changes. The modulated segmented images were then smoothed with an isotropic Gaussian kernel (σ = 2 mm–4.7 mm FWHM).
The grey matter maps were then entered into whole-brain voxel-wise Huber robust multiple regression analysis [40], [52] in the R statistical analysis package [41]. Two voxelwise regressions were conducted depending on the time period relative to combat. With respect to deployment-related experiences, main and interaction effects of combat experiences and social support subscales were investigated. For post-deployment experiences, main effects of CAPS and AUDIT severity scores were investigated. As with behavioral analyses, one participant was excluded from the post-deployment regression analyses due to not completing the self-report questionnaires. Due to the fact that three individuals had significantly higher AUDIT scores, post-deployment analysis was computed with these outliers removed. The voxelwise regression coefficients and associated t statistics were split into separate maps of positive and negative coefficients. To guard against false positives, significant voxels were required to pass a voxel-wise statistical threshold of p<0.005 and were further required to be part of a cluster of no less than 360 µl. This resulted in the following t statistic thresholds: during combat: t(28) = 3.05 and after combat: t(26) = 3.07. The volume criterion was derived from Monte Carlo simulation that together with the voxel-wise threshold resulted in a 5% probability of a cluster surviving due to chance.
Results
Behavioral Results
As expected, level of self-reported combat experiences related positively to both PTSD symptoms (CAPS score) and depressive symptoms (PHQ score). In addition, level of PTSD symptoms (CAPS) correlated significantly with level of depressive symptoms (PHQ). See Table 1 for full list of correlations amongst these measures. Regression analyses identified no significant interaction effect for combat experiences and deployment social support on PTSD symptoms (CAPS; t(28) = .973, β = −.106, p = .339) or alcohol use (AUDIT; t(25) = −.65, β = −.010, p = .519), but identified a trend effect on depressive symptoms (PHQ score; t(27) = 1.86, β = 0.05 p = .074) in which social support was protective against depressive symptoms in the case of low combat experiences but not high (see Figure 1).
Table 1. Correlations between deployment experiences and post-deployment clinical symptoms.
| Social Support | CAPS | AUDIT | PHQ | |||||
| M | SD | M | SD | M | SD | M | SD | |
| 38.87 | 10.86 | 38.84 | 29.79 | 5.68 | 5.97 | 8.39 | 6.38 | |
| rho | p | rho | p | rho | p | rho | p | |
| DRRI combat experiences | .256 | .157 | .399* | .024 | −.084 | .664 | .362* | .045 |
| DRRI deployment social support | −.228 | .209 | .087 | .653 | −.070 | .709 | ||
| CAPS total score | −.228 | .234 | .816* | <.001 | ||||
| AUDIT total score | −.350 | .068 | ||||||
Note: Statistics reported are from Spearman's rho correlation analyses with N = 30 combat veterans from Operation Iraqi Freedom or Operation Enduring Freedom (OIF/OEF). One participant did not complete the PHQ, leaving N = 31 for these analyses. Three participants representing outliers in AUDIT scores were removed from analyses involving the AUDIT, leaving N = 29 for these analyses. DRRI = Deployment Risk and Resilience Inventory; CAPS = Clinician Administered PTSD Scale; AUDIT = Alcohol Use Disorders Identification Test; PHQ = Patient Health Questionnaire; * = significant at p<.05.
Figure 1. Interaction between deployment social support and combat experiences regarding post-deployment depressive symptoms.
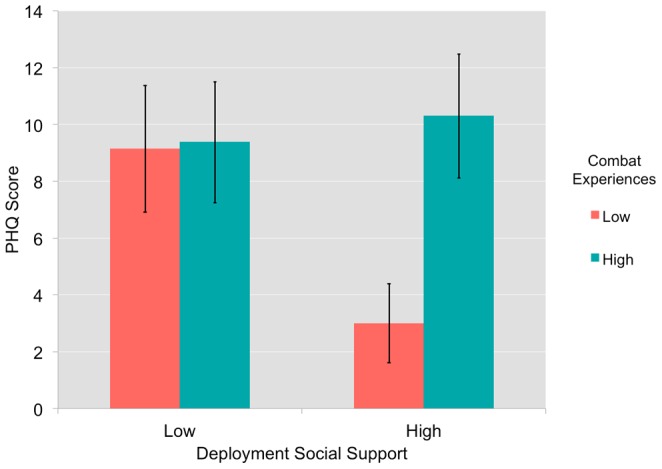
There was trend interaction effect for scores on the deployment social support and combat experiences subscales of the Deployment Risk and Resilience Inventory (DRRI) on depressive symptoms as measured by the Patient Health Questionnaire (PHQ), with a greater level of social support relating to less depressive symptoms for those with low combat experiences (t(27) = 1.86,two-tailed p = .074).
MRI Results
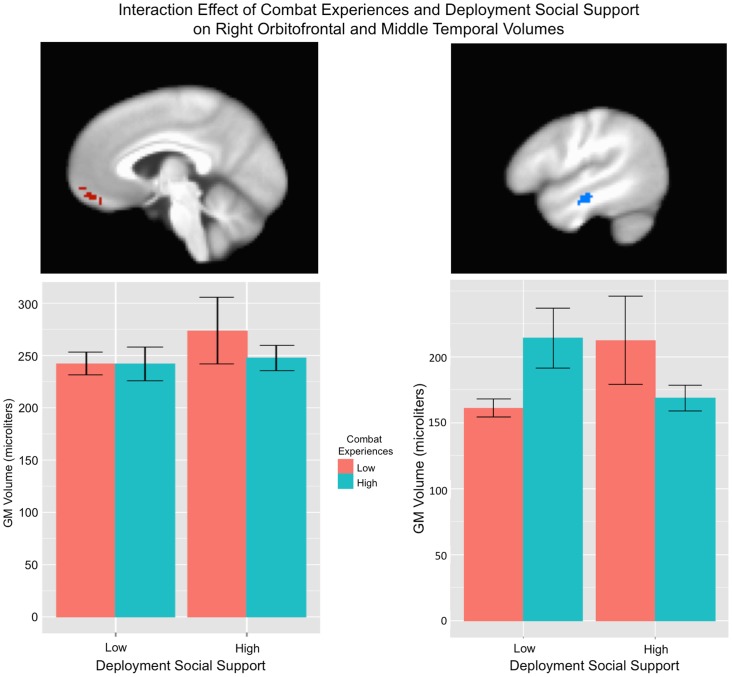
Results for deployment and post-deployment regression analyses are listed in Tables 2 and 3. There was an interaction effect of combat experiences and unit support on gray matter volume within the right orbital frontal gyrus (BA 11) and left precuneus (BA 7) in which high social support combined with low combat experiences related to greater volume. There was also an interaction effect within the right middle temporal gyrus (BA 22) in which high social support in combination with low combat experiences, or low social support in combination with high combat experiences related to greater volume (See Figure 2). For deployment experiences main effects, level of combat experiences related negatively to gray matter volume within the orbital frontal gyrus (BA 11; see Figure 3) whereas level of unit support related positively to right inferior occipital gyrus (BA 19) and negatively to right orbital frontal (BA 10/11), left precuneus (BA 7) and left cerebellum.
Table 2. Results from regression analyses investigating relationship between deployment experiences and gray matter volume.
| Structure | Side | BA | Grey Matter/Voxel | Volume (µL) | Coordinates | t | coeff | ||
| Term: Combat Experiences. Polarity Positive | |||||||||
| No Clusters found | |||||||||
| Term: Combat Experiences. Polarity Negative | |||||||||
| Orbital Frontal Gyrus | R | 11 | 0.47±0.12 [0.29–0.81] | 656 | 6 | 49 | −20 | −3.73 | −0.069 |
| Term: Deployment Social Support. Polarity Positive | |||||||||
| Inferior Occipital Gyrus | R | 19 | 0.56±0.17 [0.25–1.15] | 400 | 46 | −81 | −2 | 3.61 | 0.020 |
| Term: Deployment Social Support. Polarity Negative | |||||||||
| Orbital Frontal Gyrus | R | 10 | 0.45±0.08 [0.30–0.70] | 496 | 6 | 54 | −9 | −3.69 | −0.015 |
| Cerebellum | L | 0.80±0.25 [0.42–1.27] | 440 | −33 | −38 | −34 | −3.78 | −0.030 | |
| Precuneus | L | 7 | 0.50±0.13 [0.33–0.87] | 392 | −17 | −76 | 41 | −4.14 | −0.020 |
| Term: Interaction. Polarity Positive | |||||||||
| Orbital Frontal Gyrus | R | 10 | 0.44±0.08 [0.28–0.67] | 560 | 6 | 52 | −10 | 3.74 | 0.002 |
| Precuneus | L | 7 | 0.50±0.13 [0.33–0.86] | 384 | −17 | −75 | 41 | 3.74 | 0.002 |
| Term: Interaction. Polarity Negative | |||||||||
| Middle Temporal Gyrus | R | 21 | 0.48±0.13 [0.30–0.89] | 376 | 48 | −15 | −13 | −3.57 | −0.002 |
Note: Gray matter volume determined using FSL implementation of voxel-based morphometry. Average t statistics and unstandardized regression coefficients were extracted for significant clusters of activation. Deployment experiences included scores on the combat experiences and deployment social support subscales of the Deployment Risk and Resilience Inventory. Analyses were partial regressions investigating main and interaction effects of each predictor with N = 32 combat veterans from Operation Iraqi Freedom or Operation Enduring Freedom (OIF/OEF).
Table 3. Results from regression analyses investigating relationship between post-deployment symptoms and gray matter volume.
| Structure | Side | BA | Grey Matter/Voxel | Volume (µL) | Coordinates | t | β | |||
| Term: AUDIT Score Polarity: Positive | ||||||||||
| Thalamus | L | 0.52±0.08 [0.37–0.68] | 552 | 7.5 | 27.3 | 11.7 | 3.62 | 0.019 | ||
| Term: AUDIT Score Polarity: Negative | ||||||||||
| Precentral Gyrus | R | 6 | 0.52±0.09 [0.35–0.74] | 472 | −7.3 | 11.2 | 66.3 | −3.47 | −0.024 | |
| Postcentral Gyrus | L | 5 | 0.35±0.08 [0.21–0.53] | 440 | 2.7 | 38.9 | 66.4 | −3.44 | −0.020 | |
| Superior/MiddleTemporal Gyrus | L | 21/22 | 0.51±0.10 [0.32–0.77] | 432 | 57.9 | 7.6 | −1.6 | −3.62 | −0.025 | |
| Term: CAPS Score Polarity: Positive | ||||||||||
| Cuneus | R | 18 | 0.52±0.09 [0.35–0.73] | 552 | −1.5 | 86.1 | 13.2 | 3.45 | 0.002 | |
| Term: CAPS Score Polarity: Negative | ||||||||||
| Inferior Temporal, Fusiform and Parahippocampal Gyrus | R | 20 | 0.55±0.09 [0.39–0.73] | 3608 | −38.2 | 30.4 | −29.5 | −3.82 | −0.003 | |
| Cerebellar Tonsil/Culmen | L | 0.67±0.10 [0.48–0.93] | 472 | 31.7 | 53.3 | −30.9 | −3.51 | −0.002 | ||
| Inferior Temporal Gyrus | L | 20 | 0.58±0.11 [0.37–0.85] | 392 | 30.3 | 5.8 | −45.1 | −3.53 | −0.003 | |
| Middle Frontal Gyrus | R | 8 | 0.50±0.10 [0.35–0.73] | 368 | −42.6 | −13.8 | 44.7 | −3.76 | −0.003 | |
Note: Gray matter volume determined using FSL implementation of voxel-based morphometry. Average t statistics and unstandardized regression coefficients were extracted for significant clusters of activation. Analyses were partial regressions investigating main effects of each predictor with N = 29 combat veterans from Operation Iraqi Freedom or Operation Enduring Freedom (OIF/OEF). CAPS = Clinician Administered PTSD Scale; AUDIT = Alcohol Use Identification Test.
Figure 2. Interaction between combat experiences and deployment social support regarding gray matter volume.
Deployment experiences were measured using the Deployment Risk and Resilience Inventory (DRRI). There was a significant interaction effect on gray matter volume between the subscales corresponding to level of combat experiences and deployment social support within the right orbitofrontal gyrus (BA 11; shown at x = 4) and right middle temporal gyrus (BA 22; shown at x = 48). For the right orbitofrontal gyrus, high social support combined with low combat experiences related to greater volume. For the middle temporal gyrus, high social support in combination with low combat experiences, or low social support in combination with high combat experiences related to greater volume. Shaded areas represent 95% confidence intervals. See Table 2 for statistical results.
Figure 3. Greater combat experiences are associated with reduced gray matter volume in the orbitofrontal gyrus.
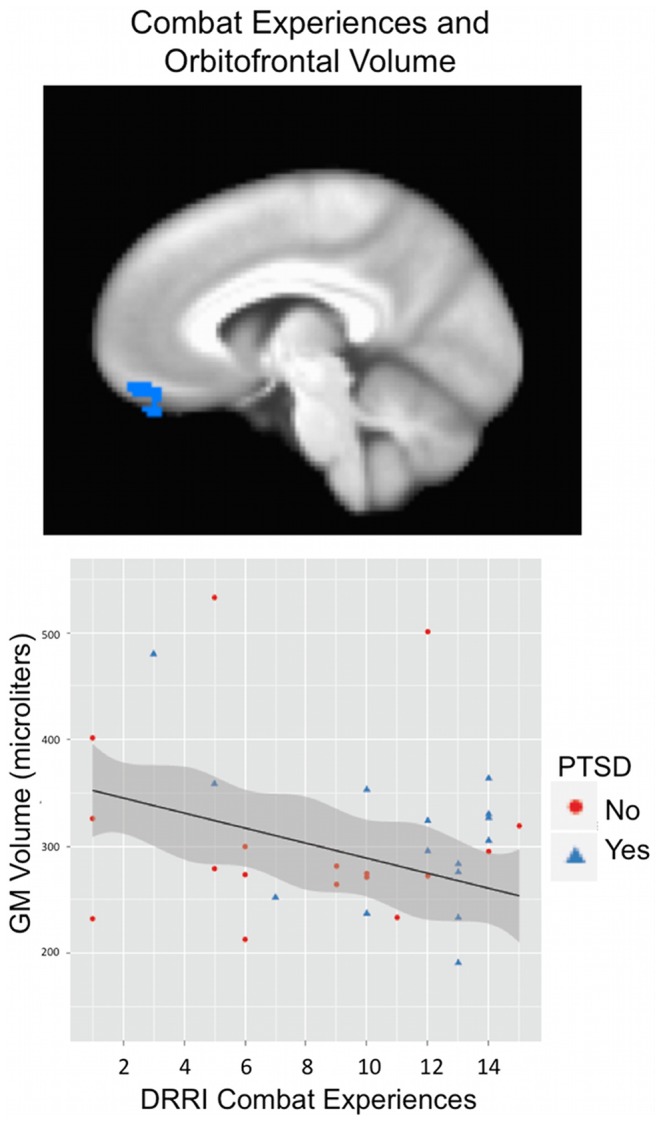
Deployment experiences were measured using the Deployment Risk and Resilience Inventory (DRRI). Scores on the subscale corresponding to level of combat experiences related negatively to gray matter volume within the orbital frontal gyrus (BA 11; shown at x = 6; see Table 2 for statistical results).
Due to three individuals having significantly higher AUDIT scores, the post-deployment fMRI analysis was computed with these outliers removed. CAPS score exhibited a positive relationship with gray matter volume in right cuneus (BA 18) and negative relationships with right inferior temporal gyrus (BA 20; spreading to parahippocampal and fusiform gyri), left inferior temporal gyrus (BA 20), left cerebellar tonsil and tuber, and right middle frontal gyrus (BA 8). See Figure 4. AUDIT score was positively related to left thalamus and negatively related to right precentral gyrus (BA 6), left postcentral gyrus (BA 5), and left superior/middle temporal gyrus (BA 21/22). See Table 3.
Figure 4. Individuals with more PTSD symptoms show reduced gray matter volume within the inferior temporal gyrus.

PTSD symptoms were measured using the total severity score from the Clinician Administered PTSD Scale (CAPS). Greater severity score on the CAPS related to reduced gray matter volume in the right inferior temporal gyrus (BA 20; spreading to parahippocampal and fusiform gyrus; shown at x = 42). See Table 3 for statistical results.
Discussion
In this study, we investigated how PTSD risk and resiliency factors relate to gray matter volume. We provide evidence that both negative (i.e., combat) and positive (i.e., unit support) deployment-related experiences relate to regional brain volume – particularly within orbitofrontal and temporal cortices. Severity of PTSD also related to reduced temporal cortex volume. Aspects of these results support study hypotheses, while others were unexpected and lead to the generation of new questions and hypotheses for future research.
We report greater combat experiences (though not PTSD symptoms) to be associated with reduced OFG volume (Figure 3). This is consistent with results of Eckart et al. [23] showing reduced bilateral OFG volume with greater extent of traumatization. Orbitofrontal regions have been proposed to play a role in object identification [21], learning of stimulus-reward associations and value-based decision-making [53], [54] or more generally for processing salient stimuli [55]. OFG volume and functioning may therefore be important for recognizing the significance of stimuli (particularly rewarding stimuli) to produce appropriate responses. OFG dysfunction could perhaps lead to generalization of affective responses to trauma-related, but currently non-relevant, stimuli (e.g., reduced contextualization) or to reduced reward processing – both of which have been implicated in trauma and PTSD [56]–[58]. Decreased OFG volume has been identified in combat-related PTSD [21] and child-abuse related PTSD [59], and has been reported to relate to severity of PTSD symptoms post-earthquake [60]. In the latter study, OFG gray matter volume post-trauma, but not pre-trauma, was related to PTSD symptoms after an earthquake – suggesting that differences in OFG volume may be acquired [60]. However, trait anxiety has been associated with reduced orbitofrontal volume as well, suggesting this could be a predisposing factor for the development of anxiety disorders, such as PTSD [61]. Future longitudinal studies are needed to establish a causal relationship between trauma and OFG volume or vice versa.
Perhaps the most unique finding of the current study was the interaction effect in which high deployment-related social support seemed to have a protective effect on OFG volume in the case of lower levels of combat experiences (Figure 2). This supports the idea that social support may exert protective effects in certain situations via influences on brain structure. While greater combat experience leads to greater risk of PTSD, combat veterans with a wide variety of severity, frequency, and type of combat experiences can develop mental health problems. We therefore believe that identifying potential mechanisms for resiliency – even in the context of lower combat experiences – is of importance. One possible interpretation of these results is that individuals with larger OFG volume seek greater social support prior to deployment or experience higher quality social interactions, and are, therefore, less impacted by combat exposure. Alternatively, it is possible that greater social support or quality of social interactions could result in greater preservation of OFG volume post-combat. Interestingly, orbitofrontal regions have been implicated in the processing of social signals and in social decision-making – perhaps relating to its role in reward processing [62]. This supports the plausibility that social aspects of individual's experiences could influence OFG function and structure. However, to our knowledge this is the first study that has directly assessed the influence of non-combat related (and positive) aspects of deployment on gray matter volume. Support for the proposition that some experiences could play a protective role concerning trauma effects on the brain is a promising and exciting one that should be followed up in future longitudinal investigations. Identifying the neural mechanisms for resiliency could help in understanding which individuals may benefit the most from programs targeting different aspects of resiliency.
A novel interaction effect of combat experiences and social support on middle temporal volume was also identified, in which high social support seemed to have a positive effect on volume in the case of low combat experiences (similar to OFG results) but a negative effect on volume in the case of high combat experiences (Figure 2). The latter relationship was not expected, but could be interpreted as high social support (or unit cohesion) being detrimental for this region in some situations (e.g., high unit cohesion when many soldiers within the unit were killed or injured, could have a greater impact on the individual).
We additionally found that level of PTSD symptoms related to reduced volume in a large right temporal region (including inferior temporal and parahippocampal, and fusiform gyri; Figure 4) and left inferior temporal gyrus. While we did not find the hypothesized relationship between PTSD symptoms and hippocampal volume, these temporal regions are highly interconnected with the hippocampus [63]. Further, reduced volume in middle or other temporal regions has been repeatedly found in previous studies as relating to PTSD diagnosis or symptom severity [20], [21], [64], [65]. The parahippocampal gyrus has been associated with representation of space and spatial context and perhaps contextual associations in general [66]–[70]. Inferior temporal and fusiform regions have been associated with visual object identification, visual associative or working memory, and face processing [71]–[77]. Thus, current results for both combat experiences and PTSD symptoms, may indicate a role for a system (orbitofrontal, temporal, parahippocampal) involved in effectively identifying salient objects within a broader visual, spatial, or memory-based context, as has been suggested by previous researchers [21].
We also attempted to identify regions for which gray matter volume related to level of alcohol use (AUDIT score). However, our sample in general had relatively low levels of alcohol use (most AUDIT scores<10), thus limiting generalizability to more significant alcohol abuse. Greater levels of alcohol use were related to reduced left superior/middle temporal volume, suggesting that comorbid alcohol use may relate to further reduced volume in temporal regions above and beyond that related to PTSD. Further research with PTSD populations with and without alcohol abuse or dependence could help clarify how these often comorbid disorders relate to differences in gray matter volumes.
Limitations
This study is one of the first to examine the relationship between gray matter volume and deployment-related risk and resilience factors. However, a primary limitation was the cross-sectional nature of the study, which prevented determination of causal relationships between deployment-related experiences, clinical symptomatology, and gray matter volume. Because of the relatively continuous distribution of PTSD symptom levels in this combat-exposed population, we chose to focus on within-subjects analyses (investigating the relationship between gray matter volume and severity of symptoms) rather than between-subjects analyses (comparing PTSD to non-PTSD groups). However, further research comparing groups who have and have not been exposed to combat and who have PTSD versus those who have not been experiencing PTSD symptoms, could be useful for replicating and extending current findings. We did not exclude for current substance abuse/dependence or use of psychotropic medications, and recognize these factors could have influenced findings. However, the current criteria may have provided for a more representative sample of returning combat veterans. Future research including PTSD with and without comorbid alcohol abuse and with and without use of psychotropic medications is needed to clarify the effects of these factors. In addition, we recognize that other factors, including pre-deployment or childhood stressors or family support also serve as risk and resiliency factors for PTSD. While the current analyses focused specifically on deployment and post-deployment factors, it could be useful for future research to investigate the effects of pre-deployment factors. Lastly, since this study focused on male combat veterans, generalization to females or to other trauma populations cannot be assumed.
Conclusion
Our findings suggest that deployment-related resiliency factors (i.e., social support) have the potential to be protective against atrophy within cortical regions (e.g., orbitofrontal, temporal), at least for those who experienced relatively low levels of combat. Consistent with previous literature, we provide evidence that extent of trauma and PTSD symptoms relate to reduced volume in frontal and temporal regions, which are thought to be important for contextual and spatial processing. Future longitudinal studies are needed to determine causal relationships between resiliency factors and brain function and structure. This research highlights the potential for resiliency factors to influence neural plasticity as a way to counteract effects of trauma exposure.
Acknowledgments
The work described in this manuscript was completed at the VASDHS and UCSD, La Jolla, CA.
Funding Statement
This work was supported by the VA Center of Excellence for Stress and Mental Health (CESAMH; MPP; http://www.mentalhealth.va.gov/coe/cesamh/) and a VA Merit Award (MPP; 1I01CX000273-01A2), which is supported by the Department of Veterans Affairs (http://www.research.va.gov). CESAMH is affiliated with VISN 22, the Veterans Affairs San Diego Healthcare System (VASDHS) and the University of California, San Diego (UCSD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, et al. (2009) Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002–2008. Am J Public Health 99: 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, et al. (2010) Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: possible explanations. J Trauma Stress 23: 59–68. [DOI] [PubMed] [Google Scholar]
- 3. Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, et al. (2011) Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend 116: 93–101. [DOI] [PubMed] [Google Scholar]
- 4. Brewin CR, Andrews B, Valentine JD (2000) Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 68: 748–766. [DOI] [PubMed] [Google Scholar]
- 5. Vasterling JJ, Proctor SP, Friedman MJ, Hoge CW, Heeren T, et al. (2010) PTSD symptom increases in Iraq-deployed soldiers: comparison with nondeployed soldiers and associations with baseline symptoms, deployment experiences, and postdeployment stress. J Trauma Stress 23: 41–51. [DOI] [PubMed] [Google Scholar]
- 6. Clancy CP, Graybeal A, Tompson WP, Badgett KS, Feldman ME, et al. (2006) Lifetime trauma exposure in veterans with military-related posttraumatic stress disorder: association with current symptomatology. J Clin Psychiatry 67: 1346–1353. [DOI] [PubMed] [Google Scholar]
- 7. Dedert EA, Green KT, Calhoun PS, Yoash-Gantz R, Taber KH, et al. (2009) Association of trauma exposure with psychiatric morbidity in military veterans who have served since September 11, 2001. J Psychiatr Res 43: 830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Voorhees EE, Dedert EA, Calhoun PS, Brancu M, Runnals J, et al. (2012) Childhood trauma exposure in Iraq and Afghanistan war era veterans: implications for posttraumatic stress disorder symptoms and adult functional social support. Child Abuse Negl 36: 423–432. [DOI] [PubMed] [Google Scholar]
- 9. King DW, King LA, Foy DW, Gudanowski DM (1996) Prewar factors in combat-related posttraumatic stress disorder: structural equation modeling with a national sample of female and male Vietnam veterans. J Consult Clin Psychol 64: 520–531. [DOI] [PubMed] [Google Scholar]
- 10. Donovan BS, Padin-Rivera E, Dowd T, Blake DD (1996) Childhood factors and war zone stress in chronic PTSD. J Trauma Stress 9: 361–368. [DOI] [PubMed] [Google Scholar]
- 11. Ozer EJ, Best SR, Lipsey TL, Weiss DS (2003) Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychol Bull 129: 52–73. [DOI] [PubMed] [Google Scholar]
- 12. Brailey K, Vasterling JJ, Proctor SP, Constans JI, Friedman MJ (2007) PTSD symptoms, life events, and unit cohesion in U.S. soldiers: baseline findings from the neurocognition deployment health study. J Trauma Stress 20: 495–503. [DOI] [PubMed] [Google Scholar]
- 13. Woon FL, Sood S, Hedges DW (2010) Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog Neuropsychopharmacol Bol Psychiatry 34: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 14. Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, et al. (2006) A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 30: 1004–1031. [DOI] [PubMed] [Google Scholar]
- 15. Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, et al. (1996) Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 40: 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, et al. (2011) Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry 69: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etkin A, Wager TD (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. A J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen S, Xia W, Li L, Liu J, He Z, et al. (2006) Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Res 146: 65–72. [DOI] [PubMed] [Google Scholar]
- 19. Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, et al. (2008) Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry 63: 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herringa R, Phillips M, Almeida J, Insana S, Germain A (2012) Post-traumatic stress symptoms correlate with smaller subgenual cingulate, caudate, and insula volumes in unmedicated combat veterans. Psychiatry Res 203: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woodward SH, Schaer M, Kaloupek DG, Cediel L, Eliez S (2009) Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry 66: 1373–1382. [DOI] [PubMed] [Google Scholar]
- 22. Geuze E, Westenberg HG, Heinecke A, de Kloet CS, Goebel R, et al. (2008) Thinner prefrontal cortex in veterans with posttraumatic stress disorder. Neuroimage 41: 675–681. [DOI] [PubMed] [Google Scholar]
- 23. Eckart C, Stoppel C, Kaufmann J, Tempelmann C, Hinrichs H, et al. (2011) Structural alterations in lateral prefrontal, parietal and posterior midline regions of men with chronic posttraumatic stress disorder. J Psychiatry Neurosci 36: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, et al. (2006) Decreased anterior cingulate volume in combat-related PTSD. Biol Psychiatry 59: 582–587. [DOI] [PubMed] [Google Scholar]
- 25. Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, et al. (2012) Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology 62: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, et al. (2006) Hippocampal volume, PTSD, and alcoholism in combat veterans. A J Psychiatry 163: 674–681. [DOI] [PubMed] [Google Scholar]
- 27. Wrase J, Makris N, Braus DF, Mann K, Smolka MN, et al. (2008) Amygdala volume associated with alcohol abuse relapse and craving. A J Psychiatry 165: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 28. Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, et al. (2008) Decreased volume of the brain reward system in alcoholism. Biol Psychiatry 64: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hedges DW, Woon FL (2010) Alcohol use and hippocampal volume deficits in adults with posttraumatic stress disorder: A meta-analysis. Biol Psychol 84: 163–168. [DOI] [PubMed] [Google Scholar]
- 30.Neigh GN, Ritschel LA, Kilpela LS, Harrell CS, Bourke CH (2012) Translational reciprocity: Bridging the gap between preclinical studies and clinical treatment of stress effects on the adolescent brain. Neuroscience. doi: 10.1016/j.neuroscience.2012.09.075. [DOI] [PMC free article] [PubMed]
- 31. Petrik D, Lagace DC, Eisch AJ (2012) The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology 62: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B (2012) PTSD and gene variants: new pathways and new thinking. Neuropharmacology 62: 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heim C, Nemeroff CB (2009) Neurobiology of posttraumatic stress disorder. CNS spectrums 14: 13–24. [PubMed] [Google Scholar]
- 34. Yehuda R, Flory JD, Southwick S, Charney DS (2006) Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci 1071: 379–396. [DOI] [PubMed] [Google Scholar]
- 35. Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, et al. (1995) The development of a Clinician-Administered PTSD Scale. J Trauma Stress 8: 75–90. [DOI] [PubMed] [Google Scholar]
- 36. Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, et al. (1994) A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol 55: 149–158. [DOI] [PubMed] [Google Scholar]
- 37. Spitzer RL, Kroenke K, Williams JB (1999) Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 282: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 38.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization.
- 39.King DW, Kind LA, Vogt DS (2003) Manual for the Deployment Risk and Resilience Inventory (DRRI): A Collection of Measures for Studying Deployment-Related Experiences of Military Veterans. Boston, MA: National Center for PTSD.
- 40. Huber PJ (1964) Robust estimation of a location parameter. Annals of Mathematical Statistics 35: 73–101. [Google Scholar]
- 41.R Development Core Team (2010) R: A Language and Environment for Statistical Computing, 2nd ed. Vienna, Austria: R Foundation for Statistical Computing. Available: http://cran.r-project.org/doc/manuals/fullrefman.pdf Accessed 07 February 2012.
- 42. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, et al. (2003) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1S208–219. [DOI] [PubMed] [Google Scholar]
- 43. Ashburner J, Friston KJ (2000) Voxel-based morphometry-the methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- 44. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, et al. (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- 45. Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Medical Imaging, IEEE Transactions on 20: 45–57. [DOI] [PubMed] [Google Scholar]
- 47. Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- 48. Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- 49.Andersson JLR, Jenkinson M, Smith S (2007) Non-linear registration, aka Spatial normalisation. Oxford, UK: FMIRB, University of Oxford. Available: http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja2/tr07ja2.pdf Accessed 07 February 2012.
- 50.Andersson JLR, Jenkinson M, Smith S (2007) Non-linear optimisation. Oxford, UK: FMRIB, University of Oxford. Available: http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja1/tr07ja1.pdf Accessed 07 February 2012.
- 51. Scorzin JE, Kaaden S, Quesada CM, Miller CA, Fimmers R, et al. (2008) Volume determination of amygdala and hippocampus at 1.5 and 3.0T MRI in temporal lobe epilepsy. Epilepsy Res 82: 29–37. [DOI] [PubMed] [Google Scholar]
- 52.Fox J (2002) An R and S-PLUS companion to applied regression. Thousand Oaks, CA: Sage Publications, Inc.
- 53. Noonan MP, Kolling N, Walton ME, Rushworth MF (2012) Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur J Neurosci 35: 997–1010. [DOI] [PubMed] [Google Scholar]
- 54. Diekhof EK, Kaps L, Falkai P, Gruber O (2012) The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50: 1252–1266. [DOI] [PubMed] [Google Scholar]
- 55. Diekhof EK, Falkai P, Gruber O (2011) The orbitofrontal cortex and its role in the assignment of behavioural significance. Neuropsychologia 49: 984–991. [DOI] [PubMed] [Google Scholar]
- 56. Elman I, Lowen S, Frederick BB, Chi W, Becerra L, et al. (2009) Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry 66: 1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, et al. (2008) Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res 42: 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Acheson DT, Gresack JE, Risbrough VB (2012) Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62: 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, et al. (2010) Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. J Clin Psychiatry 71: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 60. Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, et al. (2012) Brain structural changes as vulnerability factors and acquired signs of post-earthquake stress. Mol Psychiatry 18: 618–623. [DOI] [PubMed] [Google Scholar]
- 61. Kuhn S, Schubert F, Gallinat J (2011) Structural correlates of trait anxiety: reduced thickness in medial orbitofrontal cortex accompanied by volume increase in nucleus accumbens. J Affect Disord 134: 315–319. [DOI] [PubMed] [Google Scholar]
- 62. Watson KK, Platt ML (2012) Social signals in primate orbitofrontal cortex. Curr Biol 22: 2268–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- 64. Cardenas VA, Samuelson K, Lenoci M, Studholme C, Neylan TC, et al. (2011) Changes in brain anatomy during the course of posttraumatic stress disorder. Psychiatry Res 193: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kroes MC, Whalley MG, Rugg MD, Brewin CR (2011) Association between flashbacks and structural brain abnormalities in posttraumatic stress disorder. European Psychiatry 26: 525–531. [DOI] [PubMed] [Google Scholar]
- 66. Epstein RA (2008) Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci 12: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bar M, Aminoff E, Schacter DL (2008) Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci 28: 8539–8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aguirre GK, Detre JA, Alsop DC, D′Esposito M (1996) The parahippocampus subserves topographical learning in man. Cereb Cortex 6: 823–829. [DOI] [PubMed] [Google Scholar]
- 69. Ekstrom AD, Copara MS, Isham EA, Wang WC, Yonelinas AP (2011) Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage 56: 1803–1813. [DOI] [PubMed] [Google Scholar]
- 70. Janzen G, van Turennout M (2004) Selective neural representation of objects relevant for navigation. Nat Neurosci 7: 673–677. [DOI] [PubMed] [Google Scholar]
- 71. Hadj-Bouziane F, Liu N, Bell AH, Gothard KM, Luh WM, et al. (2012) Amygdala lesions disrupt modulation of functional MRI activity evoked by facial expression in the monkey inferior temporal cortex. Proc Natl Acad Sci U S A 109: E3640–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rolls ET (2000) Functions of the primate temporal lobe cortical visual areas in invariant visual object and face recognition. Neuron 27: 205–218. [DOI] [PubMed] [Google Scholar]
- 73. Miyashita Y (1993) Inferior temporal cortex: where visual perception meets memory. Annu Rev Neurosci 16: 245–263. [DOI] [PubMed] [Google Scholar]
- 74. Gross CG (2008) Single neuron studies of inferior temporal cortex. Neuropsychologia 46: 841–852. [DOI] [PubMed] [Google Scholar]
- 75. Woloszyn L, Sheinberg DL (2012) Effects of long-term visual experience on responses of distinct classes of single units in inferior temporal cortex. Neuron 74: 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kasahara H, Takeuchi D, Takeda M, Hirabayashi T (2011) Submodality-dependent spatial organization of neurons coding for visual long-term memory in macaque inferior temporal cortex. Brain Res 1423: 30–40. [DOI] [PubMed] [Google Scholar]
- 77. Hamame CM, Vidal JR, Ossandon T, Jerbi K, Dalal SS, et al. (2012) Reading the mind's eye: online detection of visuo-spatial working memory and visual imagery in the inferior temporal lobe. Neuroimage 59: 872–879. [DOI] [PubMed] [Google Scholar]