Abstract
GnRH agonists (GnRHa) are increasingly used for fertility preservation in women undergoing gonadotoxic chemotherapy. However, the protective mechanisms of action for these compounds have not yet been elucidated. In this study, we aimed to determine whether GnRHa have a direct effect on ovarian granulosa cells. GnRH receptor (GnRHR) expression was determined in mouse somatic and gonadal tissues including granulosa/cumulus cells and oocytes using quantitative RT-PCR and immunohistochemistry. Granulosa cells were isolated from mouse ovaries primed with pregnant mare serum gonadotropin. Response to GnRHa in cultured granulosa cells was assessed by determining the increase of intracellular cAMP and by assessing phosphorylation of downstream mediators of GnRH signaling: ERK and p38. To measure intracellular cAMP in our system, the cells were transfected with a cAMP-responsive luciferase reporter plasmid and stimulated with GnRHa. For all experiments, pituitary tissue and/or the αT3–1 mouse pituitary cell line were used as controls. GnRHR mRNA and protein were detected in mouse ovaries, granulosa/cumulus cells, and oocytes. After GnRHa stimulation at various time intervals, we were unable to detect a cAMP increase or activation of the ERK or p38 signaling pathway in cultured primary mouse granulosa cells, whereas activation was detected in the control αT3–1 mouse pituitary cells. In this study, we have not detected activation of the canonical GnRH signaling pathways in mouse ovarian somatic cells. Our findings suggest that the mechanism of action of GnRHa in the ovary is either below the detection level of our experimental design or is different from that in the pituitary.
One in every 46 women is diagnosed with cancer prior to the age of 40 years (1, 2). Due to the marked progress in detecting cancer at earlier stages and the improvement in treatment modalities within the past decades, there has been a significant increase in the 5- and 10-year survival rates of the leading causes of cancer (2). Additionally, the recent trend toward delaying childbearing has made it more common to see women considering pregnancy in their later reproductive years (2). These women, many of whom may have had a prior cancer diagnosis and undergone chemotherapy treatments, are now eager to consider fertility options.
Infertility, premature ovarian insufficiency, and menopause represent long-term consequences of cytotoxic chemotherapy used in the treatment of malignancies. Proliferating cells, such as those present in hair follicles and growing ovarian follicles, are more vulnerable to the immediate toxic effects of chemotherapy because cell division is disrupted (3). The various chemotherapeutic agents have differing effects on ovarian function. Cytotoxic agents, especially anthracyclines and alkylating agents such as cyclophosphamide, are the backbone of numerous cancer treatment regimens and are the main culprits of ovarian dysfunction (4–6). In addition to follicular atresia, these agents lead to cortical fibrosis and ovarian blood vessel damage (7). A delayed cytotoxic response to the chemotherapeutic agents is seen by the effect on the primordial cells, which lie dormant during the time of insult. The risk of ovarian insult is highly correlated to the woman's age at the time of treatment, type and dosage of drug administered, and the duration of drug exposure (4, 8–10). After chemotherapy, the long-term (3 y after diagnosis) incidence of amenorrhea is 50% in women aged 35–40 years, whereas most women over 40 years of age become amenorrheic, and their chances of restoring ovarian function become dismal (10, 11).
Currently the most widely used strategies for fertility preservation are embryo and oocyte cryopreservation. Both approaches require the patient to undergo controlled ovarian hyperstimulation in preparation for oocyte retrieval. This process is not only costly and invasive, but it also requires delaying cancer treatment for 2–4 weeks until the procedure is completed. Moreover, ovarian hyperstimulation is associated with elevated serum estradiol levels, which may be of concern in women with estrogen-sensitive cancers. Another option that is still considered experimental is the cryopreservation of ovarian tissue. With this procedure, ovarian tissue is surgically removed and the primordial follicles within the cortical tissue are cryopreserved. The tissue can then be retransplanted into the patient at a later time. Although pregnancies have been achieved, the efficiency is low and there is heightened concern with possible malignancy reseeding, thus making it an unappealing option (12, 13).
At present, the only option for fertility preservation that does not require cryopreservation of reproductive tissues (ovarian cortical tissue, oocyte, or embryo) involves the use of GnRH agonists (GnRHa). GnRH, also known as LHRH, is a decapeptide synthesized within the GnRH neurons of the hypothalamus. GnRH plays a critical role in the regulation of the gonadotropins, FSH and LH (14, 15). Gonadotropins, in turn, stimulate the gonads to produce steroids and drive follicle and oocyte maturation (14). GnRH analogs are synthetic compounds produced by amino acid substitution of the glycine at the sixth position or through replacement of the C-terminal glycine-amide (14, 16). After an initial flare upon binding of the GnRH receptors (GnRHR), the sustained action of GnRHa causes GnRHR down-regulation, leading to a hypogonadal state (14). GnRHa are widely used in clinical practice for gynecological conditions (such as uterine leiomyomata, endometriosis, precocious puberty), cancers (prostate, breast), and as part of in vitro fertilization protocols to achieve gonadal suppression.
The GnRHR is a member of the G protein-coupled receptors (GPCR) and is expressed mainly in the gonadotrope membrane of the anterior pituitary (17). Upon GnRH binding, the GnRHR undergoes a conformational change and stimulates a G protein, which in turn produces downstream activation of several signaling cascades, mainly inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG), MAPK, and adenylyl cyclase pathways (17, 18). The expression of the GnRH and its receptor has been clearly established in numerous extrapituitary tissues including cancer cell lines, prostate, ovary, placenta, and breast (18, 19). The presence of both GnRH and the GnRHR in the extrapituitary tissues implies an autocrine/paracrine role for GnRH and a potential site of action for GnRHa (19). However, the role of GnRHR in the extrapituitary tissues, particularly in the ovary, remains to be elucidated.
Recently there has been an increased interest in the role of GnRHa as an adjunct treatment for fertility preservation in women undergoing gonadotoxic chemotherapy. However, although multiple clinical studies have been performed and suggest a potential protective effect, the mechanism by which this occurs remains unclear (20–25). Resting follicles are thought to be more resistant to cytotoxic damage inflicted by chemotherapy (26). It has therefore been postulated that the protective effect of GnRHa may be due to the suppression of the pituitary-gonadal axis, resulting in an inactive or resting state of the ovaries (26). However, this scenario is unlikely due to the lack of FSH receptors in primordial follicles, which constitute the great majority of follicles in the adult ovary. Therefore, the mechanism by which GnRHa might protect fertility in women undergoing cytotoxic treatment, if any, remains uncertain.
We hypothesized that GnRHa may exert a gonadoprotective effect in women undergoing cancer treatment through a direct effect at the level of the ovaries. To test our hypothesis, we first examined whether GnRHR mRNA and protein are expressed in the mouse ovaries, somatic follicular cells, or oocytes. Then we investigated whether GnRHa stimulates the downstream mediators of GnRH activity in mouse ovarian somatic cells via the cAMP, IP3/DAG, or MAPK pathways.
Materials and Methods
Mouse tissue collection
C57BL/6J mice were purchased from Jackson Laboratories. Mice were bred and maintained according to the Yale University animal research requirements, and the Institutional Animal Care and Use Committee approval was obtained prior to the initiation of the study (protocol number 2008–11207). The mice were fed ad libitum and housed under a 12-hour light cycle. Six-week-old C57BL/6J female mice were euthanized using CO2 inhalation. The organs (heart, bladder, small intestine, liver, muscle, lung, kidney, spleen, stomach, colon, cerebellum, brain, pituitary, uterus, ovary, and testis) were dissected and kept at −80°C until RNA extraction.
Oocyte, granulosa cell, and cumulus cell collection
Mouse oocytes, granulosa cells, and cumulus cells were collected using standard protocols (27). In brief, mature C57/B6 female mice (4–6 wk old) were superovulated by ip injection of 5 IU pregnant mare serum gonadotropin (Folligon; Sigma-Aldrich) to stimulate follicle development. To obtain germinal vesicle stage oocytes, mice were euthanized 24 hours later by CO2 inhalation, the ovaries removed and placed in PBS, and cumulus oophorus complexes were isolated by puncturing the ovaries with a 26.5-gauge needle under the dissecting microscope (Olympus SZH-ILLK). The oocytes were separated from the surrounding cumulus cells via mechanical manipulation with 135- to 210-μm pipettes in HEPES-buffered medium containing 1 mg/mL hyaluronidase (Sigma-Aldrich), and oocytes and cumulus cells were collected. The granulosa cells were also collected during the same procedure, using a 0.40-μm strainer. The granulosa cells were spun down for 5 minutes at 1500 × g. For in vitro stimulation experiments, the granulosa cells were resuspended in MEMα + glutamax media (Invitrogen) supplemented with 5% fetal bovine serum (Invitrogen) and 1% antimycotic-antibiotic (Gibco). Cells were grown at 37°C with 5% CO2.
αT3–1 cells
Mouse pituitary cell line (αT3–1) was generously provided by Dr Pamela Mellon (University of California, San Diego, San Diego, California) through the Salk Institute (La Jolla, California). Cells were maintained in DMEM containing 4500 mg/L glucose, 110 mg/L pyruvate, 548 mg/L L-glutamine, 10% fetal bovine serum, and 1% penicillin/streptomycin (Invitrogen). The cells were grown at 37°C with 5% CO2 and passaged 8-fold once per week.
Total RNA extraction, reverse transcription, and PCR
Total RNA was isolated from mice tissues using Trizol (Invitrogen) according to the manufacturer's instructions and kept at −80°C until use. RNAqueous Microkit (Ambion) was used to isolate total RNA from cumulus cells, granulosa cells, and oocytes. All RNA was treated for genomic DNA contamination using deoxyribonuclease I according to the manufacturer's instructions (Ambion). The quality and concentration of the RNA was determined by measuring the absorbance at 260 and 280 nm. Equal amounts of total RNA were reverse transcribed to cDNA using oligodeoxythymidine priming at 37°C for 1 hour (Omniscript; QIAGEN). Control PCR for β-actin was performed using primers actin forward: TGC GTG ACA TCA AAG AGA AG; and actin reverse: CGG ATG TCA ACG TCA CAC TT. For GnRHR, the primers were complementary to regions on exons 2 and 3 (forward, F1: CTG GGC CCA CAG TCT TCT CGC; reverse, R1: TGA GTG GGT CGA AGC ACG GGT T), with an expected product length of 402 bp. PCR amplifications were carried out using 40 cycles of PCR, in which the initial 5 minutes for denaturation at 94°C was followed by a touchdown program for 10 cycles of 92°C for 30 seconds, 65°C for 20 seconds (−1°C per cycle) and 72°C for 30 seconds and then 20 cycles of 92°C for 30 seconds, 55°C for 20 seconds, and 72°C for 30 seconds in a 25-μL reaction mix containing 2μL cDNA, 1× PCR buffer (Ambion), 0.25 mM deoxynucleotide triphosphates, 0.5 μM primers, and 1 U of SuperTaq polymerase (Ambion). PCR products were run on a 2% agarose gel and visualized by ethidium bromide staining.
Real-time PCR
Quantitative RT-PCRs were carried out on an iCycler (Bio-Rad Laboratories). cDNA was prepared as described above and assayed in triplicate. Each 15-μL reaction contained 7.5 μL of SYBR Green supermix (Bio-Rad Laboratories), 0.4 μM of each primer, and 1 μL of cDNA. For GnRHR, the primers used for real-time PCR reactions were forward, 2.3F: GGC TGC CTC TTC ATC CCC CT, and reverse, 2.3R: CGT TCT CAG CCG AGC TCT TGG G with an expected product length of 147 bp. Expression of the target gene was normalized to β-actin levels (primers as above). A 3-step touchdown PCR was performed: 1 cycle at 95°C for 2 minutes, 10 cycles of 92°C for 30 seconds, 65°C for 20 seconds (−1°C per cycle), and 72°C for 30 seconds and then 30 cycles of 92°C for 30 seconds, 55°C for 20 seconds, and 72°C for 30 seconds, followed by a melting curve. A standard curve for each set of primers was first used to determine the linear dynamic range of each reaction and the PCR efficiency. A melting curve analysis was used to exclude nonspecific amplifications. The 2-ΔΔCΤ (cycle threshold) method was used to calculate relative gene expression.
Sequencing
For sequencing, the RT-PCR products were purified using the QIAquick gel extraction kit (QIAGEN) according to the manufacturer's protocol and eluted in 50 μL of water. Sequence analysis was carried out on Applied Biosystems 3730 capillary instruments at the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University. Sequencing results were compared with the mouse GnRHR sequences (NM_010323.1, and NC_000071) using Sequencher software version 4.8 (Gene Codes) and Gene Construction Kit 3.5 (Textco Biosoftware Inc).
Immunohistochemistry
Serial sections (5 μm thick) were cut from formalin-fixed, paraffin-embedded mouse ovarian tissue blocks and mounted on glass slides coated with 0.02% poly-L-lysine. Samples were deparaffinized in xylene, rehydrated in graded series of alcohol, and immersed in distilled water. To block endogenous peroxidase activity, samples were treated with 3% H2O2 in distilled water with 50% methanol at room temperature for 20 minutes. After rinses in PBS, slides were blocked with 10% horse serum (Vector Laboratories) in PBS in a humidified chamber for 30 minutes at room temperature. Then the excess serum was drained, and sections were incubated overnight at 4°C in a humidified chamber with the primary antibody (1:100 dilution). The primary anti-GnRHR antibodies used were goat polyclonal antimouse GnRHR antibody (1:100 dilution; Santa Cruz Biotechnology Inc), rabbit monoclonal (1:500; Epitomics), or rabbit polyclonal antibody (1:100; Proteintech). Equivalent concentration of goat IgG or rabbit IgG (Vector Laboratories) was used for negative controls. Then slides were rinsed in Tris-buffered saline and incubated with biotinylated horse antigoat or antirabbit antibody (1.5 mg/mL; Vector Laboratories) at 1:500 dilution for 30 minutes at room temperature. Avidin-biotin-peroxidase kit (LabVision) was used to detect the antigen-antibody complex, and diaminobenzidine (3,3-diaminobenzidine tetrahydrochloride dihydrate; LabVision) was used as the chromogen. Finally, sections were counterstained with hematoxylin and eosin. Sections from the pituitary, which is known to express GnRHR, were used as positive controls.
Cell stimulation
Cells (αT3–1 and primary mouse granulosa cells isolated as described above) were grown to approximately 60%-80% confluency in 24-well plates in their respective media containing fetal bovine serum and antibiotics. Prior to stimulation, the cells were incubated in 500 μL serum-free media for 2–4 hours. The cells were stimulated with recombinant FSH (follitropin-β; Follistim, Organon; 100 mIU/mL), forskolin (Fisher Scientific; 10 μM), GnRHa (leuprolide acetate; Sun Pharmaceutical Inc; 2 μg/mL to 1 ng/mL), or GnRH (Sigma; 1 μg/mL to 1 ng/mL) in serum-free media. Forskolin was used as a positive control because it causes a receptor-independent intracellular cAMP increase.
The dose of GnRHa used was calculated from the information given in the product insert provided by the manufacturer for clinical use. The bioavailability, metabolism, and elimination of the product were considered. According to the manufacturer (TAP Pharmaceuticals Inc), when a single dose of Lupron Depot 3.75 mg is administered by im injection to healthy female volunteers, a peak plasma concentration ranging from 4.6 to 10.2 ng/mL is achieved at 4 hours after dosing. After the initial rise, leuprolide concentrations started to plateau within 2 days after the dosing and remain relatively stable for about 4–5 weeks, with plasma concentrations of about 0.30 ng/mL. When Lupron Depot 30 mg is used, the mean plasma leuprolide concentration of 59.3 ng/mL is observed at 4 hours, and the mean plasma concentration of leuprolide is 0.44 ± 0.20 ng/mL (range 0.20–1.06) from weeks 3.5 to 16. We used concentrations that include peak concentrations of GnRHa achieved in clinical practice as well as 100-fold higher concentrations.
Unstimulated cells in serum-free media were used as negative controls. After 5 and 10 minutes of stimulation, the media were removed and the cells were washed with cold PBS and lysed. In a pilot experiment, we performed a time course of 0–120 minutes to determine the optimal duration of stimulation for the phosphorylation of ERK and p38.
Estradiol measurements
Mouse preluteal granulosa cells were harvested and cultured as above in 24-well plates. The cells were serum starved for 18 hours and then incubated with FSH (100 mIU/mL) or forskolin (10 μM) in the presence of androstenedione (10−6 M) for 3 days. 17β-Estradiol in the medium was determined using ELISA (Cayman Chemical Co). Measurements were performed in undiluted and 1:3 dilutions of the cell culture medium.
Western blotting
The cells were washed in cold PBS and lysed at 4°C in lysis buffer [20 mM Tris-HCL (pH 8.0), 5 mM MgCl2, 10 mM EGTA (pH 8.0), 1% Triton X-100, 1 mM Na3VO4, 50 mM NaF, one tablet of complete protease inhibitor cocktail (Roche Diagnostics) per 10 mL of buffer]. Lysates were cleared by centrifugation at 12 000 × g for 30 minutes at 4°C. Samples were mixed with 2× sodium dodecyl sulfate (SDS) sample buffer [125 mM Tris-HCL (pH 6.8), 20% glycerol, 4% SDS, 0.1% bromophenol blue, 10% mercaptoethanol], fractionated by SDS-PAGE (10% Tris-HCL Ready gels (Bio-Rad Laboratories)], and transferred to nitrocellulose membranes (Bio-Rad Laboratories) at 32 V overnight. The membrane was blocked with 5% nonfat dry milk in TBS-T (10 mM Tris-HCL, pH 7.4; and 150 mM NaCl with 1% Tween 20) for 1 hour at room temperature and incubated with primary antibodies in 1% milk in TBS-T overnight at 4°C. After washing in TBS-T, membranes were incubated with horseradish peroxidase-conjugated antirabbit secondary antibody (1:5000; Chemicon) diluted in 5% bovine serum albumin in TBS-T for 1 hour. The membranes were washed and signals were detected using SuperSignal ECL (Pierce) and exposed to film (Kodak). Antibodies against phosphorylated (p)-ERK (1:2000), p-p38 (1:1000), actin (1:5000), and glyceraldehyde-3-phosphate dehydrogenase (1:5000) were from Cell Signaling Technology. The experiments were repeated at least 3 times. Films with different exposures were scanned and band intensities were measured using ImageJ (National Institutes of Health). Actin or glyceraldehyde-3-phosphate dehydrogenase was used for normalization. At least 3 independent experiments were used to obtain relative band intensities.
Transient transfections
Cells (αT3–1 cells and primary mouse granulosa cells) were grown in 24-well plates in their respective media and transfected at 60%-80% confluency. To measure cAMP increase in response to stimulation, the cells were transiently transfected with pHTS-CRE (Biomyx Technology), which carries the luciferase gene under the control of 4 cAMP response elements. They were cotransfected with the pRL-CMV plasmid (Promega), which drives the expression of a different luciferase gene under the control of a constitutive cytomegalovirus (CMV) promoter to normalize for transfection efficiency. The cells were transfected with 200 ng pHTS-cre, 10 ng pRL-CMV, and 3 μL Lipofectamine 2000 (Invitrogen) per well. Both the DNA and Lipofectamine were mixed with 50 μL OptiMem (Invitrogen) and kept at room temperature for 5 minutes. The 2 mixes were combined and kept at room temperature for 20 minutes. During that time, the cell culture medium was removed from the cells and substituted with medium with serum but without antibiotics. The DNA-Lipofectamine-OptiMem mix was dispensed on the cells and was incubated for 16 hours at 37°C before the transfection mix was substituted with complete medium containing serum and antibiotics. Eight hours later, the cells were stimulated as above (see Cell Stimulation) for 16–18 hours. The cells were lysed and luciferase activity was measured using Dual-Glo luciferase kit (Promega) on a 20/20 Luminometer (Promega). All results presented are the means of 6 independent experiments, each performed in triplicate.
Statistical analysis
Statistical analysis was performed using Excel (Microsoft Inc). The significance of the differences between treatments was assessed using a Student t test. A value of P < .05 was considered significant for the comparisons.
Results
GnRHR mRNA is expressed in mouse extrapituitary tissues
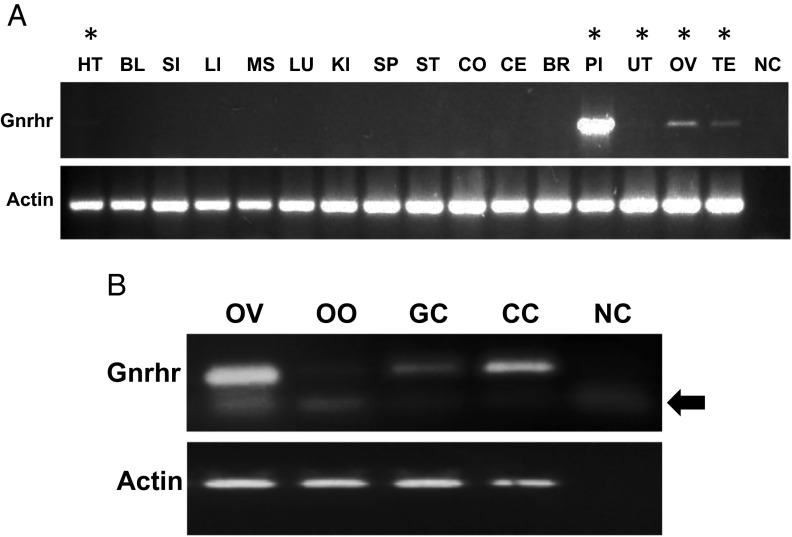
To determine GnRHR expression in mouse extrapituitary tissues, primers were designed, and RT-PCR was performed on dissected tissues from 6-week-old mice (Figure 1A). The expected size (402 bp) of the PCR product was first confirmed in the pituitary, the main target organ of GnRH, in which GnRHR is expressed in high amounts (28–30). In addition to the pituitary, the ovary, testis, uterus, and heart also showed GnRHR mRNA expression. GnRHR expression was further evaluated within the somatic and germ cells of the ovary. Cumulus cells, granulosa cells, and oocytes all showed GnRHR expression (Figure 1B). The specificity of the RT-PCR products was confirmed by sequencing (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Figure 1.
GnRHR expression in the pituitary and extrapituitary tissues. A, GnRHR expression in the pituitary and selected extrapituitary tissues. Top panel, GnRHR expression was tested by RT-PCR using primers F1–R1 in exons 2 and 3 (see Materials and Methods). Bottom panel, Control RT-PCR for β-actin. The tissues labeled with an asterisk indicate presence of GnRHR expression. BL, bladder; BR, brain; CO, colon; CE, cerebellum; HT, heart; KI, kidney; LI, liver; LU, lung; MS, muscle; NC, negative control; OV, ovary; PI, pituitary; SI, small intestine; SP, spleen; TE, testis; UT, uterus. B, GnRHR mRNA expression within the mouse ovary. Primers used were 2.3F-2.3R in exons 2 and 3 (see Materials and Methods). The top band is the specific band, and the lower band (labeled with a bold arrow) is a nonspecific band also seen in the negative control. CC, cumulus cells; GC, granulosa cells; NC, negative control; OO, oocyte; OV, ovary.
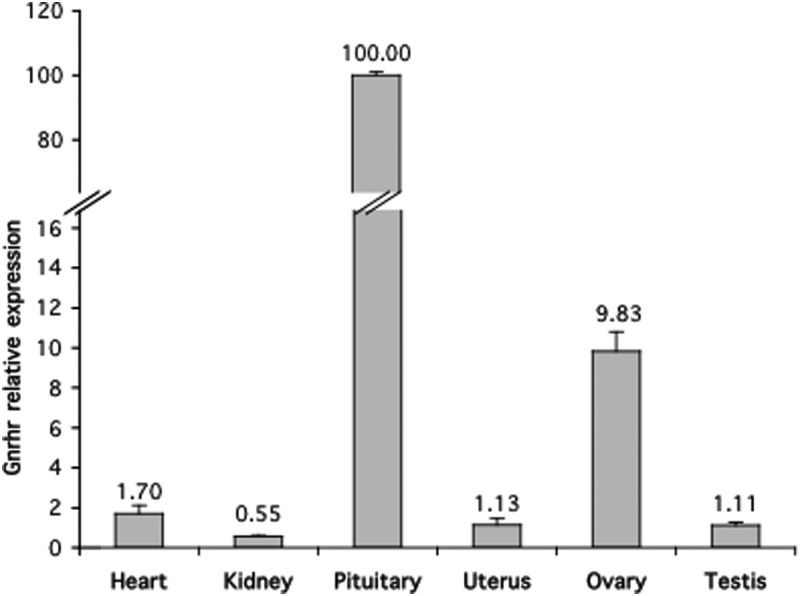
Quantitative RT-PCR was then used to compare the level of GnRHR mRNA, normalized to actin, in the tissues that express GnRHR (Figure 2). As expected, the pituitary had the highest GnRHR expression, which was nearly 10-fold greater than the next highly expressed tissue, the ovary, and nearly 100-fold greater than the other tested tissues. The ovary showed the second highest level of GnRHR expression, which was nearly 10-fold higher than that of the uterus, testis, heart, or kidney.
Figure 2.
Quantitative RT-PCR expression of GnRHR in pituitary and extrapituitary tissues. Primers used were GnRHR 2.3F-2.3R in exons 2 and 3 (see Materials and Methods). Expression levels were normalized to β-actin. The experiment was performed in triplicate. Highest expression was seen in pituitary, which is the main target organ for GnRHR. The ovary had a 10-fold higher expression when compared with the other extrapituitary tissues.
Immunohistochemistry
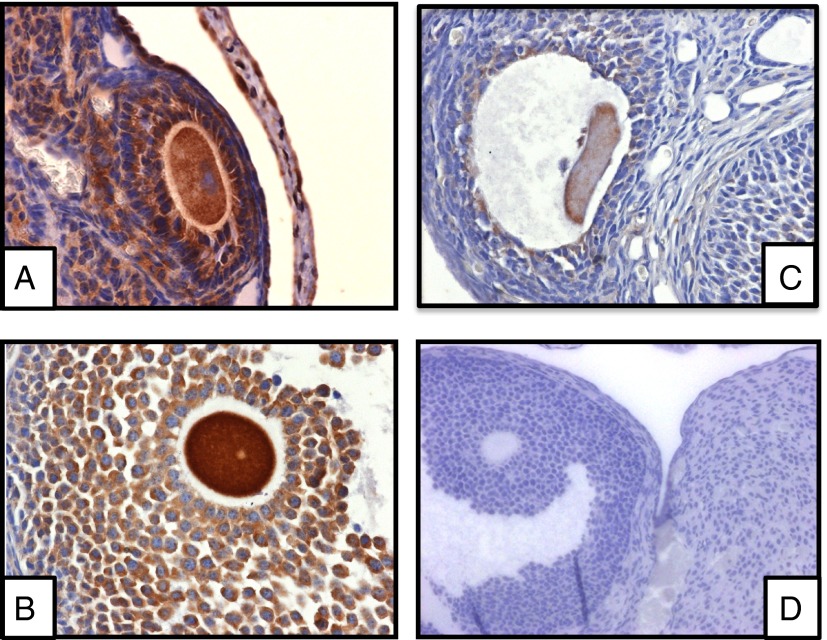
Three commercially available antibodies against GnRHR were tested using both pituitary and ovarian tissues. Cytoplasmic and membranous GnRHR immunoreactivity was detected in the cumulus and granulosa cells as well as the oocytes (Figure 3).
Figure 3.
GnRHR immunoreactivity in mouse ovarian tissue. Three commercially available antibodies (A, Santa Cruz Biotechnology; B, Epitomic; C, Proteintech) were used. D, Goat IgG negative control. Rabbit IgG (not shown) was used as a control for antibodies B and C with similar results to goat IgG. The sections were counterstained with hematoxylin and eosin.
Effect of GnRHa on the cAMP Pathway
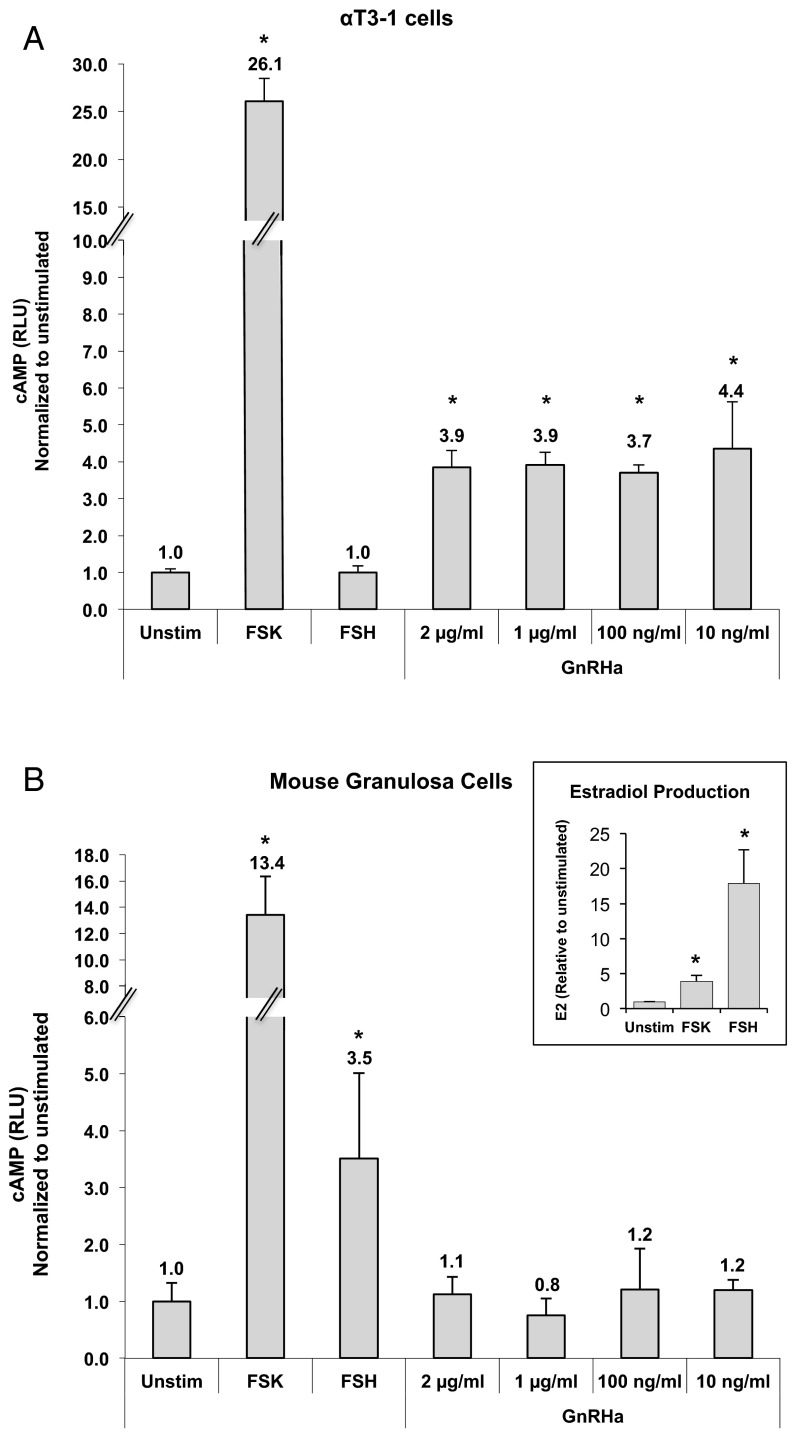
GnRHR is a GPCR (31, 32). In pituitary cells, binding of GnRH to GnRHR leads to the activation of the adenyl cyclase pathway and the subsequent elevation of intracellular cAMP (33–35). We therefore tested whether GnRH stimulation leads to an increase in cAMP levels in ovarian granulosa cells. cAMP was assessed using a luciferase reporter plasmid that responds to cAMP, and luciferase bioluminescence was measured. We first tested the cAMP response in the control mouse pituitary cell line αT3–1 (Figure 4A). Forskolin, which causes a nonreceptor-mediated cAMP increase, was used as a positive control. As expected, forskolin resulted in the highest cAMP increase compared with unstimulated cells. Stimulation with GnRHa (at all concentrations) also resulted in an increase in cAMP response in αT3–1 cells, nearly 5-fold greater than in unstimulated cells. In contrast, as expected, FSH treatment did not elicit a cAMP response in αT3–1 cells.
Figure 4.
Intracellular cAMP in response to GnRHa stimulation in αT3–1 cells (A) and mouse primary granulosa cells (B). cAMP elevation was measured using a luciferase reporter construct. Transfection efficiency was normalized with the control pRL-CMV plasmid. FSH, (50 mIU/mL); FSK, forskolin (10 μM); RLU, relative luciferase units; UNS, unstimulated. *, Significant difference. Insert, Estradiol measurements in culture media from mouse granulosa cells after forskolin (10 μM) and FSH (100 μM) stimulation. Estradiol was used to confirm that mouse granulosa cells were appropriately responding to FSH because cAMP induction routinely displays significant variability.
cAMP response to GnRHa was similarly tested in primary mouse granulosa cells (Figure 4B). Similar to the effect seen in αT3–1 cells, forskolin resulted in the highest cAMP increase in granulosa cells. FSH also resulted in a 3.5-fold and significant increase in cAMP over unstimulated cells. However, there was no detectable response to GnRHa in primary mouse granulosa cells at any of the concentrations used.
The primary granulosa cells showed a significant variability in cAMP production when stimulated with FSH, a finding that was consistent among numerous repetitions. In a parallel experiment, the cells were stimulated with FSH for 3 days and estradiol was measured. The granulosa cells showed a 15-fold increase in estradiol production, demonstrating that the downstream signaling pathways were activated (Figure 4B, insert). We believe that the main cause of this variability is that the granulosa cells were isolated from follicles of variable sizes, which are known to express different amounts of FSH receptor. Forskolin, on the other hand, which causes a nonreceptor-mediated cAMP increase, showed much less variability.
Effect of GNRHa on the ERK/MAPK pathway
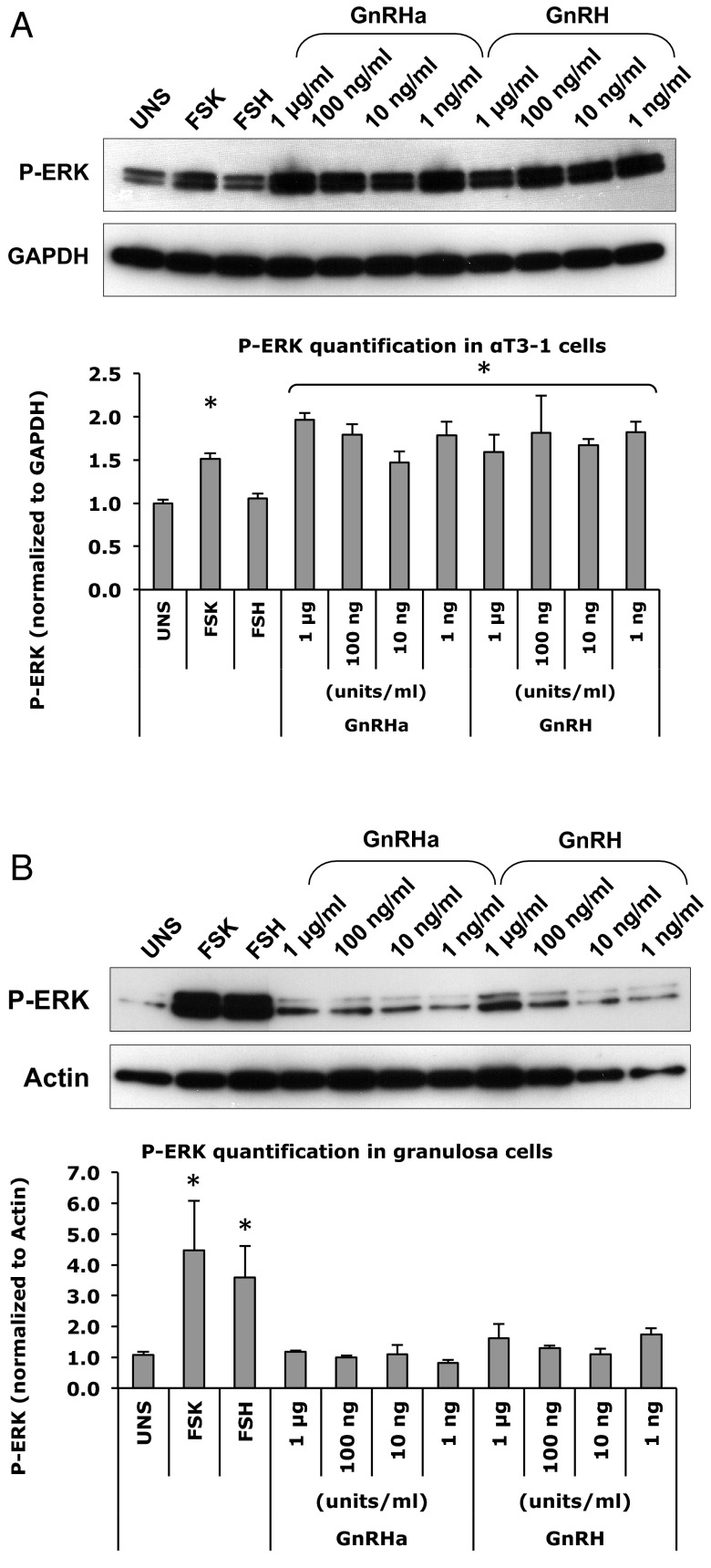
ERK is a downstream effector of both the cAMP and IP3/DAG pathways (Figure 5). ERK phosphorylation in response to GnRHa was tested in both the αT3–1 cell line and in primary mouse granulosa cells. The cells were stimulated with hormones in serum-free media for 5 and 10 minutes. In the αT3–1 cells, both GnRHa and GnRH (1 μg/mL, 100 ng/mL, 10 ng/mL, 1 ng/mL) resulted in an increase in p-ERK when compared with unstimulated cells (Figure 6A). Forskolin also resulted in a moderate increase, whereas FSH did not cause an increase in ERK phosphorylation in the αT3–1 pituitary cell line.
Figure 5.
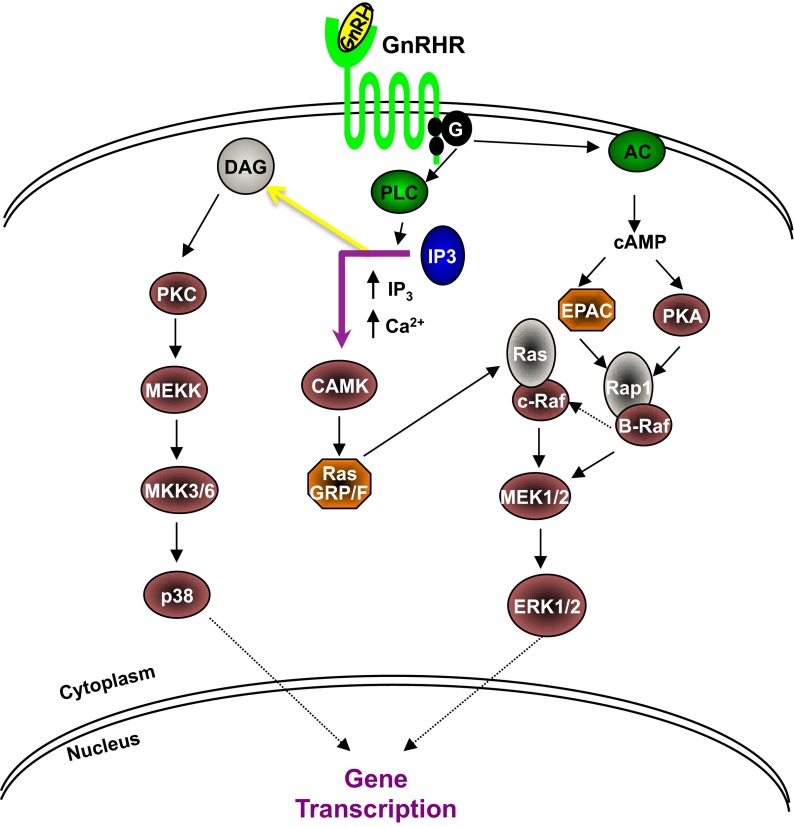
Canonical pathways of GnRH activation. AC, adenylyl cyclase; CAMK, calmodulin-dependent protein kinase; EPAC, exchange proteins activated by cAMP; MEK, MAPK kinase; MEKK, mitogen-activated protein/ERK kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C.
Figure 6.
Activation of ERK [pathway in response to forskolin (FSK; 10 μM), FSH (100 mIU/mL), GnRHa and GnRH (10−6 to 10−9 g/mL] as detected using Western blot analysis. αT3–1 cells are shown in A, and granulosa cells are shown in B. Antibody against p-ERK was used. The bar graphs depict the band intensity of the Western blots shown above, normalized for the endogenous gene. These graphs are based on 3 independent experiments, and a representative is shown here. UNS, unstimulated. *, indicates significant difference.
ERK phosphorylation in primary mouse granulosa cells was similarly assessed, and both forskolin and FSH caused an increase in ERK phosphorylation when compared with the unstimulated cells. However, treatment with different concentrations of GnRHa or GnRH did not result in elevated ERK phosphorylation in mouse primary granulosa cells (Figure 6B).
Effect of GnRHa on the IP3/DAG pathway
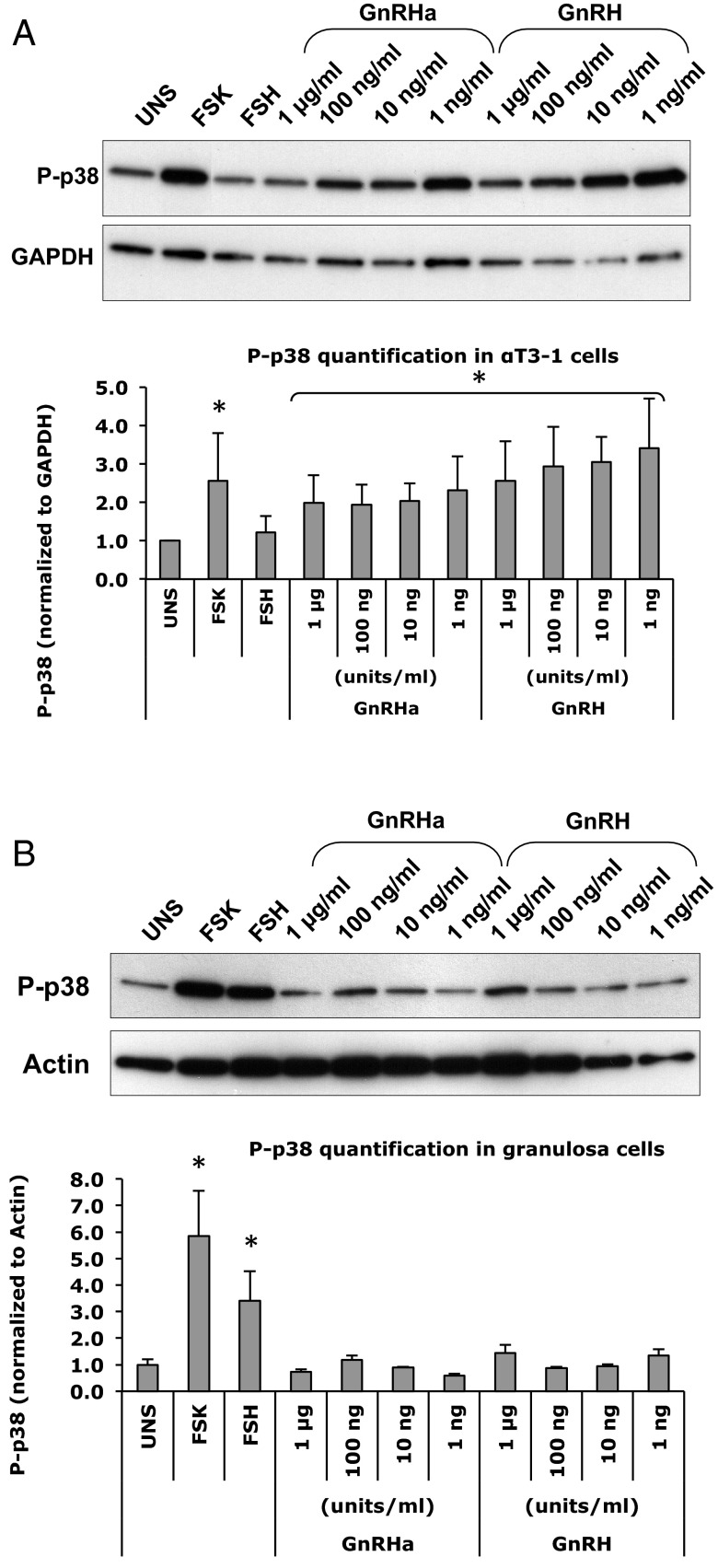
To evaluate the effect of GnRHa on the IP3/DAG pathway, phosphorylation of the downstream effector molecule, p38 MAPK (p38), was assessed. The cells were stimulated with hormone in serum-free media for 5 and 10 minutes. The control αT3–1 cells showed a small but significant increase in p38 phosphorylation with forskolin, GnRHa, and GnRH over the unstimulated cells (Figure 7A). Although all concentrations of GnRHa showed an increase in p38 phosphorylation, the greatest increase was seen at the 1 ng/mL of GnRHa. A strong effect was detected at all concentrations of GnRH used (Figure 7B). FSH, however, did not have any effect on p38 phosphorylation in the αT3–1 cells.
Figure 7.
Activation of p38 MAPK pathway in response to forskolin (FSK; 10 μM), FSH (100 mIU/mL), GnRHa and GnRH (10−6 to 10−9 g/mL) as detected using Western blot analysis. αT3–1 cells are shown in A, and granulosa cells are shown in B. Antibodies against p-p38 were used. The bar graphs depict the band intensity of the Western blots shown above, normalized for the endogenous gene. These graphs are based on 3 independent experiments, and a representative is shown here. UNS, unstimulated. *, indicates significant difference.
As expected, stimulation with FSH or forskolin increased the phosphorylation of p38 in primary mouse granulosa cells (Figure 7B). However, stimulation with GnRHa or GnRH did not elicit an increase in p38 phosphorylation.
Discussion
In this study, we investigated whether GnRHa has a direct effect on the ovaries through the known signaling pathways of GnRH action. We first confirmed the previously reported expression of GnRHR mRNA in extrapituitary tissues including the heart, uterus, ovary, and testis (18, 36–40). As expected, the presence of GnRHR was substantially higher (nearly 10-fold) in the pituitary, the main target organ for GnRH (Figure 2). The presence of GnRHR was second highest in the ovary when compared with the other somatic tissues, furthering our interest in determining whether GnRHa has any direct effect on granulosa cells (Figure 2). In particular, we showed the presence of GnRHR mRNA in mouse granulosa cells, cumulus cells, and denuded oocytes (Figure 1B and Supplemental Figure 1). These findings are consistent with previous studies showing mRNA expression within the ovary as well as within the granulosa cells of antral follicles in both rodents and primates (39, 40). GnRHR immunoreactivity was also detected in ovarian tissue using commercially available antibodies (Figure 3).
In the current study, we used the mouse model to explore the expression and function of GnRHR in the ovaries. Mouse constitutes a suitable model for these studies for several reasons. First, isolation of granulosa cells, cumulus cells, and oocytes is relatively easy from the murine ovary. In addition, biological material is more readily available compared with human, in which obtaining sufficient normal material is extremely difficult and would limit the number of performed experiments. Finally, both mouse and human express only one isoform of GnRHR (reviewed in Reference 41). However, it is noteworthy that the physiology of the murine and human ovaries differ in the number of oocytes ovulated per cycle. In humans, only one dominant follicle develops in a given cycle, as opposed to 8–12 in the mouse. It is unknown whether GnRHR may play a role in these interspecies differences.
Previous studies have shown that GnRHR mRNA is expressed in granulosa cells of antral follicles and that the ovarian receptor can bind GnRH with an affinity that is not influenced by the stage of the estrous cycle (39, 40). Therefore, we used granulosa cells to perform the signaling experiments. Specifically, we used preluteal cells that respond to FSH, which was used as the positive control. Similar to GnRH, FSH signals via activation of FSHR present on the surface of granulosa cells, which belongs to the GPCR family and stimulates adenylyl cyclase, resulting in an increase in the production of cAMP.
In this study, we tested whether the known pathways that mediate GnRH action in the pituitary are activated in ovarian granulosa cells in response GnRHa or GnRH (Figures 4, 6, and 7). The immortalized murine gonadotrope cell line αT3–1 was used to originally clone the GnRHR (32, 42). The subsequent cloning of the GnRHR from a variety of species and tissues has identified a highly conserved receptor belonging to the superfamily of 7-transmembrane-spanning GPCR (31). We used the αT3–1 cell line as our control cell line throughout our study. When the αT3–1 cells were stimulated with GnRHa, there was a cAMP response and increased phosphorylation of ERK and p38. In contrast, our primary granulosa cells did not show a cAMP response or an increase in the expression of p-ERK or p-p38 upon GnRHa or GnRH stimulation. The primary mouse granulosa cells did, however, show increased expression in cAMP, p-ERK, and p-38 with FSH stimulation. Granulosa cells contain FSH receptors and are the main target cell for FSH. FSH promotes activation of the ERKs and p38 MAPK pathways, which were tested in our study (43, 44). Therefore, the granulosa cells in our culture system showed the appropriate response to FSH, confirming the ability of our experimental system to detect the activation of ERK and p38 MAPK pathways in granulosa cells.
The GnRHR is a special type of GPCR that primarily uses the Gq protein for its downstream signaling and is capable of activating multiple signaling pathways in the pituitary and peripheral tissues. Depending on the cell type, ligand binding to GnRHRs may also activate other G proteins (Gαq, Gs, Gi) and form alternative signaling complexes. These pathways include the inositol phosphate pathway, ERK, jun-N-terminal kinase, p38 MAPK, and arrestin-dependent pathways via both protein kinase A and protein kinase C (45, 46). In pituitary gonadotropes, GnRHR preferentially stimulates the G protein Gq. The C terminus of the GnRHR is phosphorylated in response to GnRH binding, leading to receptor desensitization. Activation of the GnRHR couples the Gq/G11 proteins, which in turn activates phospholipase C and transmits its signal to DAG and IP3 (31). Downstream effectors of this pathway are p-p38 and p-ERK. In our αT3–1 cells, the phosphoinositide pathway was activated with GnRHa, evident by the increased expression of p-p38 and p-ERK (Figures 6A and 7A). In our primary granulosa cells, neither p-p38 nor p-ERK was increased with GnRHa stimulation, suggesting that this pathway is not targeted within these cells (Figures 6B and 7B).
The adenylyl cyclase pathway requires the activation of the Gs protein, which then leads to an increase in intracellular cAMP. Although not the predominant pathway in the pituitary, we observed an increase in cAMP upon stimulation with GnRHa in the αT3–1 cells. However, in our primary granulosa cells, we did not see a cAMP increase, suggesting that this pathway is not activated with GnRHa stimulation (Figure 4).
Although various ovarian protective mechanisms of GnRHa have been suggested, the understanding of its potential protective effect as an adjunct treatment during chemotherapy challenges basic research (47). Some investigators proposed that GnRHa may suppress pituitary gonadotropin secretion, resulting in a quiescent state in the ovaries (26, 48). This hypothesis has been challenged by others due to the difficulty in reliably detecting gonadotropin receptor expression in primordial follicles, which determine the ovarian follicular reserve (49). Another possible protective mechanism is decreased uteroovarian perfusion secondary to the hypoestrogenic state created by the pituitary-gonadal desensitization; the decreased perfusion could minimize the amount of chemotherapeutic agent that reaches and damages the ovarian cortex (50–53). A third proposed possibility is that GnRHa may up-regulate intraovarian antiapoptotic molecules such as sphingosine-1-phosphate (48, 54–56). Lastly, activation of the GnRHR by a direct effect of the GnRHa may decrease cellular apoptosis (48). Obviously some of the proposed mechanisms suggest a direct effect of GnRHa on ovarian cells.
In the present study, the presence of GnRHR within the ovary as well as its presence in granulosa cells, cumulus cells, and oocytes was confirmed. Treatment of primary mouse granulosa cells with GnRHa did not result in an increase in cAMP, p-ERK, or p-p38, which are downstream effectors of all G-coupled protein signaling cascades that GnRH is known to stimulate. Our positive controls (FSH/forskolin) appropriately stimulated the primary granulosa cells. Additionally, our control pituitary cell line also showed a significant increase in cAMP, p-ERK, and p-p38 in response to GnRHa. Our findings could have 3 potential explanations: 1) the action of GnRH is below the detection level of this experimental design, 2) there is no direct effect on the GnRHR at the level of the ovary, or 3) a different, yet unknown pathway may be activated in the ovary. Based on our findings, the mechanism of protective action of GnRHa, if any, remains unknown. Careful consideration weighing the risks and benefits should be considered before administering GnRHa to patients because data are conflicting and no clear mechanism can explain its benefit. Further studies are clearly needed to elucidate the possible gonadoprotective mechanism of GnRHa. Additionally, more prospective, randomized clinical studies are necessary to determine whether this experimental treatment modality should be offered in clinical practice.
Acknowledgments
We thank Dr Pamela Mellon (University of California, San Diego, San Diego, California) and the Salk Institute (La Jolla, California) for providing the mouse pituitary cell line (αT3–1). We also thank Orkan Ilbay and Aysenur Torun for technical help.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health.
E.S. is supported by Award R01HD059909 from the National Institute of Health (NIH). M.D.L. is supported by Clinical and Translational Science Award Grant KL2 RR024138 from the National Center for Research Resources and the National Center for Advancing Translational Science, components of the NIH, and NIH Roadmap for Medical Research.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CMV
- cytomegalovirus
- DAG
- diacylglycerol
- GnRHa
- GnRH agonists
- GnRHR
- GnRH receptor
- GPCR
- G protein-coupled receptor
- IP3
- inositol 1,4,5-triphosphate
- p
- phosphorylated
- TBS-T
- Tris-HCL and 150 mM NaCl with 1% Tween 20.
References
- 1. American Cancer Society Cancer facts and figures 2012; http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf
- 2. Murk W, Seli E. Fertility preservation as a public health issue: an epidemiological perspective. Curr Opin Obstet Gynecol. 2011;23(3):143–150 [DOI] [PubMed] [Google Scholar]
- 3. Fleischer RT, Vollenhoven BJ, Weston GC. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet Gynecol Surv. 2011;66(4):248–254 [DOI] [PubMed] [Google Scholar]
- 4. Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14(5):1718–1729 [DOI] [PubMed] [Google Scholar]
- 5. Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: a report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2005;23(30):7555–7564 [DOI] [PubMed] [Google Scholar]
- 6. Kiserud CE, Fossa A, Bjoro T, Holte H, Cvancarova M, Fossa SD. Gonadal function in male patients after treatment for malignant lymphomas, with emphasis on chemotherapy. Br J Cancer. 2009;100(3):455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):379–390 [DOI] [PubMed] [Google Scholar]
- 8. Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17(8):2365–2370 [DOI] [PubMed] [Google Scholar]
- 9. Padmanabhan N, Rubens RD, Howell A. Adjuvant chemotherapy in early breast cancer. Lancet. 1986;2(8519):1333–1334 [DOI] [PubMed] [Google Scholar]
- 10. Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24(7):1045–1051 [DOI] [PubMed] [Google Scholar]
- 11. Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod. 2003;18(1):90–95 [DOI] [PubMed] [Google Scholar]
- 12. Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–1410 [DOI] [PubMed] [Google Scholar]
- 13. Meirow D, Levron J, Eldar-Geva T, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–321 [DOI] [PubMed] [Google Scholar]
- 14. Speroff L, Fritz MA. Neuroendocrinology. In: Clinical Gynecologic Endocrinology and Infertility. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2011:157–197 [Google Scholar]
- 15. Oktay K, Sonmezer M, Oktem O, Fox K, Emons G, Bang H. Absence of conclusive evidence for the safety and efficacy of gonadotropin-releasing hormone analogue treatment in protecting against chemotherapy-induced gonadal injury. Oncologist. 2007;12(9):1055–1066 [DOI] [PubMed] [Google Scholar]
- 16. Conn PM, Crowley WF., Jr Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405 [DOI] [PubMed] [Google Scholar]
- 17. Sealfon SC, Weinstein H, Millar RP. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr Rev. 1997;18(2):180–205 [DOI] [PubMed] [Google Scholar]
- 18. Cheung LW, Wong AS. Gonadotropin-releasing hormone: GnRH receptor signaling in extrapituitary tissues. FEBS J. 2008;275(22):5479–5495 [DOI] [PubMed] [Google Scholar]
- 19. Aguilar-Rojas A, Huerta-Reyes M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (Review). Oncol Rep. 2009;22(5):981–990 [DOI] [PubMed] [Google Scholar]
- 20. Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91(3):694–697 [DOI] [PubMed] [Google Scholar]
- 21. Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117(3):561–567 [DOI] [PubMed] [Google Scholar]
- 22. Blumenfeld Z, Avivi I, Linn S, Epelbaum R, Ben-Shahar M, Haim N. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11(8):1620–1626 [DOI] [PubMed] [Google Scholar]
- 23. Blumenfeld Z, Avivi I, Ritter M, Rowe JM. Preservation of fertility and ovarian function and minimizing chemotherapy-induced gonadotoxicity in young women. J Soc Gynecol Investig. 1999;6(5):229–239 [DOI] [PubMed] [Google Scholar]
- 24. Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306(3):269–276 [DOI] [PubMed] [Google Scholar]
- 25. Kim SS, Lee JR, Jee BC, et al. Use of hormonal protection for chemotherapy-induced gonadotoxicity. Clin Obstet Gynecol. 2010;53(4):740–752 [DOI] [PubMed] [Google Scholar]
- 26. Ataya KM, McKanna JA, Weintraub AM, Clark MR, LeMaire WJ. A luteinizing hormone-releasing hormone agonist for the prevention of chemotherapy-induced ovarian follicular loss in rats. Cancer Res. 1985;45(8):3651–3656 [PubMed] [Google Scholar]
- 27. Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci USA. 2005;102(2):367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conn PM, Staley D, Harris C, et al. Mechanism of action of gonadotropin releasing hormone. Annu Rev Physiol. 1986;48:495–513 [DOI] [PubMed] [Google Scholar]
- 29. Kakar SS, Musgrove LC, Devor DC, Sellers JC, Neill JD. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem Biophys Res Commun. 1992;189(1):289–295 [DOI] [PubMed] [Google Scholar]
- 30. Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25(2):235–275 [DOI] [PubMed] [Google Scholar]
- 31. Anderson L. Intracellular mechanisms triggering gonadotrophin secretion. Rev Reprod. 1996;1(3):193–202 [DOI] [PubMed] [Google Scholar]
- 32. Tsutsumi M, Zhou W, Millar RP, et al. Cloning and functional expression of a mouse gonadotropin-releasing hormone receptor. Mol Endocrinol. 1992;6(7):1163–1169 [DOI] [PubMed] [Google Scholar]
- 33. Borgeat P, Chavancy G, Dupont A, Labrie F, Arimura A, Schally AV. Stimulation of adenosine 3′:5′-cyclic monophosphate accumulation in anterior pituitary gland in vitro by synthetic luteinizing hormone-releasing hormone. Proc Natl Acad Sci USA. 1972;69(9):2677–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bourne GA. Cyclic AMP indirectly mediates the extracellular Ca2+-independent release of LH. Mol Cell Endocrinol. 1988;58(2–3):155–160 [DOI] [PubMed] [Google Scholar]
- 35. Naor Z, Fawcett CP, McCann SM. Differential effects of castration and testosterone replacement on basal and LHRH-stimulated cAMP and cGMP accumulation and on gonadotropin release from the pituitary of the male rat. Mol Cell Endocrinol. 1979;14(3):191–198 [DOI] [PubMed] [Google Scholar]
- 36. Dong F, Skinner DC, Wu TJ, Ren J. The heart: a novel gonadotrophin-releasing hormone target. J Neuroendocrinol. 2011;23(5):456–463 [DOI] [PubMed] [Google Scholar]
- 37. Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98(1):57–62 [PubMed] [Google Scholar]
- 38. Peng C, Fan NC, Ligier M, Vaananen J, Leung PC. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994;135(5):1740–1746 [DOI] [PubMed] [Google Scholar]
- 39. Pieper DR, Richards JS, Marshall JC. Ovarian gonadotropin-releasing hormone (GnRH) receptors: characterization, distribution, and induction by GnRH. Endocrinology. 1981;108(4):1148–1155 [DOI] [PubMed] [Google Scholar]
- 40. Fraser HM, Sellar RE, Illingworth PJ, Eidne KA. GnRH receptor mRNA expression by in-situ hybridization in the primate pituitary and ovary. Mol Hum Reprod. 1996;2(2):117–121 [DOI] [PubMed] [Google Scholar]
- 41. Millar RP. GnRHs and GnRH receptors. Anim Reprod Sci. 2005;88(1–2):5–28 [DOI] [PubMed] [Google Scholar]
- 42. Reinhart J, Mertz LM, Catt KJ. Molecular cloning and expression of cDNA encoding the murine gonadotropin-releasing hormone receptor. J Biol Chem. 1992;267(30):21281–21284 [PubMed] [Google Scholar]
- 43. Cottom J, Salvador LM, Maizels ET, et al. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278(9):7167–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18(9):1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Caunt CJ, Finch AR, Sedgley KR, McArdle CA. GnRH receptor signalling to ERK: kinetics and compartmentalization. Trends Endocrinol Metab. 2006;17(8):308–313 [DOI] [PubMed] [Google Scholar]
- 46. Pawson AJ, McNeilly AS. The pituitary effects of GnRH. Anim Reprod Sci. 2005;88(1–2):75–94 [DOI] [PubMed] [Google Scholar]
- 47. Woodruff TK. Preserving fertility during cancer treatment. Nat Med. 2009;15(10):1124–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007;12(9):1044–1054 [DOI] [PubMed] [Google Scholar]
- 49. Meirow D, Assad G, Dor J, Rabinovici J. The GnRH antagonist cetrorelix reduces cyclophosphamide-induced ovarian follicular destruction in mice. Hum Reprod. 2004;19(6):1294–1299 [DOI] [PubMed] [Google Scholar]
- 50. Dada T, Salha O, Allgar V, Sharma V. Utero-ovarian blood flow characteristics of pituitary desensitization. Hum Reprod. 2001;16(8):1663–1670 [DOI] [PubMed] [Google Scholar]
- 51. Kitajima Y, Endo T, Nagasawa K, et al. Hyperstimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology. 2006;147(2):694–699 [DOI] [PubMed] [Google Scholar]
- 52. Meirow D, Dor J, Kaufman B, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–1633 [DOI] [PubMed] [Google Scholar]
- 53. Saitta A, Altavilla D, Cucinotta D, et al. Randomized, double-blind, placebo-controlled study on effects of raloxifene and hormone replacement therapy on plasma no concentrations, endothelin-1 levels, and endothelium-dependent vasodilation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2001;21(9):1512–1519 [DOI] [PubMed] [Google Scholar]
- 54. Morita Y, Perez GI, Paris F, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6(10):1109–1114 [DOI] [PubMed] [Google Scholar]
- 55. Paris F, Perez GI, Fuks Z, et al. Sphingosine 1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8(9):901–902 [DOI] [PubMed] [Google Scholar]
- 56. Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2(11):838–848 [DOI] [PubMed] [Google Scholar]