Abstract
The LH receptor (LHR) and FSH receptor (FSHR) are each G protein-coupled receptors that play critical roles in reproductive endocrinology. Each of these receptors has previously been shown to self-associate into homodimers and oligomers shortly after their biosynthesis. As shown herein using bioluminescence resonance energy transfer to detect protein-protein interactions, our data show that the LHR and FSHR, when coexpressed in the same cells, specifically heterodimerize with each other. Further experiments confirm that at least a portion of the cellular LHR/FSHR heterodimers are present on the cell surface and are functional. We then sought to ascertain what effects, if any, heterodimerization between the LHR and FSHR might have on signaling. It was observed that when the LHR was expressed under conditions promoting the heterodimerization with FSHR, LH or human chorionic gonadotropin (hCG) stimulation of Gs was attenuated. Conversely, when the FSHR was expressed under conditions promoting heterodimerization with the LHR, FSH-stimulated Gs activation was attenuated. These results demonstrate that the coexpression of the LHR and FSHR enables heterodimerizaton between the 2 gonadotropin receptors and results in an attenuation of signaling through each receptor.
Dimerization of G protein-coupled receptors (GPCRs) is a well-documented and accepted phenomenon (1, 2) that may also entail further receptor oligomerization (3, 4). In addition to homodimerization, many GPCRs have been shown to form heterodimers, and in some cases, this has been shown to lead to alterations in the functional properties of the heterodimer as compared with the individual GPCRs (1, 2, 4–6). The human lutropin receptor (hLHR) and human follitropin receptor (hFSHR), collectively termed the gonadotropin receptors, are GPCRs that are critically involved in various aspects of male and female reproductive physiology. The hLHR and hFSHR have each been demonstrated to form homodimers (7–10). Studies suggest that the formation of hLHR and hFSHR homodimers are obligate and constitutive processes initiating early in the biosynthetic pathway and that the extent of homodimerization is not affected by the activation status of the receptor (9, 10). In light of the finding that the properties of other GPCRs can be altered by heterodimerization and the fact that physiologically the hLHR and hFSHR are coexpressed in some instances such as in mature ovarian granulosa cells, we sought to determine whether the hLHR and hFSHR heterodimerize with each other and, if so, what the functional consequences of this might be. The studies presented herein suggest that the hLHR and hFSHR can specifically associate into heterodimers and that these interactions result in an attenuation of LH and human chorionic gonadotropin (hCG)-stimulated signaling through the hLHR and an attenuation of FSH signaling through the FSHR.
Materials and Methods
Hormones and plasmids
cDNA constructs and highly purified recombinant hormones used in the study have been described previously (9–12).
Cells and transfections
Human embryonic kidney (HEK) 293 cells were obtained from the American Type Tissue Collection (Manassas, Virginia) and were maintained, plated for experiments, and transiently transfected as previously described (9). When transfecting cells with varying concentrations of plasmid encoding receptor cDNA, the total amount of plasmid was kept constant using empty vector. Stably transfected cells were established by transfecting cells with vectors containing a neoselectable marker and selecting them in growth media supplemented with 700 μg/mL G418 (selection media). Cells were grown in selection media for at least 2 weeks prior to use in experiments and were maintained in selection media.
Bioluminescence resonance energy transfer (BRET)2, hormone binding, cAMP, and flow cytometry assays
BRET2 titration curves were performed as described previously (9–11). Cell surface expression of the hLHR and hFSHR were determined by maximal specific binding of 125I-hCG or 125I-FSH, respectively, to intact cells as described previously (13). Assays to measure 125I-FSH desorption as a function of hCG binding were performed as described elsewhere (10). Intracellular concentrations of cAMP under basal and hormone-stimulated conditions were determined as described (13). Flow cytometry to quantify relative cell surface expression of hemagglutinin (HA)-human melanocortin 3 receptor (hMC3R) on nonpermeabilized cells was performed as detailed elsewhere (11). Additional details regarding methodologies can be found in the Supplemental Materials and Methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Results
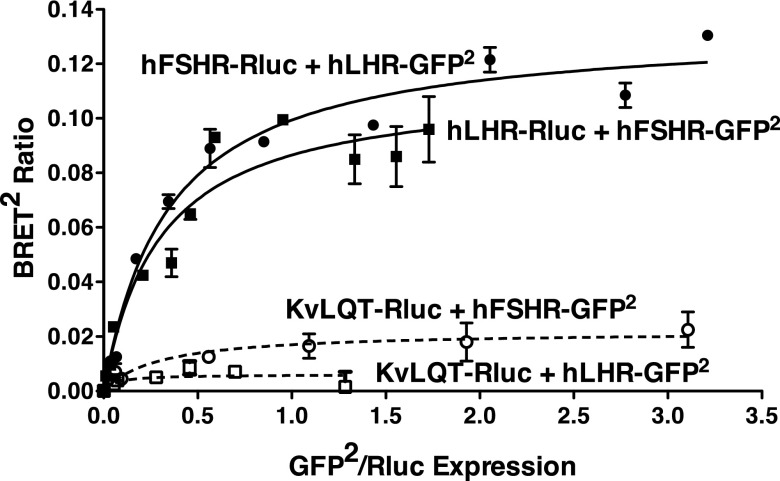
To ascertain whether hLHR and hFSHR, when coexpressed in HEK293 cells, would specifically heterodimerize with each other, quantitative BRET saturation assays were performed (14, 15). BRET2 titration curves were done in which hFSHR-Rluc was held constant and increasing amounts of hLHR-green fluorescent protein (GFP)2 were expressed or alternatively in which hLHR-Rluc was held constant and increasing amounts of hFSHR-GFP2 were expressed (Figure 1). Negative controls were performed using hFSHR-GFP2 or hLHR-GFP2 coexpressed with KvLQT-Rluc, a cell surface potassium channel (16, 17). It is important to note that within any given BRET2 saturation experiment, the cells were transfected to match the expression levels of the Rluc fusion proteins. These experiments revealed saturable hyperbolic curves indicative of specific heterodimerization between hLHR and hFSHR. The lack of similar curves between hLHR or hFSHR and KvLQT substantiate the specificity of hLHR/hFSHR heterodimerization. It has previously been reported that, BRET50's determined from BRET saturation curves done within the same experiment reflect the apparent relative affinities of the 2 proteins for each other, ie, their relative propensity for dimerization (14). Using hLHR-Rluc coexpressed with increasing concentrations of hLHR or hFSHR fused to GFP2, the BRET50's for hLHR/hLHR and hLHR/hFSHR were 0.35 ± 0.05 and 0.65 ± 0.09, respectively. Using hFSHR-Rluc coexpressed with increasing concentrations of hFSHR or hLHR fused to GFP2, the BRET50's for hFSHR/hFSHR and hFSHR/hLHR were 0.17 ± 0.004 and 0.33 ± 0.09, respectively. Thus, the apparent relative affinity of a heterodimer between hLHR and hFSHR is approximately 2-fold lower than for either homodimer.
Figure 1.
BRET2 saturation curves indicating heterodimerization of the LHR and FSHR. HEK293 cells were transiently transfected with the indicated pairs of fusion constructs under conditions in which the Rluc fusion protein was held constant and increasing amounts of the GFP2 fusion protein were expressed. Data shown are the corrected BRET2 signals as a function of GFP2/Rluc expression. Within a given experiment, the plasmid concentrations were adjusted so that all Rluc fusion proteins in the absence of GFP2 fusion proteins were expressed similarly. Data shown are from one experiment representative of 4 independent experiments.
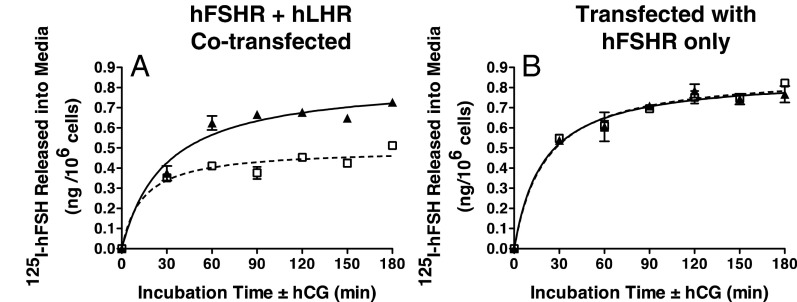
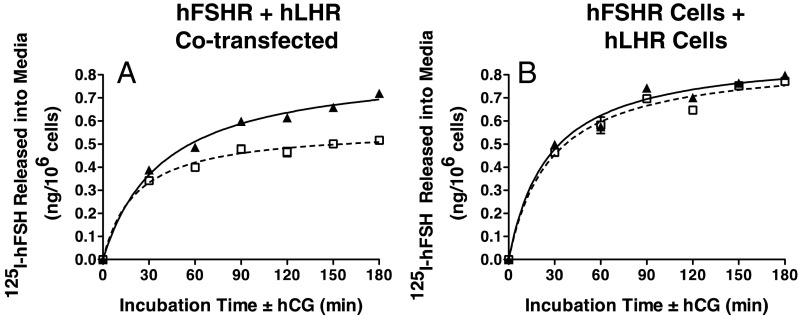
Previous studies using subcellular fractionation analyses have shown that homodimers of the LHR and FSHR are present both in the endoplasmic reticulum as well as on the cell surface (9, 10). Because BRET assays on intact cells do not distinguish the cellular localization of dimerized receptors, we performed the following experiments to validate that hLHR/hFSHR heterodimers were present on the cell surface and that they were functional. It has been shown that the association of GPCRs into dimers results in negative allosterism that can be detected by hormone desorption assays (4, 7, 18, 19). Using this experimental paradigm, we examined the ability of hCG to increase the dissociation of prebound 125I-hFSH from cells coexpressing hLHR and hFSHR. The hLHR/hFSHR cells were preincubated with 125I-hFSH and then extensively washed. At t = 0, hCG was added and the dissociation of prebound 125I-hFSH was determined by quantifying 125I-hFSH released into the media as a function of time of incubation with hCG. As shown in Figure 2A, there was an increased dissociation of prebound 125I-hFSH stimulated by the addition of hCG compared with the addition of vehicle only. Within the same experiment, the effects of hCG addition on 125I-hFSH dissociation were examined using cells expressing only hFSHR, and in this case hCG did not cause increased 125I-hFSH dissociation (Figure 2B). Therefore, both the hLHR and hFSHR must be coexpressed for hCG to stimulate hFSH desorption, consistent with hLHR/hFSHR heterodimers. Further support for this comes from studies in which, within the same experiment, we compared cells cotransfected with hLHR and hFSHR (Figure 3A) to cells in which only hLHR was transfected or only hFSHR was transfected, but then the 2 groups of cells were mixed (Figure 3B). The data in Figure 3 show that hCG-stimulated desorption of prebound 125I-hFSH can be observed only when both the hLHR and hFSHR are coexpressed in the same cells. Taken altogether, the data in Figures 2 and 3 demonstrate that hLHR/hFSHR heterodimers are detected on the cell surface by hormone desorption assays and that the heterodimers are functional with respect to hormone binding activity.
Figure 2.
Desorption of prebound 125I-hFSH by hCG addition in cells coexpressing hLHR and hFSHR but not hFSHR only. HEK293 cells were transiently cotransfected with cDNAs encoding hLHR and hFSHR at submaximal levels (A) or with the same concentrations of empty vector plus hFSHR (B). On the day of the experiment, cells were preincubated with a saturating concentration of 125I-hFSH (200 ng/mL final concentration). After washing to remove unbound 125I-hFSH, cells were incubated for the indicated times at RT with buffer only (open squares, dashed lines) or with hCG (500 ng/mL final concentration) (filled triangles, solid lines). At the end of the incubation period, the amount of acid-precipitable 125I-hFSH released into the medium was determined. Data shown are the mean ± SEM of triplicate determinations from one experiment representative of 4 independent experiments.
Figure 3.
Desorption of prebound 125I-hFSH by hCG addition is observed in cells cotransfected with hLHR and hFSHR but not in cells transfected separately and then cocultured. HEK293 cells were transiently transfected with cDNAs encoding hLHR and hFSHR at submaximal levels (A) or cells were transfected with the same concentrations of hLHR only or hFSHR only and then cocultured together (B). On the day of the experiment, cells were incubated with a saturating concentration of 125I-hFSH (200 ng/mL final concentration). After washing to remove unbound 125I-hFSH, cells were incubated for the indicated times at room temperature with buffer only (open squares, dashed lines) or with hCG (500 ng/mL final concentration) (filled triangles, solid lines). At the end of the incubation period, the amount of acid-precipitable 125I-hFSH released into the medium was determined. Data shown are the mean ± SEM of triplicate determinations from one experiment representative of 2 independent experiments.
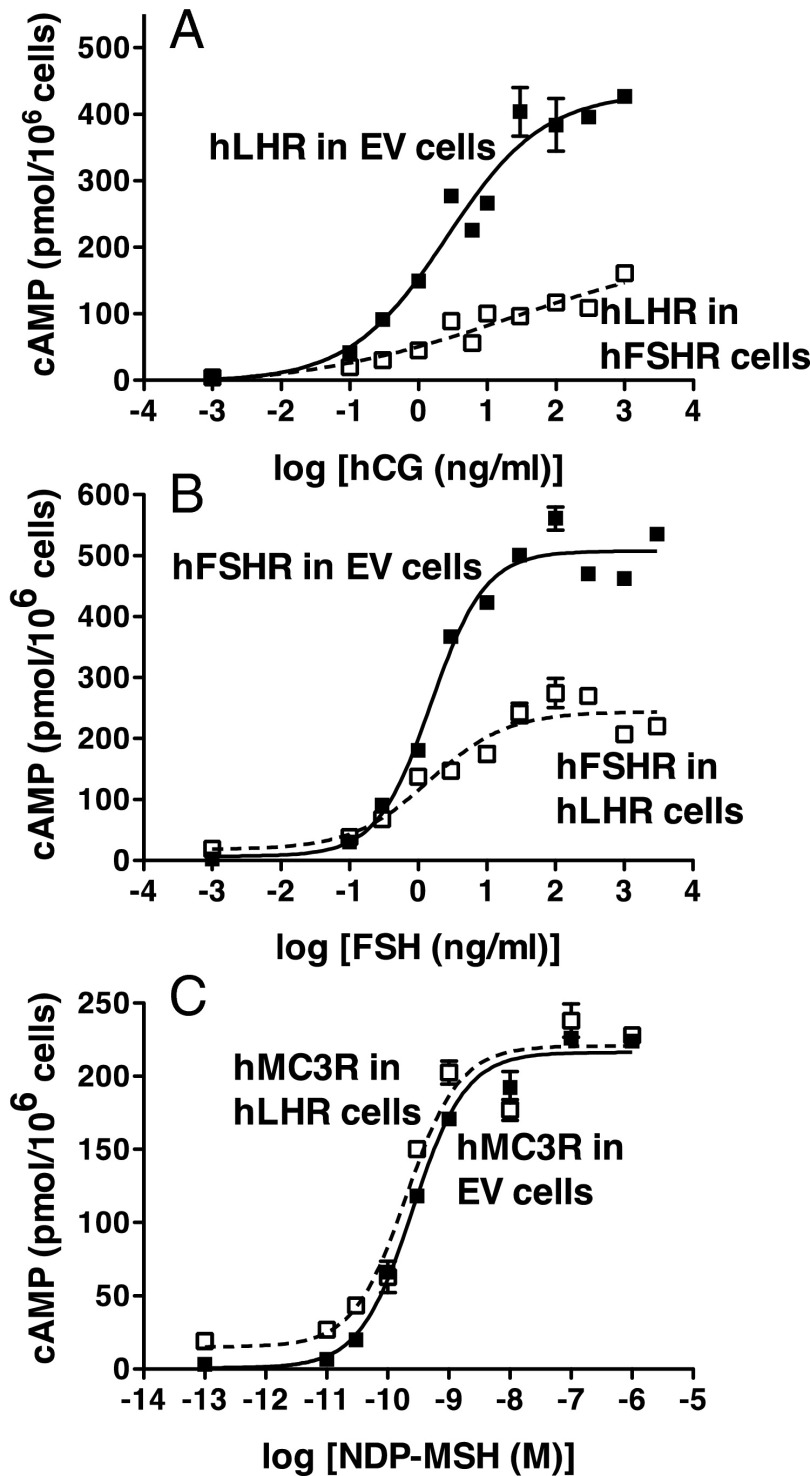
The following experiments were performed to examine potential alterations of signaling associated with the coexpression of the hLHR and hFSHR under conditions promoting their heterodimerization. Adapting a strategy we previously reported (11), cells stably transfected with the hFSHR were transiently transfected with plasmid encoding the hLHR that resulted in approximately 10-fold lower cell surface expression of the hLHR relative to hFSHR. Under these conditions of hFSHR excess, most of the hLHR would be expected to be associated with the hFSHR as opposed to being self-associated. As a control, cells stably transfected with empty vector were transiently transfected with hLHR under conditions yielding very tightly matched cell surface levels of hLHR as in the hFSHR cells. A significant attenuation of both hCG-stimulated cAMP production, as evidenced by both increased EC50's and decreased Rmax's observed when the hLHR was expressed in the hFSHR cells (Figure 4A). Similar results were observed when the cells were challenged with LH (data not shown). No difference in 125I-hCG binding affinity was observed (105 ± 4 vs 122 ± 12 ng/mL for hLHR vs hLHR/hFSHR, respectively). The converse experiment was performed in cells stably transfected with hLHR were transiently transfected with hFSHR to yield hFSHR cell surface expression approximately10-fold lower than cell surface hLHR expression. As shown in Figure 4B, a marked attenuation of FSH-stimulated cAMP production was observed in these cells as compared with empty vector cells expressing matched numbers of cell surface hFSHR. These data suggest that conditions that promote the association of the hLHR with hFSHR result in an attenuation of Gs signaling through the hLHR, and conversely, conditions that promote the association of the hFSHR with the hLHR result in an attenuation of Gs signaling through the hFSHR. The binding affinity of FSHR for 125I-FSH was not altered by the coexpression of the hLHR (76.5 ± 10.5 vs 71.4 ± 4.8 ng/mL for hFSHR vs hFSHR/hLHR, respectively). Previous studies from our laboratory showed that the hLHR does not heterodimerize with the human melanocortin 3 receptor (11). Therefore, hormone stimulated signaling through the hMC3R was examined in hLHR cells stably transfected with low levels of hMC3R (Figure 4C). The results of these experiments indicated that signaling through the hMC3R was not attenuated when the hMC3R was expressed in hLHR cells. Rather, the agonist-stimulated cAMP responses through the hMC3R when expressed in hLHR cells were similar or somewhat increased relative to the hMC3R in empty vector cells.
Figure 4.
Cross-attenuation between hLHR and hFSHR heterodimers. Panel A, HEK293 cells stably transfected with empty vector (EV) (filled squares, solid lines) or hFSHR (open squares, dashed lines) were transiently transfected with hLHR under conditions in which cell surface hLHR levels in the 2 cell lines were closely matched and were approximately 10-fold less than cell surface hFSHR. In the experiment shown, hFSHR stably transfected cells bound 15.3 ng 125I-hFSH/106 cells and hFSHR and EV cells transfected with hLHR bound 1.63 and 1.66 ng 125I-hCG /106 cells, respectively. Cells were incubated with the indicated concentrations of hCG and cAMP levels were determined. B, HEK293 cells stably transfected with empty vector (EV) (filled squares, solid line) or hLHR (open squares, dashed line) were transiently transfected with hFSHR under conditions in which cell surface hFSHR levels in the 2 cell lines were closely matched and were approximately 10-fold less than cell surface hLHR. In the experiment shown, hLHR stably transfected cells bound 23.0 ng 125I-hCG/106 cells and hLHR or EV cells transfected with hFSHR bound 2.72 and 2.73 ng 125I-hFSH/106 cells, respectively. Cells were incubated with the indicated concentrations of hFSHR and cAMP levels were determined. C, HEK293 cells were stably transfected with empty vector (EV) (filled squares, solid line) or hLHR (open squares, dashed line) were transiently transfected with a low concentration of HA-hMC3R. The relative cell surface levels of MC3R in the EV cells and hFSHR cells as determined by flow cytometry were 673 and 798 relative light units, respectively. Cells were incubated with the indicated concentrations of NDP-MSH and cAMP levels were determined. For all panels, data shown are the mean ± SEM of triplicate determinations from one experiment representative of at least 3 independent experiments.
Discussion
We show herein that the hLHR and hFSHR form heterodimers and that, when dimerized, hormone-stimulated activation of Gs by one of the gonadotropin receptors is attenuated through its association with the other gonadotropin receptor. Thus, the dimerization of the hLHR with hFSHR attenuates hCG or LH-stimulated cAMP, whereas the dimerization of the hFSHR with hLHR attenuates FSH-stimulated cAMP. Notably, it is the unliganded form of the hLHR in its basal state that attenuates FSHR activity and, conversely, the unliganded form of the hFSHR in its basal state that attenuates hLHR activity. This is in contrast to instances in which the binding of agonist or antagonist to one GPCR protomer in a heterodimer or heterooligomer alters the hormone-dependent signaling of other GPCR protomer(s) in the complex. For example, it has been shown that the CCR2, CCR5, and CXCR4 chemokine receptors oligomerize with each other and that antagonist binding to one receptor in the complex cross-inhibits functional activity of the other receptor protomers (4). Using a sophisticated experimental design to assess the functional ramification of homodimerization between dopamine receptors, Han et al (20) reported that inverse agonist binding to the secondary protomer (not engaged with Gs) enhanced signaling by the primary protomer (engaged with Gs), whereas agonist binding to the secondary protomer attenuated signaling by the secondary protomer. The seemingly contradictory effects of the activation of state of one protomer on the other protomer in the complex, as evidenced by these 2 studies, is not unusual in that allosteric effects between dimerized GPCRs appear to be very receptor dependent, and thus, no clear trend has been established in the literature.
We have previously shown that the dimerization between the wild-type (wt) hLHR and a mutant hLHR (D405N,Y546F) that is expressed on the cell surface and has normal binding affinity, but is profoundly impaired in signaling to Gs, causes an attenuation of hormone-dependent signaling through the wt receptor (11). The properties of the signaling impaired hLHR mutant were shown to resemble those of a wt hLHR stabilized in the resting state by an inverse agonist (11). Thus, in contrast to Han et al (20), we did not observe an enhancement of signaling through the wt hLHR by an associated protomer stabilized in the resting state. However, the attenuation of the wt hLHR by the signaling impaired mutant was relatively minor (∼2-fold increase in EC50 and no change in Rmax). In contrast, in this study we show that when the unoccupied hFSHR is dimerized with hLHR, it results in a much more profound attenuation of hormone-stimulated activation of Gs through the wt hLHR (∼1.4-fold increase in EC50 and ∼2–3-fold decrease in Rmax). The extent of attenuation of hormone-dependent signaling through the FSHR when it is heterodimerized with hLHR is similar. Because the basal activity of the wt hLHR or wt hFSHR is greater than that of the signaling impaired hLHR mutant, our findings agree with the general trend reported by Han et al (20). Thus, within a dimeric complex of GPCRs, a protomer with increased activity exerts more of a cross-attenuating effect on the other protomer. The further potential effects of an agonist-bound hLHR on heterocomplexed FSHR or an agonist-bound hFSHR on heterocomplexed hLHR are far more difficult to ascertain because the signaling pathways stimulated by the hLHR and hFSHR are the same. Therefore, we have not yet been able to satisfactorily address that question.
Although the hLHR and hFSHR do not coexist in the same cell type in the testes, they are coexpressed in mature ovarian granulosa cells. Therefore, gonadotropin signaling in mature granulosa cells may be modulated by the relative of expression of hLHR vs hFSHR, which would drive heterodimerization. Consequently, at low ratios of hLHR to hFSHR (such as in a less mature granulosa cell), in which much of the hLHR would be expected to be complexed with hFSHR, one would expect LH/CG-stimulated signaling through the hLHR to be attenuated; whereas at high ratios of hLHR to hFSHR (such as in the fully mature granulosa cell), in which much of the hFSHR would be expected to be complexed with hFSHR, one would expect FSH-stimulated signaling through the hFSHR to be attenuated. Interestingly, Donadeu and Ascoli et al (21) examined immature rat granulosa cells infected with adenovirus encoding recombinant hLHR and showed that increased densities of recombinant hLHR resulted in a large attenuation of FSH-stimulated cAMP production in the granulosa cells. It was speculated by these investigators that this phenomenon may have resulted from heterodimerization between the 2 gonadotropin receptors. Our studies provide experimental support for this hypothesis by demonstrating the physical association of the hLHR and hFSHR into dimeric complexes and further showing a similar pattern of cross-attenuation of signaling. Taken altogether, our data and theirs lend support toward the concept that gonadotropin signaling in granulosa cells is modulated not only by the density of each gonadotropin receptor but also by the relative cell surface expression of one to the other. Finally, because extragonadal LHR and FSHR are shown to be expressed and functional in an increasing number of tissues (22–27), potential alterations in signaling due to heterodimerization between the LHR and FSHR should be considered.
Acknowledgments
This work was supported by National Institutes of Health Grants HD022196 and DK068614.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BRET
- bioluminescence resonance energy transfer
- GFP
- green fluorescent protein
- GPCR
- G protein-coupled receptor
- HA
- hemagglutinin
- hCG
- human chorionic gonadotropin
- HEK
- human embryonic kidney
- hFSHR
- human follitropin receptor
- hLHR
- human lutropin receptor
- hMC3R
- human melanocortin 3 receptor
- wt
- wild type.
References
- 1. Dalrymple MB, Pfleger KD, Eidne KA. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol Ther. 2008;118:359–371 [DOI] [PubMed] [Google Scholar]
- 2. Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835 [DOI] [PubMed] [Google Scholar]
- 3. Guo W, Urizar E, Kralikova M, et al. Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J. 2008;27:2293–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sohy D, Yano H, de Nadai P, et al. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284:31270–31279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J. 2011;433:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fribourg M, Moreno JL, Holloway T, et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urizar E, Montanelli L, Loy T, et al. Glycoprotein hormone receptors: link between receptor homodimerization and negative cooperativity. EMBO J. 2005;24:1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tao YX, Johnson NB, Segaloff DL. Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J Biol Chem. 2004;279:5904–5914 [DOI] [PubMed] [Google Scholar]
- 9. Guan R, Feng X, Wu X, et al. Bioluminescence resonance energy transfer studies reveal constitutive dimerization of the human lutropin receptor and a lack of correlation between receptor activation and the propensity for dimerization. J Biol Chem. 2009;284:7483–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guan R, Wu X, Feng X, Zhang M, Hebert TE, Segaloff DL. Structural determinants underlying constitutive dimerization of unoccupied human follitropin receptors. Cell Signal. 2010;22:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Feng X, Guan R, Hebert TE, Segaloff DL. A cell surface inactive mutant of the human lutropin receptor (hLHR) attenuates signaling of wild-type or constitutively active receptors via heterodimerization. Cell Signal. 2009;21:1663–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang M, Guan R, Segaloff DL. Revisiting and questioning functional rescue between dimerized LH receptor mutants. Mol Endocrinol. 2012;26:655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang M, Tao YX, Ryan GL, Feng X, Fanelli F, Segaloff DL. Intrinsic differences in the response of the human lutropin receptor versus the human follitropin receptor to activating mutations. J Biol Chem. 2007;282:25527–25539 [DOI] [PubMed] [Google Scholar]
- 14. Mercier J-F, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J Biol Chem. 2002;277:44925–44931 [DOI] [PubMed] [Google Scholar]
- 15. Milligan G, Bouvier M. Methods to monitor the quaternary structure of G protein-coupled receptors. FEBS J. 2005;272:2914–2925 [DOI] [PubMed] [Google Scholar]
- 16. Ehrlich JR, Pourrier M, Weerapura M, et al. KvLQT1 modulates the distribution and biophysical properties of HERG. A novel α-subunit interaction between delayed rectifier currents. J Biol Chem. 2004;279:1233–1241 [DOI] [PubMed] [Google Scholar]
- 17. Rebois RV, Robitaille M, Gales C, et al. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818 [DOI] [PubMed] [Google Scholar]
- 18. El-Asmar L, Springael JY, Ballet S, Andrieu EU, Vassart G, Parmentier M. Evidence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol Pharmacol. 2005;67:460–469 [DOI] [PubMed] [Google Scholar]
- 19. Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M. Allosteric properties of G protein-coupled receptor oligomers. Pharmacol Ther. 2007;115:410–418 [DOI] [PubMed] [Google Scholar]
- 20. Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat Chem Biol. 2009;5(9):688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donadeu FX, Ascoli M. The differential effects of the gonadotropin receptors on aromatase expression in primary cultures of immature rat granulosa cells are highly dependent on the density of receptors expressed and the activation of the inositol phosphate cascade. Endocrinology. 2005;146:3907–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–1630 [DOI] [PubMed] [Google Scholar]
- 23. Zhang M, Shi H, Segaloff DL, Van Voorhis BJ. Expression and localization of luteinizing hormone receptor in the female mouse reproductive tract. Biol Reprod. 2001;64:179–187 [DOI] [PubMed] [Google Scholar]
- 24. Zygmunt M, Herr F, Keller-Schoenwetter S, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296 [DOI] [PubMed] [Google Scholar]
- 25. Banerjee P, Fazleabas AT. Extragonadal actions of chorionic gonadotropin. Rev Endocr Metab Disord. 2011;12:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260 [DOI] [PubMed] [Google Scholar]
- 27. Mizrachi D, Shemesh M. Expression of functional luteinising hormone receptor and its messenger ribonucleic acid in bovine cervix: luteinising hormone augmentation of intracellular cyclic AMP, phosphate inositol and cyclooxygenase. Mol Cell Endocrinol. 1999;157:191–200 [DOI] [PubMed] [Google Scholar]