Abstract
As for many human diseases, the incidence of obesity and its associated health risks are sexually dimorphic: worldwide the rate of obesity is higher in women. Sex differences in metabolism, appetite, body composition, and fat deposition are contributing biological factors. Gonadal hormones regulate the development of many sexually dimorphic traits in humans and animals, and, in addition, studies in mice indicate a role for direct genetic effects of sex chromosome dosage on body weight, deposition of fat, and circadian timing of feeding behavior. Specifically, mice of either sex with 2 X chromosomes, typical of normal females, have heavier body weights, gain more weight, and eat more food during the light portion of the day than mice of either sex with a single X chromosome. Here we test the effects of X chromosome dosage on body weight and report that gonadal females with 2 X chromosomes express higher levels of GH gene (Gh) mRNA in the preoptic area (POA) of the hypothalamus than females with 1 X chromosome and males. Furthermore, Gh expression in the POA of the hypothalamus of mice with 2 X chromosomes correlated with body weight; GH is known to have orexigenic properties. Acute infusion of GH into the POA increased immediate food intake in normal (XY) males. We propose that X inactivation–escaping genes modulate Gh expression and food intake, and this is part of the mechanism by which individuals with 2 X chromosomes are heavier than individuals with a single X chromosome.
Worldwide obesity is driving research on the biological, behavioral, and social risk factors associated with dysregulated energy homeostasis. One contributing factor is sex: the incidence of obesity is higher in women than in men (1). Sex differences in energy metabolism, cultural roles and activities, appetite, body composition, and excess fat deposition and distribution are considered among the likely contributing factors. Studies in humans and other mammals indicate that circulating gonadal hormones, such as androgens and estrogens, affect many of these sexually dimorphic body traits (2). Specifically, androgens increase lean muscle mass and lipolytic activity (3, 4), and estrogens cause fat to be distributed subcutaneously rather than viscerally (5, 6). However, metabolic differences between prepubertal boys and girls, who produce and secrete very low amounts of gonadal hormones into the circulation, implicate additional sex-specific contributing factors that could be nonhormonal in origin (7). Of relevance, mice have sex differences similar to those of humans in both the acquisition of weight gain and distribution of excess fat stores. This is in part due to the presence of 2 copies of the X chromosome in the female genome, compared with 1 copy of the X chromosome in males (8–10). This X chromosome dosage effect on adiposity is enhanced by the removal of gonads and their hormones (9). Therefore, apart from the effects of gonadal hormones, sex chromosome genes probably contribute to sex differences in energy homeostasis, fat storage, and perhaps obesity risk. Genes that escape X inactivation dosage compensation, which are more highly expressed in cells with 2 copies compared with 1 copy of the X chromosome, are one potential source of X chromosome dosage effects (11, 12).
Chen et al (9) revealed a sex chromosome effect on feeding behavior as one potential factor contributing to sex differences in adiposity of the four core genotypes (FCG) mice. In FCG mice, a nonfunctional mutation of the sex-determining region of Y (Sry) is replaced by an autosomal Sry transgene to unlink gonadal sex from sex chromosomes. Therefore, the FCG generates the following genotypes: XX females, XY females, XX males, and XY males. Both XX males and XX females in the C57BL/6 genetic background consumed more food than XY mice (males and females) during the light portion of the day. Similarly, mice with 2 X chromosomes in another mutant model (Y* mice; see Materials and Methods for details) consumed more food than mice with a single X chromosome (10). Taken together, the results of these studies suggest that the additional X chromosome in normal females contributes to sexually dimorphic feeding behavior. We recently reported a sex difference in GH gene (Gh) expression within the preoptic area (POA) and the arcuate nucleus of the hypothalamus (13), 2 brain regions that regulate metabolism and energy homeostasis. GH has orexigenic effects on feeding (14), and hypothalamic Gh expression is higher in female than in male mouse brains, especially when both sexes of mice were gonadectomized and given replacement estradiol (13). Importantly, estradiol did not change neural Gh expression in males. This result may indicate that sex-specific factors, other than gonadal hormones, influence this sex difference.
GH is a 22-kDa (190-amino acid) protein that stimulates tissue growth in the periphery. It is synthesized in somatotropic cells of the anterior pituitary, and pulsatile release of GH is regulated by GHRH and somatostatin neurosecretory cells of the hypothalamus (15). The principal target of GH signaling is the liver. In fact, most sex differences in the liver depend on GH (16, 17). Activation of cell surface GH receptors (GHRs) increases liver expression and secretion of IGF-I (18). Initially, discoveries of GHRs in brains of rats and humans were perplexing, because GH is a large protein that cannot passively diffuse across the blood-brain barrier (19–22). However, we now know that GH is expressed directly by neurons (13, 23–25). Of relevance, exogenous GH administration has orexigenic effects in rodents and humans (14, 26–28).
We tested 2 hypotheses regarding the sex difference in neural Gh expression and its possible effects on body weight and feeding behavior. First, we hypothesized that the sex difference in POA Gh expression (13) is correlated with X chromosome dosage. We measured Gh levels in the POA of an established mouse model used to determine the effects of 1 vs 2 copies of the X chromosome: the Y* model (29, 30). Next we assayed mRNA levels of genes that escape X inactivation and confirmed that X chromosome dosage affects their expression in the POA. We also found that the relative level of expression of 2 X inactivation–escaping genes, Kdm5c and Kdm6a, correlated with the level of Gh expression. Finally, we tested the hypothesis that GH in the POA affects feeding behavior and found that GH infused into the POA of normal mice increased acute food intake.
Materials and Methods
Animals and surgical procedures
Male and female mice from the Y* line on the C57BL/6EiJ background were used in experiments 1 and 2. The Y* breeding colony was originally started with B6Ei.LT-Y*/EiJ males and C57BL/6EiJ females purchased from The Jackson Laboratory (Bar Harbor, Maine; stock numbers 002021 and 000924), and maintained in the B6Ei substrain. Male breeders possess a Y chromosome variant (Y*) with an inverted duplication in the pseudoautosomal region, which shares homology with X and is important for recombination during meiosis. In addition to Y* and X, Y* male gametes produce 2 altered sex chromosomes that have undergone inverted recombination during meiosis: one chromosome consists of a pseudoautosomal region and no Y- or X-specific genes (Y*X), and a second contains both Y- and X-specific genes (XY*). The offspring from XX females and XY* males all inherit a maternal X, and 1 of the 4 paternal sex chromosomes (30). Thus, male and female offspring can have either 1 or 2 copies of X genes and only males will have Y genes. For purposes of simplicity, the 4 offspring from this cross will be referred to as X0 females (1XF), XX females (2XF), XY males (1XM), and XXY males (2XM). For more details about the genetics of this strain, see reviews and primary literature characterization (29–32). Y* mice were genotyped by reverse transcription of RNA and PCR amplification of Xist as described previously (32, 33). For experiment 3, male C57BL/6J mice were used. All mice were bred and housed at the University of Virginia and were group housed after weaning. The University of Virginia Committee on the Use and Care of Animals approved all procedures involving live animals. Adult mice were gonadectomized under general isoflurane anesthesia, and testosterone was replaced with a Silastic tubing (1.02-mm inner diameter × 2.16-mm outer diameter; Dow Corning, Midland, Michigan) capsule, filled with 1 cm of crystalline testosterone, that was sealed at both ends with silicone adhesive and placed under the skin in the back of the neck. After gonadectomy, mice were singly housed in a reverse 12-h light-dark cycle room with lights on at midnight and lights off at noon Eastern Standard Time and allowed to recover for 1 to 2 weeks before further use. For brain cannulation operations in experiment 3, mice received (0.1 mL/20-g mouse) ketamine-xylazine anesthesia (ip).
Experiment 1: X gene dosage effects on body weight over development
Body weight data were collected from pups generated in our Y* breeding colony over the course of several months (postnatal day [P] 10, n = 96; P21, n = 165). Weights were measured from 24 and 32 1XF, 28 and 52 2XF, 20 and 41 1XM, and 30 and 40 2XM mice at P10 and P21, respectively. In addition, 69 adult mice were weighed immediately before gonadectomy and testosterone replacement (P52en]P71) and again 2 weeks after surgery (n = 15 1XF, n = 13 2XF, n = 21 1XM, and n = 20 2XM mice at gonadectomy; 2 weeks later, n = 14 1XF, n = 13 2XF, n = 19 1XM, and n = 17 2XM mice).
Experiment 2: GH and X inactivation–escaping gene expression in the POA of the hypothalamus
A cohort of 60 male and female adult Y* mice were gonadectomized, and testosterone was replaced as described above. Six to 8 weeks after surgery, whole brains were collected, fresh frozen on crushed dry ice, and kept at −80°C until further processing for RNA isolation from the POA. Expression levels for 2 genes that escape X inactivation, Ddx3x and Eif2s3x, were measured by quantitative real-time PCR (qRT-PCR) from 6 mice in each of the 4 Y* genotypes (24 total mice). Two additional X inactivation–escaping genes, Kdm5c and Kdm6a, were measured from 8 1XF, 8 2XF, 10 1XM, and 15 2XM mice (41 total mice). GH gene (Gh) mRNA in the POA was quantified from all 60 mice (n = 14 1XF, n = 14 2XF, n = 13 1XM, and n = 19 2XM mice). A subset of these mice consisting of 44 total animals (10 1XF, 7 2XF, 10 1XM, and 17 2XM) were measured for body weight at the time of killing, and these values were used for correlational analysis of body weight and Gh mRNA levels in the POA.
Experiment 3: Effects of GH infusion on feeding
We tested normal (XY) males, which have low basal neural GH and no sex chromosome abnormalities, to determine whether food intake is modified by GH in the POA. Fourteen C57BL/6J adult castrated and testosterone-replaced males were used. After recovery from gonadectomy, a stainless-steel 26-gauge guide cannula (Plastics One, Roanoke, Virginia; catalog no. C235GS-5–0.8/SP) was lowered into the right POA at 0.1 mm rostral, 0.4 mm lateral, and 4.8 mm ventral to bregma and fastened to the skull with dental cement. The injector cannula (C235IS-5/SP) protruded 0.2 mm below the guide cannula and was attached to tubing connected to a syringe on an infusion pump. Two to 3 weeks after cannulation, all food was removed before the time the room lights went off, and mice were food deprived for 24 hours. Fifteen minutes before food was reintroduced, mice were infused over a 1-minute period with 0.05 μg of recombinant mouse GH in 0.5 μL of artificial cerebrospinal fluid (aCSF) or vehicle only. Mice were placed into their cages and each received preweighed powdered chow. The amount of food consumed (in grams) during refeeding was measured 2, 4, 6, and 12 hours later. One week later the infusion and refeeding experiment was repeated for a second trial, and treatments were counter-balanced. After both behavior tests were completed, the mice were killed, and brains were collected, frozen, and sectioned to confirm cannula placement just above the POA. The median infusion site in the brain was 0.04 mm caudal to bregma, with a range of 0.38 and −0.34 mm (34).
RNA isolation
Fresh frozen Y* brain tissue was cut into 120-μm coronal sections in a cryostat onto glass Superfrost Plus slides. From 8 sections, between 0.4 mm rostral and 0.6 mm caudal to bregma, 1.5-mm-wide punches of the POA were taken from the ventral surface to just below the anterior commissure (∼1 mm high). RNA was isolated from the punches using RNeasy spin column purification (QIAGEN, Valencia, California) as described previously (13, 35, 36).
qRT-PCR
cDNA was made from 100 ng of RNA using a High Capacity cDNA Kit with random primers (Applied Biosystems, Foster City, California), and qRT-PCR was performed in triplicate reactions with Fast SYBR Green Master Mix (Applied Biosystems), 1 ng of cDNA, and 100 nM concentrations of primers on an ABI StepOnePlus thermal cycler (Applied Biosystems). No reverse transcriptase control reactions ruled out amplification from genomic DNA. Oligonucleotide primers (Invitrogen, Carlsbad, California) were designed as described previously for Kdm5c, Kdm6a, Ddx3x, Eif2s3x, Gh, and Ppib (endogenous control) mRNA transcripts (Table 1). Kdm5c, Kdm6a, Ddx3x, and Eif2s3x are located on the X chromosome and are known to escape X inactivation. All primer pairs were verified as being 90% to 110% efficient in standard curve reactions and amplified a single product determined by melting-curve analysis. Relative quantifications of mRNA levels were measured by the ΔΔCt method with StepOne Software v2.2 (Applied Biosystems).
Table 1.
Primers for qRT-PCR
| Transcript | Accession No. | Direction | Nucleotide Sequence (5′ Position) |
|---|---|---|---|
| Ppib | NM_011149.2 | F | 5′-TGGAGAGCACCAAGACAGACA (642) |
| R | 5′-TGCCGGAGTCGACAATGAT (707) | ||
| Kdm5c | NM_013668.3 | F | 5′-GAGGCCCAGACAAGAGTGAAA (557) |
| R | 5′-TTGGGAATCTTTAAGGATGAGCC (642) | ||
| Kdm6a | NM_009483.1 | F | 5′-CGGGCGGACAAAAGAAGAAC (4299) |
| R | 5′-CATAGACTTGCATCAGATCCTCC (4501) | ||
| Eif2s3x | NM_012010.3 | F | 5′-GGTGAGGGTGGAGTGACTCT (28) |
| R | 5′-TTCCCATGAGCTACGTGACCA (179) | ||
| Ddx3x | NM_010028.3 | F | 5′-CAGAGTGGAGGAAGTACAGCA (170) |
| R | 5′-TCACCCCGTGATCCAAAACTG (321) | ||
| Gh | NM_008117.2 | F | 5′-AGGCCCAGCAGAGAACCGACA (333) |
| R | 5′-ACGGTCCGAGGTGCCGAACA (460) |
Oligonucleotide sequence of primers used to amplify the mRNA transcripts of the genes Kdm5c (lysine-specific demethylase 5C), Kdm6a (lysine specific demethylase 6A), Eif2s3x (eukaryotic translation initiation factor 2, subunit 3, structural gene X-linked), Ddx3x (DEAD/H box polypeptide 3, X-linked), Gh (growth hormone), and Ppib (cyclophilin B). Accession numbers are from the National Center for Biotechnology Information Reference Sequence database. Forward (F) and reverse (R) primers anneal to cDNA antisense and sense strands, respectively. Numbers in parentheses indicate the primers' 5′-nucleotide position on the reference sequence.
Statistics
Body weight and qRT-PCR measurements were analyzed with 2-way ANOVA using gonadal sex and X chromosome dose as the 2 main factors. To meet the assumption of equal variance between groups for ANOVA, the Gh values were log-transformed. Fisher least significant difference posttests were used to compare differences between groups. Nonparametric Spearmen analyses were used for all correlations. Linear modeling and tests for multicollinearity were performed in R (free software, version 2.15.1). A difference in the correlation coefficients of Gh mRNA and body weight was assessed using the Fisher r-to-z transformation calculator on the VassarStats web site (http://www.vassarstats.net/rdiff.html). To analyze the effects of GH infusion on feeding behavior, the average grams of food consumed per hour was calculated for the first 6 hours after infusion and for the next 6 hours after infusion (6–12 hours after infusion) for both treatments in all animals. A 2-factor ANOVA was used to assess the main effects of GH infusion (aCSF control vs GH) and trial (first testing trial vs second testing trial), and values were matched by subject to account for the variability among subjects.
Results
Body weights correlate with X chromosome numbers at all ages
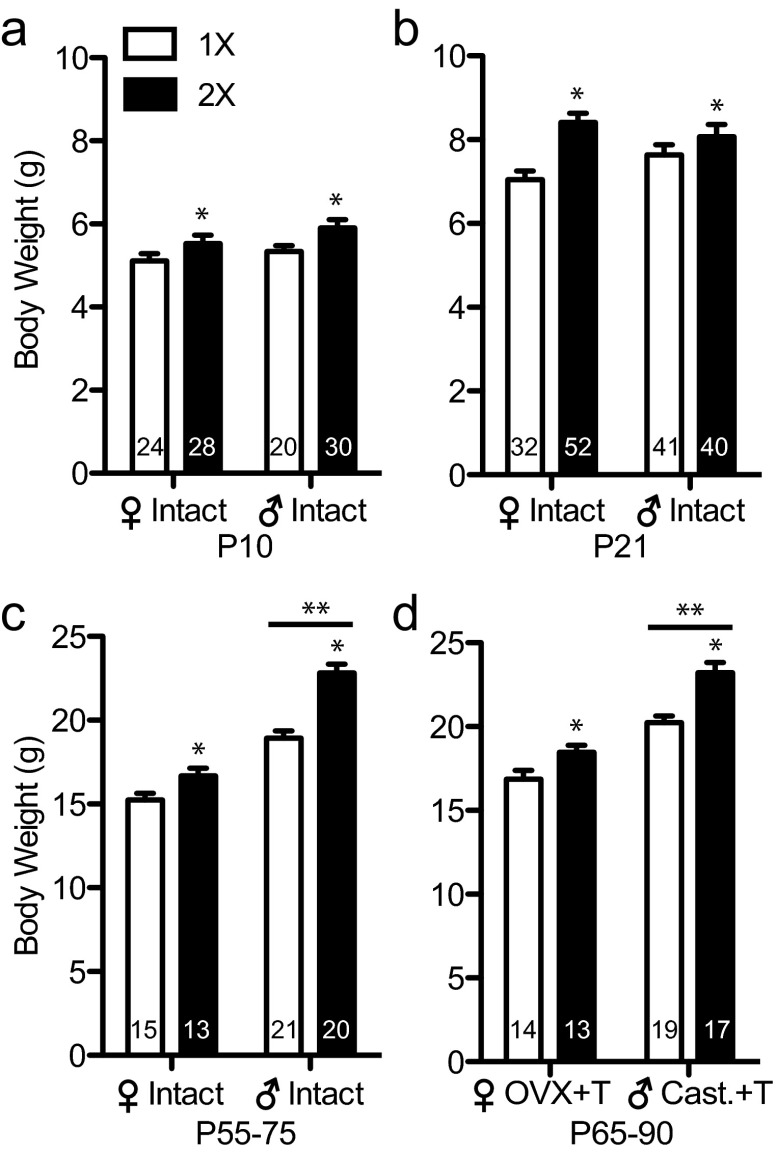
As reported previously with the FCG, Y*, and other genetic models of sex chromosome dosage (8, 9), the X chromosome dose affected the overall body weight of adult mice. We extend those earlier observations in adults to additional hormone conditions and ages. Y* mice with 2 X chromosomes (both sexes combined) are heavier than mice with a single X chromosome before puberty and as adults after testosterone treatment (Figure 1). X chromosome dosage effects on body weight were found at P10 (F1, 98 = 6.69, P < .05), P21 (F1, 161 = 13.1, P < .001), between P52 and P75 (F1, 65=31.4, P < .0001) in gonad intact mice, and between P65 and P90 (F1, 59=21.1, P < .0001) in gonadectomized and testosterone-replaced mice. There was also a main effect of gonadal sex in both gonad-intact (F1, 65=107, P < .0001) and gonadectomized (F1, 59=66.0, P < .0001) adults; males were heavier than females. A significant interaction between gonads and sex chromosomes was present in gonad-intact adults (F1, 65=6.66, P < .05). Posttests showed that testes-bearing 1XM and 2XM mice were significantly different (P < .001), but ovary-intact 1XF and 2XF mice were not significantly different (P > .05). Importantly, sex differences in body weight did not develop until after puberty, concurrent with the normal onset of adult levels of gonadal hormones.
Figure 1.
Y* mice with 2 X chromosomes are heavier than mice with 1 X chromosome. Histograms indicate the mean ± SEM grams of body weight. a, P10 mice with 2 X chromosomes are heavier than mice with 1 X chromosome. b, P21 mice with 2 X chromosomes are heavier than mice with 1 X chromosome. c, P52 to P75 gonad intact mice with 2 X chromosomes and males are heavier than mice with 1 X chromosome and females, respectively. d, After about 2 weeks of recovery from gonadectomy with testosterone replacement, adult mice with 2 X chromosomes, and males are still heavier than mice with 1 X chromosome and females, respectively. 1X, mice with 1 copy of the X chromosome (□); 2X, mice with 2 copies of the X chromosome (■); [female] Intact, females with ovaries; [male] Intact, males with testes; [female] OVX+T, adult testosterone-treated OVX females; [male] Cast.+T, adult testosterone-treated castrated males. *, Significant difference between 2X compared with 1X mice, P < .05; **, significant difference between males and females, P < .05.
Genes that escape X inactivation are highly expressed in the POA of 2 X chromosome mice
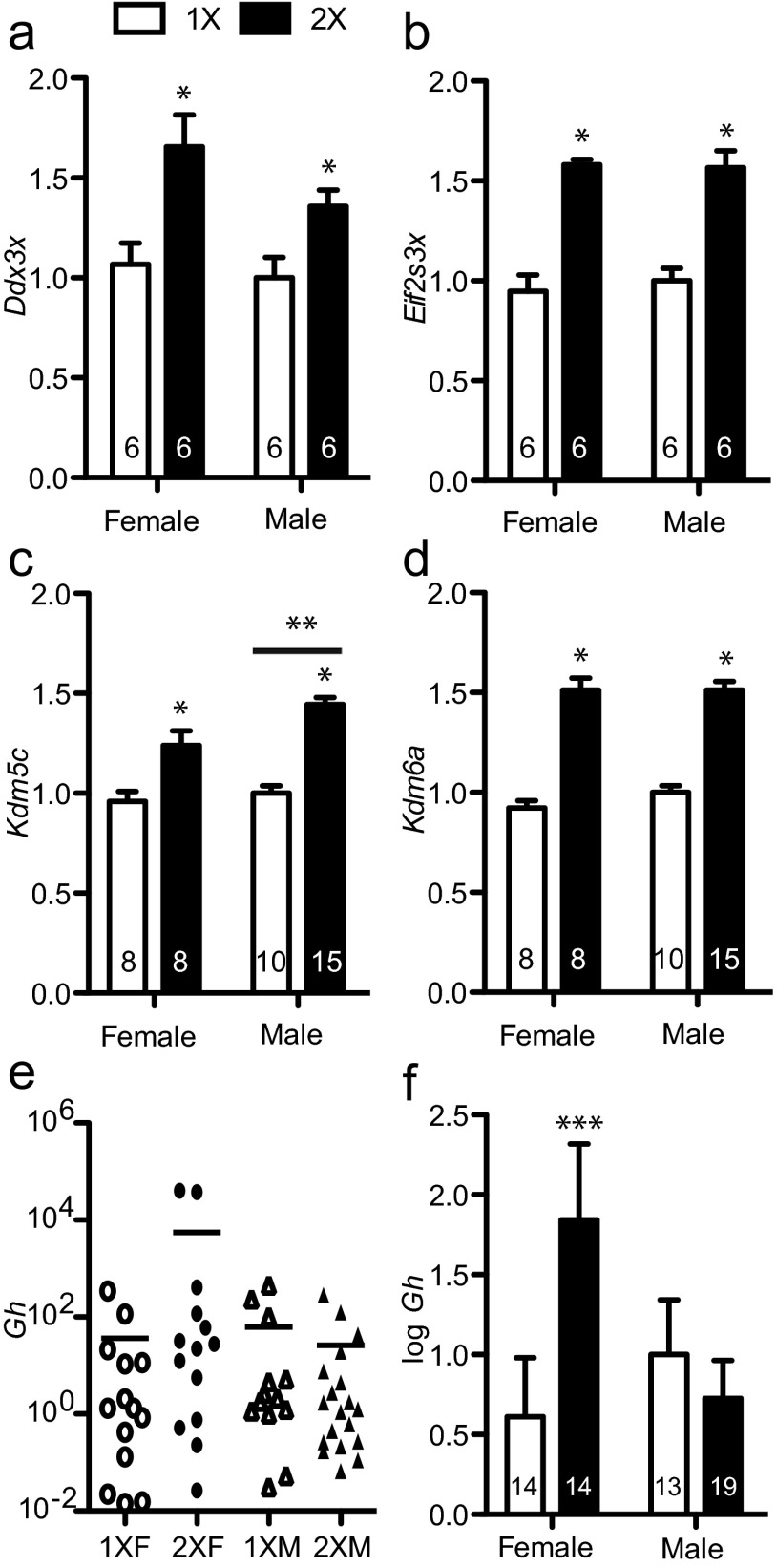
To test whether X chromosome dosage affected gene expression in the POA, qRT-PCR was performed on RNA for Kdm5c, Kdm6a, Ddx3x, and Eif2s3x (Figure 2); these are 4 genes are known to escape X inactivation in many tissues, including other brain regions. In the POA, strong effects of the X chromosome dose were found for expression of Kdm5c (F1, 37 = 57.1, P < .0001), Kdm6a (F1, 37 = 141, P < .0001), Ddx3x (F1, 20 = 16.6, P < .001), and Eif2s3x (F1, 20 = 77.4, P < .0001). As expected for genes that escape X inactivation, all these genes were more highly expressed in 2X mice than in 1X mice. A small effect of gonadal sex was found on expression of Kdm5c (F1,37 = 6.63, P < .05) with males having higher levels than females. The POA is known to play a role in modulating energy homeostasis (37), and we have previously reported a sex difference in GH in this area (13). Expression of Gh in the POA was highly variable; thus, Gh relative quantification values were log-transformed to normalize the variance. An interaction between gonadal sex and X chromosome dosage was revealed for Gh in the POA (F1, 56 = 4.50, P < .05). Females with 2 X chromosomes had the highest Gh expression and were significantly different from 1X females and 2X males (Figure 2, e and f).
Figure 2.
Genes that escape X inactivation are more highly expressed in the POA of the hypothalamus of mice with 2 X chromosomes compared with mice with 1 X chromosome. Histograms show the mean ± SEM relative quantification of mRNA concentration. All values are normalized to the average 1XM concentration that is set to a value of 1. All histograms are plotted as linear values with the exception of panel e, which is on a log scale. a–d, X inactivation escapee genes Ddx3x, Eif2s3x, Kdm5c, and Kdm6a are more highly expressed in the POA of 2X compared with 1X mice. e, Gh expression in the POA is variable. f, Log-transformed Gh expression in the POA from panel e. 1X, mice with 1 copy of X genes (□); 2X, mice with 2 copies of X genes (■); [female] OVX+T, adult testosterone-treated OVX females; [male] Cast.+T, adult testosterone-treated castrated males. *, Significant difference between 2X compared with 1X mice, P < .05; **, significant difference between males and females, P < .05; ***, 2X females are significantly different from 2XM and 1XF groups, P < .05.
Gh expression in the POA is related to Kdm5c and Kdm6a and body weights
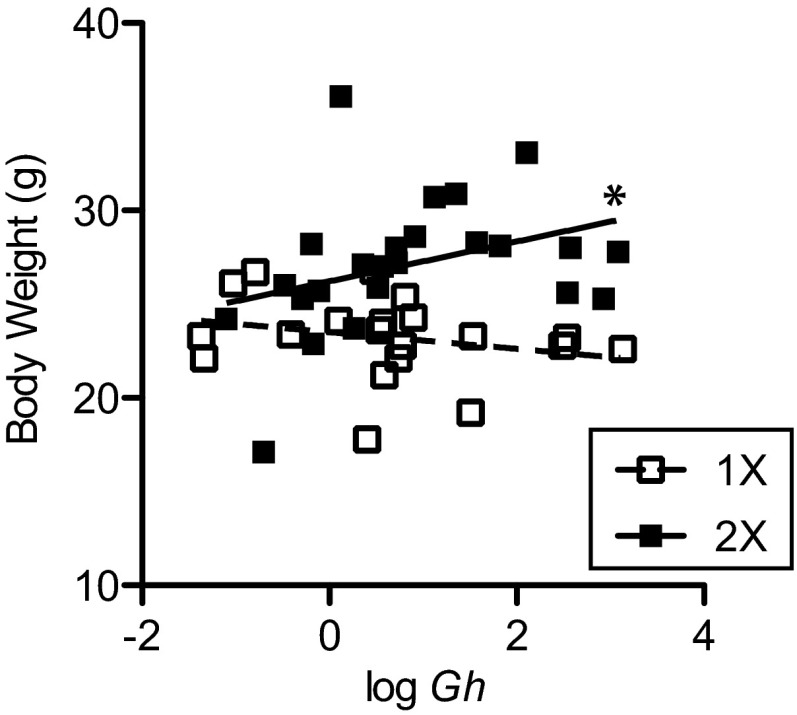
The X inactivation–escaping genes we measured above are involved in RNA transcription (Kdm5c and Kdm6a) and protein translation (Eif2s3x and Ddx3x); therefore, correlations between these genes and Gh were conducted to assess the prospect that these X chromosome genes may directly or indirectly regulate Gh gene expression in the POA of individual mice. Independently, none of the X genes significantly correlated with Gh expression (Table 2). We fitted the data to a multivariate linear model: y = b0 + b1x1 + b2x2 + b3x3 + b4x4. In this model the dependent variable “y” equals logGh expression, and the independent variables “x1” to “x4” are the expression levels of the 4 genes that escape X inactivation in the POA. Statistical indicators revealed that this model was overspecified due to the multicollinearity of Kdm6a with Eif2s3x and Ddx3x; therefore, the model was simplified to include only independent variable terms for Kdm6a and Kdm5c to eliminate the redundant information. The estimated coefficients in the reduced model where x1 = Kdm6a and x2 = Kdm5c were as follows: intercept b0 = 1.02 ± 1.00 (t = 1.02); b1 = 4.62 ± 1.12 (t = 4.12, P < .001); and b2 = −4.95 ± 1.35 (t = −3.66, P < .001). Thus, the best-fit plane through the data points of logGh in terms of Kdm6a (K6a) and Kdm5c (K5c) is defined by the equation logGh = 1.02 + 4.62K6a − 4.95K5c (multiple r2 = 0.315, residual SE = 1.3; F2, 38 = 8.72, P < .001). The positive and negative slopes associated with the K6a and K5c, respectively, show that Gh levels directly correlate with Kdm6a levels and inversely correlate with Kdm5c. We also examined whether the amount of Gh expression in the POA correlated with overall body weight for a group of 44 Y* mice. Indeed, Gh expression in the POA was correlated with body weight in 2X mice (r = 0.44, P < .05) but not in 1X mice (r = −0.27) (Figure 3). The correlation coefficients for Gh and body weight were significantly different between 2X and 1X mice (z = 2.3, P < .05).
Table 2.
Cross-Correlation Matrix of X Genes and Gh
| Ddx3x | Eif2s3x | Kdm5c | Kdm6a | |
|---|---|---|---|---|
| Eif2s3x | 0.86a | |||
| Kdm5c | 0.63b | 0.75a | ||
| Kdm6a | 0.86a | 0.88a | 0.81a | |
| Gh | 0.37 | 0.24 | −0.12 | 0.21 |
Spearman correlation coefficients and significance: aP < .001;
P < .01.
Figure 3.
GH gene expression in the POA correlates with body weight in 2X mice. Scatter plots of Gh expression in the POA are shown on the x-axis and body weight in grams on the y-axis. All animals were gonadectomized as adults and given testosterone implants. Gh expression in the POA of the hypothalamus in 2X mice correlates with body weight. □, 1X, regression and data points of 1X mice (male and female) data points; ■, 2X, regression line and data points of 2X mice (male and female) data points. *, Significant correlation for 2X POA, P < .05.
GH increases short-term food consumption
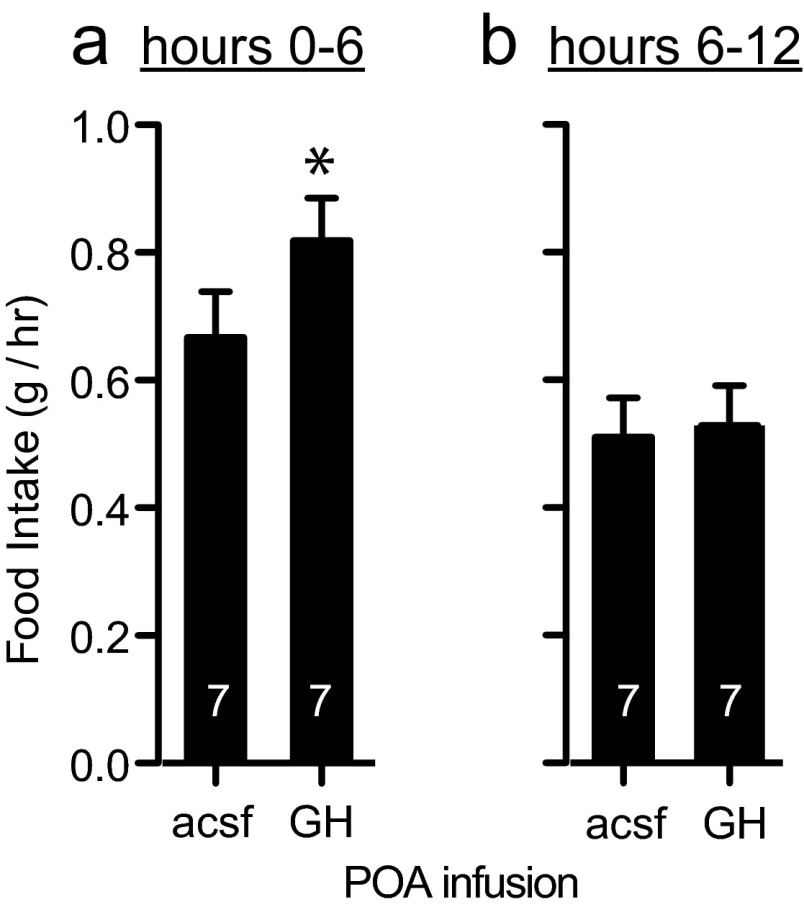
We compared food intake after a 24-hour fast in 14 normal (XY) males after infusion of either recombinant mouse GH or aCSF vehicle directly into the POA. After 24 hours of food deprivation, half the males were infused into the POA with aCSF vehicle and half were infused with 0.05 μg of GH in aCSF. Immediately and for 12 hours after infusion, food consumption was measured. One week later, the mice were tested again for feeding after 24 hours of food deprivation, but in this second trial each mouse received the opposite treatment. Therefore, each mouse was tested twice, once under each treatment, but half were tested in the order GH/aCSF and half were tested in the order aCSF/GH. Using both treatment and trial as main factors in an ANOVA matching by subjects, we found that both GH treatment (F1, 12 = 5.00, P < .05) and trial (F1, 12 = 4.91, P < .05) affected food consumption during the first 6 hours after infusion and refeeding (subject error; F1, 12 = 0.13). Infusion of GH increased the average rate of feeding by 23.0% in trial 1 and by 22.8% in trial 2 (Figure 4a). The trial effect is most likely due to experience with the task rather than carryover of GH treatment from trial 1, because both treatment groups (control and GH) had increased food intake in trial 2. Furthermore, the effects of GH infusion on the rate of feeding appeared to be acute, because food intake in hours 0 to 6 after infusion showed an effect of GH treatment, but treatment had no effect on food intake 6 to 12 hours after infusion (Figure 4b).
Figure 4.
Infusion of GH into the POA increased food intake. a, GH infusion increased food intake (grams per hour) during the first 6 hours after 24 hours of food deprivation. b, During hours 6–12 after reintroduction of food, there was no difference in food intake between GH and control infused mice. *, Significantly higher food intake after GH infusion compared to control infusion, P < .05.
Discussion
Here we report a positive correlation between body weight and Gh expression in the POA of 2X mice taken together with the short-term effects of acute infuse of GH on food intake, we suggest that neural GH is involved in the mechanism by which sex chromosome dosage affects sexually dimorphic body weight. These data have important implications for the orexigenic effects of GH and on the underlying mechanisms involved in sex differences in energy homeostasis and body deposition of excess energy stores. GH signaling in the body is well known to promote increased muscle mass and decreased fat mass and has sex-specific effects on subcutaneous adiposity in humans (38). In addition to its major trophic properties on peripheral somatic cells, GH has been reported to have neurological effects on cognition, mood, and neuroprotection (20, 39). Furthermore, GH has been shown to increase appetite (14, 26–28, 40). Peripheral GH treatments increase the appetite of GH-deficient patients and feeding in rodents (27, 28). Mice overexpressing the Gh gene, conditionally restricted to the central nervous system, eat more food per day than control mice, and intracerebral ventricular infusion of GH increases feeding over a 3-hour period (14). The increase in fat mass of mice with 2 X chromosomes (9), in a C57BL/6J background, correlated with increased food consumption during the inactive, light portion of the day. In Y* mice on an MF1 background, 2X mice consumed more food than 1X mice during the dark (10). Therefore, excessive neural Gh expression may cause mice with 2 X chromosomes to overeat. The lack of a positive correlation between Gh and body weight in 1X mice (actually a tendency for a negative correlation) could indicate that Gh levels are correctly regulated by excess energy stores in a mouse with a single X chromosome: Gh increasing when stores are low and falling when metabolic stores are high.
GH protein and GH receptors are present in the brain of humans and rodents (20, 21, 39, 41, 42). Sexually dimorphic GH signaling in the brain is indicated in humans and rats. Compared with those in males, the female hippocampus and hypothalamus binds more exogenous GH (22, 43). The production of GH in the brain was first discovered in the hippocampus and lateral hypothalamus of rats (23, 24, 44). Moreover, increased levels of GH in the hippocampus of female rats are noted on the estrus day, when estradiol is administered and after stress (24). Gh mRNA is sexually dimorphic in the C57BL/6J mouse brain, with higher levels of expression found in the POA and arcuate nucleus of females compared with those in males (13). Ovariectomized (OVX) females with estradiol replacement had higher levels of Gh than control treated OVX females; however, estradiol treatment did not increase the level of Gh mRNA in brains of castrates. The effects of androgen on Gh expression in the POA have not been tested directly; however, because Gh expression in castrates is largely unresponsive to estradiol, the androgen receptor may be involved in this sex difference. It is also possible that neonatal exposure to androgens, which normally occurs in males and not in females (45), organizes this sexual dimorphism.
In addition to gonadal hormones, an emerging principle of sexual differentiation is that sex chromosome complement is responsible for many sex differences (46). Here we measured Gh mRNA levels in the POA of Y* mice that were gonadectomized and replaced with testosterone implants to normalize hormone levels. Although 2XF mice as a group had significantly higher expression of Gh in the POA than 1XF mice, the same could not be said for the comparison of 2XM to 1XM Gh levels. Thus, it appears that gonadal sex interacts with X chromosome number in males to decrease Gh levels in the POA. Because neither activational androgens nor estrogens are capable of inducing high female levels of Gh in the POA of males, it is likely that organizational androgens during development or the presence of Y genes reduce the expression of Gh in the male POA.
X chromosome dosage effects on phenotypes are potentially caused by 3 mechanisms: genes that escape X inactivation dosage compensation, paternally imprinted X genes, and X chromosome mosaicism (29, 47). A system of dosage compensation has evolved in mammals, whereby the expression of X chromosome genes in females is reduced to the male level by inactivation of 1 of their 2 X chromosomes (48). Most X genes in the cells of XX female mammals are only expressed from the copy on the euchromatic, transcriptionally active X chromosome, but a substantial number of the genes on the heterochromatic, transcriptionally “silenced” X chromosome escape X inactivation (49–51). In humans, about 15% of X-specific genes are transcribed from both the active and inactive X chromosome (52), and about 3% also escape silencing in mice (12, 53). The consequence of X inactivation escape is that these genes are more highly expressed in the brain and other tissues of XX than in XY animals (54–58). Here we show that the X genes Ddx3x, Eif2s3x, Kdm5c, and Kdm6a were expressed at higher levels in the POA of 2X than 1X mice. These genes are known to escape X inactivation dosage compensation in mice and humans and are found at higher concentrations in specific regions of a normal XX female than in a normal XY male mouse brain (53–55, 57, 58). Therefore, similar to other brain regions and tissues examined, X chromosome dosage affects gene expression in the POA for at least a subset of genes the escape X inactivation.
The differentially expressed X genes we assayed in the POA encode proteins that are involved in processes of RNA and protein expression (11, 12). Eif2s3x (eukaryotic translation initiation factor 2, subunit 3, structural gene X-linked) encodes the γ subunit of translation initiation factor EIF-2 in mice and humans (58, 59). Ddx3x (DEAD/H [Asp-Glu-Ala-Asp/His] box polypeptide 3, X-linked) encodes an RNA helicase that is transported in an anterograde direction along dendrites and is probably involved in protein translation locally at postsynaptic terminals (60). The other 2 genes, Kdm5c and Kdm6a, encode the lysine demethylase (KDM) transcription cofactors KDM5C and KDM6A, respectively (61–65). KDMs are a class of transcriptional coregulators with enzymatic activity that remove methyl groups from specific lysine residues within the N-terminal tails of histone subunits (65, 66). KDMs in general, including KDM5C and KDM6A, are an important class of proteins involved in neural development and plasticity, neuronal survival, and brain function (65, 67–70).
We found that Gh mRNA levels in the POA varied significantly as a function of Kdm5c and Kdm6a mRNA levels in a multivariate linear regression model. Both KDM5C and KDM6A transcription factors affect expression of downstream target genes located throughout the genome (71, 72). KDM5C removes di- and trimethylation modifications from lysine residue 4 of histone H3 subunits (H3K4me2/3 modifications), a histone mark associated with active gene transcription (67). It is found in a protein complex with RE-1 silencing transcription factor (REST), which silences neuron-specific genes such as sodium channel type 2A and synapsin I (72). Furthermore, mutations of the human homolog of Kdm5c are strongly linked to forms of X-linked mental retardation (73). Another member of the KDM5 family of H3K4me2/3 demethylases (KDM5A) has been shown to interact with nuclear hormone receptors to activate transcription (65, 74, 75). KDM5C is also of interest because its specificity for H3K4 demethylase activity is shared with that of another demethylase associated with Gh expression in somatotropes. The H3K4me1/2 demethylase KDM1 is recruited to the Gh promoter, and it is important for the differentiation of GH-producing somatotropic cells in the anterior pituitary (74). The negative slope we found associated with the Kdm5c term in the Gh regression analysis could suggest that high levels of Kdm5c repress neural Gh expression in the POA and would be consistent with the role of KDM5C transcriptional repression.
Conversely, KDM6A is a transcriptional coactivator, because it specifically demethylates H3K27me2/3 chromatin modifications associated with silenced genes (76). The positive slope in the regression equation associated with Kdm6a suggests that high levels of Kdm6a may promote Gh expression in the POA. Interestingly, bivalent domains are marked by both repressive H3K27me2/3 and permissive H3K4me3 histone modification (77); under these conditions transcription may be enhanced or suppressed by histone demethylases. The fact that Gh is an autosomally encoded gene, whose expression in the POA varies as a function of Kdm5c and Kdm6a, indicates that the Gh gene is a potential direct or indirect target of X gene activity.
Two recent studies examining sex chromosome complement effects on body weight in mice found that gonadectomized mice with 2 X chromosomes gained more weight over a 10-month period, with an increase in fat deposits, than mice with a single X chromosome (9, 10). Mice with 2 X chromosomes also had a higher percentage of fat vs lean body mass when fed chow with a normal fat content and experienced more adverse metabolic consequences when fed a high-fat diet. In agreement with these reports (8–10), we show a clear X chromosome dosage effect on total body weight in adults on a C57BL/6EiJ background and extended the phenomenon to include prepubertal ages. At all ages 2X Y* mice were heavier than 1X mice of both sexes, and this X gene dosage effect was apparent by P10; earlier ages were not examined. Unlike the situation for adults, males were not heavier than females at P10 and P21. Our finding indicates that the onset of production of adult levels of gonadal hormones at puberty, in addition to sex chromosome complement, is responsible for body mass sex differences in adults. In contrast, when gonadal hormones are removed in adults, 2X mice remain heavier than 1X mice, thus demonstrating that gonadal hormones are needed to maintain the gonadal sex difference in body weight (9).
In contrast to our results in Y* mice on a C57BL/6J background, Chen et al (9, 10) found no sex chromosome effect in P21 mice of the FCG on either the random bred MF1 or the C57BL/6J backgrounds. Furthermore, the later report showed that the presence of a second sex chromosome in MF1, either X or Y, can have an impact on adult body weight and adiposity. Genetic differences between FCG and Y* and between C57BL/6 and MF1 could explain the inconsistencies between the studies. For instance, the Y chromosomes in FCG and Y* mice are derived from different genetic background strains (SV/129 and LT/Sv, respectively) (30, 78). Y genes are known to cause differences in behavior; for example, the genetic origin of the Y chromosome affects male aggression and sexual behaviors (35, 79–81). Genetic polymorphisms between C57BL/6 and MF1 X chromosomes and X gene interactions with strain autosomal polymorphisms are a second source of genetic variation. Our result that a 2 X chromosome dosage increases adult body weight in C57BL/6J mice is consistent with the previous finding in this background strain (9).
In sum, our data show that X chromosome dosage is associated with neural Gh production and that acute GH increased food intake. We hypothesize that overproduction of neural GH in mice with 2 X chromosomes is part of the mechanisms by which animals with 2 X chromosomes (typically females) are heavier than individuals with 1 X chromosome. The correlation between Gh mRNA levels and the combined levels of X inactivation escape genes Kdm5c and Kdm6a in the POA suggest a potential genetic pathway by which 2 X chromosomes promotes increased neural GH and perhaps increased food intake. Future studies using transgenic manipulation of gene expression may help determine which X chromosome genes cause higher neural GH expression. Further understanding of a direct genetic mechanism through a sex chromosome complement that causes sex differences in excess feeding and fat stores will guide approaches to development of sex-specific therapies for the obesity epidemic (6).
Acknowledgments
We thank Aileen Ryalls and Savera Shetty for technical expertise.
This work was supported by National Institutes of Health Grant R01 MH057759. P.J.B. was supported by Grant T32 GM08715 and is currently supported by Grant T32DK091317–02.
Present address for P.J.B.: Department of Neurobiology and Anatomy, University of Utah, 20 North 1900 East, Salt Lake City, Utah 84132–3401.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- FCG
- four core genotypes
- KDM
- lysine demethylase
- OVX
- ovariectomized
- P
- postnatal day
- POA
- preoptic area
- qRT-PCR
- quantitative real-time PCR.
References
- 1. James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res 2001;9(suppl 4):228S–233S [DOI] [PubMed] [Google Scholar]
- 2. Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rebuffé-Scrive M, Mårin P, Björntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991;15:791–795 [PubMed] [Google Scholar]
- 4. Frederiksen L, Højlund K, Hougaard DM, Brixen K, Andersen M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age (Dordr). 2012;34:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring). 2010;18:1875–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167 [DOI] [PubMed] [Google Scholar]
- 7. Kirkby J, Metcalf BS, Jeffery AN, et al. Sex differences in resting energy expenditure and their relation to insulin resistance in children (EarlyBird 13). Am J Clin Nutr. 2004;80:430–435 [DOI] [PubMed] [Google Scholar]
- 8. Lewejohann L, Damm OS, Luetjens CM, et al. Impaired recognition memory in male mice with a supernumerary X chromosome. Physiol Behav. 2009;96:23–29 [DOI] [PubMed] [Google Scholar]
- 9. Chen X, McClusky R, Chen J, et al. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen X, McClusky R, Itoh Y, Reue K, Arnold AP. X and y chromosome complement influence adiposity and metabolism in mice. Endocrinology. 2013;154:1092–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet. 2011;130:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Addison ML, Rissman EF. Sexual dimorphism of growth hormone in the hypothalamus: regulation by estradiol. Endocrinology. 2012;153:1898–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bohlooly-Y M, Olsson B, Bruder CE, et al. Growth hormone overexpression in the central nervous system results in hyperphagia-induced obesity associated with insulin resistance and dyslipidemia. Diabetes. 2005;54:51–62 [DOI] [PubMed] [Google Scholar]
- 15. Bluet-Pajot MT, Epelbaum J, Gourdji D, Hammond C, Kordon C. Hypothalamic and hypophyseal regulation of growth hormone secretion. Cell Mol Neurobiol. 1998;18:101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20:2613–2629 [DOI] [PubMed] [Google Scholar]
- 17. Ahluwalia A, Clodfelter KH, Waxman DJ. Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic Acid microarray analysis. Mol Endocrinol. 2004;18:747–760 [DOI] [PubMed] [Google Scholar]
- 18. Piwien-Pilipuk G, Huo JS, Schwartz J. Growth hormone signal transduction. J Pediatr Endocrinol Metab. 2002;15:771–786 [DOI] [PubMed] [Google Scholar]
- 19. Lai ZN, Emtner M, Roos P, Nyberg F. Characterization of putative growth hormone receptors in human choroid plexus. Brain Res. 1991;546:222–226 [DOI] [PubMed] [Google Scholar]
- 20. Möderscheim TA, Christophidis LJ, Williams CE, Scheepens A. Distinct neuronal growth hormone receptor ligand specificity in the rat brain. Brain Res. 2007;1137:29–34 [DOI] [PubMed] [Google Scholar]
- 21. Mustafa A, Nyberg F, Bogdanovic N, Islam A, Roos P, Adem A. Somatogenic and lactogenic binding sites in rat brain and liver: quantitative autoradiographic localization. Neurosci Res. 1994;20:257–263 [DOI] [PubMed] [Google Scholar]
- 22. Mustafa A, Adem A, Roos P, Nyberg F. Sex differences in binding of human growth hormone to rat brain. Neurosci Res. 1994;19:93–99 [DOI] [PubMed] [Google Scholar]
- 23. Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937 [DOI] [PubMed] [Google Scholar]
- 24. Donahue CP, Kosik KS, Shors TJ. Growth hormone is produced within the hippocampus where it responds to age, sex, and stress. Proc Natl Acad Sci USA. 2006;103:6031–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zearfoss NR, Alarcon JM, Trifilieff P, Kandel E, Richter JD. A molecular circuit composed of CPEB-1 and c-Jun controls growth hormone-mediated synaptic plasticity in the mouse hippocampus. J Neurosci. 2008;28:8502–8509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snel YE, Brummer RJ, Doerga ME, Zelissen PM, Koppeschaar HP. Energy and macronutrient intake in growth hormone-deficient adults: the effect of growth hormone replacement. Eur J Clin Nutr. 1995;49:492–500 [PubMed] [Google Scholar]
- 27. Azain MJ, Roberts TJ, Martin RJ, Kasser TR. Comparison of daily versus continuous administration of somatotropin on growth rate, feed intake, and body composition in intact female rats. J Anim Sci. 1995;73:1019–1029 [DOI] [PubMed] [Google Scholar]
- 28. Blissett J, Harris G, Kirk J. Effect of growth hormone therapy on feeding problems and food intake in children with growth disorders. Acta Paediatr. 2000;89:644–649 [DOI] [PubMed] [Google Scholar]
- 29. Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol. 2009;21:377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eicher EM, Hale DW, Hunt PA, et al. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230 [DOI] [PubMed] [Google Scholar]
- 31. Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40 [DOI] [PubMed] [Google Scholar]
- 32. Wolstenholme JT, Rissman EF, Bekiranov S. Sexual differentiation in the developing mouse brain: contributions of sex chromosome genes. Genes Brain Behav. 2013;12:166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park JH, Burns-Cusato M, Dominguez-Salazar E, et al. Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav. 2008;7:609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Franklin KBJ, Paxinos G. The Mouse Brain Atlas in Stereotaxic Coordinates. 3rd ed. New York: Academic Press; 2008 [Google Scholar]
- 35. Bonthuis PJ, Cox KH, Rissman EF. X-chromosome dosage affects male sexual behavior. Horm Behav. 2012;61:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JH, Bonthuis P, Ding A, Rais S, Rissman EF. Androgen- and estrogen-independent regulation of copulatory behavior following castration in male B6D2F1 mice. Horm Behav. 2009;56:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295 [DOI] [PubMed] [Google Scholar]
- 38. Münzer T, Harman SM, Hees P, et al. Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab. 2001;86:3604–3610 [DOI] [PubMed] [Google Scholar]
- 39. Nyberg F. Growth hormone in the brain: characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol. 2000;21:330–348 [DOI] [PubMed] [Google Scholar]
- 40. Meyer CW, Korthaus D, Jagla W, et al. A novel missense mutation in the mouse growth hormone gene causes semidominant dwarfism, hyperghrelinemia, and obesity. Endocrinology. 2004;145:2531–2541 [DOI] [PubMed] [Google Scholar]
- 41. Walsh RJ, Mangurian LP, Posner BI. The distribution of lactogen receptors in the mammalian hypothalamus: an in vitro autoradiographic analysis of the rabbit and rat. Brain Res. 1990;530:1–11 [DOI] [PubMed] [Google Scholar]
- 42. Hojvat S, Baker G, Kirsteins L, Lawrence AM. Growth hormone (GH) immunoreactivity in the rodent and primate CNS: distribution, characterization and presence posthypophysectomy. Brain Res. 1982;239:543–557 [DOI] [PubMed] [Google Scholar]
- 43. Lai Z, Roos P, Zhai O, et al. Age-related reduction of human growth hormone-binding sites in the human brain. Brain Res. 1993;621:260–266 [DOI] [PubMed] [Google Scholar]
- 44. Yoshizato H, Fujikawa T, Soya H, Tanaka M, Nakashima K. The growth hormone (GH) gene is expressed in the lateral hypothalamus: enhancement by GH-releasing hormone and repression by restraint stress. Endocrinology. 1998;139:2545–2551 [DOI] [PubMed] [Google Scholar]
- 45. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arnold AP. The end of gonad-centric sex determination in mammals. Trends Genet. 2012;28:55–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708 [DOI] [PubMed] [Google Scholar]
- 48. Wutz A. Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat Rev Genet. 2011;12:542–553 [DOI] [PubMed] [Google Scholar]
- 49. Craig IW, Mill J, Craig GM, Loat C, Schalkwyk LC. Application of microarrays to the analysis of the inactivation status of human X-linked genes expressed in lymphocytes. Eur J Hum Genet. 2004;12:639–646 [DOI] [PubMed] [Google Scholar]
- 50. Disteche CM, Filippova GN, Tsuchiya KD. Escape from X inactivation. Cytogenet Genome Res. 2002;99:36–43 [DOI] [PubMed] [Google Scholar]
- 51. Carrel L, Cottle AA, Goglin KC, Willard HF. A first-generation X-inactivation profile of the human X chromosome. Proc Natl Acad Sci USA. 1999;96:14440–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404 [DOI] [PubMed] [Google Scholar]
- 53. Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain Res. 2006;1126:50–55 [DOI] [PubMed] [Google Scholar]
- 54. Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One. 2008;3:e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008;28:4521–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu J. Age-related changes in Usp9x protein expression and DNA methylation in mouse brain. Brain Res Mol Brain Res. 2005;140:17–24 [DOI] [PubMed] [Google Scholar]
- 57. Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409–1419 [DOI] [PubMed] [Google Scholar]
- 58. Xu J, Watkins R, Arnold AP. Sexually dimorphic expression of the X-linked gene Eif2s3x mRNA but not protein in mouse brain. Gene Expr Patterns. 2006;6:146–155 [DOI] [PubMed] [Google Scholar]
- 59. Ehrmann IE, Ellis PS, Mazeyrat S, et al. Characterization of genes encoding translation initiation factor eIF-2γ in mouse and human: sex chromosome localization, escape from X-inactivation and evolution. Hum Mol Genet. 1998;7:1725–1737 [DOI] [PubMed] [Google Scholar]
- 60. Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525 [DOI] [PubMed] [Google Scholar]
- 61. Tsuchiya KD, Willard HF. Chromosomal domains and escape from X inactivation: comparative X inactivation analysis in mouse and human. Mamm Genome. 2000;11:849–854 [DOI] [PubMed] [Google Scholar]
- 62. Greenfield A, Carrel L, Pennisi D, et al. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet. 1998;7:737–742 [DOI] [PubMed] [Google Scholar]
- 63. Agulnik AI, Mitchell MJ, Mattei MG, et al. A novel X gene with a widely transcribed Y-linked homologue escapes X-inactivation in mouse and human. Hum Mol Genet. 1994;3:879–884 [DOI] [PubMed] [Google Scholar]
- 64. Murakami K, Ohhira T, Oshiro E, Qi D, Oshimura M, Kugoh H. Identification of the chromatin regions coated by non-coding Xist RNA. Cytogenet Genome Res. 2009;125:19–25 [DOI] [PubMed] [Google Scholar]
- 65. Nottke A, Colaiácovo MP, Shi Y. Developmental roles of the histone lysine demethylases. Development. 2009;136:879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stratmann A, Haendler B. Histone demethylation and steroid receptor function in cancer. Mol Cell Endocrinol. 2012;348:12–20 [DOI] [PubMed] [Google Scholar]
- 67. Iwase S, Lan F, Bayliss P, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–1088 [DOI] [PubMed] [Google Scholar]
- 68. Kramer JM, van Bokhoven H. Genetic and epigenetic defects in mental retardation. Int J Biochem Cell Biol. 2009;41:96–107 [DOI] [PubMed] [Google Scholar]
- 69. Froyen G, Bauters M, Voet T, Marynen P. X-linked mental retardation and epigenetics. J Cell Mol Med. 2006;10:808–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367 [DOI] [PubMed] [Google Scholar]
- 71. Agger K, Cloos PA, Christensen J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature. 2007;449:731–734 [DOI] [PubMed] [Google Scholar]
- 72. Tahiliani M, Mei P, Fang R, et al. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447:601–605 [DOI] [PubMed] [Google Scholar]
- 73. Abidi FE, Holloway L, Moore CA, et al. Mutations in JARID1C are associated with X-linked mental retardation, short stature and hyperreflexia. J Med Genet. 2008;45:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang J, Scully K, Zhu X, et al. Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature. 2007;446:882–887 [DOI] [PubMed] [Google Scholar]
- 75. Chan SW, Hong W. Retinoblastoma-binding protein 2 (Rbp2) potentiates nuclear hormone receptor-mediated transcription. J Biol Chem. 2001;276:28402–28412 [DOI] [PubMed] [Google Scholar]
- 76. Hong S, Cho YW, Yu LR, Yu H, Veenstra TD, Ge K. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc Natl Acad Sci USA. 2007;104:18439–18444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. De Gobbi M, Garrick D, Lynch M, et al. Generation of bivalent chromatin domains during cell fate decisions. Epigenetics Chromatin. 2011;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De Vries GJ, Rissman EF, Simerly RB, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maxson SC, Ginsburg BE, Trattner A. Interaction of Y-chromosomal and autosomal gene(s) in the development of intermale aggression in mice. Behav Genet. 1979;9:219–226 [DOI] [PubMed] [Google Scholar]
- 80. Shrenker P, Maxson SC. The Y chromosomes of DBA/1Bg and DBA/2Bg compared for effects on intermale aggression. Behav Genet. 1982;12:429–434 [DOI] [PubMed] [Google Scholar]
- 81. Shrenker P, Maxson SC. The DBA/1Bg and DBA/2Bg Y chromosomes compared for their effects on male sexual behavior. Behav Neural Biol. 1984;42:33–37 [DOI] [PubMed] [Google Scholar]