Abstract
Increased hepatic glucose production is a key pathophysiological feature of type 2 diabetes. Like all other cell types, hepatocytes express many G protein-coupled receptors (GPCRs) that are linked to different functional classes of heterotrimeric G proteins. The important physiological functions mediated by Gs-coupled hepatic glucagon receptors are well-documented. In contrast, little is known about the in vivo physiological roles of hepatocyte GPCRs that are linked to G proteins of the Gq family. To address this issue, we established a transgenic mouse line (Hep-Rq mice) that expressed a Gq-linked designer receptor (Rq) in a hepatocyte-selective fashion. Importantly, Rq could no longer bind endogenous ligands but could be selectively activated by a synthetic drug, clozapine-N-oxide. Clozapine-N-oxide treatment of Hep-Rq mice enabled us to determine the metabolic consequences caused by selective activation of a Gq-coupled GPCR in hepatocytes in vivo. We found that acute Rq activation in vivo led to pronounced increases in blood glucose levels, resulting from increased rates of glycogen breakdown and gluconeogenesis. We also demonstrated that the expression of the V1b vasopressin receptor, a Gq-coupled receptor expressed by hepatocytes, was drastically increased in livers of ob/ob mice, a mouse model of diabetes. Strikingly, treatment of ob/ob mice with a selective V1b receptor antagonist led to reduced glucose excursions in a pyruvate challenge test. Taken together, these findings underscore the importance of Gq-coupled receptors in regulating hepatic glucose fluxes and suggest novel receptor targets for the treatment of type 2 diabetes.
The liver plays a central role in regulating blood glucose homeostasis. Various lines of evidence suggest that an increase in the rate of hepatic glucose production (HGP) is the major contributor to fasting hyperglycemia in type 2 diabetes (T2D) (1–3). Moreover, inhibition of increased HGP is considered the key mechanism by which metformin, a widely used antidiabetic drug, exerts its therapeutic actions (3). For these reasons, studies aimed at better understanding the signaling pathways and molecules that modulate hepatic glucose metabolism are of great clinical relevance.
Like essentially all other cell types, hepatocytes are predicted to express many G protein-coupled receptors (GPCRs) on their cell surface (4). These different GPCRs are linked to distinct families of heterotrimeric G proteins (primarily Gs, Gi, or Gq, representative of GPCRs: glucagon, α2-adrenergic, and α1-adrenergic receptors, respectively), which are predicted to have multiple effects on hepatocyte function. The important metabolic roles of the glucagon receptor in maintaining normoglycemia under fasting conditions and to raise plasma glucose levels in response to hypoglycemia are well recognized (5). The glucagon receptor, which is abundantly expressed in hepatocytes, is linked to the stimulatory G protein, Gs, thus promoting the production of cAMP and ultimately triggering the release of glucose from hepatocytes by stimulating both glycogenolysis and gluconeogenesis (6, 7). Interestingly, glucagon levels are unphysiologically high in patients suffering from T2D, suggesting that increased signaling through liver glucagon receptors may play a central role in the pathophysiology of T2D (8, 9). Because GPCRs that are linked to G proteins of the Gi family exert an inhibitory effect on adenylyl cyclase, it is likely that activation of this class of receptors inhibits the hepatic actions of glucagon.
Little is known about the in vivo metabolic roles of hepatocyte GPCRs that couple to Gq-type G proteins (primarily Gq and G11). At the molecular level, agonist binding to Gq-linked GPCRs causes the activation of phospholipase Cβ, leading to the generation of 2 second messengers, diacylglycerol and inositol 1,4,5-trisphosphate (IP3) (10). Although diacylglycerol stimulates the activity of different isoforms of protein kinase C, IP3 promotes the release of Ca2+ from endoplasmic reticulum stores.
Previous studies have shown that hepatocytes express various Gq-coupled GPCRs, including different muscarinic, vasopressin, and α1-adrenergic receptor subtypes (11–13). All of these receptors are present not only on hepatocytes but also in many other peripheral and central tissues (4). For this reason, the use of classical pharmacological tools has led to ambiguous results regarding the physiological relevance of this subfamily of hepatocyte GPCRs. Another factor that has further complicated research in this field is that drugs that can activate or block these various GPCR subtypes with high selectivity in vivo are not available in most cases.
To circumvent these difficulties, we employed a novel experimental strategy that involves the use of a recently developed designer GPCR that is selectively linked to Gq-type G proteins (14–16). Specifically, we generated a transgenic mouse strain (Hep-Rq mice) that expresses this engineered GPCR (referred to as Rq) (16) selectively in hepatocytes. The Rq receptor represents a mutant M3 muscarinic receptor (Figure 1A) that no longer binds its endogenous ligand (acetylcholine) but can be selectively activated by clozapine-N-oxide (CNO), a compound that is otherwise pharmacologically inert (14, 16). As a result, CNO treatment of Hep-Rq mice allowed us to selectively stimulate a Gq-linked GPCR in hepatocytes in vivo and to monitor the resulting metabolic consequences.
Figure 1.
Liver-specific expression of the Rq designer GPCR in Hep-Rq mice. A, Schematic diagram of the transgene construct that was injected into fertilized mouse oocytes. The Rq receptor (white box) represents a mutant M3 muscarinic receptor containing the Y148C and A238G point mutations within the transmembrane receptor core (16). Rq expression was under the transcriptional control of the albumin promoter/enhancer. The 3′ untranslated region (UTR) consisted of a sequence derived from the human GH gene (hGH). To facilitate genotyping studies, a 9–amino-acid hemagglutinin (HA) epitope tag was added to the N terminus of the Rq construct. B, RT-PCR analysis of Rq transgene expression in different tissues from Hep-Rq mice. RT-PCR experiments were performed as described in Materials and Methods using an Rq-specific primer pair. As expected, Rq expression was found only with RNA derived from the liver. Control samples (indicated by the − signs above the lanes) that had not been treated with reverse transcriptase (RT) did not give any detectable signal (C. Cortex; cerebral cortex; Hypot., hypothalamus; Subm. gland, submandibular gland). C, Real-time qRT-PCR analysis of hepatic Rq and glucagon receptor (GCGR) expression in Hep-Rq mice. qRT-PCR experiments were carried out using mouse liver cDNA derived from Hep-Rq mice as described in Materials and Methods, using receptor-specific primers. Gene expression data are expressed as Ct values. Cyclophilin A expression was monitored for control purposes. The cyclophilin A and GCGR Ct values found with Hep-Rq mice did not differ significantly from the corresponding values obtained with WT mice. Data represent means ± SEM of 3 independent experiments. D, CNO-induced calcium mobilization in primary hepatocytes from Hep-Rq mice. Primary hepatocytes were isolated from WT and Hep-Rq mice, and agonist-induced changes in intracellular calcium levels were determined by using FLIPR technology (see Materials and Methods for details). AVP (10μM) served as a control agonist. CNO was used at a concentration of 100μM. Data are expressed as means ± SEM of 2 independent experiments performed in duplicate. **, P < .01; ***, P < .001, as compared with non–drug-treated mice of the same genotype. E, CNO triggers calcium release in hepatocytes from Hep-Rq but not WT mice. Primary hepatocytes prepared from Hep-Rq and WT mice were cultured on glass coverslips as described in Materials and Methods. Cells were treated with CNO (10μM) and AVP (100nM) at the indicated time points, and changes in cytosolic calcium were visualized via microscopy with fura-2. Data are expressed as means ± SEM (WT, 21 cells; Hep-Rq, 19 cells).
It should be mentioned in this context that such CNO-sensitive designer GPCRs are usually referred to as DREADDs (designer receptors exclusively activated by designer drug) or, less often, as second-generation RASSLs (receptors activated solely by synthetic ligand) (14, 15).
Metabolic studies with Hep-Rq mice demonstrated that selective stimulation of a Gq-linked GPCR expressed exclusively in hepatocytes led to striking increases in blood glucose levels and impaired glucose tolerance. Moreover, we demonstrated that the hepatic expression of the Gq-linked V1b vasopressin receptor is greatly enhanced in ob/ob mice, a mouse model of diabetes, and that pharmacological inhibition of this receptor subtype can reduce HGP in this mouse disease model. Our findings strongly suggest that pharmacological strategies aimed at inhibiting signaling through hepatocyte GPCRs that are linked to Gq-type G proteins are of potential benefit in the treatment of T2D and related metabolic disorders.
Materials and Methods
Drugs
CNO was obtained from the National Institutes of Health as part of the Rapid Access to Investigative Drug Program funded by the National Institute of Neurological Disorders and Stroke. SSR149415, a selective V1b vasopressin receptor antagonist, was a kind gift from Dr Claudine Serradeil-Le Gal (Sanofi-Aventis, Toulouse, France). Unless stated otherwise, all other drugs were from Sigma-Aldrich (St Louis, Missouri).
Mouse maintenance and diet
Mice were housed in a specific pathogen-free barrier facility maintained on a 12-hour light, 12-hour dark cycle (light period from 6:00 am to 6:00 pm). Mice were fed ad libitum with a standard mouse chow (4% [wt/wt] fat content; Zeigler, Gardners, Pennsylvania). The ob/ob mice and their wild-type (WT) littermates were obtained from The Jackson Laboratory (Bar Harbor, Maine). All animal studies were approved by the Animal Care and Use Committees of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Bethesda, Maryland) and Vanderbilt University (Nashville, Tennessee).
Generation of transgenic mice expressing a Gq-linked designer receptor selectively in hepatocytes
To generate transgenic mice that selectively express the Rq designer receptor (16) in hepatocytes, the Rq coding sequence was placed under the transcriptional control of the mouse albumin promoter/enhancer, using a plasmid described by Conner et al (17). An ∼5.0-kb SacI fragment containing the transgene (for details, see Figure 1A) was excised, purified, and microinjected into the pronuclei of fertilized ova from C57BL/6NTac mice (Taconic, Germantown, New York) using standard transgenic techniques.
Genotyping of Hep-Rq mice
The presence of the Rq transgene in the genome of Hep-Rq mice was confirmed via PCR analysis of mouse tail DNA (16).
Preparation of cDNA and RT-PCR analysis of transgene expression
cDNA was prepared from total RNA extracted from various tissues of 6-week-old Hep-Rq mice and WT littermates (16). Rq transcripts were detected via RT-PCR analysis, as described in detail previously (16). For comparison, the hepatic expression levels of the glucagon receptor were also determined (forward primer, 5′-TGCACTGCACCCGAAACTAC; reverse primer, 5′-CATCGCCAATCTTCTGGCTGT). PCR conditions were as follows: 31 cycles at 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds.
Identification of GPCRs expressed by mouse hepatocytes using array technology
Mouse hepatocytes were isolated from WT C57BL/6NTac mice (3-month-old males), as described in Ref. 18. Total RNA was prepared from hepatocytes and reverse transcribed as described above. cDNA derived from 800 ng total RNA was mixed with an equal volume of TaqMan gene expression 2× master mix (Applied Biosystems, Foster City, California). This mixture, in 100 μl-aliquots, was added to each port of an 8-port 384-well plate of a mouse GPCR array panel (Applied Biosystems). Real-time quantitative RT-PCR (qRT-PCR) measurements were carried out using the 7900HT Fast Real-Time PCR System (Applied Biosystems). PCR conditions were as follows: 40 cycles at 97°C for 30 seconds and at 59.7°C for 1 minute, respectively. Experiments were repeated 3 times.
Calcium mobilization assays
Primary hepatocytes were prepared from WT and Hep-Rq mice (17- to 19-week-old males) as described (18). Cells were resuspended in William's medium E supplemented with 10% fetal bovine serum and grown on either poly-d-lysine–coated 96-well plates (black-walled, clear bottom) for Fluorometric Imaging Plate Reader (FLIPR) (Molecular Devices, Sunnyvale, California) experiments or on glass coverslips for calcium imaging studies at 37°C with 5% CO2. For FLIPR experiments, primary hepatocytes were plated at a density of 2.5 × 104 cells per well. About 48 hours later, agonist-induced changes in intracellular calcium levels were measured by using FLIPR technology as described (19). Increases in intracellular calcium levels were expressed as changes in fluorescence (peak fluorescence activity minus basal fluorescence activity) divided by basal fluorescence levels.
In another series of experiments, hepatocytes grown on poly-d-lysine–coated glass coverslips (plating density, 5 × 105 cells per well in a 6-well dish) were loaded with 3μM fura-2 AM about 24 hours after plating, as described previously (20). Experimental compounds were added at the indicated time points, and calcium measurements were obtained by using an Olympus IX70 inverted microscope and the appropriate filter sets to record F340/F380 fluorescence ratios (for experimental details, see Ref. 20).
Real-time qRT-PCR analysis of CNO-induced changes in gene expression in Hep-Rq mice
Hep-Rq mice (freely fed 5-month-old males) received 2 ip injections of CNO (10 mg/kg) or saline, one at time 0 and the other one 2 hours later. Mice were killed 6 hours after the first injection, and cDNA was prepared from mouse livers as described above. Gene expression levels were measured by real-time qRT-PCR analysis as described previously (13). The gene-specific primers that were used are listed in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The expression of cyclophilin A served as an internal control. Four independent samples prepared from 4 different mice were analyzed. PCR were carried out in triplicate in 96-well plates. Data are expressed as fold change in gene expression in CNO- vs saline-treated mice.
Real-time qRT-PCR analysis of the expression of Gq-linked receptors in ob/ob mice
Total RNA was isolated from livers or pancreatic islets of ob/ob mice and lean littermates (3- to 5-month-old males), and real-time qRT-PCR studies were carried out using the same experimental conditions as described in the previous paragraph. The PCR primers that were used are listed in Supplemental Table 2 (these primers amplify Gq-linked receptors expressed by mouse hepatocytes). The expression of 18S rRNA served as an internal control. Data are expressed as fold change in receptor expression between ob/ob and WT mice.
In vivo physiological studies
The experiments shown in Figure 2 were carried out with 6-week-old males.
Figure 2.
CNO treatment of Hep-Rq mice leads to dose-dependent increases in blood glucose levels. A and B, Effect of increasing doses of CNO on blood glucose levels in Hep-Rq mice. Hep-Rq mice (6-week-old males) that had been fasted for 5 hours received a single ip injection of increasing doses of CNO or vehicle (saline), and blood glucose levels were measured at the indicated time points. WT littermates received a single high dose of CNO (10 mg/kg ip). Data are expressed as percent increase in blood glucose levels above basal levels (100%) measured before injections. Basal blood glucose levels were as follows: WT, 132 ± 4 mg/dL; Hep-Rq, 129 ± 7 mg/dL. Blood glucose levels were significantly increased (P < .05) at 15 and 30 minutes in Hep-Rq mice treated with 0.1, 1, and 10 mg/kg CNO (ip), as compared with saline-injected Hep-Rq mice. B, Plot illustrating that the acute effects of CNO on blood glucose levels in Hep-Rq mice are dose-dependent. Data were taken from A (15-minute time point). C, CNO treatment of Hep-Rq mice has no significant effect on serum insulin levels. Hep-Rq mice and WT littermates (6-week-old males) that had been fasted for 5 hours received a single ip injection of CNO (10 mg/kg) or vehicle (saline), and serum insulin levels were measured at the indicated time points. Data are given as means ± SEM (n = 5 per group).
Glucose tolerance tests were carried out with 10-week-old male mice that had been subjected to an overnight (12-hour) fast. Mice received 2 mg glucose/g body weight either alone or in combination with CNO (10 mg/kg) via ip injection.
In insulin tolerance (sensitivity) tests, 10-week-old male mice that had been fasted for 5 hours were injected ip with human insulin (0.75 U/kg; Eli Lilly, Indianapolis, Indiana), either alone or in combination with CNO (10 mg/kg). In pyruvate challenge tests, mice that had been deprived of food for 12 hours were injected ip with sodium pyruvate (1.5 or 2 mg/g; Sigma). To study the effect of glucagon on HGP in vivo, mice were fasted for 5 hours and then injected with human glucagon (16 μg/kg ip) (13, 21).
Blood samples were collected via the tail vein. Blood glucose and serum insulin levels were determined as described previously (13).
Measurement of hepatic gluconeogenesis and glycogenolysis in vivo
Experiments were conducted in chronically catheterized (carotid artery and jugular vein) conscious Hep-Rq mice (4-month-old females) (22–24). Catheters were implanted 5 days before a study. On the day of the study (7:30 am), conscious, unrestrained mice were placed in a plastic container with bedding (without access to food). Micro-Renathane (0.033-in outer diameter) tubing was connected to the catheter leads and infusion syringes. Three hours later (time = −120 minutes), a primed (1.0 μCi) continuous (0.05 μCi/min) infusion of [3-3H]glucose (NET331c; PerkinElmer, Waltham, Massachusetts) was initiated into the jugular vein catheter. After this, baseline arterial blood samples were drawn (at −10 and 0 minutes) for the measurement of arterial blood glucose, glucose specific activity, and plasma insulin. Mice received heparinized saline-washed erythrocytes from donors at 5 μL/min to prevent a fall of hematocrit. At time 0, mice were injected ip with a bolus of saline or CNO (200 μg in 100 μL). Blood glucose concentrations and specific activity were monitored at 5, 10, 15, 20, and 30 minutes. At 20 minutes, the [3-3H]glucose infusion rate was increased to 0.15 μCi/min, and a primed (1.4 μCi) continuous (0.14 μCi/min) infusion of [U-14C]alanine (NEC 266; PerkinElmer) was initiated and continued for the duration of the study. Mice were then anesthetized, and tissues were excised and rapidly freeze-clamped in liquid nitrogen. Liver samples were subsequently stored at −80°C until further analysis. Analysis of radioactivity was performed in the Vanderbilt Mouse Metabolic Phenotyping Center and Diabetes Research and Training Center analytical cores. Plasma glucose was analyzed as previously described (22). Glucose flux rates were assessed using non–steady-state equations (25), assuming a volume of distribution of 130 mL/kg. The liver content of UDP-glucose (UDPG) and phosphoenolpyruvate (PEP) were obtained through 2 sequential chromatographic separations, and the radioactivity of 3H and 14C in each fraction was measured (26, 27). Under the chosen experimental conditions, gluconeogenesis is defined as the rate of glucose appearance × [14C]UDPG specific activity/[14C]PEP specific activity × 2, whereas glycogenolysis is equal to the difference between the rates of glucose appearance and gluconeogenesis.
Statistics
Data are expressed as means ± SEM for the indicated number of observations. For comparisons between two groups, the paired or unpaired Student's t test (two-tailed) was used, as appropriate. For multiple comparisons, the one-way ANOVA was applied. A P value < .05 was considered statistically significant.
Results
Generation of transgenic mice expressing the Rq designer GPCR selectively in hepatocytes
Our initial goal was to generate a transgenic mouse line that expressed the Rq construct, a CNO-sensitive designer GPCR that is selectively linked to Gq-type G proteins (16), in mouse hepatocytes. To ensure that the Rq receptor was selectively expressed by hepatocytes, transgene expression was placed under the transcriptional control of the albumin promoter (Figure 1A). By using standard transgenic techniques, we obtained several transgenic founder mice (for details, see Materials and Methods). One of these lines, referred to as Hep-Rq mice, showed selective expression of the Rq designer receptor in the liver (Figure 1B). These mice were maintained on a pure C57BL/6NTac background.
To examine the relative expression of Rq in the liver of Hep-Rq mice, we carried out real-time qRT-PCR studies using PCR primers specific for the Rq receptor and the endogenously expressed glucagon receptor (GCGR). This analysis showed that Rq was expressed at considerably lower levels (∼20-fold) than the GCGR (the expression of Rq and GCGR was characterized by cycle threshold [Ct] values of ∼30 and ∼26, respectively; Figure 1C).
To compare Rq expression levels with those of other GPCRs expressed by mouse hepatocytes, we first prepared total RNA from hepatocytes of C57BL/6NTac mice (3-month-old males). We then measured receptor expression levels via real-time qRT-PCR using mouse GPCR array plates containing specific primers and TaqMan probes for all mouse GPCRs, except for the odorant, taste, and pheromone receptors. This analysis showed that mouse hepatocytes express many GPCRs, including 8 receptors that are known to be linked to G proteins of the Gq family (Supplemental Table 3). These receptors include GPR91 (succinate receptor 1), the AT1a angiotensin II receptor, the V1a and V1b vasopressin receptor subtypes, and the α1b-adrenergic, PAR1 thrombin, S1P2 lysophospholipid, and M3 muscarinic receptors. A comparison of Ct values indicated that these receptors were expressed at similar or higher levels, as compared with the hepatic expression of Rq in Hep-Rq mice (note that the Ct value characterizing GCGR expression in the array assay was similar to that shown in Figure 1C). These observations indicated that the hepatic expression of Rq in Hep-Rq mice was rather low and certainly not unphysiologically high.
We next wanted to demonstrate the presence of CNO-sensitive, functional Rq receptors in hepatocytes of Hep-Rq mice. To address this issue, we incubated primary hepatocytes prepared from WT and Hep-Rq mice with CNO (100μM) or arginine vasopressin (AVP, 10μM; for control purposes) and determined changes in intracellular calcium levels ([Ca2+i]) by using FLIPR technology (Gq activation triggers profound increases in [Ca2+i]). Control experiments showed that AVP treatment led to significant increases in [Ca2+i]) in both WT and Hep-Rq hepatocytes (Figure 1D). In contrast, CNO treatment of WT hepatocytes had no effect on [Ca2+i] but triggered a robust increase in [Ca2+i] in hepatocytes prepared from Hep-Rq mice (Figure 1D). We obtained identical results when we monitored changes in [Ca2+i] in individual hepatocytes using a fura-2-based microscopic imaging technique (Figure 1E).
CNO treatment of Hep-Rq mice causes dose-dependent increases in blood glucose levels
Hep-Rq mice and their WT littermates did not differ in body weight and basal blood glucose and serum insulin levels (Supplemental Figure 1).
To determine the in vivo effects of acute activation of Rq, Hep-Rq mice that had been fasted for 5 hours received a single ip injection of either saline (control) or increasing doses of CNO (0.001–10 mg/kg). Blood glucose levels were monitored over a 60-minute period. Strikingly, CNO treatment of Hep-Rq mice led to dose-dependent increases in blood glucose levels (Figure 2, A and B), indicating that acute activation of Rq signaling in hepatocytes strongly promotes HGP in vivo. The stimulatory effect of CNO on blood glucose levels plateaued at 1 mg/kg (Figure 2, A and B). CNO treatment (10 mg/kg ip) of WT mice caused only very minor elevations in blood glucose levels, similar to those observed with saline-injected Hep-Rq mice (Figure 2A), probably resulting from the injection stress.
To examine whether CNO treatment of Hep-Rq mice affected in vivo insulin release, we monitored serum insulin levels in Hep-Rq mice and WT littermates after ip injection of a high dose of CNO (10 mg/kg) or saline (mice were fasted for 5 hours before injection). CNO- or saline-treated mice showed only minor changes in serum insulin levels that were not significantly different between the two genotypes (Figure 2C).
CNO-treated Hep-Rq mice show impaired glucose tolerance, unchanged insulin sensitivity, and increased gluconeogenesis in vivo
To examine CNO effects on glucose tolerance, we injected Hep-Rq mice and WT littermates with glucose (2 mg/g ip), either alone or in combination with CNO (10 mg/kg ip). We found that CNO-treated Hep-Rq mice showed significantly impaired glucose tolerance, as compared with the 3 control groups (saline-treated transgenic and WT mice and CNO-treated WT mice; Figure 3A).
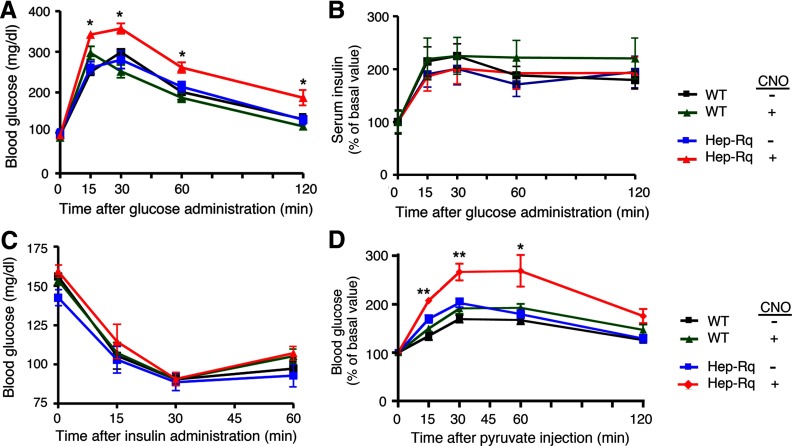
Figure 3.
Physiological analysis of Hep-Rq mice and WT littermates. A, Glucose tolerance test. Hep-Rq mice and WT littermates were injected with either glucose alone (2 mg/g ip) (−CNO) or together with CNO (10 mg/kg ip). Blood glucose levels were measured at the indicated time points. B, Glucose-induced insulin release. Serum insulin levels were measured at the indicated time points, after treatment of Hep-Rq mice and WT littermates with either glucose alone (2 mg/g ip) (−CNO) or in combination with CNO (10 mg/kg ip). Basal (preinjection) serum insulin levels were set equal to 100%. Actual basal serum insulin levels were as follows: WT, 0.80 ± 0.12 ng/mL (n = 12); Hep-Rq, 1.01 ± 0.15 ng/mL (n = 12). C, Insulin tolerance test. Hep-Rq mice and WT littermates were injected with either insulin alone (0.75 U/kg ip) (−CNO) or together with CNO (10 mg/kg ip), and blood glucose levels were measured at the indicated time points. *, P < .05, as compared with the other 3 groups of mice. D, Pyruvate challenge test. Hep-Rq mice and WT littermates received a single ip injection of sodium pyruvate (2 mg/g) either alone or in combination with CNO (10 mg/kg). Blood glucose levels were measured at the indicated time points. All experiments were carried out with 8- to 10-week-old male mice. Data are given as means ± SEM (n = 6 per group). *, P < .05; **, P < .01, as compared with the other 3 groups of mice.
In parallel, we also measured serum insulin levels in the glucose- (or glucose plus CNO)-injected animals. As shown in Figure 3B, all 4 groups of mice (CNO- or saline-injected Hep-Rq and WT mice) showed comparable increases in serum insulin levels. Basal insulin levels (preinjection values) did not differ significantly between Hep-Rq mice and WT littermates (WT, 1.01 ± 0.15 ng/mL; Hep-Rq, 0.80 ± 0.12 ng/mL; n = 6).
Moreover, after injection of a single dose of insulin (0.75 U/kg), all 4 groups of mice (CNO- or saline-injected Hep-Rq and WT mice) showed similar reductions in blood glucose levels (Figure 3C), indicating that CNO-mediated activation of Rq in Hep-Rq mice had no significant effect on overall insulin sensitivity.
Finally, we treated Hep-Rq mice and WT littermates with a single ip injection of the gluconeogenic substrate sodium pyruvate (2 mg/g), either alone or in combination with CNO (10 mg/kg) (pyruvate challenge test; Figure 3D). We found that pyruvate-induced increases in blood glucose levels were significantly greater in CNO-treated Hep-Rq mice, as compared with the other 3 groups of mice (Figure 3D), suggesting that activation of hepatic Rq receptors stimulates gluconeogenesis in vivo.
CNO and glucagon treatment of Hep-Rq mice increases blood glucose levels to a similar degree
Glucagon, the major counterregulatory hormone of insulin, plays a key role in maintaining physiological blood glucose levels, primarily by promoting HGP via activation of hepatocyte glucagon receptors. Thus, our next goal was to compare the hyperglycemic effects that CNO induced in Hep-Rq mice with those observed after treatment of mice with glucagon. Specifically, we injected Hep-Rq mice and WT littermates with ip saline (control), glucagon (16 μg/kg ip), or CNO (10 mg/kg ip) (mice were fasted for 5 hours before injections). As shown in Figure 4, glucagon triggered robust increases in blood glucose levels that did not differ significantly between the two genotypes. Injection of a higher dose of glucagon (300 μg/kg instead of 16 μg/kg) did not lead to greater hyperglycemic effects (in fact, the higher dose resulted in smaller increases in blood glucose levels; data not shown). Strikingly, CNO treatment of Hep-Rq mice led to hyperglycemic effects that were similar in magnitude to those observed with glucagon-injected Hep-Rq or WT mice (Figure 4).
Figure 4.
Comparison of the effects of CNO and glucagon on blood glucose levels in WT and Hep-Rq mice. Hep-Rq mice and WT littermates received a single ip injection of saline (control), glucagon (16 μg/kg), or CNO (10 mg/kg) (mice were fasted for 5 hours before injections). Blood glucose levels were determined at the indicated time points. All experiments were carried out with 8-week-old males. Data are given as means ± SEM (n = 6 per group).
Hepatic gluconeogenesis and glycogenolysis
Our next goal was to examine to which extent hepatic gluconeogenesis and glycogenolysis contributed to the Rq-mediated increases in blood glucose levels in Hep-Rq mice. To address this issue, we carried out isotope labeling studies using chronically catheterized conscious Hep-Rq mice, as described in Materials and Methods (22–27). The contribution of gluconeogenesis to the rate of glucose appearance was estimated from the specific activities of 14C-labeled hepatic UDPG (this is assumed to reflect the specific activity of hepatic glucose 6-phosphate) and hepatic PEP after the infusion of [U-14C]alanine. [3-3H]Glucose was used to measure the rate of glucose appearance (see Materials and Methods for details).
Using this strategy, Hep-Rq mice were injected with an ip bolus of either saline (vehicle) or CNO (200 μg; ∼10 mg CNO/kg) after a 5-hour fast (for details, see Materials and Methods). CNO treatment of Hep-Rq mice increased arterial glucose concentrations and the rate of glucose appearance to a significantly greater extent than vehicle (saline) alone (Figure 5A). The CNO-mediated increase in the rate of glucose appearance (ie, HGP) was due to a combined increase in the rates of both gluconeogenesis and glycogenolysis (Figure 5B). Arterial plasma insulin concentrations were similar in both groups (1.2 ± 0.2 vs 1.3 ± 0.1 ng/mL, vehicle vs CNO; n = 6 per group).
Figure 5.
Effect of CNO on hepatic glucose fluxes in conscious Hep-Rq mice in vivo. The time course of arterial blood glucose concentrations and the rate of glucose appearance (A) and the rates of gluconeogenesis and glycogenolysis (B) were determined as described in detail in Materials and Methods. At time 0, chronically catheterized conscious Hep-Rq mice (4-month-old females) that had been fasted for 5 hours were injected with an ip bolus of CNO (200 μg per mouse) or saline (control). Data are given as means ± SEM (n = 6 per group). *, P < 05 vs saline.
It should be noted that the saline-injected mice also showed significant increases in the rate of glucose appearance (Figure 5A), most likely as a result of the stress response associated with the ip injection. However, as shown in Figure 5B, the rates of hepatic glycogenolysis and gluconeogenesis were significantly greater in CNO-treated than in saline-injected Hep-Rq mice.
Effects of CNO treatment of Hep-Rq mice on liver gene expression levels
We used real-time qRT-PCR analysis to examine whether acute CNO treatment of Hep-Rq mice affected the expression levels of a series of genes important for hepatic glucose and lipid metabolism. Hep-Rq mice that had free access to food received 2 ip injections of CNO (10 mg/kg) or saline (injections were given 2 hours apart). Because we had demonstrated previously that the CNO plasma half-life is rather short (<1 hour) (16), we decided to inject CNO repeatedly to maintain high CNO plasma levels for a longer period of time, thus facilitating the detection of changes in gene expression levels. Six hours after the first injection, total hepatic RNA was prepared and subjected to real-time qRT-PCR analysis (Figure 6). Using this strategy, we identified several genes that showed CNO-dependent changes in hepatic expression levels (Figure 6). Interestingly, the genes that displayed the most pronounced increases in expression levels were genes coding for proteins involved in fatty acids synthesis, including ATP citrate lyase (ACLY), acetyl-CoA carboxylase 1 (ACC1), and fatty acid synthase (FAS). On the other hand, CNO treatment resulted in significantly reduced hepatic expression levels of stearoyl-CoA desaturase 1 (SCD1). The transcript levels of PEP carboxykinase (PEPCK), one of the rate-limiting enzymes of gluconeogenesis, were also significantly increased in CNO-treated mice (∼1.7-fold; Figure 6).
Figure 6.
Effects of CNO treatment of Hep-Rq mice on liver gene expression levels. Hep-Rq mice (freely fed 5-month-old males) received 2 ip injections of CNO (10 mg/kg) or saline that were given 2 hours apart. Livers were harvested for the preparation of total RNA 6 hours after the first injection. Gene expression was studied by real-time qRT-PCR using total hepatic RNA as a template. Data were normalized relative to the expression of cyclophilin A RNA (internal control) and are presented as fold change in gene expression in CNO-treated vs saline-treated Hep-Rq mice. CNO had no significant effect on the expression of cyclophilin A. Data are given as means ± SEM (n = 4 for all genes except PEPCK [n = 8]). *, P < .05; **, P < .01 vs saline-treated Hep-Rq mice. Full gene names are given in Supplemental Table 1.
To rule out the possibility that the changes in gene expression levels observed with CNO-treated Hep-Rq mice were caused by nonspecific effects of CNO, we carried out analogous experiments with WT mice, focusing on the genes that showed significantly changed expression levels after CNO treatment of Hep-Rq mice. This analysis showed that CNO had no significant effect on hepatic transcript levels in WT mice, as compared with the corresponding levels observed with saline-injected WT mice (Supplemental Figure 2).
The ob/ob mice show a striking increase in V1b vasopressin receptor expression levels in the liver
Enhanced HGP represents a key factor contributing to fasting hyperglycemia in T2D (1, 2). The ob/ob mouse diabetes model mimics many of the key pathophysiological features of T2D including increased HGP (28). Prompted by the finding that CNO-mediated activation of Rq in Hep-Rq mice promoted HGP, we next examined whether the hepatic expression of endogenous GPCRs linked to Gq-type G proteins (Supplemental Table 2) was altered in ob/ob mice. We prepared total RNA from livers of ob/ob and WT control mice (4- to 5-month-old males) and then carried out real-time qRT-PCR analysis to determine the hepatic expression levels of these receptor genes. We identified several Gq-linked receptors, including the AT1a angiotensin II, α1b-adrenergic, S1P2 lysophospholipid, and V1b vasopressin receptor subtypes, that were expressed at significantly higher levels in livers from ob/ob mice, as compared with their WT littermates (Figure 7). Most strikingly, the hepatic expression of the V1b vasopressin receptor was ∼6-fold higher in ob/ob mice than in WT control mice (Figure 7). In contrast, the hepatic expression of the V1a vasopressin receptor was significantly reduced (by ∼50%) in ob/ob mice.
Figure 7.
The ob/ob mice show a pronounced increase in V1b vasopressin receptor expression in the liver. Total hepatic RNA was prepared from livers of ob/ob and WT lean control mice (4- to 5-month-old males). The expression of GPCRs linked to Gq-type G proteins expressed in mouse liver was studied by real-time qRT-PCR using total hepatic RNA as a template. Primers specific for the following receptors were used (for primer sequences, see Supplemental Table 2): GPR91 (succinate receptor 1), AT1a angiotensin II receptor (AT1a), V1a and V1b vasopressin receptors (V1a and V1b), α1b-adrenergic receptor (α1b), PAR1 thrombin receptor (PAR1), S1P2 lysophospholipid receptor (S1P2), and M3 muscarinic receptor (M3). Data were normalized relative to the expression of 18S RNA (internal control) and are presented as fold change in gene expression in ob/ob vs WT mice. Ct values for 18S RNA expression were not significantly different between ob/ob mice and control (lean) littermates. Data are given as means ± SEM (n = 4 per group). *, P < .05; **, P < .01; ***, P < .01 vs WT.
Treatment of ob/ob mice with a selective V1b vasopressin receptor antagonist leads to reduced glucose excursions in a pyruvate challenge test
To test the contribution of V1b receptor signaling to elevated HGP in ob/ob mice in vivo, we subjected ob/ob mice and their WT littermates to a pyruvate challenge test (13, 21), either in the absence or the presence of a selective V1b vasopressin receptor antagonist. We injected mice with the gluconeogenic substrate pyruvate (1.5 mg/g, ip) and monitored changes in blood glucose levels over a 60-minute period. We found that pyruvate-injected ob/ob mice showed significantly more pronounced increases in blood glucose levels than pyruvate-injected WT mice (Figure 8A), confirming that HGP is enhanced in ob/ob mice (28). Strikingly, co-injection of ob/ob mice with pyruvate and SSR149415 (20 mg/kg ip), a selective V1b vasopressin receptor antagonist (29, 30), resulted in considerably smaller increases in blood glucose levels (Figure 8A). In fact, the blood glucose excursions observed with the co-injected ob/ob mice were similar to those observed with the 2 WT control groups, either injected with pyruvate alone or co-injected with pyruvate and SSR149415 (Figure 8A). These data suggest that V1b receptor signaling makes a critical contribution to the elevated HGP in ob/ob mice.
Figure 8.
A selective V1b vasopressin receptor antagonist reduces glucose excursions in ob/ob mice in a pyruvate challenge test. A, Pyruvate challenge test. The ob/ob and WT lean control mice (12-week-old males) received an ip injection of 1.5 mg/g sodium pyruvate, either alone or in combination with SSR149415 (20 mg/kg ip), a selective V1b vasopressin receptor antagonist. Blood glucose levels were measured at the indicated time points. Data are given as means ± SEM (n = 4 per group). *, P < .05; **, P < .01 vs the other 3 groups of mice. B, Glucose tolerance test. The ob/ob mice and WT lean littermates (16-week-old males) received an ip injection of glucose (2 mg/g), either alone or in combination with SSR149415 (20 mg/kg ip). Blood glucose levels were measured at the indicated time points. Data are given as means ± SEM (n = 4 per group).
On the other hand, treatment of ob/ob mice with SSR149415 (20 mg/kg ip) had no significant effect on blood glucose levels in an ip glucose tolerance test (Figure 8B).
V1b vasopressin receptor expression levels are unchanged in islets from ob/ob mice
Besides their presence in the liver, V1b receptors are expressed in several other tissues including pancreatic islets where these receptors promote the release of insulin (31, 32). Real-time qRT-PCR studies using islet RNA prepared from ob/ob mice and lean littermates (3-month-old males) showed that islet V1b receptor transcript levels were not significantly different between the two groups of mice (Supplemental Figure 3), excluding the possibility that changes in islet V1b receptor expression levels contributed to the beneficial metabolic effects observed with SSR149415-treated ob/ob mice (see previous paragraph).
Discussion
In this study, we generated a transgenic mouse line that expressed a Gq-coupled designer receptor (Rq) (16) selectively in hepatocytes. Due to 2 point mutations, the Rq receptor can no longer bind endogenous ligands such as acetylcholine (Rq is derived from the M3 muscarinic receptor) but can be efficiently activated by CNO, a synthetic drug that is pharmacologically inert (14, 16). Thus, CNO treatment of Hep-Rq mice provided us with the unique opportunity to study the in vivo metabolic consequences of activating a Gq-coupled receptor selectively in hepatocytes. In the present study, we focused on acute effects on HGP and glucose homeostasis in general.
We first demonstrated that hepatic Rq receptors were expressed at physiological levels in Hep-Rq mice and that CNO stimulation of Hep-Rq hepatocytes led to pronounced increases in intracellular calcium levels. The expression data suggested that the Rq receptor was expressed at comparable or lower levels, as compared with Gq-coupled receptors endogenously expressed by mouse hepatocytes.
A single ip injection of CNO into Hep-Rq mice caused pronounced, dose-dependent increases in blood glucose levels. CNO treatment of Hep-Rq mice did not lead to any significant changes in serum insulin levels, consistent with a direct action of CNO on hepatic Rq receptors. Moreover, CNO-injected Hep-Rq mice showed impaired glucose tolerance and significantly increased glucose excursions in a pyruvate challenge test, most likely due to the ability of activated Rq to facilitate HGP (see below). Hep-Rq mice, however, displayed normal glucose-induced insulin release and normal insulin sensitivity in vivo.
Glucagon plays a central role in maintaining blood glucose levels in a physiological range, primarily by activating hepatic glucagon receptors that are coupled to the stimulatory G protein Gs (6, 7). Activated Gs, via a series of cellular events that includes the generation of cAMP and the activation of protein kinase A, then triggers enhanced HGP by stimulating both glycogenolysis and gluconeogenesis (6, 7). To directly compare the magnitudes of the hyperglycemic effects induced by glucagon with those caused by ligand activation of a Gq-coupled receptor, we injected Hep-Rq mice with maximally effective doses of glucagon and CNO, respectively. Strikingly, we found that CNO treatment of Hep-Rq mice resulted in increases in blood glucose levels that were similar in magnitude to those observed after glucagon administration. This observation clearly indicates that hepatocyte GPCRs that signal through Gq-type G proteins are likely to play important roles in regulating blood glucose levels. These data may also be of potential pathophysiological relevance, because enhanced HGP is one of the hallmarks of T2D.
To determine to which extent hepatic gluconeogenesis and glycogenolysis contributed to the Rq-mediated hyperglycemic effects in Hep-Rq mice, we carried out isotope labeling experiments using chronically catheterized conscious Hep-Rq mice. These studies demonstrated that the CNO-dependent increase in blood glucose levels were due to the stimulation of both gluconeogenesis and glycogenolysis.
Gene expression analysis demonstrated that CNO-mediated activation of Rq in Hep-Rq mice led to a significant increase in the expression levels of PEPCK, one of the rate-limiting enzymes of gluconeogenesis. Similarly, the hepatic expression of several genes coding for proteins involved in fatty acid synthesis, including ACLY, ACC1, and FAS, was strongly increased, suggesting that Gq signaling pathways may play a role in the regulation of hepatic lipid metabolism. However, the physiological relevance of these findings remains to be explored in future studies. It should also be noted that the lack of CNO-induced changes in hepatic gene expression levels in Hep-Rq mice needs to be interpreted with caution because the activity of many key metabolic enzymes is also regulated at the posttranslational level.
Using GPCR array technology, we demonstrated that mouse hepatocytes express various Gq-coupled receptors including GPR91 (succinate receptor 1), the AT1a angiotensin II receptor, the V1a and V1b vasopressin receptor subtypes, and the α1b-adrenergic, PAR1 thrombin, S1P2 lysophospholipid, and M3 muscarinic receptors. The hepatic expression of some of these receptors, including different α1-adrenergic, angiotensin II, and V1 vasopressin receptor subtypes (11) and the M3 muscarinic receptor (13), has been reported previously. It is well documented that the activation of Gq-coupled receptors leads to the generation of IP3, which triggers the release of Ca2+ from intracellular stores (10). Previous studies have demonstrated that activation of hepatocyte GPCRs that cause increases in intracellular Ca2+ concentrations, including different α1-adrenergic, angiotensin II, and vasopressin receptor subtypes, stimulates hepatic glycogenolysis via Ca2+-dependent allosteric activation of phosphorylase kinase (11, 33, 34). It is therefore highly likely that the stimulatory effect of CNO on glycogenolysis in Hep-Rq mice involves a similar mechanism. However, little is known about the potential mechanisms through which Gq-coupled receptors can stimulate hepatic gluconeogenesis.
Increased HGP plays a key role in the pathophysiology of T2D (1–3). In T2D, ∼90% of the increase in HGP above basal is predicted to be caused by an enhanced rate of gluconeogenesis (35). This metabolic deficit can also be observed in animal models of diabetes, including the ob/ob mouse (28). In the present study, we demonstrated that the hepatic expression of several Gq-coupled receptors was elevated in ob/ob mice. Most notably, the V1b vasopressin receptor gene was expressed at ∼6-fold higher levels in ob/ob mice than in WT littermates. We therefore speculated that enhanced signaling through the V1b vasopressin receptor and other Gq-coupled receptors might contribute to maintaining enhanced HGP in this mouse diabetes model. To test this hypothesis, we examined whether treatment of ob/ob mice with a selective V1b vasopressin receptor antagonist (SSR149415) (29, 30) affected in vivo gluconeogenesis in a pyruvate challenge test. When mice were injected with the gluconeogenic substrate pyruvate alone, ob/ob mice showed significantly more robust increases in blood glucose levels than WT littermates, confirming that gluconeogenesis is enhanced in the mutant animals (28). In contrast, when mice were treated with pyruvate in combination with SSR149415, ob/ob mice showed significantly smaller blood glucose excursions that were similar in magnitude to those observed with WT mice. This finding raises the possibility that pharmacological blockade of V1b vasopressin receptors may represent a novel approach to reduce HGP in T2D. A key issue that needs to be addressed in future studies is whether human T2D involves similar changes in GPCR expression levels.
In the body periphery, V1b receptors are expressed not only by hepatocytes but also by other tissues and cell types including pancreatic islets (31, 32). In the central nervous system, pituitary V1b receptors act as important modulators of the physiological stress response by promoting the release of ACTH (32). Thus, the potential use of V1b receptor antagonists for the suppression of HGP would benefit from the development of agents that do not cross the blood-brain barrier and are therefore devoid of central side effects.
Although the Rq construct, like the M3 muscarinic receptor, preferentially activates G proteins of the Gq family (14, 16), it can also activate additional signaling cascades including arrestin-dependent pathways (36, 37). Thus, it remains to be explored whether non–Gq-dependent signaling molecules also contribute to the metabolic phenotypes displayed by the Hep-Rq mice. Moreover, endogenous GPCRs are known to form GPCR signaling complexes that are often restricted to distinct membrane subdomains, including lipid rafts and caveolae (38). Thus, it remains to be demonstrated to which extent designer GPCRs expressed from a transgene show a similar subcellular distribution pattern as native GPCRs.
Although the Hep-Rq mice analyzed in the present study showed striking, CNO-dependent metabolic phenotypes, transgenic mice overexpressing the M3 muscarinic receptor in hepatocytes did not display any obvious metabolic changes (13). One possible explanation for this observation is that CNO treatment of Hep-Rq mice leads to a continuous stimulation of hepatic Rq receptors. In contrast, in transgenic mice overexpressing M3 receptors in hepatocytes, receptor activation depends on the endogenous release of acetylcholine, which is likely to cause a different temporal pattern of receptor activation (compared with CNO-stimulated Rq receptors).
In the present study, we exclusively focused on the acute metabolic effects caused by activation of a Gq-coupled receptor selectively expressed in hepatocytes. In future studies, we are planning to explore how chronic treatment of Hep-Rq mice with CNO affects liver glucose fluxes and whole-body glucose homeostasis. Such studies should help address the question of whether enhanced signaling through Gq-coupled receptors contributes to the pathophysiology of T2D.
In conclusion, the development and phenotypic analysis of a novel mouse model (Hep-Rq mice) allowed us to explore the in vivo metabolic consequences after drug-induced activation of a Gq-coupled receptor selectively expressed in hepatocytes. Given the critical role of increased HGP in the pathophysiology of T2D, our findings may lead to new avenues to modulate liver glucose fluxes for therapeutic purposes.
Acknowledgments
We thank Dr Snorri Thorgeirsson (National Cancer Institute, Bethesda, Maryland) for providing the albumin promoter plasmid used for the generation of the Hep-Rq transgene construct. We are also very grateful to Dr Tamas Balla (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland) for carrying out the fura-2 experiments shown in Figure 1E.
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases, and NIH Grants DK059637 and DK020593. Metabolic flux studies were performed at the Vanderbilt Mouse Metabolic Phenotyping Center (U24 DK059637). UDPG and PEP analyses were performed in the Hormone Assay and Analytical Services Core of the Vanderbilt Diabetes Research and Training Center (P60 DK020593) and Mouse Metabolic Phenotyping Center.
Disclosure Summary: The authors have nothing to declare.
For editorial see page 3495
- AVP
- arginine vasopressin
- [Ca2+i]
- intracellular calcium level
- CNO
- clozapine-N-oxide
- Ct
- cycle threshold
- FLIPR
- Fluorometric Imaging Plate Reader
- GPCR
- G protein-coupled receptor
- HGP
- hepatic glucose production
- IP3
- inositol 1,4,5-trisphosphate
- PEP
- phosphoenolpyruvate
- PEPCK
- phosphoenolpyruvate carboxykinase
- qRT-PCR
- quantitative RT-PCR
- T2D
- type 2 diabetes
- UDPG
- UDP-glucose
- WT
- wild-type.
References
- 1. Taylor SI. Deconstructing type 2 diabetes. Cell. 1999;97:9–12 [DOI] [PubMed] [Google Scholar]
- 2. Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004;30:398–408 [DOI] [PubMed] [Google Scholar]
- 3. Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678 [DOI] [PubMed] [Google Scholar]
- 6. Estall JL, Drucker DJ. Glucagon and glucagon-like peptide receptors as drug targets. Curr Pharm Des. 2006;12:1731–1750 [DOI] [PubMed] [Google Scholar]
- 7. Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther. 2012;135:247–278 [DOI] [PubMed] [Google Scholar]
- 8. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Alessio D. The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab. 2011;13(Suppl 1):126–132 [DOI] [PubMed] [Google Scholar]
- 10. Wess J. Molecular basis of receptor/G-protein-coupling selectivity. Pharmacol Ther. 1998;80:231–264 [DOI] [PubMed] [Google Scholar]
- 11. Exton JH, Blackmore PF, El-Refai MF, et al. Mechanisms of hormonal regulation of liver metabolism. Adv Cyclic Nucleotide Res. 1981;14:491–505 [PubMed] [Google Scholar]
- 12. Clair C, Tran D, Boucherie S, Claret M, Tordjmann T, Combettes L. Hormone receptor gradients supporting directional Ca2+ signals: direct evidence in rat hepatocytes. J Hepatol. 2003;39:489–495 [DOI] [PubMed] [Google Scholar]
- 13. Li JH, Gautam D, Han SJ, et al. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes. 2009;58:2776–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conklin BR, Hsiao EC, Claeysen S, et al. Engineering GPCR signaling pathways with RASSLs. Nat Methods. 2008;5:673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guettier JM, Gautam D, Scarselli M, et al. A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A. 2009;106:19197–19202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Conner EA, Lemmer ER, Omori M, Wirth PJ, Factor VM, Thorgeirsson SS. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene. 2000;19:5054–5062 [DOI] [PubMed] [Google Scholar]
- 18. Jaruga B, Hong F, Kim WH, Gao B. IFN-γ/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–G1052 [DOI] [PubMed] [Google Scholar]
- 19. Li B, Scarselli M, Knudsen CD, Kim SK, Jacobson KA, McMillin SM, Wess J. Rapid identification of functionally critical amino acids in a G protein-coupled receptor. Nat Methods. 2007;4:169–174 [DOI] [PubMed] [Google Scholar]
- 20. Korzeniowski MK, Manjarrés IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2010;3:ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic α-cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397 [DOI] [PubMed] [Google Scholar]
- 23. Ayala JE, Samuel VT, Morton GJ, et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayala JE, Bracy DP, Malabanan C, et al. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp. 2011;e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wall JS, Steele R, De Bodo RC, Altszuler N. Effect of insulin on utilization and production of circulating glucose. Am J Physiol. 1957;189:43–50 [DOI] [PubMed] [Google Scholar]
- 26. Giaccari A, Rossetti L. Isocratic high-performance liquid chromatographic determination of the concentration and specific radioactivity of phosphoenolpyruvate and uridine diphosphate glucose in tissue extracts. J Chromatogr. 1989;497:69–78 [DOI] [PubMed] [Google Scholar]
- 27. Fujimoto Y, Donahue EP, Shiota M. Defect in glucokinase translocation in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2004;287:E414–E423 [DOI] [PubMed] [Google Scholar]
- 28. Turner SM, Linfoot PA, Neese RA, Hellerstein MK. Sources of plasma glucose and liver glycogen in fasted ob/ob mice. Acta Diabetol. 2005;42:187–193 [DOI] [PubMed] [Google Scholar]
- 29. Serradeil-Le Gal C, Wagnon J, Simiand J, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Ther. 2002;300:1122–1130 [DOI] [PubMed] [Google Scholar]
- 30. Griebel G, Stemmelin J, Gal CS, Soubrié P. Non-peptide vasopressin V1b receptor antagonists as potential drugs for the treatment of stress-related disorders. Curr Pharm Des. 2005;11:1549–1559 [DOI] [PubMed] [Google Scholar]
- 31. Oshikawa S, Tanoue A, Koshimizu TA, Kitagawa Y, Tsujimoto G. Vasopressin stimulates insulin release from islet cells through V1b receptors: a combined pharmacological/knockout approach. Mol Pharmacol. 2004;65:623–629 [DOI] [PubMed] [Google Scholar]
- 32. Roper J, O'Carroll AM, Young W, 3rd, Lolait S. The vasopressin Avpr1b receptor: molecular and pharmacological studies. Stress. 2011;14:98–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blackmore PF, Hughes BP, Shuman EA, Exton JH. α-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982;257:190–197 [PubMed] [Google Scholar]
- 34. Reinhart PH, Taylor WM, Bygrave FL. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984;223:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835, ix [DOI] [PubMed] [Google Scholar]
- 36. Alvarez-Curto E, Prihandoko R, Tautermann CS, et al. Developing chemical genetic approaches to explore G protein-coupled receptor function: validation of the use of a receptor activated solely by synthetic ligand (RASSL). Mol Pharmacol. 2011;80:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol. 2012;82:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel HH, Murray F, Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008;186:167–184 [DOI] [PubMed] [Google Scholar]