Abstract
IGFs play key roles in regulating vertebrate development, growth, reproduction, and aging. In extracellular fluids, IGFs are bound and regulated by a family of IGF-binding proteins (IGFBPs). Although all known IGFBPs are secreted proteins, some are also found in the nucleus and possess IGF-independent activities. When and how these distinct modes of biological actions have evolved is unknown. In this study, we identified and analyzed an IGFBP gene from amphioxus. Amphioxus shares a common ancestor with the modern vertebrate lineage that dates back to more than 520 million years ago. The amphioxus IGFBP shares all major structural characteristics of vertebrate IGFBPs. Phylogenetic analyses place it in a basal position in the IGFBP lineage. Ligand blot analysis reveals that amphioxus IGFBP does not bind to IGF-I or -II. Changing its Phe70 into Leu, however, is sufficient to convert it into a functional IGF binder. When tested in cultured cells, amphioxus IGFBP is localized in the nucleus, and this is attributed to 2 redundant nuclear localization sequences in its L domain. Furthermore, the amphioxus IGFBP N-terminal domain has strong transcriptional activation activity. Forced expression of amphioxus IGFBP in zebrafish embryos results in dorsalized phenotypes. This action requires nuclear localization. These results suggest that the nuclear localization and transcription activation activity of IGFBPs are ancient functions and the IGF-binding function may have been acquired by opportunistic gain-of-functional mutations later in evolution.
IGFs are evolutionarily conserved peptide growth factors that play fundamental roles in regulating development, growth, reproduction, and aging. The biological actions of IGFs are mediated by the IGF-1 receptor (IGF1R) and its downstream signaling network (1). In extracellular fluids, IGFs are bound by several high-affinity and specific binding proteins known as IGFBPs (1). Human and other vertebrate genomes all contain multiple IGFBP genes (2). These proteins share similar domain structures. They all consist of a highly conserved and highly cysteine-rich N-terminal domain, a variable linker domain in the middle, and a conserved and cysteine-rich C-terminal domain (3, 4). There is a characteristic IGFBP motif in the N domain and a thyroglobulin type-1 domain in the C domain in all known IGFBPs (2, 4).
IGFBPs were initially thought to be simple carrier proteins for circulating IGFs. Indeed, the vast majority of IGFs in the bloodstream are found in a ternary complex containing an IGF-I or -II, an IGFBP-3 or -5, and another protein called the acid labile subunit (3). The ternary complex prolongs the half-lives of circulating IGFs and prevents potential cross binding of IGFs to the insulin receptor (IR) (1–5). Furthermore, the ability of IGFBPs to bind to IGFs with equal or even greater affinities than the IGF1R has placed them in a key position in regulating the IGF ligand and receptor interaction (3, 4). Numerous in vitro and in vivo studies have shown that IGFBPs can inhibit IGF actions and/or potentiate IGF actions (1–5). More recent studies suggest that some human/mammalian IGFBPs also possess intrinsic biological activities that are independent of IGF binding (6–8). Intriguingly, several IGFBPs have been detected in the nucleus and can interact with nuclear proteins (3, 7, 9–14). The nuclear localization of human IGFBP-3 and -5 has been attributed to a highly conserved bipartite nuclear localization sequence (NLS) in their C domain (3, 9, 15, 16). IGFBP-6 has a unique and functional NLS in the C domain (17). Furthermore, some IGFBPs have been reported to possess intrinsic transcription activation activity and can regulate gene expression directly or indirectly (9, 10, 15, 18, 19). These findings suggest that IGFBPs are multifunctional proteins. However, when and how these distinct modes of biological action have evolved is unclear.
To gain insights into the structural and functional evolution of the IGFBP family, we identified and analyzed amphioxus IGFBP (amphiBP) in this study. Amphioxus shares a common ancestor with the modern vertebrate lineage that dates back to more than 520 million years ago (20). Its phylogenetic position and conserved morphology has promoted its use in studies of vertebrate evolution (21, 22). The amphioxus genome is a good surrogate for the ancestral chordate genome with respect to gene content, exon-intron gene structure, and chromosomal organization (23). Amphioxus did not go through the 2–3 rounds of whole-genome duplication seen in modern vertebrates (21, 24). Consequently, its genome often contains a single gene in a given vertebrate gene family. Indeed, a previous in silico study identified one putative IGFBP sequence in the amphioxus genome database. Its true identity, however, could not be confirmed because the predicted central region was highly divergent (2). In this study, we provide evidence that the amphioxus genome indeed contains a single IGFBP gene. Our phylogenetic, structural, and functional analyses suggest that amphioxus IGFBP is localized in the nucleus and has strong transcription activation activity. When tested in vivo, amphioxus IGFBP exhibited IGF-independent biological action that requires its nuclear presence. Interestingly, amphioxus IGFBP does not bind to IGF-I or -II, but a single amino acid change is sufficient to convert it into a functional IGF binder.
Materials and Methods
All chemicals and reagents were purchased from Fisher Scientific (Pittsburgh, Pennsylvania) unless otherwise stated. Cell culture media and oligonucleotide primers were purchased from Invitrogen (Carlsbad, California). Restriction enzymes were purchased from New England Biolabs (Ipswich, Massachusetts) or Promega Corp. (Madison, Wisconsin). Recombinant human IGF-I and IGF-II were purchased from GroPep (Adelaide, Australia). Bovine insulin was purchased from Sigma (St Louis, Missouri). The Dual-luciferase reporter assay kit was purchased from Promega. The anti-green fluorescent protein (GFP) antibody was purchased from Torrey Pines Biolabs (Secaucus, New Jersey), and the anti-Gal4 antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, California).
Experimental animals
Amphioxus (Branchiostoma belcheri tsingtauense) was collected from the seashore in Qingdao City, Shandong Province, China. Wild-type zebrafish (Danio rerio) were raised and kept in the Duan laboratory Fish facility, University of Michigan, on a 14-hour light/10-hour dark cycle. Fertilized eggs were obtained by natural cross and raised in embryo-rearing medium at 28.5°C and staged according to Kimmel et al. (25). All experiments were conducted at the University of Michigan in accordance with the guidelines approved by the University Committee on the Use and Care of Animals.
Molecular cloning and sequence analysis
To identify the amphioxus IGFBP gene and clone its cDNA for functional analysis, we performed TBLASTN searches against the Branchiostoma Floridae genome database and the transcripts database (http://genome.jgi-psf.org/cgi-bin/runAlignment?db=Brafl1&advanced = 1) using human IGFBP-1∼6 sequences as queries. A single putative IGFBP (est number: estExt_fgenesh2_pg.C_1700020; genomic location: Bf V1_scaffold_170) was identified. Its full-length cDNA sequence was determined by 5′-and 3′-rapid amplification of cDNA ends (RACE) using the SMART RACE kit (CLONTECH, Mountain View, California). Total RNA was extracted from adult B. belcheri using TRIzol reagent (Invitrogen) and reverse transcribed into RACE-Ready first-strand cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen). The RACE products were subcloned into the pGEM-T Easy Vector (Promega) and sequenced at the DNA Sequencing Core, University of Michigan. These sequence data have been submitted to the DDBJ/EMBL/GenBank databases (Accession no. FJ971406).
The amphioxus IGFBP genomic structure was determined by comparing the B. belcheri IGFBP open reading frame (ORF) sequence with the available B. Floridae genome sequence (version 2.0). For sequence alignment and comparison, known IGFBP sequences were obtained from the GenBank or Ensembl database and aligned with amphioxus IGFBP sequence by Clustal X. Phylogeneic tree was constructed by Bayesian Markov chain Monte Carlo analysis using MrBayes version 3.0b4 by integrating over protein models, assuming a 4-category γ among-site rate variation distribution, with uniform priors over trees, branch lengths (0.5), and the ASRV α parameter (0.05–10). Three independent analyses, each with 4 chains (1 cold and 3 heated), were run for 1 000 000 generations and sampled every 100 generations. The first 250 samples from each run, a point well past stationarity, were discarded as burn-in. All analyses converged on the same tree and found the Jones-Taylor-Thornton protein model had 100% posterior probability. Maximum Likelihood was performed using PhyML 3.0 with the Jones-Taylor-Thornton model selected by ProtTest. Several IGFBP-related proteins, which are structurally related to IGFBPs (26), were used as out groups.
Quantitative real-time RT-PCRs (qRT-PCRs)
Total RNA was isolated from amphioxus using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, California). cDNAs were reverse-transcribed using oligo(dT) (Sangon, Shanghai, China) and SuperScript II reverse transcriptase according to the manufacturer's instructions. qRT-PCR was carried out using the iCycler iQ5 Multicolor real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, California) according to the manufacturer's instructions. Primer sequences for qRT-PCR were listed in supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, California). β-Actin mRNA levels were used as the internal control. Amphioxus IGFBP amplification efficiency was similar to that of β-actin. Results were analyzed using the 2-ΔΔCT method (27). Each experiment was performed in duplicate and repeated 3 times.
Plasmid construction
The construction of the human IGFBP-2-GFP and IGFBP-5-GFP plasmids was reported previously (9, 10). DNA encoding the amphioxus IGFBP ORF was amplified by PCR, subcloned into the pCS2-enhanced GFP (eGFP) vector, resulting in pCS2-amphiBP-eGFP. The primers used are shown in Supplemental Table 1. To map the domain responsible for nuclear localization, several amphioxus IGFBP/human IGFBP-2 chimera constructs were engineered. For this, DNA fragments corresponding to amphioxus IGFBP signal peptide + N domain (22–101 amino acids [aa]), L domain (102–232 aa), C domain (233–309 aa), N+L domains, L+C domains, as well as human IGFBP-2 L domain (135–229 aa), and C domain (230–328 aa) were generated by PCR using primers shown in Supplemental Table 1. The fragments were subcloned into the pCS2-eGFP vector. To further map the critical residues for nuclear localization, 157–170 aa and 185–214 aa, 2 basic residue-rich regions in the amphioxus IGFBP L domain, were deleted, resulting in ΔNLS1 and ΔNLS2, respectively. Next, a number of point mutants were made. The primers used are shown in Supplemental Table 1. To test whether the amphioxus IGFBP N domain has transcription activation activity, DNA encoding amphioxus IGFBP N domain (22–101 aa) was amplified by PCR and subcloned into the pBIND vector (Promega), resulting in pBIND-amphiBPN. Two transcription activation dead mutants (E43L/E52A and Q8A/E11S/E43L/E52A) were generated by site-directed mutagenesis using pBIND-amphiBPN as the template. The primers used are shown in Supplemental Table 1. All plasmids engineered were sequenced at the University of Michigan DNA Sequencing Core Facility.
Cell culture, subcellular localization, Western blot, ligand blot, and transcription activation assay
Human U2 osteosarcoma (U2OS) and human embryonic kidney (HEK) 293T cells were purchased from American Type Culture Collection (Manassas, Virginia) and cultured in Macoy 5A medium and DMEM supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin in a humidified-air atmosphere containing 5% CO2. For transfection, 0.5 × 106 cells were seeded into each well in 6-well cell culture plates (Falcon, Corning, New York). Plasmid DNA was transfected into cells using Lipofectamine 2000 (Invitrogen). The subcellular localization and transcription activation activities of various IGFBPs were determined, and Western blot analysis was conducted as described previously (10).
The activity of amphioxus IGFBP and its mutants to bind to IGFs or insulin was determined by ligand blot following a published procedure (28). Briefly, 50 μg human IGF-I, IGF-II, and bovine insulin were labeled with digoxingenin (DIG) as reported elsewhere (28). The DIG-labeled IGF-I, IGF-II, and insulin were stored at −80°C until use. Conditioned media were prepared from HEK 293T cells transfected with various IGFBP expression plasmids and concentrated from 800 μl to 60 μl as previously reported (15). Concentrated condition media (15 μl) were analyzed by ligand blot analysis using 200 ng DIG-labeled ligands and Western blot analysis using a GFP antibody as previously reported (15).
Mammalian one-hybrid transcription assays were carried out to determine the possible transcription activation activity of the amphioxus IGFBP N domain. Briefly, 2 plasmids were used; the pG5-luc plasmid (Promega) contains a Gal 4-binding site driving the expression of the firefly luciferase gene. The pBIND plasmid (Promega) contains the Gal4 DNA-binding domain (without a transactivation domain). The pBIND-amphiBPN and its mutants were described earlier in this paper. The pBIND-humanBP5N plasmid, which has strong transcription activation activity (9), was used as a positive control. The pBIND-humanBP1N was used as a negative control (9). HEK 293T cells were cotransfected with pG5-luc and a pBIND-IGFBPN construct. Cells were washed and lysed 24 hours after the transfection. The lysates were measured for firefly and Renilla luciferase activities using the Dual-Luciferase Reporter assay system (Promega). The results were expressed as fold changes over the pBIND empty vector group. Transfection efficiency was normalized by Renilla luciferase activity.
Microinjection experiment
Capped mRNA synthesis was performed using linearized plasmid DNA as template and the mMESSAGE mMACHINE kit (Ambion, Inc., Austin, Texas). Capped mRNA (1 ng per embryo) was microinjected into zebrafish embryos at the 1- to 2-cell stage as reported previously (16). GFP mRNA-injected embryos were used as controls. After microinjection, embryos were raised as described above. Live embryos were imaged.
Statistics
All values were presented as means ± SEM. Statistical analyses were performed by Tukey post hoc ANOVA using GraphPad Prism 5 (GraphPad Software, La Jolla, California).
Results
Amphioxus has a single IGFBP that is expressed throughout early development
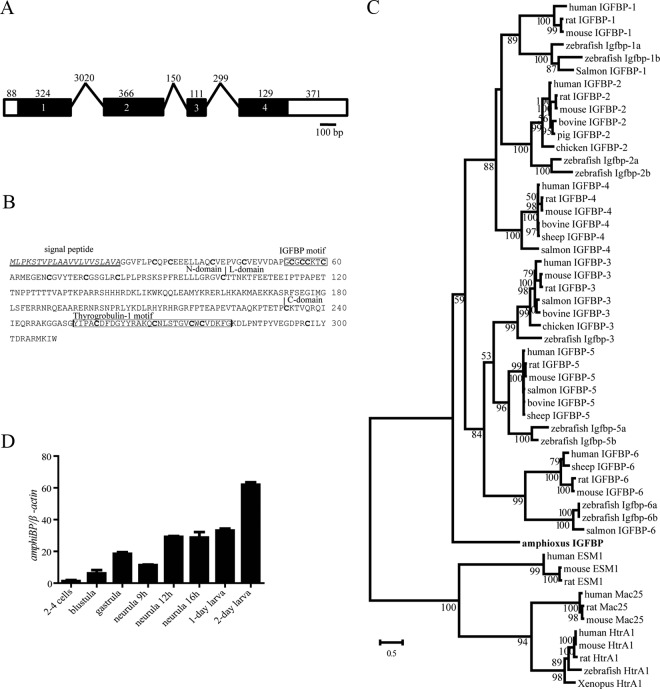
We searched the public databases using human IGFBP-1 through 6 sequences as independent queries. All searches identified the same putative IGFBP gene. Its full-length cDNA was obtained and genomic structure determined. The amphioxus IGFBP gene is 4.8 kb long and contains 4 exons and 3 introns (Figure 1A). The full-length cDNA is 1422 bp containing 1 ORF (Genbank identification no. FJ971406). The encoded protein is 309 aa with a putative signal peptide of 21 aa and mature protein of 288 aa. This protein shares significant amino acid identity with human IGFBPs and a similar domain structure, including an N domain containing 12 cysteine residues, a C domain containing 6 cysteine residues, and an L domain with no cysteine (Figure 1B). Amphioxus IGFBP has a characteristic IGFBP motif in the N domain and a thyroglobulin domain in the C domain (Figure 1B). Phylogenetic analyses suggest that amphioxus IGFBP occupies a basal position in the IGFBP lineage (Figure 1C and Supplemental Figure 1) (26, 29). These results suggest that amphioxus has a single IGFBP gene encoding a bona fide IGFBP. Amphioxus IGFBP mRNA was easily detected in all developmental stages examined. Its levels were relatively low at the 2- to 4-cell stage and gradually increased from the blastula to the neurula stage (Figure 1D). There was a further increase at the 2-day larva stage (Figure 1D).
Figure 1.
Amphioxus Has a Single IGFBP Expressed throughout Early Development. A, Schematic diagram of the amphioxus IGFBP gene. Black boxes represent the protein-coding region, open boxes represent the untranslated regions (UTR), and lines represent the introns. Numbers inside the boxes represent the exon number. The size (bp) of each exon and intron, respectively, is shown on the top. B, The amino acid sequence of amphioxus IGFBP. The predicted signal peptide is underlined. Vertical lines mark the boundaries between the N, L, and C domains. The characteristic IGFBP motif and thyroglobulin domain are boxed, and the conserved cysteine residues are marked in bold letters. C, Phylogenetic tree of IGFBPs. The full-length amino acid sequence of the indicated IGFBPs was aligned and analyzed by the Maximum Likelihood method. Mac25, HtrA1, and EMS1 were used as out groups. Numbers indicate bootstrap values for each node. D, Developmental expression profile of the amphioxus IGFBP gene. The amphioxus IGFBP mRNA levels were measured by qRT-PCR. The values are expressed as relative values to the β-actin mRNA levels. Values shown are means ± SE; n = 3.
Amphioxus IGFBP does not bind to IGFs but gains IGF-binding ability with a single amino acid change
To determine whether amphioxus IGFBP can bind IGF, an amphioxus IGFBP expression construct was engineered and introduced into HEK 293T cells. A human IGFBP-5 expression plasmid was used as a control (9). Western immunoblot analysis of the conditioned media collected from these cells showed the successful expression and secretion of human IGFBP-5 and amphioxus IGFBP (Figure 2A). Although the predicted mass of amphioxus IGFBP-GFP is approximately 63 kDa, its apparent molecular mass on a sodium dodecyl sulfate gel was about 98 kDa. Vertebrate IGFBPs are known to be modified in the variable L domain by glycosylation, phosphorylation, and/or proteolysis (3). Amphioxus IGFBP has a highly divergent and long L domain compared with its human orthologs. Analysis of this sequence using Expasy program indicated that there 19 predicted O-glycosylation sites and 1 predicted N-glycosylation site. Although both IGF-I and IGF-II bound to human IGFBP-5 in ligand-blot analysis, neither was able to bind to amphioxus IGFBP (Figure 2A). Similarly, amphioxus IGFBP was not able to bind to insulin (Supplemental Figure 2). The high-affinity IGF-binding site in human IGFBP-3 and -5 is 69R/KPLXXLL75 (30–32). This sequence is conserved in all known vertebrate IGFBP-3 and -5 (Figure 2B). However, the corresponding sequence in mature amphioxus IGFBP is 68SPFXXLL74 (Figure 2B). To determine whether either or both changes are responsible for the lack of IGF binding, 3 amphioxus IGFBP mutants were engineered and tested. Whereas mutant S/K had little IGF-binding activity, Mutant F/L and Mutant F/L+S/K both had IGF-binding activity (Figure 2C). These data suggest that changing residue 70F into L in amphioxus IGFBP is sufficient to gain the IGF-binding function.
Figure 2.
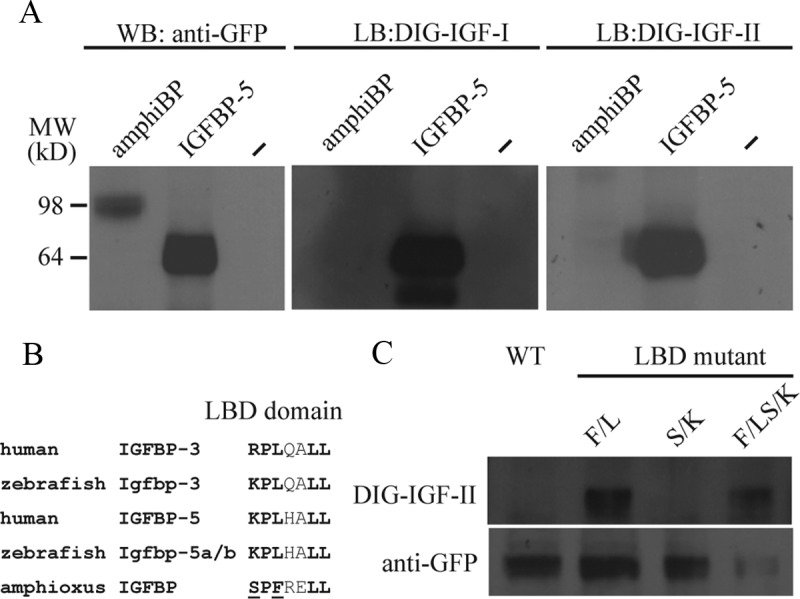
Amphioxus IGFBP Does Not Bind to IGF-I or -II but Gains the IGF-Binding Function with a Single Amino Acid Change. A, Western immunoblot and ligand-blot analysis of amphiBP and human IGFBP-5. Conditioned media from HEK 293T cells transfected with expression plasmids for the indicated IGFBP were collected and analyzed by Western immunoblot (WB) and ligand immunoblot (LB) using the indicated antibody or DIG-labeled IGFs. B, Alignment of the IGF-binding site sequence in the indicated IGFBPs. Bold letters indicate the residues known to be critical for ligand binding. The 2 residues unique to amphioxus and tested are underlined. C, Western immunoblot and ligand-blot analysis of amphioxus IGFBP and the indicated mutants. Conditioned media prepared from HEK 293T cells transfected with expression plasmids for amphiBP or the indicated mutants were analyzed by Western immunoblot using a GFP antibody and ligand blot using DIG-labeled IGF-II. LBD, ligand-binding domain; WT, wild type.
Amphioxus IGFBP is localized in the nucleus and has 2 redundant NLS motifs
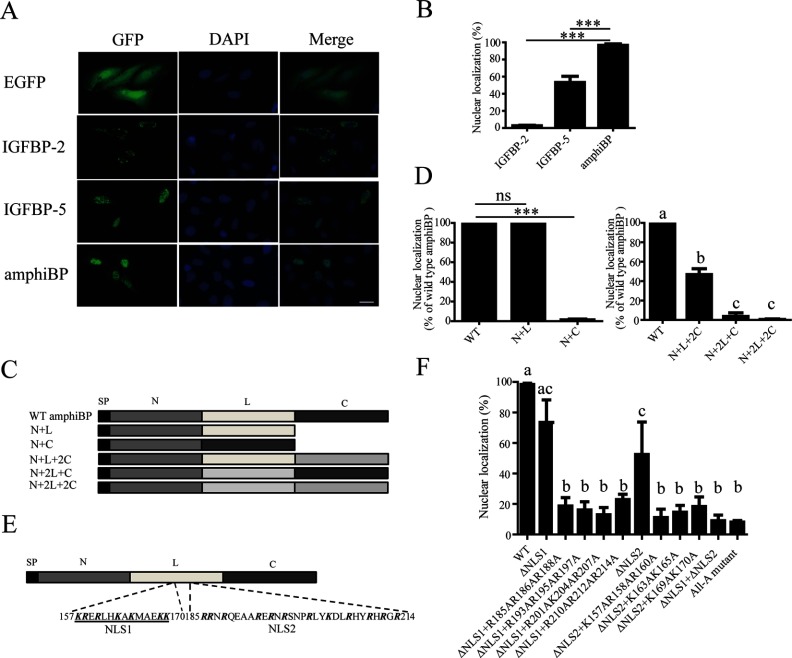
We next investigated whether amphioxus IGFBP is localized in the nucleus. Because there is no amphioxus cell line or primary cell culture system available, we used human U2OS cells instead. These cells have large and easily visible nuclei and have previously been used to investigate the nuclear localization of human and fish IGFBPs (10, 15, 16). When expressed in cultured U2OS cells, amphioxus IGFBP-eGFP signal was detected in the nucleus (Figure 3A). As reported previously (9), human IGFBP-5-eGFP was found in the nuclei of transfected cells, whereas human IGFBP-2-eGFP was not localized in the nucleus (Figure 3A). The expression levels of human IGFBP-2, IGFBP-5, and amphioxus IGFBP were similar. The subcellular localization experiments were repeated 3 times. In each experiment, more than 200 cells were counted and the percentage of cells showing nuclear eGFP signal was calculated and shown in Figure 3B. Amphioxus IGFBP had a greater level of nuclear localization compared with human IGFBP-5 (Figure 3B, P < .001).
Figure 3.
Amphioxus IGFBP Is Present in the Nucleus and Has Two Functional NLSs. A, Subcellular localization of amphioxus IGFBP. U2OS cells were transfected with the pC2-EGFP plasmid containing the indicated protein. eGFP signal was visualized (left panels) at 24 hours after transfection. Corresponding 4′,6-diamidino-2-phenylindole (DAPI) staining is shown in the middle panels and merged views in the right panels. Scale bar, 50 μm. B, Quantitative results of experiments described in panel A. In each experiment, more than 200 cells were counted and the percentage of cells showing nuclear eGFP signal was calculated and is shown. Values shown are means ± SEM; n = 3. ***, P < .001. C, Schematic diagrams of the amphioxus IGFBP/human IGFBP-2 chimeras tested. D, Nuclear localization of the chimeric proteins shown in panel C. Values are means ± SEM; n = 3. ***, P < .001. ns, not significant (P > .05). In the right panel, groups labeled with different letters are significantly different from each other (P < .05). E, Two NLS motifs are present in the L domain. Bold letters indicate basic residues. F, Subcellular localization of the indicated amphioxus IGFBP mutants. Values are means ± SEM; n = 3. Groups labeled with different letters are significantly different from each other (P < .05). WT, wild type.
Previous studies have shown that a bipartite NLS in the C domain of IGFBP-3/5 is responsible for their nuclear localization (9, 15, 16, 33). Although there are several basic residues in the amphioxus IGFBP C domain, deletion of the amphioxus IGFBP C domain had little effect on its nuclear localization. In contrast, deletion of its L domain abolished the nuclear localization (Figure 3, C and D). Next, 3 amphioxus IGFBP/human IGFBP-2 chimera constructs were generated by replacing the L and/or C domain of amphioxus IGFBP with that of the human IGFBP-2. These chimeras are named IGFBP-NL2C (which consisted of the amphioxus IGFBP N and L domains and the human IGFBP-2 C domain), N2LC (amphioxus IGFBP N domain+human IGFBP-2 L domain+amphioxus IGFBP C domain), and N2L2C (amphioxus IGFBP N domain+human IGFBP-2 L domain and C domain) (Figure 3C). The NL2C mutant had significant levels of nuclear localization, albeit lower compared with those of the wild-type amphioxus IGFBP. In contrast, both N2LC and N2L2C showed negligible nuclear localization (Figure 3D). These results suggest that the amphioxus IGFBP L domain is critical for its nuclear localization. There are two R/K-rich sequences in the amphioxus IGFBP L domain (157–170 aa and 185–214 aa referred as NLS1 and -2, respectively, in Figure 3E). Deletion of both NLS1 and NLS2, ie, ΔNLS1+ΔNLS2 mutant, nearly abolished its nuclear presence (Figure 3F). Likewise, changing all R and K in these 2 motifs into A (All-A mutant) resulted in a 91% reduction in the levels of nuclear localization (Figure 3F). To clarify the functional relationship between NLS1 and NLS2, they were deleted individually. Deletion of NLS1 or NLS2 individually decreased the nuclear localization levels to 73% and 52% of the control group level, respectively (Figure 3F). Next, 185R/186R/188R, 193R/195R/197R, 201R/204K/207R, and 210R/212R/214R were mutated in the ΔNLS1 background. Likewise, 157K/158R/160R, 163K/165K, and 169K/170K were mutated in the ΔNLS2 mutant background. All of these 7 mutants had significantly lower levels of nuclear localization comparable to the original ΔNLS1 mutant or ΔNLS2 mutant (Figure 3F). These results suggest that amphioxus IGFBP is capable of entering the nucleus via 2 functionally redundant NLS motifs in its L domain.
Amphioxus IGFBP N domain contains a functional transcription activation domain
Several human and fish IGFBPs contain a transcription activation domain in their N domain and have strong transcription activation activity (9, 10, 15, 16). We tested whether this activity is conserved in amphioxus IGFBP. As shown in Figure 4A, the amphioxus IGFBP N domain had a transcription activation activity comparable to the human IGFBP-5 N domain. In comparison, human IGFBP-1 N domain had no such activity (Figure 4A). There are 8 residues in the human IGFBP-5 N domain known to be crucial for its transcription activation activity (10). Five of them, ie, Q8, E11, E12, E43, and E52, are conserved in amphioxus IGFBP. Importantly, these 5 residues are not present in the closely related human IGFBP-1. Replacing Q8, E11, E12, E43, and E52 in amphioxus IGFBP with the corresponding residues of human IGFBP-1 resulted in a significant reduction in the transcription activation activity (Figure 4B). Likewise, mutation of E43/E52 into L43/A52 also significantly decreased the transcription activation activity. Western blot analysis showed that all of these fusion proteins were successfully expressed. These results suggest that the transcription activation domain is conserved and functional in amphioxus IGFBP.
Figure 4.
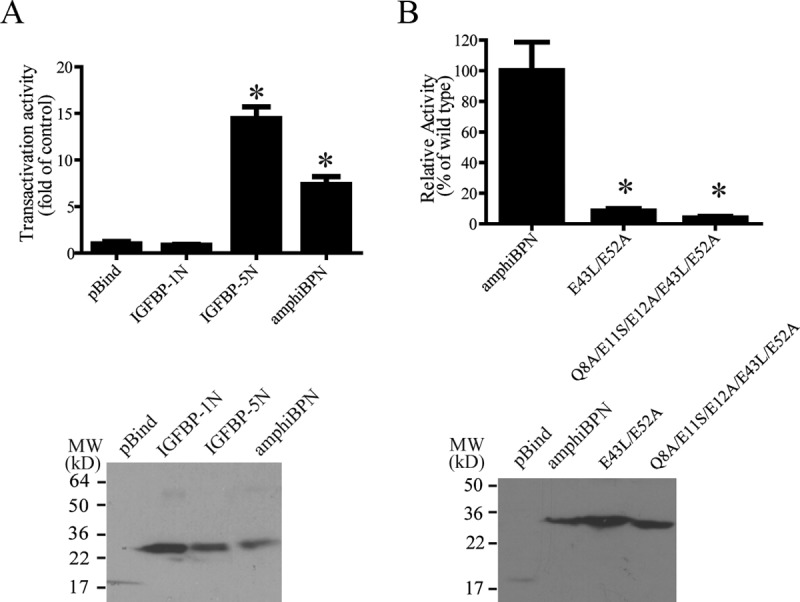
Amphioxus IGFBP Has Transcriptional Activation Activity. A, The N domain of amphioxus IGFBP was fused to the Gal4-DBD and introduced into HEK 293T with a Gal4 reporter plasmid. The human IGFBP-5 N domain and the human IGFBP-1 N domain were fused to the Gal4-DNA-binding domain (DBD) and used as positive and negative controls. The expression of the indicated Gal4 fusion proteins was verified by immunoblot, and this is shown in the lower panel. B, The indicated amphioxus IGFBP N domain mutants were fused to the Gal4-DBD and their transcriptional activities were determined and are expressed as relative values of the control group. Transfection efficiency was normalized by Renilla luciferase activity. Values are means ± SEM; n = 3. *, P < .05 compared with the pBind group. The expression of the indicated Gal4 fusion proteins was verified by immunoblot and this is shown in the lower panel. MW, molecular weight.
Amphioxus IGFBP has in vivo biological activity that requires its nuclear presence
Because it is technically difficult to perform transgenic studies in amphioxus and there is no amphioxus cell culture system available, we examined the biological actions of amphioxus IGFBP in zebrafish embryos. Previous studies have shown that forced expression of human or fish IGFBP-1 or -2 in zebrafish embryos resulted in smaller but morphologically normal embryos and these actions are IGF dependent (34, 35). In comparison, expression of IGFBP-3 or -5 resulted in dorsalized embryos, which occurred through an IGF-independent mechanism (16). As shown in Figure 5A and 5B, forced expression of amphioxus IGFBP caused a shortening or complete loss of the tail and a truncated body in approximately 36% of the embryos, whereas all GFP mRNA-injected control embryos were indistinguishable from the intact embryos. In the human IGFBP-5 expressing embryos, more that 50% showed this abnormal phenotype, whereas the rest of the embryos were smaller but morphologically normal (Figure 5B). In comparison, nearly all embryos expressing human IGFBP-2 were smaller but morphologically normal (Figure 5B). Next, the biological actions of wild-type amphioxus IGFBP and its ΔNLS1+ΔNLS2 mutant were examined and compared. Compared with wild-type amphioxus IGFBP, the ΔNLS1+ΔNLS2 mutant had markedly reduced activity (Figure 5C), suggesting that this action of amphioxus IGFBP requires its nuclear presence.
Figure 5.
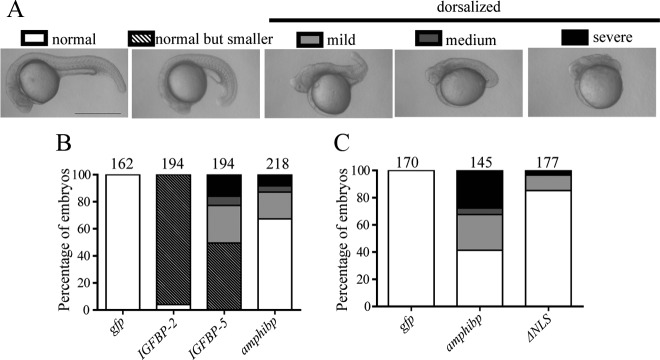
Amphioxus IGFBP Has NLS-Dependent Biological Actions Similar to IGFBP-5 but Different from IGFBP-2. A, Classification of phenotypes caused by forced expression of various IGFBPs. 1- to 2-cell stage zebrafish embryos were injected with capped mRNA encoding human IGFBP-2, human IGFBP-5, and amphioxus IGFBP or GFP. They were raised to 24 hours postfertilization (hpf). Lateral views are shown. Scale bar, 0.5 mm. B, Quantitative results. Embryos described in panel A were scored accordingly and the percentage in each category is shown. Total embryo number is shown on the top of each column. C, Nuclear localization is required. Embryos injected with amphioxus IGFBP, its ΔNLS1+ΔNLS2 mutant, or GFP mRNA were raised to 24 hpf and scored and are presented as described above.
Discussion
In this study, we have discovered that amphioxus has a single IGFBP gene with a typical exon-intron organization seen in vertebrate IGFBP genes. Structural and phylogenetic analyses indicate that the amphioxus IGFBP protein is a bona fide member of the IGFBP family and it occupies a basal position in the IGFBP phylogenetic tree. The identification of an IGFBP in amphioxus suggests that the IGFBP family is evolutionarily ancient and emerged before the appearance of vertebrates.
IGFBPs, as their name indicates, were discovered based on their physical association with IGF-I and/or -II. To date, studies show all vertebrate IGFBPs bind to IGF-I and/or IGF-II with high affinity (36). In this study, we show that amphioxus IGFBP bound neither to mammalian IGF-I or IGF-II in ligand-blotting assays nor to mammalian insulin. The lack of IGF ligand binding is not likely to be an experimental artifact because a single amino acid change can convert amphioxus IGFBP into a functional IGF binder. This finding is also supported by the structural analysis results. The primary IGF-binding site is R/KPLXXLL in the N domain in human IGFBP-3 and -5 (30–32). This sequence and the IGF-binding property are conserved in zebrafish Igfbp-3 and -5 (15, 16). The corresponding sequence in amphioxus IGFBP, however, has 2 critical changes. Likewise, among the 4 critical residues in the B and A chain of mammalian IGF-I (E3, T4, Q15, and F16) and IGF-II (E6, F48, R49, and S50) that are important for IGFBP binding, only 2 of them are found in amphioxus insulin-like peptide (ILP)-1 (E3 and F16) and one in ILP-2 (F62). Future studies are needed to address this issue using pure and biologically active amphioxus ILPs and amphioxus IGFBP. It should also be mentioned that we used ligand-blot analysis to examine the ligand-binding function of amphioxus IGFBP. Although this assay is useful in demonstrating the direct binding between an IGF and an IGFBP, it is not quantitative.
The vertebrate IGFBP family can be divided into 2 main subgroups. One branch includes IGFBP-3, -5, and -6, whereas the other includes IGFBP-1, -2, and -4 (2). Interestingly, all members in the IGFBP-3/5/6 lineage have been found in the nucleus and have IGF-independent actions (3, 9, 15–17). Among the IGFBP-1/2/4 lineage, published evidence indicates that IGFBP-1 and -4 are not localized in the nucleus (9, 16). Inconsistent evidence has been reported regarding IGFBP-2 (11, 18, 44, 45). Azar et al. (18) have provided experimental evidence suggesting that IGFBP-2, when overexpressed, can enter the nucleus and stimulate vascular endothelial growth factor gene promoter activity. On the other hand, Sun et al. (45) reported that endogenous IGFBP-2 is not found in chondrocyte nuclei. In this study, we found that amphioxus IGFBP, when fused to GFP and tested in cultured human U2OS cells, had a greater level of nuclear localization than that of human IGFBP-5-GFP. Our further structural and functional experiments revealed that the amphioxus IGFBP L domain is critical for its nuclear localization. There are 2 NLS motifs in this region and they act in a functionally redundant manner. This is different from vertebrate IGFBP-3, -5, and -6, which have a functional NLS in their C domain (3, 9, 15–17). Our functional analyses also suggested that amphioxus IGFBP N domain has strong transcription activation activity. The transcription activation domain is most extensively studied in human IGFBP-5 (9, 10, 15). Five of the 8 residues critical for the transcription activation activity in human and zebrafish IGFBP-5 N domain are present in amphioxus IGFBP. Changing of these residues resulted in a significant reduction in the transcription activation activity. These data suggest that nuclear localization and transactivation activity are ancient features of the IGFBP family.
Several mammalian and fish IGFBPs have been shown to have biological actions that are independent of IGF binding (3, 7). Forced expression of human or fish IGFBP-1 and -2 in zebrafish embryos results in morphologically normal but smaller embryos, and these growth-inhibitory actions are IGF-dependent (34, 35). In comparison, forced expression of human and fish IGFBP-3 and -5 resulted in dorsalized phenotypes, and these actions have been shown to be IGF independent (16). In this study, we used this in vivo bioassay system to test the biological activity of the amphioxus IGFBP. Our results showed that amphioxus IGFBP has in vivo biological actions similar to those of IGFBP-5 but distinct from those of IGFBP-2. These results, together with the ligand blot data, suggest that amphioxus IGFBP possesses biological activities that are independent of IGF binding. Intriguingly, although amphioxus IGFBP has a greater level of nuclear localization compared with human IGFBP-5, it appeared to have a weaker activity in zebrafish embryos. Amphioxus IGFBP N domain had a weaker transcriptional activity compared with that of human IGFBP-5 N domain. This may partially explain the weaker biological activity of amphioxus IGFBP. Moreover, these activities are evaluated in a heterogeneous bioassay system using zebrafish embryos. Because the evolutionary distance between zebrafish and amphioxus is greater than that between zebrafish and humans (both are vertebrates), it is possible that a human protein may “function” more efficiently when expressed in zebrafish. The IGF-independent actions of IGFBP-3/5 have been well documented, and several plausible mechanisms have been suggested for the IGF-independent actions, including 1) possible cell surface receptors; 2) nuclear localization and nuclear actions; and 3) intracellular (but nonnuclear) actions (46). Our results suggest that the IGF-independent activity of amphioxus IGFBP requires its nuclear presence. However, the possibility that deleting the basic residues may have some effects on the protein conformation and function cannot be ruled out at present.
IGF-I and IGF-II belong to the insulin/IGF superfamily (36). This superfamily includes insulin, IGFs, relaxin, several insulin-related peptides in vertebrates, and many ILPs in invertebrates (36). It is believed that members of this superfamily are derived from an ancestral insulin-type gene (36). This view is supported by the fact that no IGF-like molecule has been found in the fully sequenced fly and nematode genomes, whereas these genomes contain many insulin-like molecules (37). In the amphioxus genus, Chan et al. (38) identified an ILP in B. californiensis. The A and B chains of this peptide are similar to those of human insulin and IGFs, and it also contains sequences resembling the D and E domains of IGFs (38). Amphioxus ILP contains a C chain that is flanked by paired basic residues, as in human proinsulin. A homologous peptide was identified from another amphioxus species, B. belcheri tsingtauense (39), and this ILP stimulated cell proliferation and/or differentiation in cultured teleost and mammalian cells, albeit at pharmacologically high concentrations (39, 40). Holland and colleagues (21) surveyed the fully sequenced amphioxus (B. floridae) genome and found 5 additional insulin/relaxin-related molecules. Upon further analysis, we found that only 1 of these 5 is an ILP. Among the other 4, 2 are relaxin-like molecules, 1 is an ATP-binding protein, and the other is an annotation error. Phylogenetic analysis also placed both amphioxus ILPs in the basal position of the vertebrate insulin and IGF branches (Supplemental Figure 3). Therefore, amphioxus genome contains 2 ILPs and 2 relaxin-like peptides. These 2 amphioxus ILPs are clearly more similar to mammalian insulin than IGFs. The putative mature ILP-1 is 36% identical to human insulin and 33% and 30% identical to human IGF-I and IGF-II. The sequence identities between ILP-2 to human insulin, IGF-I, and IGF-II are 31%, 25%, and 27%, respectively. Importantly, the C chain of both amphioxus ILPs is flanked by paired basic residues, suggesting that the mature ILPs are probably double-chain molecules, like mature insulin. Future studies will be needed to produce amphioxus ILPs and related peptides and test whether they have the ability to bind to amphioxus IGFBP directly or indirectly.
Molecular and genomic studies suggest that there is a single ILP receptor gene in amphioxus (21, 41). This ILP receptor can be activated by both mammalian insulin and IGF-I, albeit at very high concentrations (41). In mammals, there are 3 structurally related receptors, the IGF1R, the insulin receptor (IR), and the IR-related receptor (42, 43). The amphioxus ILP receptor shares similar sequence identity with the human IGF1R, IR, and IR-related receptor (21). These studies suggest that the emergence of IGF ligands and IGF1Rs likely occurred after the divergence of the vertebrates from their invertebrate ancestors. The identification of an IGFBP in amphioxus lacking IGF-binding capacity supports this notion.
In summary, we have shown that amphioxus IGFBP has all of the major structural features of vertebrate IGFBPs. Functional analyses suggest amphioxus IGFBP lacks the ability to bind to human IGFs. Instead, it can enter the nucleus and has transcriptional activation activity. These results suggest that nuclear localization and transcription activation activity are evolutionarily ancient functional features of IGFBPs. It is intriguing that the amphioxus IGFBP F/L mutant was able to bind IGFs. The genetic code for 70F in amphioxus IGFBP is UUC and that for Leu is UUA/G. Therefore, a single nucleotide change from C to A or G in the amphioxus IGFBP gene is sufficient to introduce a functional change and convert amphioxus IGFBP into a functional IGF binder. It is plausible that an ancient IGFBP, similar to the modern amphioxus IGFBP, may have acquired IGF-binding function by a similar opportunistic gain-of-function mutation during evolution. There are precedents for such a scenario. The nuclear receptor first emerged as an orphan receptor, and its ligand binding was acquired later during the course of evolution (47). An alternate scenario is the IGF-binding function was lost in the lineage leading to modern amphioxus IGFBP. This has been proposed for the case of rodent liver receptor homolog-1. Its loss of ligand dependence has been attributed to a single amino acid replacement that stabilizes liver receptor homolog-1 in the transcriptionally active conformation (48). It is also possible that sequence changes during the evolution from the ancestral IGFBP to the modern amphioxus IGFBP may have resulted in the inability of amphioxus IGFBP to bind to mammalian IGFs. Future quantitative binding studies using amphioxus ILPs and IGFBPs and characterization of IGFBPs from other chordates, especially hagfish and lamprey, will help clarify these possibilities and help determine more precisely when the shift in IGFBP function might occur during vertebrate evolution. The discovery of an IGFBP possessing IGF-independent activity also raises the question about its functional role in amphioxus. To this end, we found that amphioxus IGFBP is expressed throughout early development, with the highest levels in larval stages. Recent studies in zebrafish suggested that IGFBP-3 and IGFBP-5 exert IGF-independent actions in early development (15, 16). Future studies are needed to determine whether IGFBP may play a similar role in regulating amphioxus development.
Acknowledgments
We thank Mr John Allard (Department of Molecular, Cellular and Developmental Biology, University of Michigan) for proofreading this manuscript.
This work was supported by National Science Foundation Grant IOS-1051034 and National Institutes of Health Grant 1R21AG040604–01A1 (to C.D.). J.Z. was supported by Major Science Programs of China (2011CB043800).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aa
- amino acids
- amphiBP
- amphioxus IGFBP
- DIG
- digoxingenin
- eGFP
- enhanced green fluorescent protein
- GFP
- green fluorescent protein
- HEK
- human embryonic kidney
- IGFBP
- IGF-binding protein
- IGF1R
- insulin-like growth factor 1 receptor
- ILP
- insulin-like peptide
- IR
- insulin receptor
- NLS
- nuclear localization sequence
- ORF
- open reading frame
- qRT-PCR
- quantitative real-time RT-PCR
- RACE
- rapid amplification of cDNA ends
- U2OS
- U2 osteosarcoma.
References
- 1. Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev Drug Discov. 2007;6:821–833 [DOI] [PubMed] [Google Scholar]
- 2. Daza DO, Sundström G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152:2278–2289 [DOI] [PubMed] [Google Scholar]
- 3. Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854 [DOI] [PubMed] [Google Scholar]
- 4. Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52 [DOI] [PubMed] [Google Scholar]
- 5. Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen P. Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology. 2006;147:2109–2111 [DOI] [PubMed] [Google Scholar]
- 7. Jogie-Brahim S, Feldman D, Oh Y. Unraveling insulin-like growth factor binding protein-3 actions in human disease. Endocr Rev. 2009;30:417–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol. 2009;296:C954–976 [DOI] [PubMed] [Google Scholar]
- 9. Xu Q, Li S, Zhao Y, Maures TJ, Yin P, Duan C. Evidence that IGF binding protein-5 functions as a ligand-independent transcriptional regulator in vascular smooth muscle cells. Circ Res. 2004;94:E46–E54 [DOI] [PubMed] [Google Scholar]
- 10. Zhao Y, Yin P, Bach LA, Duan C. Several acidic amino acids in the N-domain of insulin-like growth factor-binding protein-5 are important for its transactivation activity. J Biol Chem. 2006;281:14184–14191 [DOI] [PubMed] [Google Scholar]
- 11. Miyako K, Cobb LJ, Francis M, et al. PAPA-1 Is a nuclear binding partner of IGFBP-2 and modulates its growth-promoting actions. Mol Endocrinol. 2009;23:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Iosef C, Vilk G, Gkourasas T, et al. Insulin-like growth factor binding protein-6 (IGFBP-6) interacts with DNA-end binding protein Ku80 to regulate cell fate. Cell Signal. 2010;22:1033–1043 [DOI] [PubMed] [Google Scholar]
- 13. Cui J, Ma C, Qiu J, et al. A novel interaction between insulin-like growth factor binding protein-6 and the vitamin D receptor inhibits the role of vitamin D3 in osteoblast differentiation. Mol Cell Endocrinol. 2011;338:84–92 [DOI] [PubMed] [Google Scholar]
- 14. Grkovic S, O'Reilly VC, Han S, Hong M, Baxter RC, Firth SM. IGFBP-3 binds GRP78, stimulates autophagy and promotes the survival of breast cancer cells exposed to adverse microenvironments. Oncogene. 2013;32:2412–2420 [DOI] [PubMed] [Google Scholar]
- 15. Dai W, Kamei H, Zhao Y, Ding J, Du Z, Duan C. Duplicated zebrafish insulin-like growth factor binding protein-5 genes with split functional domains: evidence for evolutionarily conserved IGF binding, nuclear localization, and transactivation activity. FASEB J. 2010;24:2020–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong Y, Lu L, Zhou J, et al. IGF binding protein 3 exerts its ligand-independent action by antagonizing BMP in zebrafish embryos. J Cell Sci. 2011;124:1925–1935 [DOI] [PubMed] [Google Scholar]
- 17. Iosef C, Gkourasas T, Jia CY, Li SS, Han VK. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–1226 [DOI] [PubMed] [Google Scholar]
- 18. Azar WJ, Azar SH, Higgins S, et al. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology. 2011;152:3332–3342 [DOI] [PubMed] [Google Scholar]
- 19. Kuo YS, Tang YB, Lu TY, Wu HC, Lin CT. IGFBP-6 plays a role as an oncosuppressor gene in NPC pathogenesis through regulating EGR-1 expression. J Pathol. 2010;222:299–309 [DOI] [PubMed] [Google Scholar]
- 20. Gibson-Brown JJ, Osoegawa K, McPherson JD, et al. A proposal to sequence the amphioxus genome submitted to the joint genome institute of the US department of energy. J Exp Zool B Mol Dev Evol. 2003;300:5–22 [DOI] [PubMed] [Google Scholar]
- 21. Holland LZ, Albalat R, Azumi K, et al. The amphioxus genome illuminates vertebrate origins and cephalochordate biology. Genome Res. 2008;18:1100–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertrand S, Escriva H. Evolutionary crossroads in developmental biology: amphioxus. Development. 2011;138:4819–4830 [DOI] [PubMed] [Google Scholar]
- 23. Putnam NH, Butts T, Ferrier DE, et al. The amphioxus genome and the evolution of the chordate karyotype. Nature. 2008;453:1064–1071 [DOI] [PubMed] [Google Scholar]
- 24. Dehal P, Boore JL. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 2005;3:e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 26. Rosenfeld RG, Hwa V, Oh Y. Nomenclature of the insulin-like growth factor-binding protein superfamily. J Clin Endocrinol Metab. 2001;86:946. [DOI] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)) method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 28. Shimizu M, Swanson P, Fukada H, Hara A, Dickhoff WW. Comparison of extraction methods and assay validation for salmon insulin-like growth factor-I using commercially available components. Gen Comp Endocrinol. 2000;119:26–36 [DOI] [PubMed] [Google Scholar]
- 29. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787 [DOI] [PubMed] [Google Scholar]
- 30. Buckway CK, Wilson EM, Ahlsén M, Bang P, Oh Y, Rosenfeld RG. Mutation of three critical amino acids of the N-terminal domain of IGF-binding protein-3 essential for high affinity IGF binding. J Clin Endocrinol Metab. 2001;86:4943–4950 [DOI] [PubMed] [Google Scholar]
- 31. Hong J, Zhang G, Dong F, Rechler MM. Insulin-like growth factor (IGF)-binding protein-3 mutants that do not bind IGF-I or IGF-II stimulate apoptosis in human prostate cancer cells. J Biol Chem. 2002;277:10489–10497 [DOI] [PubMed] [Google Scholar]
- 32. Imai Y, Moralez A, Andag U, Clarke JB, Busby WH, Jr., Clemmons DR. Substitutions for hydrophobic amino acids in the N-terminal domains of IGFBP-3 and -5 markedly reduce IGF-I binding and alter their biologic actions. J Biol Chem. 2000;275:18188–18194 [DOI] [PubMed] [Google Scholar]
- 33. Schedlich LJ, Young TF, Firth SM, Baxter RC. Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells. J Biol Chem. 1998;273:18347–18352 [DOI] [PubMed] [Google Scholar]
- 34. Kamei H, Lu L, Jiao S, et al. Duplication and diversification of the hypoxia-inducible IGFBP-1 gene in zebrafish. PloS one. 2008;3:e3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou J, Li W, Kamei H, Duan C. Duplication of the IGFBP-2 gene in teleost fish: protein structure and functionality conservation and gene expression divergence. PloS one. 2008;3:e3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wood AW, Duan C, Bern HA. Insulin-like growth factor signaling in fish. Int Rev Cytol. 2005;243:215–285 [DOI] [PubMed] [Google Scholar]
- 37. Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351 [DOI] [PubMed] [Google Scholar]
- 38. Chan SJ, Cao QP, Steiner DF. Evolution of the insulin superfamily: cloning of a hybrid insulin/insulin-like growth factor cDNA from amphioxus. Proc Natl Acad Sci USA. 1990;87:9319–9323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo B, Zhang S, Wang S, Liang Y. Expression, mitogenic activity and regulation by growth hormone of growth hormone/insulin-like growth factor in Branchiostoma belcheri. Cell Tissue Res. 2009;338:67–77 [DOI] [PubMed] [Google Scholar]
- 40. Liu M, Zhang S. Amphioxus IGF-like peptide induces mouse muscle cell development via binding to IGF receptors and activating MAPK and PI3K/Akt signaling pathways. Mol Cell Endocrinol. 2011;343:45–54 [DOI] [PubMed] [Google Scholar]
- 41. Pashmforoush M, Chan SJ, Steiner DF. Structure and expression of the insulin-like peptide receptor from amphioxus. Mol Endocrinol. 1996;10:857–866 [DOI] [PubMed] [Google Scholar]
- 42. Renteria ME, Gandhi NS, Vinuesa P, Helmerhorst E, Mancera RL. A comparative structural bioinformatics analysis of the insulin receptor family ectodomain based on phylogenetic information. PloS one. 2008;3:e3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hernández-Sanchez C, Mansilla A, de Pablo F, Zardoya R. Evolution of the insulin receptor family and receptor isoform expression in vertebrates. Mol Biol Evol. 2008;25:1043–1053 [DOI] [PubMed] [Google Scholar]
- 44. Hoeflich A, Reisinger R, Schuett BS, et al. Peri/nuclear localization of intact insulin-like growth factor binding protein-2 and a distinct carboxyl-terminal IGFBP-2 fragment in vivo. Biochem Biophys Res Commun. 2004;324:705–710 [DOI] [PubMed] [Google Scholar]
- 45. Sun T, Hunziker EB, Morales TI. Subcellular distribution of the insulin-like growth factor (IGF) binding proteins (IGFBPs) 2 and 3 in articular chondrocytes. J Orthop Res. 2008;26:1421–1427 [DOI] [PubMed] [Google Scholar]
- 46. Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167:344–351 [DOI] [PubMed] [Google Scholar]
- 47. Escriva H, Safi R, Hänni C, et al. Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci USA. 1997;94:6803–6808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krylova IN, Sablin EP, Moore J, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355 [DOI] [PubMed] [Google Scholar]