Abstract
Aims
The clinical syndrome of heart failure includes exercise limitation that is not directly linked to measures of cardiac function. Quadriceps fatigability may be an important component of this and this may arise from peripheral or central factors.
Methods and results
We studied 10 men with CHF and 10 healthy age-matched controls. Compared with a rest condition, 10 min after incremental maximal cycle exercise, twitch quadriceps force in response to supramaximal magnetic femoral nerve stimulation fell in both groups (CHF 14.1% ± 18.1%, p = 0.037; Control: 20.8 ± 11.0%, p < 0.001; no significant difference between groups). There was no significant change in quadriceps maximum voluntary contraction voluntary force. The difference in the motor evoked potential (MEP) response to transcranial magnetic stimulation of the motor cortex between rest and exercise conditions at 10 min, normalised to the peripheral action potential, also fell significantly in both groups (CHF: 27.3 ± 38.7%, p = 0.037; Control: 41.1 ± 47.7%, p = 0.024). However, the fall in MEP was sustained for a longer period in controls than in patients (p = 0.048).
Conclusions
The quadriceps is more susceptible to fatigue, with a similar fall in TwQ occurring in CHF patients at lower levels of exercise. This is associated with no change in voluntary activation but a lesser degree of depression of quadriceps motor evoked potential.
Keywords: Brain, Exercise, Muscles, Transcranial magnetic stimulation
1. Introduction
The clinical syndrome of congestive heart failure (CHF) is not fully explained by measures of cardiac function [1] and the classic haemodynamic model does not adequately explain the exercise intolerance which is characteristic. In particular, exercise tolerance does not correlate with ejection fraction at rest, [2] or central haemodynamics at rest or on exercise [3], though some echocardiographic measures of left ventricular function fare better [4]. Models incorporating adaptations to organs other than the heart have been more successful [5] and there is growing interest in exercise and rehabilitation as therapy for heart failure patients [6].
Leg discomfort is a symptom which limits exercise in some patients with CHF. The underlying mechanisms implicated in this sensation are multifactorial but include increased fatigability, where fatigue is defined as a reversible loss of the capacity to generate force resulting from activity under load [7]. The quadriceps muscle in CHF displays a shift away from fatigue resistant type I fibres, loss of oxidative enzymes and reduced capillarity, similar to that observed in COPD [8]. Quadriceps muscle fatigue can be identified by measuring the fall in isometric twitch tension (TwQ) in response to a single supramaximal stimulus applied to the femoral nerve occurring after exercise [9]. In addition to this peripheral mechanism, a reduction in the ability to generate force can be caused by a fall in neural drive termed “central fatigue”. The excitability of the motor cortex is also influenced by exercise. In healthy subjects, exercise induces a reversible fall in the amplitude of the electrical signal (Motor Evoked Potential, MEP) elicited by transcranial magnetic stimulation (TMS) of the motor cortex area controlling the quadriceps [10].
In the present study we wished to examine whether peripheral and central fatigue mechanisms were similar in patients with CHF and healthy age-matched controls by measuring response to magnetic femoral nerve stimulation assessing the level of voluntary activation using twitch interpolation and measuring quadriceps motor cortical excitability before and after symptom limited cycle ergometry.
2. Methods
2.1. Subjects
The study was conducted in accordance with The Helsinki Declaration. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology. All subjects gave their written informed consent and the experiment was approved by the Royal Brompton Hospital ethics committee. Subjects were requested to refrain from exercise in the 24 hours before the study, from significant alcohol consumption on the night before the study, and from caffeine on the day.
A total of 12 controls and 16 patients with CHF participated. One control subject, and 4 CHF patients, did not complete the protocol because they found it too demanding. One CHF patient developed angina during his exercise test and the experiment was stopped. In one further CHF patient and control subject we were unable to stimulate the motor cortex satisfactorily on a second occasion. Thus 10 subjects from each group completed both stages of the protocol and their data is presented here.
The 10 male patients, aged 54.8 ± 12.3 years, height 175 ± 7 cm, weight 90 ± 13 kg, ejection fraction 38 ± 9% were recruited from specialist CHF clinics. Diagnosis was based upon clinical history and echocardiography. Exclusion criteria included any history of lung disease, neuromuscular disease limiting exercise, symptomatic peripheral vascular disease, uncontrolled hypertension, exercise-induced angina, or exercise-induced ventricular arrhythmias as well as the presence of a permanent pacemaker, or previous neurosurgery, which are contra-indications to magnetic stimulation. All patients had been stable for the previous month. One patient was in NYHA class I, 7 were in class II and 2 were in class III. The aetiology of left ventricular dysfunction was ischaemic in 3 and idiopathic dilated cardiomyopathy in 7 patients. One was in atrial fibrillation and one had type II diabetes. All were taking loop diuretics and ACE-inhibitors or angiotensin II receptor antagonists. Nine took beta-blockers; four spironolactone and two digoxin.
The control group was recruited from healthy volunteers who were known to the laboratory. It also consisted of 10 men, matched for age (52.6 ± 13.2 years, p = 0.62), height (178 ± 6 cm, p = 0.38) and weight (88 ± 14 kg, p = 0.65).
2.2. Measurements
Quadriceps maximum voluntary contraction (QMVC) was measured as previously described with subjects supine [9]. Subjects performed at least 5 sustained maximal isometric contractions of between 5 and 10 s duration with simultaneous visual feedback and vigorous verbal encouragement. There was at least a 30‐s pause between contractions.
Transcranial magnetic stimulation (TMS) was performed using a double Magstim 200 magnetic stimulator, discharging both units simultaneously (Magstim Co. Ltd, Whitlands, Dyfed, Wales, UK) through a 120 mm double cone coil (type 9902, Magstim Co. Ltd.). The coil was centred over the vertex, which was marked on the subject at the start of the experiment [11–15]. Unpotentiated, twitch quadriceps force (TwQ) in response to supramaximal, magnetic femoral nerve stimulation was measured as described previously, with subjects supine on the bench described above using the same double stimulator and a 70 mm ‘branding iron’ coil (Magstim Co. Ltd.) [9].
2.2.1. Electromyography
Surface recordings of the rectus femoris response to magnetic stimulation (EMG) were obtained using electrodes placed over the belly of the muscle in its longitudinal axis [16]. EMG signals, whether evoked by peripheral nerve (compound muscle action potential, CMAP) or cortical (motor evoked potential, MEP) stimulation were amplified, recorded at 20 kHz, band-pass filtered between 0.3 and 3 kHz. The MEP was normalised in a linear fashion for variations in the CMAP.
2.2.2. Cardiopulmonary exercise testing
Subjects performed symptom limited incremental cycle ergometry with measurement of metabolic parameters with increments set at 10 W per minute for patients with CHF and 20 W per minute for healthy controls. The percent predicted VO2 for each subject was also calculated using published formulae [17].
2.3. Protocols and timings
Both CHF and control subjects underwent an exercise session and a control session on separate days, in a random order (Fig. 1). All sessions started at the same time of day, and took approximately 4 hours. Subjects rested supine for 20 min at the start of each session. To elicit the motor threshold for the quadriceps, the lowest stimulator output, in steps of 5%, that elicited at least a 50 μV MEP in the rectus femoris in at least 5 of 10 TMS stimulations, was sought. Seven TMS at 140% of that threshold level or 100% stimulator output (whichever was the lower) were then delivered.
Fig. 1.
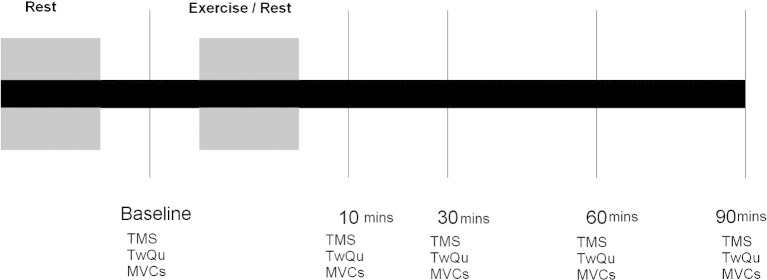
Schematic of the protocol that all subjects underwent. The rest period lasted 30 min. Assessments were made at baseline and at 10, 30, 60 and 90 min after the intervention (either rest or exercise). TMS transcranial magnetic stimulation; TwQu Quadriceps twitch in response to femoral nerve stimulation; MVC quadriceps maximum voluntary contraction.
The optimal coil position for femoral nerve stimulation was determined as previously described [9]. A 30‐s gap between stimulations was required to prevent ‘twitch on twitch’ potentiation and a stimulus response curve constructed to ensure supramaximality, with 4 stimuli at 80%, 85%, 90%, 95% and 100% of stimulator output, delivered in random order. 2 further stimuli were then delivered at maximal stimulator output.
Finally, five QMVC's were recorded. To determine the degree of voluntary activation of the quadriceps, stimulation was performed at the peak of the voluntary contraction to produce an interpolated twitch, and approximately 4 s after the end of the contraction, to produce a potentiated twitch. The interpolated and potentiated twitches were compared to determine the percent activation during the QMVC manoeuvre (100 − interpolated twitch / potentiated twitch × 100) [18].
Subjects then either sat quietly resting in the muscle laboratory for 30 min or underwent a maximal exercise test in random order. After this period subjects were requested to lie supine on the bench for the remainder of the experiment. At 10, 30, 60 and 90 min seven TMS, five TwQ and five QMVC'd with interpolated and potentiated twitches were recorded.
2.4. Statistics
Analyses were performed using SPSS 11.0.0 and R 2.0.1 software. All tests were 2-sided and p values under 0.05 were considered significant. Simple comparisons between groups were assessed with t tests (paired or unpaired), Mann–Whitney U tests, or Wilcoxon Signed Rank tests as appropriate. Values recorded over time were normalised to baseline to ensure equivalent contribution from each subject. Areas under the curves between 10 min and 90 min were chosen as summary measures to describe the differences between the rest and exercise conditions; the units are therefore % minute. Mixed model analysis of variance (ANOVA) with random subject effects was performed where appropriate to evaluate changes from baseline.
3. Results
3.1. Exercise data
Patients exercised for a similar duration to controls (775 ± 203 s vs. 704 ± 117 s, p = 0.35), but the maximum workload achieved the CHF patients was markedly lower (127 ± 34 W vs. 226 ± 38 W, p = < 0.001). The peak RQ in both groups was high, confirming the motivation of the subjects taking part in this experiment (CHF: 1.24 ± 0.07; Controls: 1.32 ± 0.14, p = 0.16). Both absolute (17.9 ± 4.3 ml kg− 1 min− 1 vs. 29.2 ± 3.6 ml kg− 1 min− 1, p < 0.001) and percent predicted (66.0 ± 13.3% vs. 102.8 ± 18.1%, p < 0.001) peak VO2 were significantly lower in the CHF group.
3.2. Muscle force data
Baseline quadriceps strength did not differ significantly between patients and controls; TwQ: 93 ± 27 N vs. 108 ± 31 N, (p = 0.26); QMVC: 428 ± 91 N vs. 522 ± 124 N, (p = 0.09). TwQ fell significantly after exercise (Fig. 2) in both groups (Control: 898% min, p = 0.002; CHF: 867% min, p = 0.019). At 10 min, the mean fall in the control group was 20.8 ± 11.0% (p < 0.001) vs. 14.1 ± 18.1% (p = 0.037) in the CHF group. There was no significant difference in either the overall response or that at 10 min between groups.
Fig. 2.
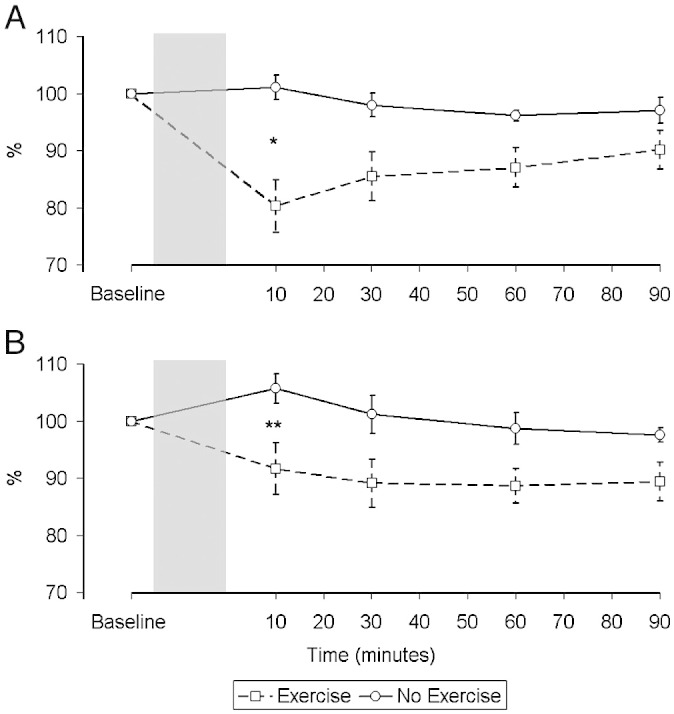
Change in force of peripheral quadriceps twitches over time in control subjects (A) and patients (B) relative to baseline expressed as a percentage. There was a significant difference in the pattern of the response between the two interventions in both groups (Controls: p = 0.002, CHF: p = 0.019). Difference between interventions at 10 min: *p < 0.001, **p = 0.037.
In the control group QMVC increased after exercise (Fig. 3), whereas in the CHF group force fell. Neither change achieved significance (− 310% min, p = 0.44 vs. 647% min, p = 0.084; difference between groups p = 0.076). Potentiated twitch responses are larger than unpotentiated ones and a number of subjects did not tolerate them, meaning that the degree of voluntary activation could only be calculated in 9 control subjects and 5 patients. The degree of voluntary activation at baseline was numerically higher in the control group at rest but this difference was not significant (Controls 85.3 ± 6.3% vs. CHF 79.3 ± 2.9%, p = 0.071). The degree of voluntary activation did not vary over the course of the experiment in either group or between groups.
Fig. 3.
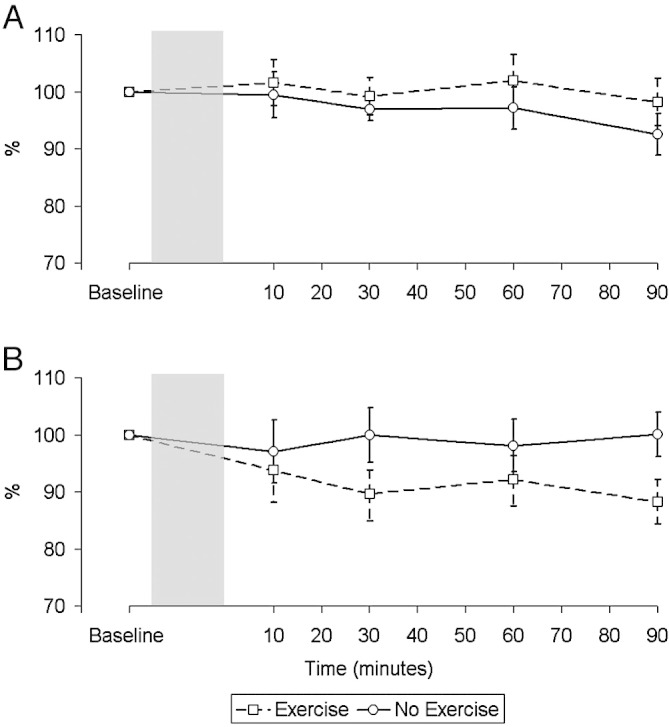
Change in magnitude of the force of maximal voluntary contractions (MVC) over time in control subjects (A) and patients (B) (n = 9 in both groups) relative to baseline expressed as a percentage. There was no significant difference in the pattern of the response between the two interventions in both groups (Controls p = 0.44, CHF p = 0.084).
3.3. EMG data
The response to TMS of the quadriceps motor area after exercise differed between patients and controls (Fig. 4). In both groups there was a significant fall in the MEP amplitude (normalised for CMAP against baseline) at 10 min (Control: Rest 112.1 ± 32.0%, Exercise 73.7 ± 28.1%, Difference 41.1 ± 47.7%, p = 0.024; CHF: Rest 107.1 ± 36.1%, Exercise 79.8 ± 36.0%, Difference 27.3 ± 38.7%, p = 0.037). However, whereas this separation was maintained in the control group it was not in the CHF group (Control: Area 2365% min, p = 0.029, CHF: Area 214% min, p = 0.96). Using a mixed model analysis of variance this differential was significant (p = 0.048).
Fig. 4.
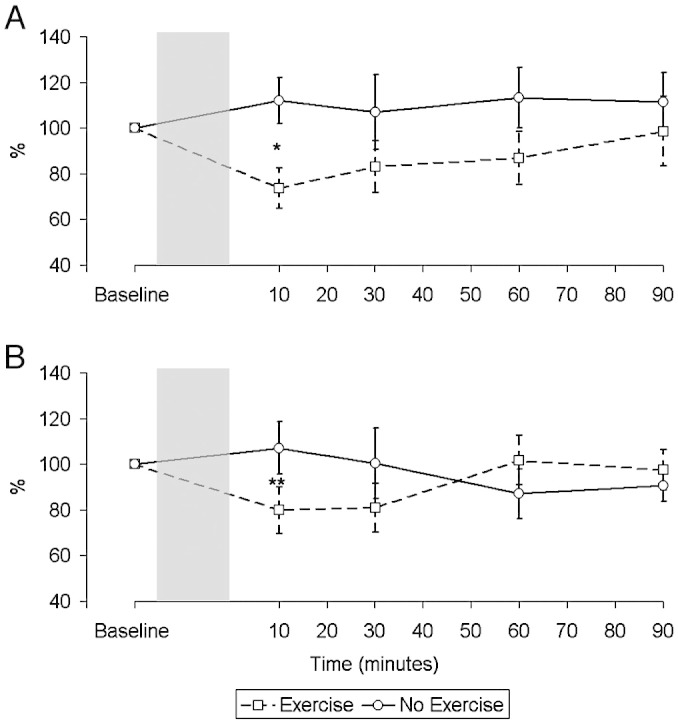
Change in motor evoked potential (MEP) over time in control subjects (A) and patients (B), corrected for changes in the compound muscle action potential (CMAP) relative to baseline expressed as a percentage. There was a significant difference in the pattern of the response between the control subjects and patients (p = 0.048). Difference between interventions at 10 min: *p = 0.024, **p = 0.037.
There was no significant change in either group over time in the CMAP response to femoral nerve stimulation in either condition (Fig. 5) (Rest, Controls, p = 0.17; Exercise, Controls, p = 0.95; Rest, CHF, p = 0.72; Exercise, CHF, p = 0.88). There was no significant difference in the MEP latency at baseline between groups or conditions (Rest, Controls, 23.1 ± 1.1 ms; Exercise, Controls, 22.4 ± 1.4 ms; Rest, CHF, 23.3 ± 1.8 ms; Exercise, CHF, 23.0 ± 1.6 ms; overall p = 0.60) and MEP latency also remained constant throughout the experiment for each group and condition (Fig. 6) (Rest, Controls, p = 0.99; Exercise, Controls, p = 0.86; Rest, CHF, p = 0.97; Exercise, CHF, p = 0.99).
Fig. 5.
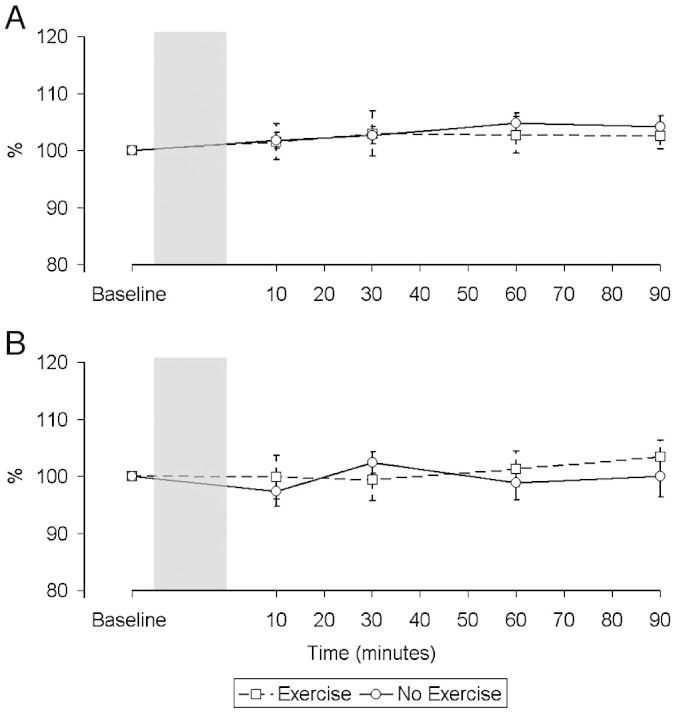
Changes in the compound muscle action potential (CMAP) over time in control subjects (A) and patients (B) expressed as a percentage. There was no significant change in the electrical response to peripheral stimulation in either group in either condition.
Fig. 6.
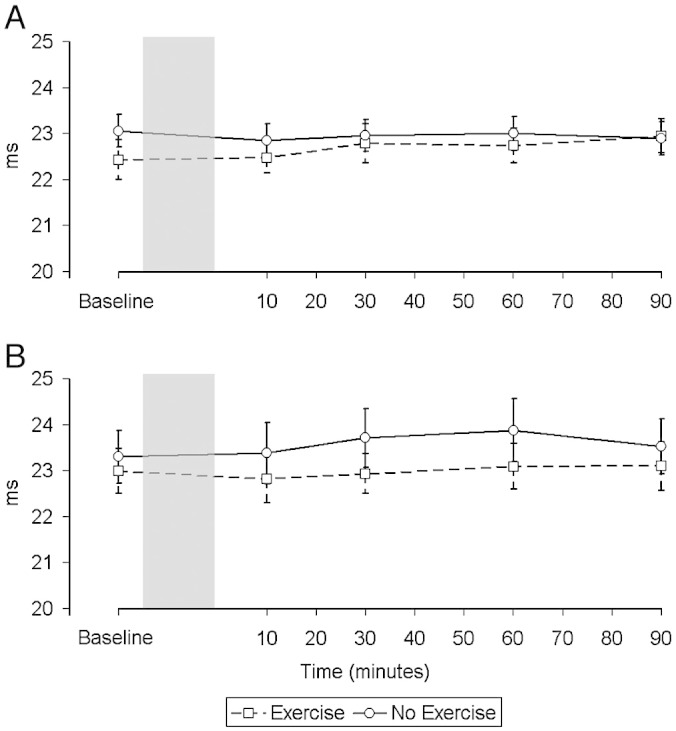
Changes in the latency in milliseconds (ms) of the motor evoked potential (MEP) over time in control subjects (A) and patients (B). There was no significant change in the latency in either group in either condition.
4. Discussion
The main finding of this study is that the quadriceps of patients with CHF displays increased susceptibility to fatigue, with a similar fall in TwQ occurring despite patients exercising at a workload approximately half of the control subjects. The healthy subjects experienced a greater fall in MEP response to TMS than CHF patients but there was no difference between groups in the degree of voluntary activation.
4.1. Significance of the findings
There have now been a number of studies documenting wasting and a variety of histological and biochemical abnormalities of striated muscle in patients with the clinical syndrome of heart failure including a reduction in the proportion of fatigue resistant type I fibres, reduced oxidative enzymes, reduced capillarity and reduced single fibre myosin content [8,19–21]. The clinical correlates of these changes are reduced muscle strength and endurance, with a reduced force per unit of cross sectional area associated with reduced exercise capacity [22]. Increased quadriceps fatigability has been observed in patients with chronic obstructive pulmonary disease, a disorder with many similarities to CHF with respect to the underlying muscle abnormalities [8]. Studies in this condition have demonstrated both low frequency fatigue, as in the present study [23] and more rapid decline in force in response to repetitive magnetic stimulation [24].
Although twitch force declined at 10 min after exercise, the force of the maximal voluntary contraction did not, in keeping with other studies [25] and the known differences in physiology between high and low frequency fatigue [26,27]. During a maximal voluntary contraction, if central nervous system drive is maximal, then an additional stimulus to the peripheral nerve will elicit no additional response. We found no significant change in either the force generated by a maximal voluntary contraction of the quadriceps or the degree of activation as assessed by the interpolated twitch in either the control or heart failure groups after exercise. The second finding of our study, therefore, is that exhaustive exercise does not lead to prolonged functional impairment in the ability to generate force in patients with CHF.
4.1.1. The cortical response to exercise
In addition to skeletal muscle abnormalities, the ability to generate force might be influenced by central factors, reviewed in detail here [28]. Central nervous system perturbation is a feature of CHF. The characteristic sympathetic and parasympathetic abnormalities may in part have a central origin [29], there is a high incidence of cognitive deficits [30], cerebrovascular reactivity may be altered [31], as may cerebral metabolism [32]. Several studies have documented structural abnormalities [33–35], which cannot entirely be explained by either hypotension or embolism. Rosen et al. documented distinct patterns of cortical activity between patients with heart failure and controls in association with exercise-induced breathlessness [36].
Both patients and controls exhibited a depressed response to TMS after exercise. The mechanisms and significance of the depression of the MEP after whole body exercise have not yet been determined. The change in the MEP with exercise is complex and most TMS studies have focused on exercise of single muscle groups. During a fatiguing contraction of a single muscle group, MEP amplitude is increased [37]. Following a fatiguing isometric contraction, the MEP is briefly increased, but this is followed by a longer-lasting depression [38].
Several studies have examined the effect of whole body exercise on cortical excitability in healthy subjects. A depression in the quadriceps MEP after exhaustive treadmill exercise has been described from 5 min after exercise which recovered slowly over time [10]. After rowing, a reduction in the erector spinae MEP which was more pronounced in non-rowers than elite rowers and persisted for 16 min post exercise has been observed [39]. A short-lived potentiation in the MEP in the 2 min after exercise was only present in the non-rowers. Hollge et al. noted a significant reduction in a number of muscles' MEP response to intense exercise [40]. Finally a paper by Jonville et al. [41] examined the impact of an inspiratory load on the evolution of the diaphragmatic MEP after constant load exercise set just below the anaerobic threshold noting that, in the absence of an additional load, this level of exercise resulted in a significant fall in the MEP amplitude at 10 min; it remained lower, but not significantly so, at 20 and 40 min. The present findings that whole body exercise results in a sustained depression of MEP amplitude are therefore consistent with these studies.
This persistent MEP depression has been frequently referred to as central fatigue, although it has been unclear whether the changes in the electromyographic response to transcranial magnetic stimulation contribute to the loss of force [42]. In this study we specifically looked at the ability to generate force after exercise and conclude that the changes seen in the MEP are not related to impairment of the ability to generate force. The lesser reduction observed in CHF patients may be adaptive or maladaptive. Since the skeletal muscles develop low frequency fatigue more readily (at a lower workload) it may be that less cortical compensation is required for adaptations in peripheral neuromuscular activation. Alternatively it could be that the cortical changes occur in response to signalling through some neurohumoral mechanism related to the work done in an absolute sense rather than as a proportion of the individuals maximum exercise capacity. Myokines produced during exercise such as IL6, which is known to have central effects might be implicated [43].
4.2. Critique of the method
The subjects in the two groups were well matched and patients were typical of those found in heart failure clinics. Patients had numerically lower quadriceps strength than controls, though these differences were not statistically significant due to the relatively small sample size. They were optimally treated, as evidenced by the high use of ACE inhibitors/angiotensin II receptor antagonists and beta-blockers. Cycle ergometry was chosen as it provides a stable platform for taking metabolic measurements and allows workload to be measured directly. The quadriceps muscle was our principal focus and compared with walking, biomechanics data confirm that the locomotor limb muscles are more activated during cycling [44]. Strong verbal encouragement was given to all subjects, which is reflected in the high RERs seen in both groups; the lowest obtained by any of the 20 subjects was 1.13. Importantly, to enable comparisons between the groups, the subjects exercised for a similar length of time and to a similar level of discomfort, with comparable Borg scale ratings of leg discomfort and RERs.
4.2.1. Stimulus reproducibility
For the results of this study to be valid stimulus reproducibility is imperative. The position of the body and head of the subjects was kept constant as far as possible throughout the experiment. The position of the coils relative to the head and femoral nerve was carefully controlled for by the use of ink marks. The relative stability of the MEP amplitudes and CMAP amplitudes, as well as forces produced, in the control condition makes us confident about our ability to reproduce a consistent stimulation from one series to another. The consistency of the CMAP amplitudes confirms that there was not a significant change in peripheral factors that may have contributed to the observed changes in the MEP. Furthermore, the fact that correction of the TMS data for any variation in the CMAP did not significantly change the pattern or significance of the results suggests that any changes we saw in MEP amplitudes were genuinely the result of a supraspinal phenomenon.
4.2.2. Magnetic twitch quadriceps tension as a measure of fatigue
Skeletal muscle fatigue is defined as a reversible loss of the capacity to generate force resulting from activity under load [45]. Low frequency fatigue (LFF) results in loss of force generated in response to low stimulation frequencies (10–20 Hz). These are the typical motor neurone firing frequencies during human skeletal muscle contraction. The conventional method to detect LFF is to construct force frequency curves using tetanic stimulation. This is uncomfortable and unacceptable to most subjects. An alternative is to measure the force elicited from a single stimulus. A number of investigators have confirmed that a twitch elicited by magnetic stimulation of the femoral nerve can be used to detect fatigue [23]. Magnetic stimulation of the femoral nerve is not as specific as electrical stimulation, but it is more comfortable, and supramaximal stimulation is easier to achieve. It is important to note that a fall in the twitch quadriceps force only indicates contractile fatigue as a consequence of excitation–contraction coupling if there is no documented change in the CMAP amplitude, otherwise it may be due to transmission failure. Our data clearly show that the CMAP amplitude remained remarkably constant across groups and conditions.
4.2.3. Factors relating to the motor evoked potential
The motor evoked potential is the electrical response measured in a target muscle in response to stimulation of the motor cortex. Clearly the exercise-induced depression of the MEP could occur from any site from the cortex to the muscle. We believe that we have excluded peripheral transmission failure by recording the CMAP and correcting for fluctuations in its values. The data we present cannot localise the source of this change within the central nervous system. However, we believe that the changes do reflect changes in motor cortical excitability. Experiments using transcranial electrical stimulation, transmastoid electrical stimulation, and double or triple stimulation suggest that the changes seen after exercise are likely to be intracortical rather than spinal [38,46–48].
5. Conclusion
The quadriceps is more susceptible to fatigue in CHF patients with reduction in twitch force, a non-volitional measure, occurring following a lower level of exercise. This is associated with no change in voluntary activation but a lesser degree of depression of quadriceps motor evoked potential.
Acknowledgements
The authors would like to thank the subjects for their participation in this demanding experiment. We would also like to thank Professor Martin Cowie and Dr Hugh McIntyre for their support and advice. Professor Philip Poole Wilson supervised MJD but died before he was able to contribute to this version of the MS.
The work was supported by The National Institute for Health Research Biomedical Research Unit at Royal Brompton and Harefield NHS Foundation Trust and Imperial College, London. Mark Dayer was supported by a British Heart Foundation project grant.
Footnotes
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Mann D.L., Bristow M.R. Mechanisms and models in heart failure. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 2.Carell E., Murali S., Schulman D., Estrada-Quintero T., Uretsky B. Maximal exercise tolerance in chronic congestive heart failure. Relationship to resting left ventricular function. Chest. 1994;106:1746–1752. doi: 10.1378/chest.106.6.1746. [DOI] [PubMed] [Google Scholar]
- 3.Lipkin D.P., Poole-Wilson P.A. Measurement of cardiac output during exercise by the thermodilution and direct Fick techniques in patients with chronic congestive heart failure. Am J Cardiol. 1985;56:321–324. doi: 10.1016/0002-9149(85)90857-4. [DOI] [PubMed] [Google Scholar]
- 4.Duncan A.M., Francis D.P., Gibson D.G., Henein M.Y. Limitation of exercise tolerance in chronic heart failure: distinct effects of left Bundle–Branch block and coronary artery disease. J Am Coll Cardiol. 2004;43:1524–1531. doi: 10.1016/j.jacc.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Coats A.J., Clark A.L., Piepoli M., Volterrani M., Poole-Wilson P.A. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J. 1994;72:S36–S39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung C.J., Schulze P.C. Exercise as a nonpharmacologic intervention in patients with heart failure. Phys Sportsmed. 2011;39:37–43. doi: 10.3810/psm.2011.11.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NHLBI Workshop Respiratory muscle fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am Rev Respir Dis. 1990;142:474–480. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]
- 8.Gosker H.R., Wouters E.F., van der Vusse G.J., Schols A.M. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr. 2000;71:1033–1047. doi: 10.1093/ajcn/71.5.1033. [DOI] [PubMed] [Google Scholar]
- 9.Polkey M.I., Kyroussis D., Hamnegard C.H., Mills G.H., Green M., Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve. 1996;19:549–555. doi: 10.1002/(SICI)1097-4598(199605)19:5<549::AID-MUS1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Verin E., Ross E., Demoule A. Effects of exhaustive incremental treadmill exercise on diaphragm and quadriceps motor potentials evoked by transcranial magnetic stimulation. J Appl Physiol. 2004;96:253–259. doi: 10.1152/japplphysiol.00325.2003. [DOI] [PubMed] [Google Scholar]
- 11.Sharshar T., Hopkinson N.S., Jonville S. Demonstration of a second rapidly conducting cortico-diaphragmatic pathway in humans. J Physiol. 2004;560:897–908. doi: 10.1113/jphysiol.2004.061150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkinson N.S., Sharshar T., Ross E.T. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2004;141:1–12. doi: 10.1016/j.resp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Hopkinson N.S., Sharshar T., Dayer M.J., Lofaso F., Moxham J., Polkey M.I. The effect of acute non-invasive ventilation on corticospinal pathways to the respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2012;183:41–47. doi: 10.1016/j.resp.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharshar T., Ross E., Hopkinson N.S. Effect of voluntary facilitation on the diaphragmatic response to transcranial magnetic stimulation. J Appl Physiol. 2003;95:26–34. doi: 10.1152/japplphysiol.00918.2002. [DOI] [PubMed] [Google Scholar]
- 15.Sharshar T., Ross E.T., Hopkinson N.S. Depression of diaphragm motor cortex excitability during mechanical ventilation. J Appl Physiol. 2004;97:3–10. doi: 10.1152/japplphysiol.01099.2003. [DOI] [PubMed] [Google Scholar]
- 16.Winter D.A., Yack H.J. EMG profiles during normal human walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol. 1987;67:402–411. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 17.Wasserman K., Hansen J.E., Sue D.Y., Casaburi R., Whipp B.J. 3rd ed. Lippincott Williams & Wilkins; Baltimore: 1999. Principles of exercise testing and interpretation. [Google Scholar]
- 18.Bellemare F., Bigland-Ritchie B. Central components of diaphragmatic fatigue assessed by phrenic nerve stimulation. J Appl Physiol. 1987;62:1307–1316. doi: 10.1152/jappl.1987.62.3.1307. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan M.J., Green H.J., Cobb F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 20.Mancini D.M., Walter G., Reichek N. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 21.Miller M.S., VanBuren P., LeWinter M.M. Mechanisms underlying skeletal muscle weakness in human heart failure/clinical perspective. Circ Heart Fail. 2009;2:700–706. doi: 10.1161/CIRCHEARTFAILURE.109.876433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrington D., Anker S.D., Chua T.P. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 23.Man W.D.-C., Soliman M.G.G., Gearing J. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:562–567. doi: 10.1164/rccm.200302-162OC. [DOI] [PubMed] [Google Scholar]
- 24.Swallow E.B., Gosker H.R., Ward K.A. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol. 2007;103:739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- 25.Mador J.M., Kufel T.J., Pineda L. Quadriceps and diaphragmatic function after exhaustive cycle exercise in the healthy elderly. Am J Respir Crit Care Med. 2000;162:1760–1766. doi: 10.1164/ajrccm.162.5.2001005. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon P., Saey D., Vivodtzev I. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol. 2009;107:832–840. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]
- 27.Polkey M.I., Kyroussis D., Hamnegard C.H. Paired phrenic nerve stimuli for the detection of diaphragm fatigue in humans. Eur Respir J. 1997;10:1859–1864. doi: 10.1183/09031936.97.10081859. [DOI] [PubMed] [Google Scholar]
- 28.Gandevia S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein R.E., Beiser G.D., Stampfer M., Epstein S.E. Impairment of autonomically mediated heart rate control in patients with cardiac dysfunction. Circ Res. 1975;36:571–578. doi: 10.1161/01.res.36.5.571. [DOI] [PubMed] [Google Scholar]
- 30.Almeida O.P., Flicker L. The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Intern Med. 2001;31:290–295. doi: 10.1046/j.1445-5994.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 31.Georgiadis D., Sievert M., Cencetti S. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J. 2000;21:407–413. doi: 10.1053/euhj.1999.1742. [DOI] [PubMed] [Google Scholar]
- 32.Lee C.W., Lee J.H., Lim T.H. Prognostic significance of cerebral metabolic abnormalities in patients with congestive heart failure. Circulation. 2001;103:2784–2787. doi: 10.1161/01.cir.103.23.2784. [DOI] [PubMed] [Google Scholar]
- 33.Woo M.A., Macey P.M., Fonarow G.C., Hamilton M.A., Harper R.M. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt R., Fazekas F., Offenbacher H., Dusleag J., Lechner H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke. 1991;22:195–199. doi: 10.1161/01.str.22.2.195. [DOI] [PubMed] [Google Scholar]
- 35.Dusleag J., Klein W., Eber B. Frequency of magnetic resonance signal abnormalities of the brain in patients aged less than 50 years with idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1446–1450. doi: 10.1016/0002-9149(92)90899-a. [DOI] [PubMed] [Google Scholar]
- 36.Rosen S.D. Is central nervous system processing altered in patients with heart failure. Eur Heart J. 2004;25:952–962. doi: 10.1016/j.ehj.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Taylor J.L., Butler J.E., Gandevia S.C. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res. 1999;127:108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- 38.McKay W.B., Tuel S.M., Sherwood A.M., Stokic D.S., Dimitrijevic M.R. Focal depression of cortical excitability induced by fatiguing muscle contraction: a transcranial magnetic stimulation study. Exp Brain Res. 1995;105:276–282. doi: 10.1007/BF00240963. [DOI] [PubMed] [Google Scholar]
- 39.Fulton R., Strutton P., McGregor A., Davey N. Fatigue-induced change in corticospinal drive to back muscles in elite rowers. Exp Physiol. 2002;87:593–600. doi: 10.1113/eph8702409. [DOI] [PubMed] [Google Scholar]
- 40.Hollge J., Kunkel M., Ziemann U., Tergau F., Geese R., Reimers C.D. Central fatigue in sports and daily exercises. A magnetic stimulation study. Int J Sports Med. 1997;18:614–617. doi: 10.1055/s-2007-972691. [DOI] [PubMed] [Google Scholar]
- 41.Jonville S., Jutand L., Similowski T., Denjean A., Delpech N. Putative protective effect of inspiratory threshold loading against exercise-induced supraspinal diaphragm fatigue. J Appl Physiol. 2005;98:991–998. doi: 10.1152/japplphysiol.00528.2004. [DOI] [PubMed] [Google Scholar]
- 42.Taylor J.L., Gandevia S.C. Transcranial magnetic stimulation and human muscle fatigue. Muscle Nerve. 2001;24:18–29. doi: 10.1002/1097-4598(200101)24:1<18::aid-mus2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Wardyn G.G., Rennard S.I., Brusnahan S.K. Effects of exercise on hematological parameters, circulating side population cells, and cytokines. Exp Hematol. 2008;36:216–223. doi: 10.1016/j.exphem.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Ericson M.O., Bratt A., Nisell R., Arborelius U.P., Ekholm J. Power output and work in different muscle groups during ergometer cycling. Eur J Appl Physiol Occup Physiol. 1986;55:229–235. doi: 10.1007/BF02343792. [DOI] [PubMed] [Google Scholar]
- 45.NHLBI Workshop summary Respiratory muscle fatigue: report of the Respiratory Muscle Fatigue Workshop Group. Am Rev Respir Dis. 1990;142:474–480. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]
- 46.Gandevia S.C., Petersen N., Butler J.E., Taylor J.L. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;3:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tergau F., Geese R., Bauer A., Baur S., Paulus W., Reimers C.D. Motor cortex fatigue in sports measured by transcranial magnetic double stimulation. Med Sci Sports Exerc. 2000;32:1942–1948. doi: 10.1097/00005768-200011000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Andersen B., Westlund B., Krarup C. Failure of activation of spinal motoneurones after muscle fatigue in healthy subjects studied by transcranial magnetic stimulation. J Physiol. 2003;551:345–356. doi: 10.1113/jphysiol.2003.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]


