Summary
Background
House screening should protect people against malaria. We assessed whether two types of house screening, full screening of windows, doors and closing eaves or installing netting ceilings in local houses, could reduce malaria vector house entry and anaemia in children, in an area of seasonal transmission.
Methods
500 occupied houses in and near Farafenni town in The Gambia were randomly assigned to receive full screening, screened ceilings, or no screening, in an area where coverage of insecticide-treated nets was low. Screening was not treated with insecticide. Exposure to mosquitoes indoors was assessed by fortnightly light trap collections, and haemoglobin (Hb) concentration and the prevalence of anaemia and parasitaemia measured in children, aged 6 months to 10 years old, at the end of the transmission season.
Findings
The mean number of Anopheles gambiae sensu lato mosquitoes, the principal malaria vector, was reduced by 59% in houses with full screening (95% CI 46, 69; p<0.001) and 47% in houses with screened ceilings (95% CI 30, 60; p<0.001) compared with unscreened houses (37.5/trap/night). Anaemia prevalence (Hb <80g/L) was 19.0% among children in unscreened houses, compared to 12.3% with full screening, adjusted odds ratio (OR) 0.53 (0.29, 0.97; p=0.04), and 11.7% with screened ceilings, adjusted OR 0.51, (0.27, 0.96; p=0.04). Mean Hb concentration was higher in children living in fully screened houses and in houses with screened ceilings (104g/L in both groups), than those in unscreened houses (100g/L); adjusted estimates of the differences 3.7g/L (0.3, 7.2; p=0.03) and 4.2g/L (0.6, 7.7; p=0.02) respectively. There was no evidence of an effect on the prevalence of malaria infection.
Interpretation
House screening substantially reduced the number of mosquitoes inside houses and can contribute to prevention of anaemia in children.
Funding
Medical Research Council
Introduction
For the first time in a generation malaria is declining in many parts of tropical Africa1, which has led to renewed calls for malaria elimination. The reduction is mainly due to the extensive use of long-lasting insecticide-treated nets (LLIN) and artemisinin-based combination therapy (ACT). However, the emergence of vectors resistant to insecticides used for net impregnation2 and parasites resistant to Artemisinin derivatives3 will ultimately compromise these hard-won gains and impede efforts to eliminate the disease. Malaria remains one of the world’s greatest childhood killers4, uses almost half of the clinical services in Africa (www.rbm.who.int), and is a substantial obstacle to social and economic development5. It is therefore of considerable strategic importance to focus on sustainable, environmentally friendly and easily-integrated methods of control that can be added to the existing arsenal. Environmental management (EM) provides several tools for malaria control that have been effective in the tropics in the past6-9, and could be again if incorporated into Integrated Vector Management (IVM) programmes10.
Mosquito-proofing homes is one of the principal tools of EM that has been associated with protection against malaria11, 12; yet it has been ignored during long term anti-malarial drug- and insecticide-driven campaigns. House screening works by reducing exposure to malaria-transmitting mosquitoes and has the added benefit of protecting everyone in the room, avoiding issues of inequity within the household. In The Gambia we anticipated that house screening might be particularly effective since the primary vector, Anopheles gambiae s.l., bites predominantly at night and indoors. Our intervention study was thus designed to demonstrate this that house screening is effective against malaria in an African setting. We tested two types of screening: (1) full screening of doors, windows and closed eaves, based on established WHO criteria13 and (2) screened ceilings, effective in experimental hut trials12, where mosquitoes that enter the house through open eaves are denied access to the room space by the screening.
Methods
Study site
The trial was based at the Medical Research Council (MRC) laboratories at Farafenni field station in The Gambia, and carried out in 2006 and 2007. The characteristics of the area have been described in detail elsewhere14. Briefly, the study area was situated approximately 170 km from the mouth of the Gambia River and covered 70 km2 of the north bank, an area of open Sudan savanna. The climate consists of a single rainy season from June to October followed by a long dry season. There was 808mm of rain in 2006 and 751mm in 2007. Malaria cases are almost entirely attributable to Plasmodium falciparum. Members of the A gambiae s.l. complex are the main vectors and the entomological inoculation rate (EIR) varies from 0-166 infective bites per person per rainy season15. Combination therapy based on chloroquine and sulfadoxine pyrimethamine was the first-line treatment for uncomplicated malaria throughout the trial. An effectiveness study testing artesunate/lumefantrine combined, at the Armed Forces Provisional Ruling Council (AFPRC) General Hospital in Farafenni and at other health centres in the north bank region, started at the end of the trial, in December 2007. The study area population was composed of 7852 people, with roughly equal numbers of men (53%) and women, and dominated by three ethnic groups; Wolof (38%), Mandinka (28%), and Fula (27%).
Study design
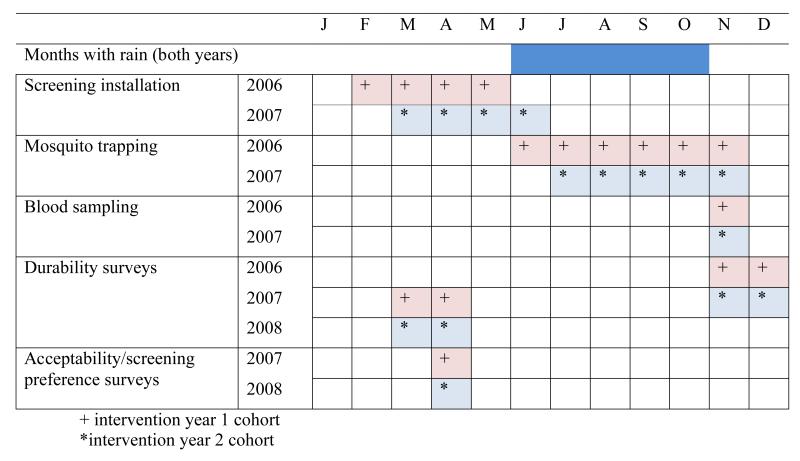
The study was a three-arm randomised controlled trial (trial registration: ISRCTN51184253 – Screening-homes to prevent malaria) to assess the efficacy and acceptability of two types of house screening designed to reduce house entry by A gambiae s.l., and anaemia and parasitaemia prevalence in children sleeping in those houses. In each year of the study we aimed to install full screening i.e. screened doors, windows and closed eaves (with a mixture of sand, rubble and cement as is normal local practice), in 100 houses, and screened ceilings in a further 100 houses. 50 different houses each year served as a control group. The screening was made from local timber and PVC coated fibreglass netting (1.2m wide for doors, 2.4m wide for ceilings and 1.0m wide for windows), with a mesh size of 42 holes/cm2 (Vestergaard Frandsen group, Denmark).
A detailed description of the intervention arms is published in the trial protocol16. The primary objective was to estimate the efficacy of house screening against A gambiae s.l. house entry. The trial was designed to detect straight superiority of the interventions over the control; having in excess of 90% power to detect a 50% reduction in either intervention arm. Further, the trial was designed to compare the two types of screening, with 90% power to discriminate between a 50% reduction and 67% reduction in mosquitoes/trap/night between the two intervention groups. We considered that this difference would make little, if any, appreciable change to the clinical pattern of malaria in the study area.
The trial was also designed to examine the efficacy of house screening in preventing anaemia and reducing malaria infection at the end of the transmission season in November each year. The clinical endpoints were haemoglobin (Hb) density, prevalence of anaemia (<80g/L), severe anaemia (<50g/L), presence of malaria parasites, parasite density and high parasitaemia (≥5000 parasites/μL). The study was designed to have 90% power to detect a difference of 5g/L or more in the mean Hb concentration of children in the intervention arms compared with the control group assuming a standard deviation of 17g/L, an average of 2.5 children per house, and an intraclass correlation of between 0.04 to 0.08 from earlier studies. Children were selected since they are most at risk from anaemia in this population17.
Guidelines for recommending either type of intervention were established before the trial began. Full screening or screened ceilings would be recommended if they reduced house entry by malaria mosquitoes by 50% and were considered acceptable by more than 67% of householders. If both interventions satisfied those criteria, the intervention which was statistically more protective, or, if there was no difference, the cheapest, would be selected.
The protocol was approved by the Health Services and Public Health Research Board of MRC, UK and, The Gambia Government and MRC Laboratories Joint Ethics Committee and the Ethics Advisory Committee of Durham University. Two independent panels, a Trial Steering Committee and a Data Monitoring and Ethics Committee, reviewed the conduct and results of the trial. The only incentives given to households that participated were provision of screening and treatment of study children during the clinical survey at the end of the transmission season.
Participants
MRC Farafenni ran a demographic surveillance system (FDSS) in the study area, which includes 46 residential blocks in Farafenni town and 23 surrounding villages. Lists of potentially eligible houses, and children sleeping in those houses, were generated from this census and visited to check criteria for recruitment. Houses had to be (1) single-storey buildings, (2) have open eaves, (3) have <five rooms, (4) have no existing ceilings, (5) have no existing screening and (6) have at least one child aged between 6 months and 10 years sleeping there at night. There were no other exclusion criteria for children. Village and urban block sensitisation meetings were held to explain to the residents the purpose of the study and the benefits from participation in the trial. Subsequently, information sheets were read to individual house owners and to parents or guardians of children. Comprehension was checked before written consent was sought. Participants were invited to sign (or thumbprint if not literate) the consent documents, which were countersigned by the fieldworker present. Separate consent forms were filled if the house owner was not the parent or guardian of the resident eligible children. Houses were enrolled between the December and February prior to the intervention. Eligible children were enrolled at the same time, and a second round of enrolment of children was conducted in September of each year to include all children born during the screening installation phase (February to April), that would be eligible for the survey in November. In September each enrolled child was given a unique study number and individual photographic identification card.
Procedures
Eligible houses were sorted by (1) rural (village) or urban (Farafenni) location, (2) residential block or village and (3) the number of children in each house, to achieve implicit stratification, before assigning treatment group in permuted blocks of 5 (2 full screening interventions: 2 ceiling screening interventions 1: control) generated using Stata 7 (StataCorp., College Station, TX, USA).
Exposure to mosquitoes was measured by routine surveillance with CDC light traps (Model 512, John Hock Co., Gainsville, FL) positioned 1m above the ground, 1-2m from the foot end of a bed protected with an untreated net used on that night only. Each study house was sampled every two weeks during this surveillance period (26 June to 2 November 2006 or 16 July to 5 November 2007). Sub-samples of A gambiae mosquitoes from each trial arm and each month of the surveillance period were taken for species identification by PCR18 and identification of infective mosquitoes by ELISA. Heads and thoraces of mosquitoes were homogenized in pools of 10 individuals and the presence of sporozoites identified by ELISA19.
A clinical cross-sectional survey of children was conducted at the end of each transmission season, at least six months after the screening was installed. The clinical team was not involved in any other study procedures and was blind to the intervention status of each child that attended. Axillary temperature was measured and a rapid diagnostic test (ICT malaria P.f Cassette Test, ICT Diagnostics, South Africa) performed for children with temperature ≥37.5°C and/or history of fever in the preceding 48h, to allow on-the-spot treatment of unwell children with detectable malaria. A finger-prick blood sample was taken from each child to measure Hb using a portable β-Hb photometer (Hemocue®, Ängelholm, Sweden), and to make thin and thick films for detection and quantification of malaria parasites. To establish parasite presence and density (asexual stages/μL, assuming a blood volume per HPF of 0.002μL), Giemsa-stained blood slides were examined under 1000-fold magnification and 200 fields examined before a slide was declared negative. Parasite prevalence is defined as the proportion of children with parasites detectable by microscopy.
Children with Hb <80g/L were classified as anaemic and given iron supplementation. Chloroquine and Sulfadoxine/Pyrimethamine (Fansidar) were given to any child ICT-positive, and to those who were ICT-negative or not tested but who were shown positive on subsequent blood-slide examination. The parents of any child treated for malaria were asked to take their child to the nearest Maternal and Child Health clinic if the child did not recover from the symptoms of malaria within 48h. Children with Hb<50g/L were taken to the AFPRC General Hospital at Farafenni for blood transfusion and treatment of any underlying illnesses. They were followed up at MRC for repeat Hb and general clinical review two weeks after discharge from hospital. Socioeconomic status was based on nine household characteristics, including household commodities, livestock and house structure20
After the end of season survey, house owners were given the choice of keeping the screening they had been given or having it removed, with the option of having the other screening type installed. Those in the control group were given the choice of having either screening type installed. The relative acceptability of each intervention was measured as the proportion of residents that continue the use of each intervention after they have been given the choice of changing to the other intervention type or having no screening. Two durability surveys, carried out at 6 and 12 months after the screening was installed, recorded data specific to each type of screening.
Two costings incorporating materials and labour were calculated for both interventions on a per person basis: the cost incurred during the trial, and a cost incorporating locally available netting. Each costing was based on the average study house 22.2m2 with 2.6 doors and 0.3 windows, with four residents.
Statistical analysis
Analysis of this trial adhered, as far as possible, to a detailed analytical plan established before the investigators had access to the finalized data.
Primary analysis
The primary analysis considered two end-points only, (1) the number of female A. gambiae s.l./house/night and (2) Hb density/house in children, without adjustment for covariates. When comparing each intervention arm with the control arm we adopted a modified intention to treat (ITT) approach. This incorporated all houses that were randomized for which there were some outcome data (excluding collections when the light trap was not working, houses that were vacated by residents or destroyed, and houses for which the occupier withdrew consent), and all children recruited before the clinical survey who were sampled for Hb and parasitology at that survey. When comparing the two intervention arms, it was useful make an entomological comparison in an according to protocol (ATP) analysis that excluded all houses from either intervention group (and study children that slept there) that had screening scored as ‘badly damaged’ in a durability study conducted 6 months after screening was installed. The definition of ‘badly damaged’ screens was determined separately for homes in the two treatment arms. In full screened houses, ‘badly damaged’ was defined as 5 or more holes in the screening and/or doors not closing tightly. This number of holes was shown to be important in a bednet study21. In screened ceiling houses, ‘badly damaged’ was defined as 5 or more holes in the screening and/or a house in which the netting had come away from the battens that secured it to the walls.
We estimated the relative reduction in mean mosquito count for each intervention group compared to the control using Poisson regression models. In addition we incorporated a variable for household in these models as a gamma-distributed random effect; this provided a means of accounting for dependence among counts made at the same house, and was also a way of modeling overdispersion in the distribution of mosquito counts. To estimate the effect of the ceiling intervention, data from the control and ceiling intervention were selected and the outcome was regressed on an indicator for the ceiling intervention. We report the exponentiated coefficient which is interpreted as the ratio of the mean mosquito count for the group receiving the ceiling intervention relative to the mean count of the control group. The statistical significance of the effect was tested using the p-value obtained from this regression. To adjust for multiple comparisons a significance level of α=0.025 was used. Poisson regression was also used to determine the efficacy of full screening, and to compare the relative efficacies of ceiling versus full. For the latter comparison, a non-inferiority analysis was undertaken in which we considered the two treatments to be equivalent if the lower bound of the confidence interval for the ratio of full/ceiling exceeded 2/3.
For the primary clinical analysis we report mean Hb for each of the trial arms. Differences in mean Hb between trial arms were estimated from a regression of Hb on intervention type in which the effect of household on Hb level was included as a random effect.
Secondary analysis
In the secondary analysis of the entomology data, efficacy was estimated using negative binomial regression models for the number of A gambiae s.l. and total number of culicine mosquitoes caught per house. Multiple imputation was used to reduce bias due to missing mosquito counts and missing covariate data. Ratios of the mean mosquito count (screened ceiling: control and full screening: control) were adjusted for covariates specified in the analysis plan that were shown to be associated with mosquito catch size in this area14: (1) presence of horse(s) tethered near the house at night, (2) number of people sleeping in the trapping room, (3) wall material (mud brick or concrete), (4) household socio-economic status (SES). Where variables were recorded at each visit (1-3) the mean value was used. SES scores were computed using the first component of a principal components analysis of 9 household characteristics (wall material, metal roof, radio, iron bed, cart, bicycle, car or motor bike, livestock, literacy of mother)20.
The secondary analysis of clinical data was based on a complete case analysis (i.e., only individuals with complete outcome and covariate data were included). Differences in mean Hb densities between trial arms were estimated using a normal model in which household was included as a random effect. Adjusted estimates of mean difference were based on a model that incorporated the full set of covariates used for the analysis of the entomological outcome, plus age of study subject. Unadjusted and adjusted odds ratios (OR) were estimated for anaemia (Hb<80 g/L), severe anaemia (Hb<50g/L), the presence of malaria parasites and high parasitaemia (≥5000 parasites/μL). In each case, a logistic regression was used in which the household was modelled as a random effect. Adjusted ORs were obtained by including the covariates used in the model for Hb density described above.
For all adjusted analyses we explored the effect on estimates of including variables that were potentially on the causal pathway between the intervention and outcome, namely: churai incense burnt at night, net use and net condition. By including these mediator variables in the regression models it was possible to estimate the direct effect of the intervention i.e., the effect in households where these characteristics are the same.
Contingency tables were used to compare: the durability between years, sporozoite rates between years and trial arms, and the relative acceptability of each type of screening Analyses were conducted using SPSS version 15.0 (SPSS Inc., Chicago, IL) and Stata version 10.1 (StataCorp).
Role of the funding source
MRC, the sponsor of the study, had no role in study design, data collection, analysis, interpretation or writing of the report.
Results
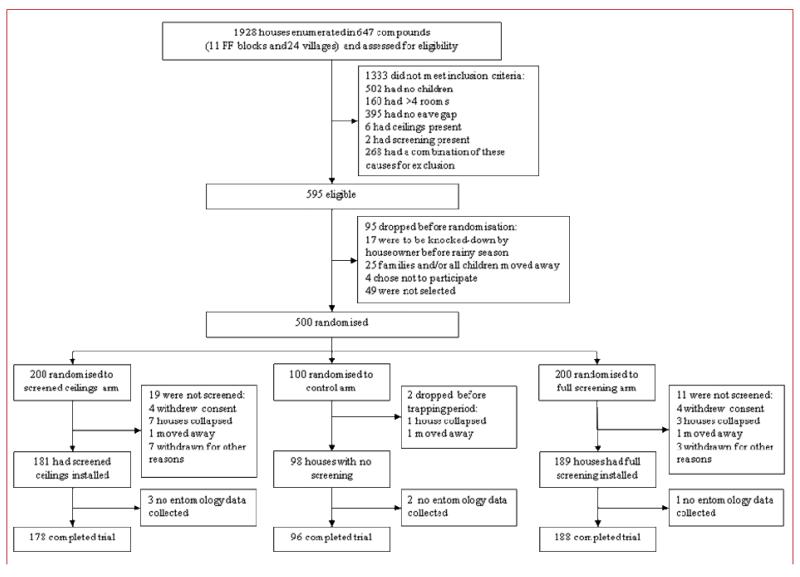
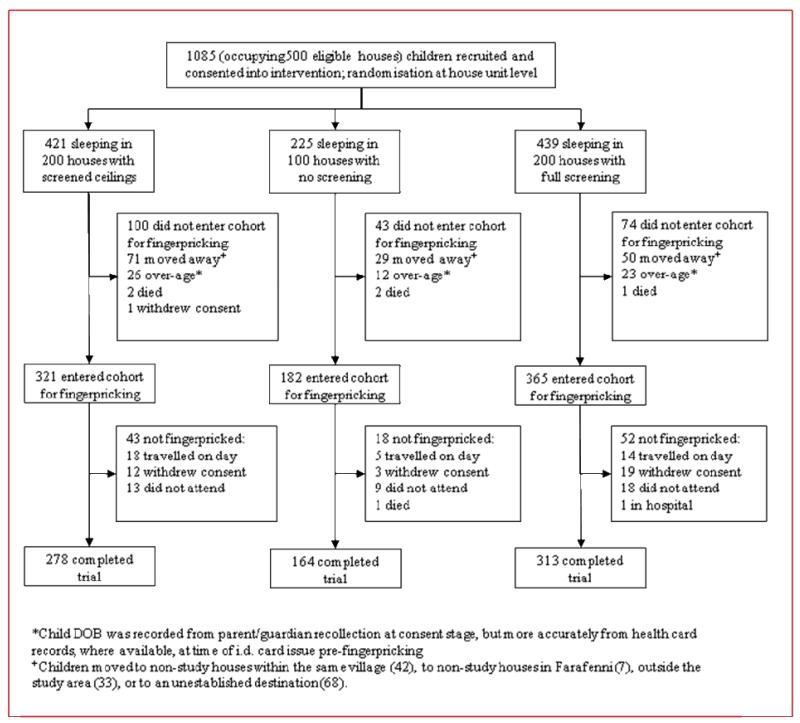
1928 houses were assessed for eligibility, of which 1333 did not satisfy the inclusion criteria. 500 houses were recruited and randomized to the intervention and control arms. Two teams, each of one and three assistants, installed full screening into 2-3 houses, or screened ceilings into 4-5 houses, per day. 462 houses completed the trial (figure 2). 1085 children age 6 months to 10 years old resided in the 500 houses, of which 755 were surveyed (164/225 in the control arm, 313/439 in the full screening arm and 278/421 in the ceiling arm; figure 3). The characteristics of the houses and children were similar in each arm of the trial, though both treated and untreated bednet coverage was slightly higher at the end of the transmission season in the control group than in the two intervention groups (table 1). Table 2 describes the entomological, clinical and acceptability data by treatment allocation before analysis.
Figure 2.
Trial profile for study houses
Figure 3.
Trial profile for study children
Table 1. Characteristics of study arms.
| Factors averaged over trapping seasons | Control (n=96) | Screened Ceiling (n=178) | Full Screening (n=188) |
|---|---|---|---|
| Number of trapping room occupants | 4.2 (4.0-4.4) | 4.1 (3.9-4.2) [2] | 4.1 (4.0-1.2) [1] |
| Use of incense a | 28% (21-36) | 29% (23-34) | 28% (23-34) |
| Mosquito coil use | 2% (0-3) | 2% (1-3) | 1% (0-2) |
| Number of horses tethered near house | 0.9 (0.7-1.1) | 0.9 (0.7-1.0) [2] | 0.9 (0.8-1.0) [1] |
| Time to bed b | 21:50h (21:34-22:06) | 21:49h (21:36-22:02) | 21:47h (21:34-22:00) |
| Factors measured at end of trapping seasons | Control (n=163) | Screened Ceiling (n=277) | Full Screening (n=315) |
| Females | 48% [1] | 47% [5] | 49% [2] |
| Ethnicity | 49% Wollof 41% Fula 10% Mandinka [1] |
46% Wollof 42% Fula 9% Mandinka 3% Other [5] |
54% Wollof 34% Fula 8% Mandinka 4% Other [2] |
| Age (months) | 68 (63-74) [2] | 66 (62-70) [5] | 69 (65-73) [2] |
| Socio-economic status score | 3.6 (3.4-3.9) [1] | 3.5 (3.3-3.7) [12] | 3.8 (3.6-3.9) [9] |
| Use of untreated bednet in good condition c | |||
| 18% | 12% | 13% | |
| Use of treated bednet | 35% | 25% | 31% |
Data are arithmetic mean (95% CI) or % frequency, [missing cases].
churai,
control n=69, screened ceiling n=89, full screening n=94.
net intact or with no more than 5 ≤2cm diameter holes, that was long enough to tuck under mattress.
Table 2. Descriptive characteristics by treatment allocation.
| Variable | Control | Screened ceiling | Full screening |
|---|---|---|---|
| Entomological | |||
| Mean number of Anopheles gambiae s.l. /trap/night |
37.5 (31.6-43.3) | 19.1 (16.1-22.1) | 15.2 (12.9-17.4) |
| Estimated entomological inoculation rate (EIR) |
|||
| 2006 | 2.27 (1.38-3.16) |
1.14 (0.85-1.42) |
0.77 (0.57-0.96) |
| 2007 | 1.35 (0.74-1.97)) |
0.90 (0.22-1.57) |
0.42 (0.24-0.63) |
| Clinical | |||
| Haemoglobin density (g/L) | |||
| 2006 | 98 (93-102) | 103(99-106) | 103(100-106) |
| 2007 | 103(98-109) | 105(102-108) | 105(102-108) |
| Prevalence of moderate anaemia (<8 g/L) | |||
| 2006 | 19.1% | 11.4% | 11.0% |
| 2007 | 17.6% | 12.4% | 14.6% |
| Prevalence of severe anaemia (<5 g/L) | |||
| 2006 | 2.2% | 0.7% | 0.6% |
| 2007 | 2.7% | 0% | 0.7% |
| Parasite prevalence (all parasitaemias) | |||
| 2006 | 32.6% | 32.1% | 28.7% |
| 2007 | 9.5% | 8.0% | 8.6% |
| Parasite prevalence (high parasitaemias, ≥5000 parasites/μL) |
|||
| 2006 | 10.1% | 5.0% | 3.7% |
| 2007 | 1.4% | 2.9% | 4.6% |
| Crude mortality rates (deaths/100 children) | |||
| 1.53 (3/196) |
0.57 (2/350) |
0.26 (1/389) |
|
| Acceptability | |||
| Proportion of residents willing to continue use of intervention |
1% | 46% | 94% |
| Proportion of control arm residents opting for intervention installation |
1% a | 17% | 82% |
Data are arithmetic mean (95% CI) or % frequency unless otherwise indicated. EIRs are mean number of sporozoite-infected A gambiae/person/trapping season. Clinical data were recorded at the end of the rainy season in both years.
Chose not to have either screening type installed.
Entomological
180472 mosquitoes were trapped, of which 48% were anophelines and 52% culicines. A gambiae represented 87% of all anophelines caught. A sub-sample of 3% of A gambiae caught were identified to species by PCR. 50% of these were A gambiae sensu stricto, 46% A melas and 4% A arabiensis. Overall levels of transmission were lower in 2007 than 2006 (table 5). Both screening interventions reduced house entry. The mean number of A gambiae caught in unscreened houses was 37.5 per trap per night (95% CI 31.6, 43.3), compared to 15.2 in houses with full screening (12.9, 17.4; p<0.001) and 19.1 in screened ceiling houses (16.1, 22.1; p<0.001, table 2).
Table 5. Prevalence and odds ratios (OR) for the effect of screening on anaemia and parasitaemia as estimated from logistic regression models incorporating house as a random effect and adjusting for covariate imbalance.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Cases | N | % | OR | OR* | 95%C.I. | p | |
|
|
|||||||
| Anaemia (<80g/L) | |||||||
| Control | 30 | 158 | 18.99 | 1.00 | 1.00 | - | - |
| Ceiling | 31 | 264 | 11.74 | 0.57 | 0.51 | 0.27-0.96 | 0.035 |
| Full | 38 | 309 | 12.30 | 0.59 | 0.53 | 0.29-0.97 | 0.040 |
| Parasitaemia | |||||||
| Control | 34 | 158 | 21.52 | 1.00 | 1.00 | - | - |
| Ceiling | 54 | 264 | 20.45 | 0.91 | 0.96 | 0.54-1.70 | 0.877 |
| Full | 58 | 309 | 18.77 | 0.79 | 0.94 | 0.53-1.66 | 0.827 |
Adjusted for location, year, child’s age, SES, wall material and, measured each night, the no. of horses in compound, people sleeping in the house.
The primary analysis revealed a reduction of 59% (95% CI 46%, 69%, p<0.001) A gambiae s.l./trap/night in full screening houses and 47% (95% CI 30%, 60%, p<0.001) in screened ceiling houses compared with control houses (table 3). The ATP analysis revealed that there was no significant difference between mean catches in the two intervention arms. However, the confidence intervals were too wide to demonstrate equivalence between the two interventions (table 3).
Table 3. Comparison of mosquito densities between treatments.
| Anopheles gambiae s.l. | All Culicinae mosquitoes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary analysis * | N | Mean | Ratio | 95%C.I. | p | N | Mean | Ratio | 95%C.I. | p |
| ITT | ||||||||||
| Control | 731 | 37.46 | - | - | - | |||||
| Ceiling | 1,376 | 19.12 | 0.53 | 0.40-0.70 | <0.001 | |||||
| Full | 1,463 | 15.15 | 0.41 | 0.31-0.54 | <0.001 | |||||
| ATP | ||||||||||
| Ceiling | 693 | 18.87 | - | - | - | |||||
| Full | 826 | 15.45 | 0.81a | 0.56-1.15 | 0.23 | |||||
| Secondary analysis ** | ||||||||||
| Control | 96 | 309.61 | - | - | - | 96 | 376.27 | - | - | - |
| Ceiling | 178 | 169.39 | 0.60 | 0.46-0.80 | <0.001 | 178 | 286.93 | 0.75 | 0.60-0.93 | 0.010 |
| Full | 188 | 133.21 | 0.46 | 0.34-0.63 | <0.001 | 188 | 133.18 | 0.34 | 0.25-0.46 | <0.001 |
Rate ratios of mean counts for Anopheles gambiae s.l./trap/night obtained by Poisson regression, with house included as a random effect, where ITT is intention to treat and ATP is according to protocol.
Ratios of means for the total A gambiae s.l. and all culicines counts over all trapping visits. Data fitted to negative binomial regression models adjusting for location, year, SES, wall material and, measured each night, the no. of horses in compound, people sleeping in the house .
ratio vs ceiling, all other ratios vs control
Collections of A gambiae s.l. were made on at least 7 occasions from 90% of households. Covariate data were complete with the exception of SES where 103 (22%) households had missing data. Both mosquito count and covariate data were imputed for the secondary analysis. Parameter estimates from the secondary analysis revealed a 54% reduction in the A gambiae s.l., catch/house/night and 66% reduction in culicines in full screened houses compared with control houses. Similarly there were reductions of 40% and 25% in screened ceiling houses compared to the control (table 3).
There were no significant differences in sporozoite rates between locations in either year or between trial arms in year 2 and so these data were pooled. However the sporozoite rate in 2006 was nearly twice that of 2007 (2006 = 60/25180 (0.24%), 2007 = 19/13146 (0.14%); χ2=4.3, df=1, p=0.04). The resulting estimates of EIR for each season for 2006 and 2007 are described in table 2.
Clinical
The analysis of clinical outcomes was based on 731 children with complete data (158 children in houses without screening, 309 children in fully screened houses and 264 children from houses with screened ceilings). Mean Hb concentration was higher in children living in fully screened houses and in houses with screened ceilings (104g/L in both groups), than in children in unscreened houses (100g/L). Unadjusted estimates (primary analysis) of the differences were 3.7 (95% CI −0.4, 7.8; p=0.08) and 3.6 (95% CI −0.6, 7.8; p=0.09), adjusted estimates were 3.7 (95% CI 0.3, 7.2; p=0.03) and 4.2 (95% CI 0.6, 7.7; p=0.02) respectively (table 4). 30/158 (19.0%) children in unscreened houses had anaemia (Hb<80g/L), compared to 38/309 (12.3%) in houses with full screening, adjusted OR 0.53 (95% CI 0.29, 0.97; p=0.04), and 31/264 (11.7%) with screened ceilings, adjusted OR 0.51, (95% CI 0.27, 0.96; p=0.04, table 5). 7 children had severe anaemia (Hb<50g/L); 4 (2.53%) among children in houses without screening, 2 (0.65%) in the full screening group and 1 (0.38%) in the ceiling group (differences not significant). 3/573 (0.52%) children in both screened intervention groups had severe anaemia, a significantly lower proportion than in the control group (p = 0.043, Fisher’s exact test 2 tailed value).
Table 4. Differences in mean haemoglobin between interventions and control estimated using regression models with house as a random effect, with and without adjusting for covariate imbalance.
| Haemoglobin density (g/L) |
||||||
|---|---|---|---|---|---|---|
| N | Mean hb | Difference from control |
Difference from control* |
95%C.I. | p | |
|
|
||||||
| Control | 158 | 100.3 | - | - | - | - |
| Ceiling | 264 | 104.3 | 3.6 | 4.2 | 0.6-7.7 | 0.021 |
| Full | 309 | 104.1 | 3.7 | 3.7 | 0.3-7.2 | 0.034 |
Adjusted for location, year, child’s age, SES, wall material and, measured each night, the no. of horses in compound, people sleeping in the house.
The prevalence of microscopy-detectable parasitaemia was highest among children in houses without screening (21.5%). It was slightly lower in the full screening group (18,8%) and the group that received screened ceilings (20.5%), although the differences between the control and intervention arms were not statistically significant (table 5). Similarly there were no differences in the prevalence of high parasitaemia (≥5000 parasites/μL): 6.3% in the control group, 4.2% in the full screening group and 3.8% in the screened ceiling group. Whilst the crude mortality rates were lower in the screened intervention groups than in the control this was not statistically significant (Fisher’s exact test 2 tailed value, table 2).
Acceptability
At the end of the trial 94% of householders opted to continue using full screening whilst only 47% were willing to continue use of screened ceilings. There was a significant association between the type of screening that participants received and whether or not they would opt to change to the other type (χ2=94.1, df=1, p<0.001). The odds of changing intervention type were 18.5 times greater for households that received screened ceilings compared with those that received full screening. Full screening was also the preferred choice of the participants in the control arm (82% compared to 17% opting for ceilings).
Durability
The extent of damage to fully screened houses was highly varied. Screened windows suffered little or no damage; even after 12 months 80% (36/45) of windows were still intact. The mortar blocking the eaves was similarly durable, with 90% remaining intact after 12 months (220/245). The screened doors suffered the greater damage with only 29% (105/ 365) being intact after 12 months. Nonetheless there were more intact doors in the second year of the study (37%, 68/186) than the first year (21%, 68/186; χ2 =10.5, p = 0.001), suggesting that people in the second year had learnt about the advantages of screening from the neighbours in the first year of the study and looked after the screens better. It is important to appreciate that this damage was minor with the median number of holes being only 4 (IQR 1, 8) in year 1 and 2 (0, 5) in year 2. 89% (347/390) of doors still closed tightly in the frame after 12 months, with no gaps large enough for mosquitoes to pass through. In screened ceilings houses the main type of damage was also holes in the netting, with very little damage to the battens or the masonry. Though only 15% of screens were intact after 12 months, the median number of holes was again low: 6 (IQR 2, 11) in year 1 and 4 (2, 7) in year 2.
Cost
Given the average house occupancy of 4 individuals, the cost of full screening/person protected in the trial (netting donated free of charge) was US$9.98, compared with $8.69 for screened ceilings/person protected. If locally available netting was used, the average cost/person would be $11.11 for full screening and $21.17 for screened ceilings.
Discussion
We have shown that screened ceilings and full screening reduced indoor exposure to A gambiae s.l., the principal vector of malaria in Africa, and reduce anaemia prevalence in children occupying those houses.
Though house screening as an intervention against malaria is itself not a novel idea11, the trial reported here was the first designed to meet the standards of a clinical randomized controlled trial and addressed methodological criticisms of previous screening studies. For example, house screening was trialed as a preventative measure in Italy over a century ago22, but there was no random allocation of the interventions. And while there have been other investigations that have demonstrated an association between house architecture and malaria transmission, infection and morbidity23, 24, many have been conducted during observational studies, and the focus is often on the quality of walls, ceilings and floorboards rather than screening per se. It is difficult therefore to quantify the degree of protection offered by screening alone from these studies and perhaps for this reason they failed to convince implementers in the public health sector of the importance of screening.
House screening proved an effective barrier against both anopheline and culicine mosquitoes. Whilst both methods worked well, full screening was more protective than screened ceilings, suggesting that doors and windows were important routes of entry for many mosquitoes. It is perhaps surprising that even in full screened houses we trapped on average an unadjusted total of 30 mosquitoes (all species) each night. This is probably because screened doors were often propped open during daylight hours, only being closed at 19:00-20:00h. Mosquitoes that were active earlier in the day could enter homes before doors were closed, and therefore even greater reductions in transmission could be achieved by persuading home owners to shut doors earlier in the evening. At best house screening should protect people from the 80% of bites that occur indoors 25
Few entomological interventions that cause a significant reduction in adult mosquitoes also demonstrate that this leads to a significant reduction in malaria or anaemia prevalence. In this study house screening reduced anaemia in children, since those living in screened homes had higher Hb densities and were less likely to be anaemic than children in unscreened homes. These findings are important since anaemia is a clinically relevant measure of malaria in children in this setting. Many intervention trials have examined anaemia only in children aged up to 24-36 months because anaemia, and thus the impact of malaria control, is often greatest in the youngest children26. For this reason it was critical to adjust the house screening efficacy estimates by age.
The significant reduction in anaemia prevalence associated with house screening compares favourably with the RTS,S/AS02A vaccine which failed to reduce anaemia prevalence27. The adjusted increase in Hb of 3.7g/L in the full screened group and 4.2g/L in the screened ceiling group is similar to the weighted mean increase of 1.7 PCV%, the equivalent of approximately 5.7g/L28, across six randomized controlled trials of ITNs against no net use control groups29, and to the 7.2g/L increase after indoor residual spraying with Lambdacyhalothrin in Tanzania30. It is also similar to the increase in PCV% generated by chemoprophylaxis, using pyrimethamine-dapsone (1.5%) or chlorproguanil (1.0%) in the same study area31. Although there were no significant differences in cases of severe anaemia between the arms of the trial, the rates were lower than expected, which limited our ability to detect significant screening efficacy for this endpoint. Nevertheless, the proportion of children with severe anaemia was significantly lower in the intervention arms combined than in the control arm.
Unsurprisingly neither screening intervention was associated with a reduction in the prevalence of parasitaemia since this can only be achieved if the infection level in the intervention groups were substantially suppressed 32-34. Thus the introduction of major interventions such as ITNs29, indoor residual spraying (IRS)35 and intermittent preventative treatment of infants36 have all had limited impact on parasite prevalence within six months of introduction. Our interpretation is that house screening, whilst not reducing parasite prevalence, reduced malaria superinfection of children which leads to anaemia. We would also expect that screening would reduce clinical episodes of malaria since any reduction in EIR will reduce malaria incidence37. Malaria prevalence was much lower in the second year and probably reflects a lower exposure to malaria parasites experienced in 2007. It cannot be explained by an increase in ITN coverage between years (35% coverage in cohort subjects in 2006, 24% in cohort subjects in 2007) nor the use of ACT, as this was introduced after the 2007 transmission season. No reduction in parasite density was seen in either intervention arm but again this is not uncommon for otherwise effective prophylactic interventions27 and can be hard to detect owing to large variation between slide readers in the estimates of parasite density by microscopy38.
Both screening interventions were well tolerated and safe to use. Only 2% from either group withdrew consent during the study because of problems relating to the screening itself. The most common concern expressed by participants was that the netting was hard to keep clean (55% of respondents from fully screened houses and 68% from ceilings). This was outweighed by advantages common to both screening types, including reducing dust (100% and 97%) and beautifying the house (100% and 88%). Occupants of fully screened houses reported more often than those in screened ceiling houses that their screening stopped mosquitoes (92% vs 62%) and other animals (100% vs 32%), and improved privacy (100% vs 74%). These are likely to be the reasons that full screening was the more acceptable intervention.
One possible concern is that installing screening might have reduced the use of bednets in those houses. Children in both screening groups were less likely to be under any sort of net than those in control houses, which may reflect a belief among some participants that the screening operated as a replacement of bednets. For children in fully screened houses it was the coverage of untreated nets that was lower (18% vs 31%, M-Hχ2=9.2, p = 0.002), and in ceiling houses it was ITN coverage (25% vs 35%, M-Hχ2=5.2, p = 0.08. Thus introducing screened ceilings in areas where ITNs coverage is high might increase transmission risk to individuals. We advocate house screening to augment, rather than replace, ITN use. However, we note that estimates of the direct effect of the intervention (obtained by including net use and net condition in models for clinical and entomological outcomes) were almost identical to estimates of the combined effect (direct and indirect effects); this suggests that indirect effects mediated through bed net use are of limited significance.
Our results indicate the feasibility of developing an effective house screening design against malaria. Both techniques offered satisfactory protection against A gambiae s.l. and anaemia, but only the full screening met the acceptability criteria for recommendation, and offered added protection against culicine mosquitoes, including some species that are arbovirus vectors. This is important because vector control activities that do not reduce nuisance biting will lack community support. Full screening can be a sustainable control method: the interventions were largely made using locally available materials and installed by local carpenters to a standard screening blueprint, at a reasonable cost, particularly if one considers that the screening can be protective for a number of years. Whilst most screening on the doors and ceilings were damaged after 12 months, this was relatively minor. As with many new technologies it is likely that durability can be improved by changes in materials and design. The use of insecticide-impregnated screening may also help create an even more effective barrier, especially when the screening is damaged. At a cost of ~$10/person, these are comparable with ITNs and IRS39, providing screening can remain effective for 3-4 years. Where the resources are available, there is also the opportunity to improve the durability of the interventions by using longer-lasting materials such as metal frames for doors.
Although screening should be tailored to local house designs, the general principles involved in this trial should help inform screening for malaria control in other African settings and elsewhere in the tropics. It is most likely to be successful in areas of low transmission where a large reduction in indoor biting could have a significant effect on reducing malaria morbidity, especially where people prefer not to use bednets, or have stopped using them because nuisance biting is fairly low. House screening could be readily incorporated into IVM programmes10, and because it does not rely on insecticides it may be particularly beneficial in areas where insecticide resistance develops. The results of this trial contribute to the evidence base for malaria control programmes, local administrations and non-governmental organisations throughout sub Saharan Africa to make an informed decision about house screening. We would encourage undertaking a larger trial to assess whether this intervention reduces clinical episodes of malaria in diverse settings. We also hope it will stimulate development of additional sustainable methods that in combination with improved health care and access to treatment can protect the poor and vulnerable and help drive malaria towards elimination.
Figure 1.
Study design
Acknowledgements
We are grateful for the helpful comment made by the referees. We acknowledge Paul Emerson and Amy Ratcliffe for their help designing an early version of the study. We are grateful for the donations of netting from The Vestergaard-Frandsen group and in particular the efforts of the director of development, Torben Frandsen, and associates in getting the netting to The Gambia. We thank Rob Hutchinson who helped develop methods for erecting the screening and the carpentry team that installed it; MRC and Durham University associates of the STOPMAL trial and other support staff at MRC Farafenni and MRC Fajara, in particular Chloe Day, Erin Dilger, Margaret Pinder, Pateh Bah, Ensa Touray, Pa Chebou Saine, Mamkumba Sanneh and Roger Conteh; Momodou Jasseh and Sarah Crozier as members of the Data Monitoring and Ethics Committee, the late Prof Chris Curtis, chairman of the TSC and the other committee members Robin Bailey and Peter Billingsley; Ann Kelly and Caroline Jones of London School of Tropical Medicine and Hygiene for help with the acceptability studies. We also thank the study children and their parents for their continuous support for this work. This study was funded by the Medical Research Council, UK.
Footnotes
Conflict of interest statement
We declare that we have no conflict of interest.
References
- 1.WHO . World Malaria Report 2008. World Health Organisation; Geneva: 2008. p. 190. [Google Scholar]
- 2.Kerah-Hinzoumbé C, Péka M, Nwane P, et al. Insecticide resistance in Anopheles gambiae from south-western Chad, Central Africa. Malaria J. 2008;7:192. doi: 10.1186/1475-2875-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jambou R, Legrand E, Niang M. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet. 2005;366:1960–3. doi: 10.1016/S0140-6736(05)67787-2. [DOI] [PubMed] [Google Scholar]
- 4.Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am J Trop Med Hyg. 2004;71(suppl. 2):1–15. [PubMed] [Google Scholar]
- 5.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 6.Takken W, Snellen WB, Verhave JP, et al. Environmental measures for malaria control in Indonesia - an historical review on species sanitation. Wageningen Agricultural University Papers; Wageningen: 1990. [Google Scholar]
- 7.Spielman A, D’Antonio M. Mosquito. A natural history of our most persistent and deadly foe. Faber & Faber; London: 2001. [Google Scholar]
- 8.Killeen GF, Fillinger U, Kiche I, et al. Eradication of Anopheles gambiae from Brazil: lessons for malaria control in Africa? Lancet Inf Dis. 2002;2:618–27. doi: 10.1016/s1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- 9.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Inf Dis. 2005;5:695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- 10.WHO . Global strategic framework for Integrated Vector Management. World Health Organisation; Geneva: 2004. [Google Scholar]
- 11.Lindsay SW, Emerson PM, Charlwood JD. Reducing malaria by mosquito-proofing houses. Trends Parasit. 2002;18:510–4. doi: 10.1016/s1471-4922(02)02382-6. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay SW, Jawara M, Paine K, et al. Changes in house design reduce exposure to malaria mosquitoes. Trop Med Int Hlth. 2003;8:512–7. doi: 10.1046/j.1365-3156.2003.01059.x. [DOI] [PubMed] [Google Scholar]
- 13.WHO . Manual on environmental management for mosquito control, with special emphasis on malaria vectors. World Health Organisation; Geneva: 1982. WHO Offset Publication No. 66. [PubMed] [Google Scholar]
- 14.Kirby MJ, Green C, Milligan P, et al. Risk factors for house-entry by malaria vectors in a rural town and satellite villages in The Gambia. Malaria J. 2008;7 doi: 10.1186/1475-2875-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bøgh C, Lindsay SW, Clarke SE, et al. High spatial resolution mapping of malaria transmission risk in The Gambia, West Africa, using LANDSAT TM satellite imagery. Am J Trop Med Hyg. 2007;76:875–81. [PubMed] [Google Scholar]
- 16.Kirby MJ, Milligan PJ, Conway D, et al. Study protocol for a three-armed randomized controlled trial to assess whether house screening can reduce exposure to malaria vectors and reduce malaria transmission in The Gambia. Trials. 2008;9:33. doi: 10.1186/1745-6215-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gambia RoT . Nationwide survey on the prevalence of vitamin A and iron deficiency in women and children in The Gambia. The National Nutrition Agency (NaNA) and the Medical Research Council; Banjul (The Gambia): 2001. [Google Scholar]
- 18.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the Polymerase Chain Reaction. Am J Trop Med Hyg. 1993;49:520–9. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 19.Burkot TR, Williams JL, Schneider I. Identification of Plasmodium falciparum-infected mosquitoes by a double antibody enzyme-linked immunosorbent assay. American Journal of Tropical Medicine and Hygiene. 1984;33:783–8. doi: 10.4269/ajtmh.1984.33.783. [DOI] [PubMed] [Google Scholar]
- 20.Clarke S. Variation in malaria risk and response in rural Gambia. University of Copenhagen; Copenhagen: 2001. PhD thesis. [Google Scholar]
- 21.Clarke SE, Bogh C, Brown RC, et al. Do untreated bednets protect against malaria? Trans Roy Soc Trop Med. 2001;95:457–62. doi: 10.1016/s0035-9203(01)90001-x. [DOI] [PubMed] [Google Scholar]
- 22.Celli A. The new preventative treatment of malaria in Latium. London School of Hygiene & Tropical Medicine; London: 1901. pp. 1–12. Collected papers on malaria. Angelo Celli, 1899-1912. [Google Scholar]
- 23.Butraporn P, Sornmani S, Hungsapruek T. Social, behavioural, housing factors and their interactive effects associated with malaria occurrence in East Thailand. SE Asian J Trop Med Publ Hlth. 1986;17:386–92. [PubMed] [Google Scholar]
- 24.Ghebreyesus TA, Haile M, Witten KH, et al. Household risk factors for malaria among children in the Ethiopian Highlands. Trans Roy Soc Trop Med. 2000;94:17–21. doi: 10.1016/s0035-9203(00)90424-3. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay SW, Armstrong Schellenberg JRM, Zeiler HA, et al. Exposure of Gambian children to Anopheles gambiae malaria vectors in an irrigated rice production area. Med Vet Entomol. 1995;9:50–8. doi: 10.1111/j.1365-2915.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 26.Brewster DR, Greenwood BM. Seasonal variation of paediatric diseases in The Gambia, West Africa. Ann Trop Paed. 1993;13:133–46. doi: 10.1080/02724936.1993.11747637. [DOI] [PubMed] [Google Scholar]
- 27.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 28.Korenromp EL, Armstrong Schellenberg JRM, Williams BG, et al. Impact of malaria control on childhood anaemia in Africa - a quantitative review. Trop Med Int Hlth. 2004;9:1050–65. doi: 10.1111/j.1365-3156.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- 29.Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database System Rev. 2004 doi: 10.1002/14651858.CD000363. Art. No: CD000363. [DOI] [PubMed] [Google Scholar]
- 30.Curtis CF, Maxwell CA, Finch RJ, et al. A comparison of use of a pyrethroid either for house spraying or for bednet treatment against malaria vectors. Trop Med Int Health. 1998;3:619–31. doi: 10.1046/j.1365-3156.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 31.Greenwood BM, Greenwood AM, Smith AW, et al. A comparative study of Lapudrine (chlorproguanil) and Maloprim (pyrimethamine and dapsone) as chemoprophylactics against malaria in Gambian children. Trans Roy Soc Trop Med. 1989;83:182–8. doi: 10.1016/0035-9203(89)90635-4. [DOI] [PubMed] [Google Scholar]
- 32.Smith D, Dushoff J, Snow R, et al. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature. 2005;438:492–5. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bødker R, Msangeni HA, Kisinza W, et al. Relationship between the intensity of exposure to malaria parasites and infection in the Usambara mountains, Tanzania. Am J Trop Med Hyg. 2006;74:716–23. [PubMed] [Google Scholar]
- 34.Beier JC, Killeen GF, Githure JI. Short report: entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–13. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 35.Rowland M, Mahmood P, Iqbal J, et al. Indoor residual spraying with alphacypermethrin controls malaria in Pakistan: a community-randomized trial. Trop Med Int Hlth. 2000;5:472–81. doi: 10.1046/j.1365-3156.2000.00581.x. [DOI] [PubMed] [Google Scholar]
- 36.Kobbe R, Kreuzberg C, Adjei S, et al. A randomized controlled trial of extended intermittent preventative antimalarial treatment in infants. Clin Infect Dis. 2007;45:16–25. doi: 10.1086/518575. [DOI] [PubMed] [Google Scholar]
- 37.Smith T, Charlwood JD, Kitua AY, et al. Relationships of malaria morbidity with exposure to Plasmodium falciparum in young children in a highly endemic area. Am J Trop Med Hyg. 1998;59:252–7. doi: 10.4269/ajtmh.1998.59.252. [DOI] [PubMed] [Google Scholar]
- 38.Kilian AHD, Metzger WG, Mutschelknauss EJ, et al. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Hlth. 2000;5:3–8. doi: 10.1046/j.1365-3156.2000.00509.x. [DOI] [PubMed] [Google Scholar]
- 39.Goodman CA, Mnwava AEP, Dlamini SS, et al. Comparison of the cost-effectiveness of insecticide-treated bednets and residual house-spraying in KwaZulu-Natal, South Africa. Trop Med Int Hlth. 2001;6:280–95. doi: 10.1046/j.1365-3156.2001.00700.x. [DOI] [PubMed] [Google Scholar]