Abstract
Malaria is still one of the major public health threats in sub-Saharan Africa. An effective vaccine could be a sustainable control measure that can be integrated into existing health infrastructures. The malaria vaccine candidate GMZ2 is a recombinant fusion protein of conserved parts of Plasmodium falciparum Glutamate Rich Protein and Merozoite Surface Protein 3 adjuvanted with aluminium hydroxide. GMZ2 is immunogenic and well tolerated in malaria-naive adults from Germany. To assess safety and immunogenicity in malaria exposed individuals, 40 adults from Lambaréné, Gabon were randomly assigned to receive either 100 μg GMZ2 or a rabies control vaccine three times in monthly intervals. Both vaccines were well tolerated. GMZ2 induced antibodies and memory B-cell responses, despite a high prevalence of GMZ2-specific immune reactivity due to previous intense exposure to P. falciparum.
Keywords: malaria vaccine, phase I clinical trial, malaria immunity
Introduction
Governments and international agencies are committed to reduce morbidity and mortality caused by malaria. Nevertheless, it remains a major public health problem in Sub-Saharan Africa. The search for effective and sustainable control tools continues and the goals are set high, since elimination has reappeared on the research agenda [1, 2]. Within this setting vaccines are particularly attractive medical interventions, because they can be integrated into an existing healthcare infrastructure that targets infants before they are at high risk to develop malaria. Development of vaccines for parasitic diseases is challenging because of complex life cycles, large numbers of potential target molecules, and adaptive potential to changing environments [3]. Despite these challenges, several recombinant and attenuated organism vaccines have been developed and some are licensed for veterinary use [4].
Currently, malaria vaccine candidates can broadly be categorized according to i) the targeted life stage or ii) the approach to pass information about the pathogen to the immune system. Vaccines in advanced clinical development either target the pre-erythrocytic stage or the disease-causing asexual blood stage. The distinction is not absolute since pre-erythrocytic vaccines seemingly attenuate subsequent blood stages [5]. Altruistic vaccines that decrease transmission to the mosquito are another area of research [6]. In most current malaria vaccine candidates, information about the pathogen is passed to the host’s immune system in form of recombinant proteins or attenuated organisms. Within this classification GMZ2 is a recombinant protein vaccine that targets the asexual blood stage of Plasmodium falciparum. It is a fusion protein of peptides of P. falciparum glutamate rich protein (GLURP27-500) and merozoite surface protein 3 (MSP3212-380) which attempts to mimic pathogen components that induce semi-immunity, a state naturally acquired after numerous malaria episodes. The pharmacophore of GMZ2 are immunoglobulins (Ig) with the same activity as “therapeutic” preparations of sera from semi-immune individuals that have been used to treat malaria patients [7]. GLURP27-500 is conserved as well as immunogenic and elicits antibodies that mediate antibody dependent cellular inhibition (ADCI) [8]. MSP3212-380 is a conserved part of the otherwise highly polymorphic MSP3 and was identified by systematic analysis of sera from semi-immune adults for Western blot and ADCI reactivity [9]. High concentration of Ig against both peptides are associated with less clinical malaria [10, 11] and a pre-clinical study of GMZ2 in splenectomised Saimiri sciureus monkeys showed partial protection against blood stage challenge and a good safety profile [12]. These results prompted a first-in-man phase I clinical trial in individuals with no previous exposure to malaria, which showed good safety and immunogenicity [13]. Here, we present the results of a second clinical trial, where safety and immunogenicity of GMZ2 in malaria exposed adults from a highly endemic area in Central Africa was tested [14]. It was a double blind phase I clinical trial with random assignment of subcutaneously administered 100 μg GMZ2 or rabies vaccine in healthy adult men from Lambaréné, Gabon. Adults from Gabon are expected to have high Ig concentrations against the vaccine antigens because of natural exposure to the pathogen. This has important consequences on the interpretation of the data from this clinical trial: i) special emphasis was laid on the occurrence of severe immunological reactions towards the vaccine antigen in those with high level of pre-existing immune responses and ii) it was explored if GMZ2 vaccination is capable to boost the existing level of immune reactivity against the vaccine antigens.
Participants and Methods
Objectives of the clinical trial
The primary objective of the clinical trial was to assess safety of GMZ2, given three times in monthly intervals at a dose of 100 μg each, in healthy, malaria exposed, and adult men compared to a registered rabies vaccine (Verorab, Sanofi Pasteur). Secondary objectives were the assessment of immune responses against the vaccine antigens by enzyme linked immunosorbent assay (ELISA) and memory B-cell enzyme linked immunospot assay (ELISPOT).
Vaccines
GMZ2 is expressed in Lactococcus lactis as a secreted recombinant protein and purified from the supernatant following good manufacturing practice (GMP) to obtain one batch for clinical use (Henogen S.A., Belgium). The lyophilized product was reconstituted in water and mixed with aluminium hydroxide immediately before subcutaneous injection. Rabies vaccine was administered according to the manufacturer’s specifications.
Participants and study design
The study took place at the Medical Research Unit of the Albert Schweitzer Hospital in Lambaréné, Gabon between July 2007 and August 2008. It was a double blinded randomized phase I clinical trial Of GMZ2 against rabies vaccine in 40 healthy men between 18 and 45 years of age (clinicaltrials.gov ID: NCT00424944). Twenty participants received 100 μg GMZ2 adjuvanted with aluminium hydroxide (Alhydrogel) subcutaneously on enrolment (Day 0) and after one (Day 28) and two (Day 56) months.
The 20 participants in the control group received rabies vaccine intramuscularly at the same time points (enrolment, Day 28 and Day 56). The list of eligible subjects was sorted by age and then treatment allocations were assigned in randomly permuted blocks of four. An additional list of eligible subjects was prepared at randomization, indicating which person should be enrolled should a participant withdraw before receiving the first dose of vaccine. A sealed copy of the randomization list was retained by the local safety monitor. Vaccines were administered alternately into the left or right deltoid muscle by a trained nurse who played no other role in the trial. Participants and clinical investigators were kept blinded to the vaccine group throughout the study. After each injection, participants were observed for 30 minutes. One, 3, and 14 days after vaccination subjects were examined by a physician at the study site. On Days 5, 8 and 11 after each vaccination, house visits were performed to record adverse events. A 24 hourly operated telephone line was maintained throughout the whole study period to ease passive reporting of unsolicited adverse reactions or events. Blood samples were taken during screening and on Days 0, 3, 28, 31, 56, 59, 84 and 365 for routine laboratory assessments. For immunological assays blood from Days 0, 28, 56, 84, and 365 was analyzed.
The clinical trial was performed according to the Declaration of Helsinki (5th revision) and International Conference on Harmonization – Good Clinical Practice (ICH-GCP) guidelines. The study received approval from the regional Ethics Committee (Comité d’Ethique Régional Indépendant de Lambaréné; CERIL) and the Gabonese ministry of health. After explaining the aims and procedures of the study, signed consent was obtained from the participant or, if the participant was unable to sign, from an impartial witness. Biochemical and haematological measurements were done according to standard procedures. Plasmodia were detected by microscopy using the Lambaréné method [15].
Immunological methods
All immunological assays were performed as described previously [13]. Since all participants are semi-immune we did not perform immunofluorescence assays on whole parasites, an assay that does not discriminate between vaccine-induced and other anti-plasmodial antibodies in this population. GMZ2-, GLURP-, and MSP3-specific Ig concentrations were measured by ELISA against GMZ2 as well as recombinant GLURP and MSP3. Optical densities were compared to a standard curve of a purified, serially diluted human polyclonal IgG (The Binding Site, UK). As negative control, a pool of malaria-naïve Europeans was used. Pooled sera from semi-immune individuals served as positive control. Anti-GMZ2 IgG subclasses were measured against purified human polyclonal IgG1-IgG4 (The Binding Site, UK). GMZ2-specific memory B-cells were quantified by memory B-cell ELISPOT as described [13, 16]. Briefly, peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation (Ficoll-Paque PLUS, GE Healthcare, USA) and cryopreserved at −150 °C in 10% DMSO, 90% fetal calf serum (FCS Gold, PAA, Germany). For ELISPOT assays, cells were thawed, seeded at 1 × 106 cells per ml, and incubated with 2.5 μg per ml CpG-2006 (TIB-MOLBIOL, Germany) and 10 ng per ml interleukin 15 (R&D Systems, USA) for 6 days to allow maturation of memory B-cells into antibody secreting cells (ASC). Following maturation, 2.5 × 105 cells were serially diluted on GMZ2-coated 96-well plates. The number of total IgG producing cells was measured by serial dilution of 2 × 104 cells on anti-human Ig-γ chain specific Ig (Sigma, Germany) coated wells. PBS-coated wells were used as negative control. Plates were read using a stereoscopic microscope by two independent and blinded investigators. Results are reported as a fraction of Ag specific ASC per total ASC.
Data analysis
Analysis of immunogenicity and safety included all vaccinated subjects. Ninety-five percent confidence intervals (95% CI) for the difference in concentration were calculated on the log scale and back transformed to give the ratio to the pre-vaccination value. Zero IgG values were treated as left-censored observations, and were replaced with a value equal to half the minimum non-zero value for that variable in the dataset. Geometric mean concentrations were compared between vaccine groups using analysis of covariance, with the pre-vaccination value as a covariate. Analyses were done using Stata version 10 (Statacorp, College Station, Texas). Ninety-five percent CI for the proportions experiencing at least one adverse event were calculated. Analysis followed an analytical plan that was written before unblinding.
Results
Clinical and biological safety
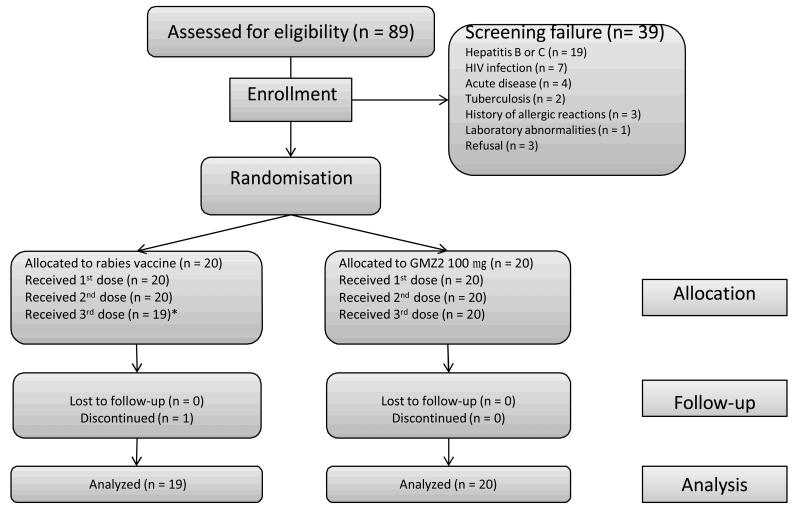
Eighty-nine individuals were screened, of those, 39 were not eligible (Figure 1). Forty individuals were randomized at a ratio of 1:1 to receive either 100 μg GMZ2 or rabies vaccine. The first participant was enrolled on the 18th of July 2007 and the last participants’ follow up ended on the 11th August 2008. Twenty participants received all three doses of GMZ2, whereas only 19 got all doses of the rabies vaccine, since one participant was discontinued because he developed clinical signs of tuberculosis after the second vaccination (see below). Baseline clinical and laboratory data were similar in both groups (Table 1). A total of 10 serious adverse events (SAE) in 7 participants were recorded; 6 SAE (hospitalisation due to trauma, tuberculosis, overdosing of self-medication, snake bite, elective surgery, hepatocellular carcinoma) from 4 subjects in the rabies vaccine and 4 SAE (hospitalisation due to trauma [3 times] and eosinophilia) in 3 individuals in the GMZ2 group. One SAE was initially considered possibly related to GMZ2: an increased eosinophil count that developed 7 days after the 3rd vaccination and led to hospitalization 44 days after the third vaccination to identify the cause of the abnormal laboratory finding. A persistent Loa loa infection was diagnosed as a likely cause of the elevated eosinophil count and treated accordingly. All other SAE were judged not to be related to the intervention. One death due to hepatocellular carcinoma occurred in the rabies vaccine group. The participant presented with cough that was diagnosed as tuberculosis 4 days before the third vaccination. Therefore no third injection was given and further clinical investigations were undertaken. A hepatocellular carcinoma was diagnosed 155 days after inclusion into the study. The participant succumbed to the disease. A total of 7 malaria episodes in 6 individuals occurred; 3 episodes in 3 participants in the rabies vaccine and 4 episodes in 3 participants in the GMZ2 group. After the third vaccination 4 participants experienced 4 malaria episodes (2 in the rabies vaccine and 2 in the GMZ2 group). No grade III solicited adverse event was reported throughout the study. Within 30 minutes after injection, grade II pain at the injection site was reported after rabies vaccination on 4 and after GMZ2 administration on 9 occasions with no increase in frequency or severity due to previous vaccination. Only one grade I immediate systemic reaction was encountered (headache). The pattern of grade I and II adverse drug reactions during active and passive follow up is shown in Table 2.
Figure 1.
Study flow of the GMZ2 phase Ib clinical trial.
Table 1.
Baseline characteristics of the study population
| GMZ2 | Rabies vaccine | |
|---|---|---|
| Age (in years) * | 26.8(18.0 – 42.6) | 25.8(18.3 – 44.2) |
| Weight (in kg) * | 65.0 (55.6 – 78.6) | 61.4(44.4 – 77.8) |
| BMI* | 22.3 (18.6 – 6.9) | 22.0 (18.4 – 28.3) |
| Hemoglobin (in g/dl)* | 13.8(11.6 – 17.9) | 14.1(10.9 – 16.7) |
| anti-GMZ2 (in mg/dl)** | 0.29 (0.05 – 2.00) | 0.22 (0.03 – 2.06) |
| anti-GLURP (in mg/dl)** | 0.10 (0.02 – 0.35) | 0.10 (0.04 – 0.37) |
| anti-MSP3 (in mg/dl)** | 0.25 (0.07 – 1.42) | 0.20 (0.07 – 1.09) |
Mean (Min - Max)
Geometric mean (Min - Max)
Table 2.
The number of subjects with any adverse reaction within 14 days of vaccination; all reactions were mild or moderate in severity (Grades 1 or 2).
| Rabies | GMZ2 | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Vacc 1 (n = 20) |
Vacc 2 (n = 20) |
Vacc 3 (n = 19) |
Vacc 1 (n = 20) |
Vacc 2 (n = 20) |
Vacc 3 (n = 20) |
|
| Local Reactions | ||||||
| Pain | 4 | 6 | 9 | 12 | 18 | 11 |
| Erythema | 0 | 2 | 5 | 2 | 1 | 2 |
| Induration | 3 | 3 | 5 | 4 | 5 | 8 |
| Edema | 0 | 2 | 5 | 2 | 1 | 2 |
| Pruritus | 2 | 2 | 5 | 1 | 2 | 2 |
| Local heat | 0 | 2 | 5 | 1 | 1 | 2 |
| Systemic reactions | ||||||
| Fever | 0 | 0 | 0 | 0 | 0 | 0 |
| Contra-lateral reaction | 0 | 2 | 5 | 1 | 1 | 2 |
| Fatigue | 4 | 4 | 5 | 4 | 3 | 4 |
| Drowsiness | 0 | 3 | 5 | 2 | 3 | 3 |
| Malaise | 1 | 4 | 9 | 1 | 2 | 2 |
| Headache | 3 | 5 | 6 | 4 | 3 | 9 |
| Joint pain | 0 | 2 | 5 | 1 | 1 | 2 |
| Myalgia | 0 | 3 | 6 | 3 | 1 | 3 |
| Loss of appetite | 0 | 4 | 6 | 4 | 1 | 4 |
| Nausea | 0 | 2 | 5 | 3 | 2 | 3 |
| Vomiting | 0 | 2 | 5 | 1 | 1 | 3 |
| Diarrhea | 0 | 4 | 7 | 2 | 2 | 5 |
| Tachycardia | 0 | 2 | 5 | 1 | 1 | 2 |
Immunogenicity
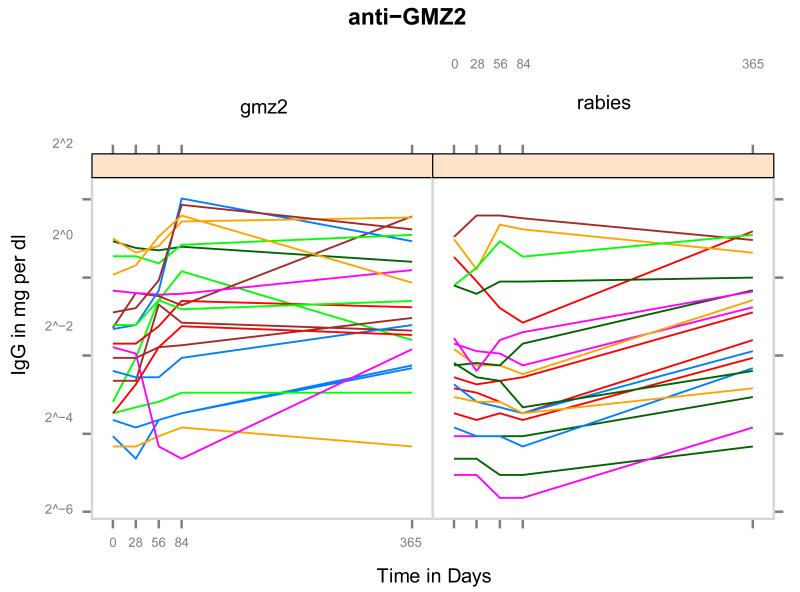
As expected for semi-immune individuals, antigen specific Ig against GMZ2 and its constituents were present before vaccination (Figure 2). At baseline, concentration of anti-GMZ2 IgG was slightly higher in the GMZ2 group than in the rabies vaccine group (Table 1). Geometric mean concentration of IgG to GMZ2 increased 1.14 (95% CI: [0.99, 1.32]) fold after two and 1.29 (95% CI: [1.06, 1.57]) fold after 3 doses whereas no such increase was observed in the rabies group. After 3 doses there was a 1.28 (95% CI: [1.11, 1.48]) fold increase in geometric mean concentration of GLURP antibodies in the GMZ2 group compared to baseline. Concentration of Ig to MSP3 did not increase significantly after vaccination in both groups.
Figure 2.
Course of anti-GMZ2 IgG concentrations from inclusion to Day 365. Note the wide range of anti-GMZ2 Ig on inclusion and the difference between Day 0 and Day 84 in the two treatment groups.
After one, two and three injections GMZ2 vaccinated individuals had significantly higher anti-GMZ2 Ig (1.09, 1.20 and 1.40 fold, respectively) compared to control vaccinees. When multiple testing was accounted for, the difference after three vaccinations remained significant. On Day 365 no significant difference between GMZ2 and rabies vaccinated individuals was present.
Vaccine specific IgG subclasses were analyzed on admission (Day 0) and one month after the full course of vaccinations (Day 84). As for total IgG, a wide scatter of baseline concentrations was present for all IgG subclasses. The boost of anti-GMZ2 and anti-GLURP specific subclass Ig attributed to GMZ2 vaccination was statistically significant for IgG1 and IgG3 but not for IgG2 and IgG4. No statistically significant increase of the different subtypes was present for anti-MSP3.
GMZ2-specific memory B-cells were detected by memory B-cell ELISPOT. On enrolment, 15 out of 39 participants had detectable GMZ2-specific memory B-cells (8 out of 20 in the GMZ2- and 7 out of 19 in the rabies-immunized group, respectively). This proportion increased to 31 out of 39 on Day 84 (18 and 13 in the GMZ2- and rabies-immunized groups, respectively). On Day 365, 26 out of 39 still had detectable GMZ2-specific spots (13 in each group). Rabies vaccinated individuals had a significantly lower baseline corrected number of spots on Day 84 (0.23 fold; 95% CI: [0.08, 0.70]). On Day 365, no significant difference to baseline or between groups was observed. The correlation between GMZ2-specific Ig concentration and memory B-cells was not significant.
Discussion
GMZ2 was developed based on the rationale to mimic naturally acquired immunity to malaria. This natural immunity is not sterile but protects from severe disease and complications. Passive transfer experiments have shown that this protection is not dependent on regional differences of parasite strains, since Ig preparations from West Africans were effective in Thai adults [7]. Clinical development of such a malaria vaccine candidate implies that efficacy is preferably tested in naturally exposed individuals since current biomarkers and challenge protocols do not represent validated surrogates of efficacy and sterile protection cannot be anticipated. The first-in-man clinical trial of GMZ2 in malaria-naive Europeans showed good safety, tolerability, and immunogenicity [13]. In that clinical trial 10, 30, and 100 μg of alum adjuvanted GMZ2 were administered in exactly the same schedule as in the current clinical trial (3 injections in monthly intervals). Since no safety concern at any of the 3 doses was present, we decided to advance in clinical development with the maximum tested dose (100 μg). To accelerate development in the target population (African children), we proceeded with the present phase I clinical trial in malaria-exposed African adults to be able to age de-escalate the exposed population subsequently. The primary objective of the clinical trial was to investigate if GMZ2 is well tolerated and safe when solid, naturally acquired immunity is present. Secondarily, it was of interest if immune responses to GMZ2 can be boosted in pre-exposed individuals. Rabies vaccine was chosen as the comparator because it is generally well tolerated and not part of the regular vaccinations in Gabon, although rabies is present in the country. We found that GMZ2 is well tolerated, immunogenic, and safe. In contrast to the first-in-man clinical trial [13] no grade three adverse events were present and local as well as systemic tolerability was unexpectedly good. The level of anti-GMZ2 Ig and memory B-cells at baseline was highly variable and vaccine-responses were difficult to deduce by inspection of raw data. After correction for baseline values a similar response-pattern as in non-exposed Europeans was present: I) GMZ2 boosted antigen-specific Ig responses against GMZ2 and GLURP (one of its constituents), II) GMZ2-induced antigen-specific IgG subclasses were of cytophilic nature and, in contrast to the previous clinical trial, included IgG3, and III) the number of GMZ2-specific memory B-cells one month after the last vaccination (Day 84) was higher in GMZ2 compared to rabies vaccinated individuals. Six individuals experienced 7 malaria episodes during the trial. The individuals were equally distributed among the two interventional groups and no distinctive pattern was observed. Since all individuals in the clinical trial have naturally acquired immunity to malaria and the vaccine is intended to induce exactly this type of immunity, it was not surprising that no difference between the groups was present. In conclusion, GMZ2 is well tolerated, safe and immunogenic in a sample of healthy, malaria-exposed men from Central Africa. These results encourage further clinical development of GMZ2. A clinical phase Ib trial in young children is ongoing and efficacy trials are planned to start in 2010.
Acknowledgments
We thank all participants and all who have been involved in the clinical trial. Special thanks go to Anne-Marie Nkoma Mouima, Judith Kammer, Ferdinand Gnansounou, Sonja Killinger, Philemon Koumba Koumba, and all field workers. We would like to acknowledge contributions of Alphonse Ouedraogo from the Swiss Tropical Institute, Brian Faragher from the Liverpool School of Tropical Medicine, and Gregory Adzoda from the Albert Schweitzer Hospital, Lambaréné, Gabon. GMP production of the GMZ2 candidate vaccine was funded by the European Malaria Vaccine Initiative (now Euopean Vaccine Initiative) through a grant received from the Directorate General for International Cooperation/Netherlands Ministry of Foreign Affairs – DIGIS. We thank Terkel Olsen, BioCare Nordic ApS for supply of the VanishPoint® syringes (Retractable Technologies, USA). The work was supported by the African Malaria Network Trust (AMANET) through a grant from the European Commissions’ Europe Aid Co-operation office, sub-Saharan Africa, Caribbean, Pacific, B-1049 Brussels, Belgium.
References
- [1].Mendis K, Rietveld A, Warsame M, Bosman A, Greenwood B, Wernsdorfer WH. From malaria control to eradication: The WHO perspective. Trop Med Int Health. 2009;7:802–809. doi: 10.1111/j.1365-3156.2009.02287.x. [DOI] [PubMed] [Google Scholar]
- [2].Targett GA, Greenwood BM. Malaria vaccines and their potential role in the elimination of malaria. Malar J. 2008;(Suppl 1):S10. doi: 10.1186/1475-2875-7-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mordmüller B, Kremsner PG. Malarial parasites vs. antimalarials: never-ending rumble in the jungle. Curr Mol Med. 2006;2:247–251. doi: 10.2174/156652406776055122. [DOI] [PubMed] [Google Scholar]
- [4].Dalton JP, Mulcahy G. Parasite vaccines - a reality? Vet Parasitol. 2001;1-3:149–167. doi: 10.1016/s0304-4017(01)00430-7. [DOI] [PubMed] [Google Scholar]
- [5].Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;9443:1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- [6].Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;7:e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, Chantavanich P, et al. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg. 1991;3:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- [8].Theisen M, Soe S, Oeuvray C, Thomas AW, Vuust J, Danielsen S, et al. The glutamate-rich protein (GLURP) of Plasmodium falciparum is a target for antibody-dependent monocyte-mediated inhibition of parasite growth in vitro. Infect Immun. 1998;1:11–17. doi: 10.1128/iai.66.1.11-17.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, et al. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in killing by cooperation with blood monocytes. Blood. 5:1594–1602. 194. [PubMed] [Google Scholar]
- [10].Soe S, Theisen M, Roussilhon C, Aye K, Druilhe P. Association between protection against Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect Immun. 2004;1:247–252. doi: 10.1128/IAI.72.1.247-252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Meraldi V, Nebié I, Tiono AB, Diallo D, Sanogo E, Theisen M, et al. Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol. 2004;6-7:265–272. doi: 10.1111/j.0141-9838.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- [12].Carvalho LJM, Alves FA, Bianco C, Oliveira SG, Zanini GM, Soe S, et al. Immunization of Saimiri sciureus monkeys with a recombinant hybrid protein derived from the Plasmodium falciparum antigen glutamate-rich protein and merozoite surface protein 3 can induce partial protection with Freund and Montanide ISA720 adjuvants. Clin Diagn Lab Immunol. 2005;2:242–248. doi: 10.1128/CDLI.12.2.242-248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Esen M, Kremsner PG, Schleucher R, Gässler M, Imoukhuede EB, Imbault N, et al. Safety and immunogenicity of GMZ2 - a MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine. 2009;49:6862–6868. doi: 10.1016/j.vaccine.2009.09.011. [DOI] [PubMed] [Google Scholar]
- [14].Wildling E, Winkler S, Kremsner PG, Brandts C, Jenne L, Wernsdorfer WH. Malaria epidemiology in the province of Moyen Ogoov, Gabon. Trop Med Parasitol. 1995;2:77–82. [PubMed] [Google Scholar]
- [15].Planche T, Krishna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, et al. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg. 2001;5:599–602. doi: 10.4269/ajtmh.2001.65.599. [DOI] [PubMed] [Google Scholar]
- [16].Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;5601:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]