Abstract
Background
Cryptococcal meningitis (CM) in Africa is associated with up to 70% mortality at 3 months and 500,000 deaths annually. We examined strategies to improve on fluconazole monotherapy: addition of flucytosine (5-FC); and/or addition of short-course amphotericin B (AmB).
Methods
In step 1, previously reported, patients were randomized to receive FLU 1200 mg/d with or without 5-FC 100 mg/kg/day for 14 days. In step 2, 43 patients were similarly randomised, with addition of AmB 1 mg/kg/d for 7 days to both arms. After 2 weeks, patients received FLU monotherapy and were followed to 10 weeks. The primary endpoint was rate of clearance of infection (early fungicidal activity, EFA). Secondary endpoints related to safety and mortality.
Results
40 patients (25% with Glasgow Coma Scale < 15) were analyzed. EFA for the triple combination arm was greater than for AmB+FLU: −0.50 ± 0.15 log CFU/day vs. −0.38 ± 0.19 log CFU/day (p = 0.03); and greater than step 1 with FLU+5-FC (−0.28 ± 0.17) or FLU alone (−0.11 ± 0.09). Combined analysis across steps revealed that addition of 5-FC and AmB had significant, independent additive effects on EFA, with trends toward fewer early deaths with addition of 5-FC (4/41 vs. 11/39, p = 0.05) and fewer deaths overall with addition of AmB (13/39 vs. 20/40, p = 0.1).
Conclusions
Addition of 5-FC and short course AmB to high-dose FLU significantly enhance EFA and may be associated with favourable trends in survival. Both these strategies should be tested in a larger phase III study.
Keywords: Cryptococcal, Meningitis, AIDS, Fluconazole, Flucytosine, Amphotericin B
Background
Cryptococcal meningitis (CM) is a common, often fatal, AIDS-related infection in areas of high HIV-prevalence. In many areas of Sub-Saharan Africa it has become the leading cause of adult meningitis (1-3) and is one of the commonest causes of death among people living with HIV.(4, 5) Despite roll-out of antiretroviral programs, high numbers of patients still progress to advanced immunocompromise,(6) at which point they are at risk of clinical cryptococcal infection.
Mortality in patients with CM in Africa has recently been estimated to be 70% at 3 months (7) and an urgent need exists to develop more effective antifungal regimens in areas of low resource and high HIV prevalence. Prior dose escalation studies in Uganda demonstrated increased rates of clearance of infection with a higher dose of FLU (1200 mg/d),(8) but the rates observed still did not approach those seen with gold standard 2 week induction therapy with AmB.(9, 10)Identifying better antifungal regimens, feasible in settings with limited medical resources and staffing, may improve outcomes. Both the combination oral therapy of FLU plus 5-FC, and a short course therapy of AmB, should be more sustainable induction treatments in resource limited settings when compared to 2 weeks AmB induction. A substantial reduction in cryptococcal organism load can be achieved with 1 week of high-dose AmB,(11) while the major dose-dependent toxicities of AmB usually become clinically significant only in the second week of therapy.(10) Therefore, limiting AmB duration to one week might achieve clinical benefit while reducing drug toxicity, monitoring requirements, duration of intravenous access, and length of hospitalization.
Early fungicidal activity (EFA), or rate of fungal clearance, is an endpoint that correlates with clinical outcome.(12) Using EFA as our primary endpoint we have studied 2 additional approaches to improve on the efficacy of high dose FLU: addition of 5-FC and addition of short-course AmB. In step 1 of this study, patients were randomised to FLU alone at 1200 mg/d or FLU 1200 mg/d plus 5-FC;(13) in step 2, a short course (7 days) of AmB was added to both of these arms. The results of step 1 have been published.(13) Herein we focus on the results of step 2 and present a combined analysis of both steps, acknowledging that the comparison between step 1 to step 2 is not randomised.
Methods
The study was conducted in Lilongwe, Malawi, at Kamuzu Central Hospital (KCH), a government-run, tertiary referral hospital serving the Central Region of Malawi, which has a population of 5.5 million people.(14) The adult prevalence of HIV in Malawi is approximately 12%.(15) The University of North Carolina Project has a research facility located on the campus of KCH, which provides clinical and laboratory support to the main hospital. The Lighthouse Trust Clinic, also co-located on the KCH campus, provides free HIV care and antiretroviral therapy (ART) to approximately 14,000 out-patients.
This study was conducted by the UNC Project in collaboration with St George’s University of London. Ethical approval was obtained from the Malawi National Health Sciences Research Committee, the University of North Carolina Review Board, and the Research Ethics Committee for St George’s University of London. The trial was registered (ISRCTN02725351) at http://www.controlled-trials.com.
Participants and procedures
We enrolled ART-naïve, HIV-1 infected adults with a first episode of cryptococcal meningitis diagnosed by cerebrospinal fluid (CSF) India Ink (later confirmed by culture or cryptococcal antigen) or CSF cryptococcal antigen. We excluded patients who were pregnant or breast-feeding, those with alanine aminotransferase > 1200 iu/L, platelets < 50,000 × 103/mL, neutrophils < 500 × 103/mL, or with any contraindication to the study medications. In step 2 a creatinine > 2.5 mg/dL was also an exclusion criterion. Written informed consent was obtained from the patient, or from the next of kin if the patient was incapable of consent. Patients were stratified according to Glasgow Coma Scale (GCS=15, or GCS<15) and then randomized to intervention groups using a random computer-generated list.
The treatment groups for step 1 were: (1) 14 days of FLU 1200mg/day (Pfizer Diflucan Partnership program), (2) 14 days of FLU 1200mg/day plus 14 days of 5-FC 100 mg/kg/day (rounded down to the nearest gram per day, given over 4 doses per day; Valeant Pharmaceuticals). In step 2 patients were randomised to either (1) 14 days of FLU 1200 mg/day plus 7 days of AmB 1 mg/kg/day (Fungizone, Bristol-Meyers Squibb) or (2) 14 days of FLU 1200mg/day plus 14 days of 5-FC 100 mg/kg/day plus 7 days of AmB (1 mg/kg/day). While receiving AmB, all patients received an extra 1 L normal saline with 20 mmol KCl per day intravenously and extra potassium supplementation as required. AmB was held if creatinine was greater than 2.5 mg/dL, and restarted if the creatinine dropped to below 2.5 mg/dL.
After 2 weeks all patients were given FLU 800 mg/d until ART was started at 4 weeks, then 400 mg/d until week 10, and 200 mg/d thereafter. FLU and 5-FC doses were adjusted for renal function. 5-FC was reduced by 50% for grade III neutropenia or thrombocytopenia and discontinued for grade IV neutropenia or thrombocytopenia. After the first 2 weeks FLU doses were increased 50% for patients on concomitant rifampicin.
After 4 weeks of antifungal therapy, patients attended the Lighthouse Clinic for initiation of antiretrovirals (stavudine, lamivudine, and nevirapine) according to Malawi national guidelines.
Evaluation and outcomes
Lumbar punctures were performed on days 1, 3, 7, and 14 to obtain CSF for quantitative culture and to document CSF opening pressure. Additional therapeutic LPs, with additional quantitative culture, were done on patients with previously demonstrated or clinically suspected raised CSF pressure (>30 cm H2O) in accordance with guidelines.
For quantitative cultures, CSF was plated in serial 10-fold dilution, as previously described.(16) The dilution with the least colonies, but at least 30 colony-forming units (CFU) per plate, was used to calculate CFU per millilitre of CSF. Laboratory personnel calculating quantitative cryptococcal culture results were masked to the treatment group. The decrease in log CFU/ml CSF/d was calculated using the slope of the linear regression of log CFU counts against time for each patient.(16) All data points were analyzed except sterile cultures in the second week if these values lessened the slope, because sterility would have been achieved before that day’s lumbar puncture and this value would therefore underestimate the true slope.
Laboratory tests were processed in the University of North Carolina Project laboratory (which, with its multiple network studies, undergoes regular quality assurance through international regulatory bodies). Testing included blood count with differential, aspartate aminotransferase, alanine aminotransferase, potassium, and creatinine at baseline and then three times per week in the first 2 weeks. CD4+ cells and HIV load were determined at baseline. Aspartate aminotransferase and alanine aminotransferase measurements were performed again at weeks 4, 6, and 10 after study enrolment. Clinical and laboratory adverse events were graded using the toxicity table from the Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases, National Institutes of Health.(17) Percentage changes in laboratory values were calculated using the following formula: (final value-baseline value/ baseline value) × 100.
The primary outcome measure was the mean rate of decrease in CSF cryptococcal counts or early fungicidal activity (EFA) for each treatment arm. Secondary outcome measures were serious adverse events, laboratory toxicities, and mortality at 2 and 10 weeks.
Statistical analysis
We compared baseline characteristics of the treatment groups using Fisher exact or chi-squared tests for categorical variables and the Mann-Whitney U test or Student’s t-test, as appropriate, for continuous variables. Linear regression was used to compare early fungicidal activity by treatment group, adjusting where indicated for other variables, giving summary differences with 95% confidence intervals (CIs) and significance levels. Mortality was examined by Fishers exact (with cells of frequency ≤5) and chi squared tests and Cox regression.
It was initially planned to include 40 patients per arm in step 1, giving >90% power to detect an increase in early fungicidal activity with addition of 5-FC to high dose fluconazole.(13) However, at the pre-planned analysis after 40 patients enrolled, the Data Monitoring Committee (DMC) recommended termination as there was already a statistically significant difference in EFA, the study’s primary outcome, favouring the combination regimen. Therefore, the study was modified and completed as step 2, with addition of short course AmB to both arms, yielding two closely related clinical trials. It was assumed that AmB would lead to an increase in EFA at least as great as with 5-FC, and that there would be no significant interaction between AmB and 5-FC and therefore power to detect independent effects of addition of 5-FC and AmB would be maintained.
Results
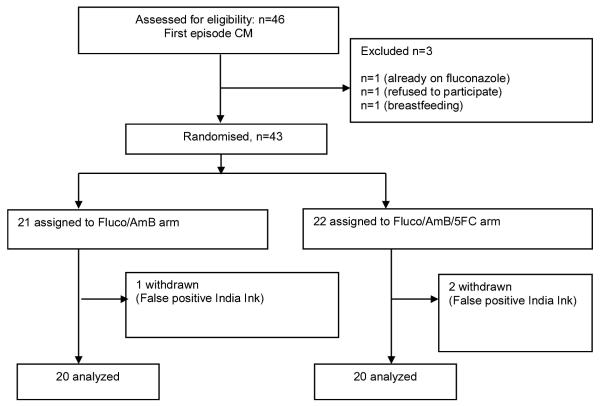
Forty-three patients were enrolled in step 2 between May 2009 and March 2010 (Figure 1). Three patients were withdrawn due to initial false-positive India Ink tests that could not be later confirmed by culture or CSF cryptococcal antigen. Baseline characteristics were similar between the arms (Table 1): there were no significant differences between the arms treated with and without 5-FC in step 2, or when combining step 1 and step 2. When considering patients enrolled in step 2 compared to step 1, there were no significant differences, apart from both CD4+ cell count and HIV viral load being lower in step 1 compared to step 2. One patient in the triple combination arm, who relocated to her home district, was lost to follow up at 4 weeks.
Figure 1.
Trial Profile.
Fluco = fluconazole; AmB = amphotericin B; 5-FC = flucytosine
PHASE 1 – before amphotericin amendment
Table 1.
Baseline laboratory characteristics.
| Baseline Characteristics | All patients in step 1a n = 41 |
All patients in step 2 n = 40 |
p value (Step 1 vs. Step 2) |
FLU + AmB (Step 2) n = 20 |
FLU + 5-FC + AmB (Step 2) n = 20 |
p value (Arms in Step 2) |
|---|---|---|---|---|---|---|
| Clinical data | ||||||
| No. (%) of male patients | 27 (66) | 26 (65) | 0.94 | 13 (65) | 13 (65) | 1 |
| Age, years | 36 (23 – 73) | 35 (19 – 52) | 0.2 | 35 (23 – 52) | 34 (19 – 50) | 0.17 |
| Weight, mean kg +/− SD | 54 +/− 11 | 52 +/− 7 | 0.32 | 50 +/− 7 | 54 +/− 7 | 0.2 |
| No. (%) of patients with a GCS score <15 |
17 (41) | 10 (25) | 0.12 | 6 (30) | 4 (20) | 0.72 |
| No. (%) of patients taking tuberculosis medication |
10 (24) | 6 (15) | 0.41 | 4 (20) | 2 (10) | 0.66 |
| Laboratory data | ||||||
| CD4+ cell count, ×109 cells/L | 21 (1 – 101) | 41 (2 – 258) | 0.02 | 47 (3 – 189) | 38 (2 – 258) | 0.93 |
| HIV load | 98752 (2258 – 1606740) |
265897 (6873 – 750000) |
0.01 | 259180 (46347 – 750000) |
336435 (6873 – 750000) |
0.82 |
| CSF data | ||||||
| Opening pressure, cm H2O | 34 (5 – 100) | 32 (3 – 80) | 0.53 | 37 (3 – 80) | 22 (4 – 68) | 0.65 |
| White blood cell count, cells/mL |
11 (0 – 1307) | 47 (0 – 1575) | 0.17 | 43 (0 – 1575) | 49 (0 – 640) | 1 |
| QCC, CFU/mL | 185000 (265 – 30950000) |
105250 (0 – 2845000) |
0.09 | 136250 (1100 – 2845000) |
48250 (0 – 1320000) | 0.48 |
Values are given in median (range) unless stated. 5-FC, flycytosine; AmB, amphotericin B; CFU, colony forming unit; GCS, Glasgow coma scale.
Data from [13].
Early fungicidal activity
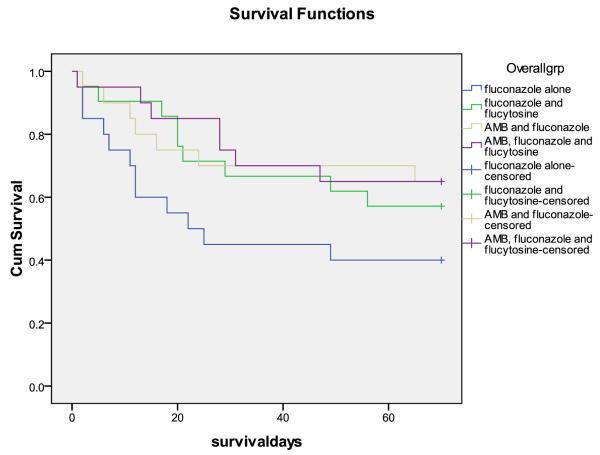
In step 2, the rate of clearance of infection was more rapid in the triple combination arm compared with AmB plus FLU: −0.50 +/− 0.15 log CFU/ml/d vs. −0.38 +/− 0.19 CFU/ml/d, p=0.03) (Figure 2). Fifteen patients in the triple combination arm had sterile CSF at 2 weeks, compared to 8 in the AmB plus FLU arm (p = 0.05, fishers exact). In step 2, none of the other variables examined affected rate of clearance of infection (baseline CFU count, altered mental status at presentation, CSF opening pressure, tuberculosis treatment, gender, age, weight, CD4 cell count, viral load).
Figure 2.
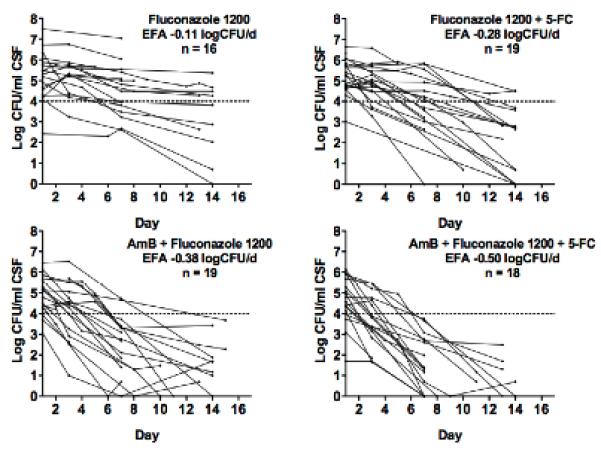
Decrease in Cryptococcus neoformans CFU over time, by treatment group. Dotted lines represent a threshold of 1 × 104 CFU/mL to aid visual comparison. Each line represents results from a single patient. In combined analysis, the mean rate of decrease of log CFU, or early fungicidal activity (EFA), was significantly more rapid for patients receiving vs. not receiving 5-FC, and for patients receiving vs. not receiving short course AmB (p < 0.001, both comparisons).
We next analysed data from steps 1 and 2 together, in order to examine the separate and combined effects of adding 5-FC and/or short course AmB to high dose fluconazole. In univariate analyses, 5-FC and short course AmB were each associated with a very significant increase in the rate of clearance of infection: For 5-FC, the difference was 0.14 log CFU/day (95%CI 0.04-0.23; p = 0.004); for short course AmB the difference was 0.24 log CFU/day (95%CI 0.16-0.32; p < 0.001). In multivariate analysis including both 5-FC and short course AmB, these estimates of effect were hardly altered suggesting independent additive effects of the two agents: For 5-FC, the difference was 0.15 log CFU/day (95%CI 0.08-0.22; p <0.001); for short course AmB the difference was 0.25 log CFU/day (95%CI 0.17-0.32; p < 0.001). In the combined data set, only CD4+ cell count was also associated with rate of clearance of infection (the difference for each increment in CD4+ cell count quartile 0.05 log CFU/day, 95%CI 0.01-0.09; p = 0.02). Adjusting for CD4 cell count made no difference to the estimates of the independent effects of AmB and 5-FC.
Mortality
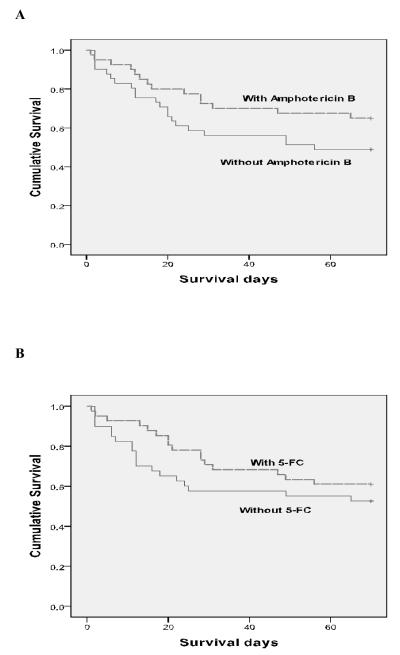
The study was not powered for mortality differences, and there were no statistically significant differences in survival at 2 and 10 weeks between the arms. However, combining steps 1 and 2, consistent with the EFA results, there were fewer early deaths with addition of 5-FC (4/41 vs. 11/39, p = 0.05, and 15/40 vs. 18/39, p = 0.4, at 2 and 10 weeks respectively; hazard ratio (HR) at 2 weeks 0.33 95%CI 0.10-1.03, p = 0.05) and fewer total deaths in step 2, with addition of short course AmB, compared with step 1 (6/40 vs. 9/40, p = 0.4, and 13/39 vs. 20/40, p = 0.1, at 2 and 10 weeks respectively; HR at 10 weeks 0.58 95%CI 0.29-1.17, p = 0.1), although these differences did not reach statistical significance. In step 2, there were fewer presumed cryptococcal meningitis-related deaths in the triple combination arm compared to the AmB/fluconazole arm (1 vs. 6, p = 0.09). Combining steps 1 and 2, there were also fewer presumed cryptococcal meningitis-related deaths with vs. without 5-FC (5 vs. 13, p = 0.03). Kaplan-Meier survival curves for step 1 vs. step 2 (effect of short course AmB) and for addition of 5-FC (both steps) are shown in figure 3.
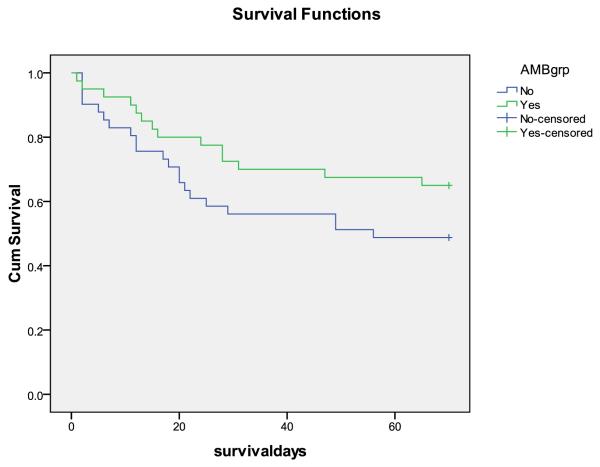
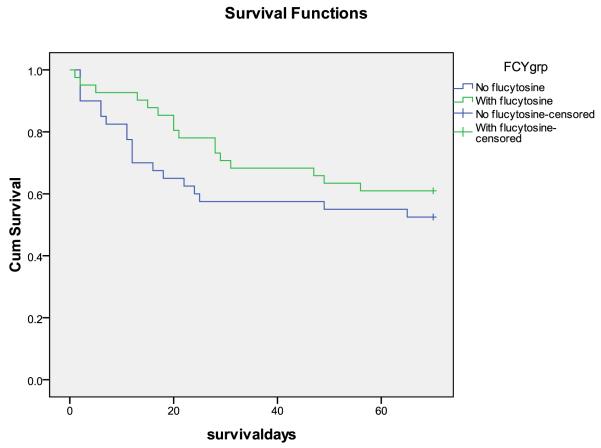
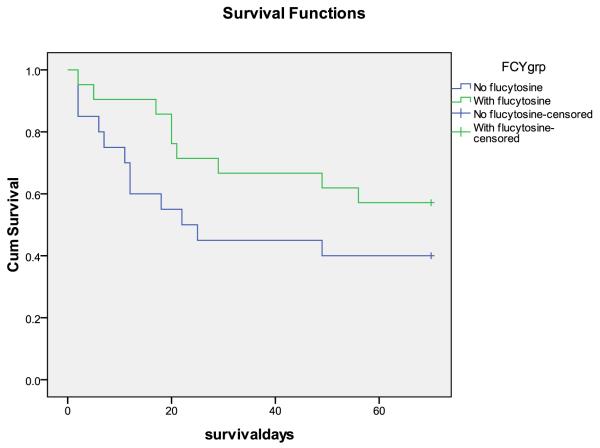
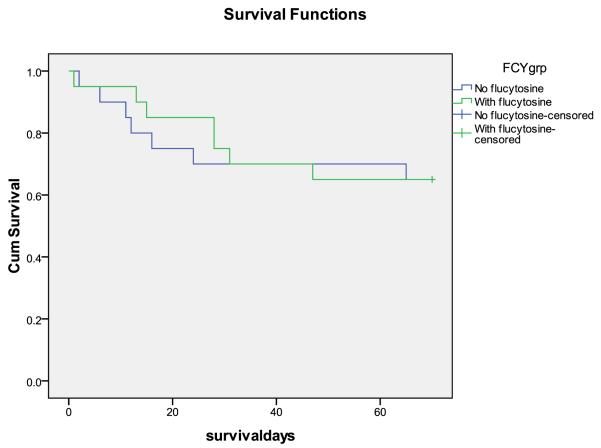
Figure 3.
Survival curves, A. Step 1 vs. step 2 (effect of short course AmB). B. With and without 5FC (steps combined). There were trends towards fewer deaths at 10 weeks with AmB (HR 0.58 95%CI 0.29-1.17, p = 0.1), and fewer early deaths with 5FC (HR 0.33 95%CI 0.10-1.03, p = 0.05)
Kaplan Meier by study arm
Effect of AmB (step 1 compared to step 2)
Effect of flucytosine on survival – across all steps
Effect of flucytosine in step 1 only
Effect of flucytosine in step 2 only
Safety
Safety for step 1 has previously been reported.(13) Safety for step 2 is discussed here. Laboratory adverse events in the first 2 weeks are shown in table 2. There were 2 instances of grade IV neutropenia in patients on 5-FC. One of these occurred on Day 14, so the 14 days of 5-FC therapy had already been given. This patient had a grade II neutropenia at enrolment (837/mm3), and had recovered to grade I neutropenia (1120/mm3) by day 37. The other occurred on Day 13, so doses for day 14 were held. Counts for this patient recovered to grade II neutropenia (750/mm3 – 999/mm3) by the next blood draw. In an additional 5 patients with grade III neutropenia, the 5-FC dose was reduced, as per protocol. There was no increase in infection-related adverse events among patients receiving 5-FC compared to those receiving AmB plus fluconazole alone (9 vs. 10 events, with and without 5-FC).
Table 2.
Laboratory adverse events in the first 2 weeks.
| Adverse event | Fluconazole + AmB | 5FC + Fluconazole + AmB | ||
|---|---|---|---|---|
| Grade III | Grade IV | Grade III | Grade IV | |
| Thrombocytopenia | 1 | |||
| Neutropenia | 4 | 3 | 2 | |
| Anemia | 5 | 2 | 4 | |
| Hypokalemia | 2 | 1 | 2 | |
| Hyperkalemia | 1 | |||
| Hyponatremia | 1 | 1 | ||
| Renal Impairment | 5 | 4 | ||
| Transaminitis | 1 | 1 | ||
Cut-offs used to define grade III and grade IV laboratory abnormalities respectively were as follows: Thrombocytopenia: Platelet count: 25,000–49,999 platelets/mm3 and < 25,000 platelets/mm3; Neutropenia: Absolute neutrophil count, 500–749 cells/mm3 and < 500 cells/mm3; Anemia: Hemoglobin concentration: 6.5–7.4 g/dL and < 6.5 g/dL; Transaminitis: Alanine aminotransferase level, 175–350 IU/L and >350 IU/L; Aspartate aminotransferase level, 190–380 IU/L and > 380 IU/L; Hyponatremia: Sodium level: 121–124 mmol/L and < 120mmol/L; Renal impairment: Creatinine level: 2.2–4.1 mg/dL and > 4.1 mg/dL for men and 2.3-4.4 mg/dL and > 4.4 mg/dL for women.
As expected with AmB, there were more instances of anemia and renal impairment in step 2 compared to step 1,(13) but few grade IV events, and only one patient missed more than one of the planned 7 doses of AmB (in this instance due to raised creatinine). In step 1 the mean week 1 percentage fall in hemoglobin was 7% compared to 16% in step 2 (p < 0.001).
In step 1 there was a negligible percent rise in creatinine after 1 and 2 weeks, 1% and 2%, respectively. By comparison, in step 2, when AmB was used, there were significantly greater percentage increases in creatinine, 58% after 1 week and 25% after 2 weeks (both p < 0.05). There was only one instance of grade IV hypokalemia, which occurred in a patient in the dual therapy arm on day 11 (4 days after completing AmB) and was partly attributed to persistent vomiting. Levels responded to fluid and potassium replacement.
FLU 1200 mg/d was well tolerated. There were 2 instances of grade III hepatic transaminase elevation during the first 2 weeks in step 2 (with none in step 1), both of which occurred at times of concurrent bacterial infection and resolved without interruption of FLU. One additional patient developed grade IV hepatic transaminitis on day 18 (FLU dose 800 mg/d) at the time of concurrent pneumonia. Fluconazole was held temporarily, and upon restarting there was no further liver test abnormality leading us to consider this unlikely related to FLU. No significant rises in transaminases were seen when ART with nevirapine was started at 4 weeks.
Two patients had a syndrome compatible with cryptococcal immune reconstitution inflammatory syndrome, both in the triple therapy arm. Both of these patients improved with therapeutic LPs and survived to the end of follow-up.
Discussion
Addition of either 5-FC or short course AmB to high dose FLU treatment for CM led to statistically significant and substantial increases in the rate of clearance of infection from the CSF. The magnitudes of the increases are such that would be expected to translate into clinical benefit, (12) a conclusion supported by favourable trends, although not statistically significant, in 2 and 10 week survival,. Furthermore, in the longer term in resource limited settings, both of these interventions could be more sustainable than 2-week AmB induction, given that the rapid and reliable laboratory monitoring and extra costs required to give AmB safely for 2 weeks are often not available.
Addition of short course AmB to FLU had a larger impact on rate of clearance of infection over the first 2 weeks than did addition of 5-FC, with the caveat that this comparison, between steps 1 and 2, was not based on randomized groups, but sequential groups in the same study setting. The rates of clearance seen with 7 days of AmB were similar to those previously measured with full 2 weeks courses (10, 16, 18). As previously observed in Cape Town and in Uganda, (11, 19) there was no detectable reduction in clearance rate in the second week, after AmB had been discontinued, which is consistent with fact that AmB has a relatively long half-life, particularly in the brain and meningeal tissues. Renal impairment and anemia appeared less severe than in prior studies with 2 week courses of AmB at 1 mg/kg/d, (10) and with regular potassium supplementation severe hypokalemia was rare. AmB side effects were more frequent than in an observational cohort from Uganda where a shorter course of 5 days of AmB combined with fluconazole 1200mg/day for 2 weeks was also associated with a marked increase in fungicidal activity (to −0.30 ± 0.11 log CFU/day) compared with historical controls from that site treated with fluconazole monotherapy (19). While some AmB induction is likely better than none, the maximum duration that can administered safely in the absence of reliable laboratory monitoring may be less than one week. AmB had not previously been used as part of routine induction therapy in Malawi, and thus step 2 provided invaluable experience with its use in one of the country’s principal referral centers. At Kamuzu Central Hospital, with monitoring support through the UNC Project, one week AmB plus FLU was adopted as the standard treatment for CM.
There were 2 instances of grade IV neutropenia in patients on 5-FC in step 2. Overall, in studies by our group, including both steps of this study, 5-FC at this dosage and duration has been associated with grade IV neutropenia in 8/183 (4.4%) of patients (13, 16, 18, 20, 21). In Thailand, the bioavailability of oral 5-FC in patients with CM was only around 50%, and the levels of 5-FC were well below those usually associated with toxicity. 5-fluorouracil was infrequently detected in serum.(20) However, given the probable importance of the gut flora in conversion of 5-FC to 5-fluorouracil, the pharmacokinetics of 5-FC and 5-fluorouaracil may be different across geographically and ethnically diverse populations. Planned analyses of 5-FC and 5-fluorouracil levels in serum and CSF from our patients will help determine whether the pharmacokinetics of 5-FC in HIV patients are different in Africa compared to Asia and will help determine whether the neutropenia observed in this study was causally related to 5-FC.
Finally, although this was not a true factorial study design, the combined analysis of steps 1 and 2 suggests that, in the context of high dose FLU, 5-FC and AmB have largely independent, additive effects on the rate of clearance of infection. Unlike a 2004 study in Thailand in which FLU was dosed at 400 mg/d,(16) the triple combination of these agents at our protocol doses gave the most rapid clearance of all the regimens. Data since 2004 have convincingly shown that higher induction doses (1200 mg/d) of FLU are more effective and appear safe.(8, 13, 22, 23) The nature of the interaction between agents in combination may be different at different dosages of the individual components. Our results are consistent with the mouse model data in which optimal responses were seen when high doses of AmB and FLU were combined with a moderate dose of 5-FC.(24)
While both of our experimental strategies improve EFA compared to FLU alone, the implementation of the oral only combination of FLU/5-FC may have broader application across rural health centers. Although previously registered and marketed in South Africa, 5-FC is not generally available in Sub Saharan Africa. However, it is a simple molecule and could be produced at low cost by generic manufacturers. The addition of Amphotericin based therapy, with reduced duration, may also be feasible at rural centers based on the safety profile we observed, though we acknowledge our research setting is well resourced compared to rural health centers.
In summary, the results from this study lend strong support to a planned phase III trial in resource limited centres comparing both the optimal oral combination of high dose FLU plus 5-FC and short course AmB with standard 2 week induction therapy with AmB. In addition, further studies are warranted with triple therapy, and efforts should continue to increase the availability of 5-FC in Africa and Asia, where the burden of cryptococcal disease remains unacceptably high.
Acknowledgements
Funding was provided through grants from the Medical Research Council (G0501476) and from the UK government through the Department For International Development in Malawi. Dr. van der Horst and Dr. Hosseinipour were supported by the UNC Center for AIDS Research NIAID P30-AI50410. Dan Namarika is a previous NIH Fogarty AITRP Trainee (FIC 2-D43 TW01039-06).
Footnotes
Potential conflicts of interest: All authors: no conflicts
References
- 1.Scarborough M, Gordon SB, Whitty CJ, French N, Njalale Y, Chitani A, et al. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N Engl J Med. 2007 Dec 13;357(24):2441–50. doi: 10.1056/NEJMoa065711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000 Jul 7;14(10):1401–7. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- 3.Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67. doi: 10.1186/1471-2334-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okongo M, Morgan D, Mayanja B, Ross A, Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 (HIV-1) natural history cohort in Uganda. Int J Epidemiol. 1998 Aug;27(4):698–702. doi: 10.1093/ije/27.4.698. [DOI] [PubMed] [Google Scholar]
- 5.French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002 May 3;16(7):1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis JN, Boulle A, Loyse A, Bicanic T, Rebe K, Williams A, et al. High ongoing burden of cryptococcal disease in Africa despite antiretroviral roll out. AIDS. 2009 Jun 1;23(9):1182–3. doi: 10.1097/QAD.0b013e32832be0fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009 Feb 20;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 8.Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. 2008;47(12):1556–61. doi: 10.1086/593194. [DOI] [PubMed] [Google Scholar]
- 9.van der Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. N Engl J Med. 1997 Jul 3;337(1):15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 10.Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis. 2008 Jul 1;47(1):123–30. doi: 10.1086/588792. [DOI] [PubMed] [Google Scholar]
- 11.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007 Jul 1;45(1):76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 12.Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. 2009 Sep 1;49(5):702–9. doi: 10.1086/604716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clin Infect Dis. 2010 Feb 1;50(3):338–44. doi: 10.1086/649861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Population and Housing Census. National Statistics Office; Malawi: 2008. [Google Scholar]
- 15.WHO . Epidemiological Fact Sheet on HIV and AIDS. Core data on epidemiology and response. Malawi: 2008. [Google Scholar]
- 16.Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004 May 29;363(9423):1764–7. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 17.Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases, National Institutes of Health [Accessed 2 July 2011];Tables for grading severity of adult and pediatric adverse events. Available at http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/Safety.htm.
- 18.Loyse A, Wilson D, Meintjes G, Jarvis J, Bicanic T, Bishop L, et al. Comparison of the early fungicidal activity of high dose fluconazole, voriconazole, and flucytosine, as second drugs given in combination with amphotericin B, for the treatment of HIV-associated cryptococcal meningitis. Poster-131; Conference on Retroviruses and Opportunistic Infections; Boston, USA. February, 2011; 2011. [DOI] [PubMed] [Google Scholar]
- 19.Muzoora C, Kabanda T, Ortu G, Ssentamu J, Hearn P, Mwesigye J, et al., editors. Short Course Amphotericin B with High Dose Fluconazole for HIV-Associated Cryptococcal Meningitis. Poster-120; Conference on Retroviruses and Opportunistic Infections; Boston, USA. 2011 February; 2011. [DOI] [PubMed] [Google Scholar]
- 20.Brouwer AE, van Kan HJ, Johnson E, Rajanuwong A, Teparrukkul P, Wuthiekanun V, et al. Oral versus intravenous flucytosine in patients with human immunodeficiency virus-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2007 Mar;51(3):1038–42. doi: 10.1128/AAC.01188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarvis J, Meintjes G, Rebe K, Williams N, Bicanic T, Williams A, et al. Adjunctive IFN-gamma Immunotherapy for the Treatment of HIV-Associated Cryptococcal Meningitis: A Randomized Controlled Trial; Conference on Retroviruses and Opportunistic Infections; Boston, USA. February, 2011; 2011. [Google Scholar]
- 22.Milefchik E, Leal MA, Haubrich R, Bozzette SA, Tilles JG, Leedom JM, et al. Fluconazole alone or combined with flucytosine for the treatment of AIDS-associated cryptococcal meningitis. Med Mycol. 2008 Jun;46(4):393–5. doi: 10.1080/13693780701851695. [DOI] [PubMed] [Google Scholar]
- 23.Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T, et al. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis. 2009 Jun 15;48(12):1775–83. doi: 10.1086/599112. [DOI] [PubMed] [Google Scholar]
- 24.Diamond DM, Bauer M, Daniel BE, Leal MA, Johnson D, Williams BK, et al. Amphotericin B colloidal dispersion combined with flucytosine with or without fluconazole for treatment of murine cryptococcal meningitis. Antimicrob Agents Chemother. 1998 Mar;42(3):528–33. doi: 10.1128/aac.42.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]