Abstract
Genetic factors determine the asymmetrical position of vertebrate embryos allowing asymmetric environmental stimulation to shape cerebral lateralization. In birds, late-light stimulation, just before hatching, on the right optic nerve triggers anatomical and functional cerebral asymmetries. However, some brain asymmetries develop in absence of embryonic light stimulation. Furthermore, early-light action affects lateralization in the transparent zebrafish embryos before their visual system is functional. Here we investigated whether another pathway intervenes in establishing brain specialization. We exposed chicks' embryos to light before their visual system was formed. We observed that such early stimulation modulates cerebral lateralization in a comparable vein of late-light stimulation on active retinal cells. Our results show that, in a higher vertebrate brain, a second route, likely affecting the genetic expression of photosensitive regions, acts before the development of a functional visual system. More than one sensitive period seems thus available to light stimulation to trigger brain lateralization.
Asymmetry along the left-right axis is a feature common to all vertebrates. Heart and liver are placed to the left and right side respectively1,2,3,4 and even paired-symmetric organs display some degree of asymmetry (e.g. lungs differ in the number of lobes). The brain exhibits profound anatomical and functional asymmetries (review5). How anatomical asymmetry is imposed on a seemingly bilaterally symmetric structure, the vertebrate neural tube, is however still largely obscure. Selective expression of the transforming growth factor (TGF) family member Nodal, a signal transduction pathway, on the left side of the early embryo seems to mediate the asymmetrical morphogenesis and placement of the internal organs through activation of a signaling cascade6,7. Whilst such a Nodal cascade controls ventral forebrain development, its effect on lateralization is on epiphyseal gene expression in the dorsal forebrain (e.g. in zebrafish, the asymmetry of the diencephalic habenular nuclei and the photoreceptive pineal complex8,9).
Lateralization mediated by Nodal cascade seems to operate in the brain, triggered by asymmetric sensory stimulation in embryo. The processes underlying the asymmetric morphology and positioning of the viscera are accompanied by a slight torsion of the embryo whose forehead points to the right10. Such a rightward spinal torsion seems to occur in all amniotes11, including human embryos, which also display a right-turn of their head12. Asymmetric turning associated with Nodal signals may set the stage for either direct asymmetrical sensory stimulation of the embryo (because of its placement in utero or in ovo13,14) or by constrained motor patterns that in turn may promote asymmetrical stimulation (for instance, a slight preference to move the right arm because of turning of the embryo can then be enhanced by an increased eye-hand contact on the right side15).
This has been investigated in detail in the avian brain. During incubation, the birds' embryos bend so that the head is asymmetrically tilted with the right eye placed below the egg surface and the left eye leant below the wing16. In this position, environmental light penetrating the eggshell acts on the retina of the right eye only, producing structural asymmetries on the ascending visual projections and thus modulating functional cerebral specialization14,17,18,19. Environmental illumination during latest stages of embryonic development is crucial in the determination of brain asymmetries through the selective action on the fully-formed visual receptors20: domestic chicks hatched from dark incubated eggs lack any asymmetry in the visual pathways21,22. Furthermore, swapping the exposed eye by withdrawing the embryo's head from the egg (i.e., making the left rather than the right eye to receive illumination) reverses the pattern of asymmetry in both chicks23,21 and pigeons24,25. Environmental illumination affects also functions of the left (unstimulated) eye and associated contralateral brain structures (as shown in attack, copulation and detection of predator23 and visuo-spatial abilities26) by modifying inter-hemispheric cross-talk27,28.
Despite light being such a strong environmental trigging factor, some asymmetries in birds are unaffected by embryonic light exposure. For instance, uni-hemispheric sleep patterns29, lateralized mechanisms of social recognition30, and the neural mechanisms underlying imprinting31,32 are apparent also in dark-incubated birds. Besides, work on zebrafish has shown only partial correspondence between the reversal of the visceral situs and diencephalic asymmetries and the reversal of lateralized behaviours33. Similarly, in rare cases of spontaneous situs inversion in humans only some of the brain and behavioural lateralities change the direction of their asymmetry (e.g. language dominance continued to be a feature of the left hemisphere, i.e., not reversed34,35). All this is suggestive of multiple genetic/environmental routes to brain lateralization.
In zebrafish environmental illumination applied early in development (at day one after fertilization) is needed to generate left/right cerebral asymmetries. After light stimulation, the left eye shows more interest in motivating stimuli36,37,38. At this early stage of development, light reaching the embryos is not acting over photoreceptive cells in the retina, because these have not yet differentiated39. Rather, since larval zebrafish are transparent, light may influence the genetic expression of undifferentiated cells of photosensitive regions40.
The evidence discussed insofar is suggestive of at least two different developmental pathways for determination of laterality in the vertebrate brain. The first pathway may involve genes of the Nodal cascade which determine a rightward torsion of the embryo that allows asymmetric light stimulation to trigger anatomical and functional asymmetries. A second pathway, never demonstrated in higher vertebrates (birds and mammals), may act directly by light stimulating the embryo at an age before the development of a functional visual system. The effects of such a second pathway, if proved, may explain why some forms of laterality seem to be unaffected by embryonic late stimulation.
Here we show for the first time that early environmental illumination provided to chicks' embryo at an age in which light cannot exert any effect on fully-formed retinal receptors which seem not to be in place41 may nonetheless cause cerebral lateralization, likely involving the second developmental pathway, i.e., by affecting the genetic expression of undifferentiated cells of photosensitive regions.
Results
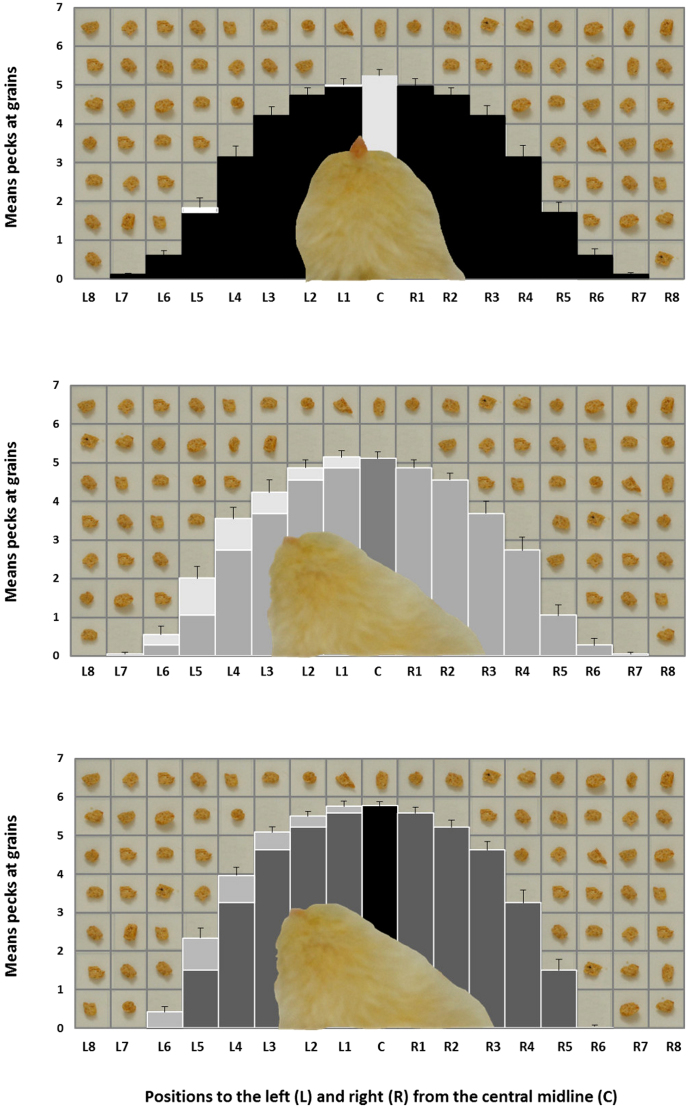
In Experiment 1, chicks incubated in darkness (Di-chicks), exposed to light during the first 3 days after fertilization (EarlyLi-chicks) and the last 3 days before hatching (LateLi-chicks) were free to peck at food grains, scattered in an array of identical vertical sectors: a central one, 8 left and 8 right sectors (Figure 1). The total amount of pecks in each sector over a 3 minutes period was scored for each chick. In a repeated measures ANOVA, Hatch (Di-, EarlyLi- and LateLi-chicks) Side (Left and Right) and Distance (1 to 8 sectors) were analyzed as factors. The ANOVA showed a significant effect of Hatch (F(2,85) = 4.046, P = 0.021) and a significant effect of Distance (F(7,595) = 1093.597, P < 0.001) with decreased pecking with increasing distance from the center; there was also a significant main effect of Side (F(1,85) = 10.657, P = 0.002). The interaction between Side and Hatch was significant (F(2,85) = 4.841, P = 0.010) with Di-chicks choosing equally for the left and the right side of the grid (Di- toward left: 2.353 ± 0.128; toward right: 2.427 ± 0.156, t(28) = 0.533, P = 0.598, Paired Samples t-Test) and both EarlyLi- and LateLi-chicks preferring significantly the left side (respectively, EarlyLi-: toward left: 2.531 ± 0.133; toward right: 2.165 ± 0.133, t(27) = −3.731, P = 0.001; LateLi-: toward left: 2.907 ± 0.067; toward right: 2.573 ± 0.100, t(30) = −3.599, P = 0.001) as shown in Figure 2. The interaction between Distance and Hatch was significant (F(14,595) = 3.322, P < 0.001), as well as the interaction between Side and Distance (F(7,595) = 4.173, P < 0.001).
Figure 1. The chick's head and neck protruding from the window of the confining box and oriented toward its left during the activity of pecking at the cancellation grid.
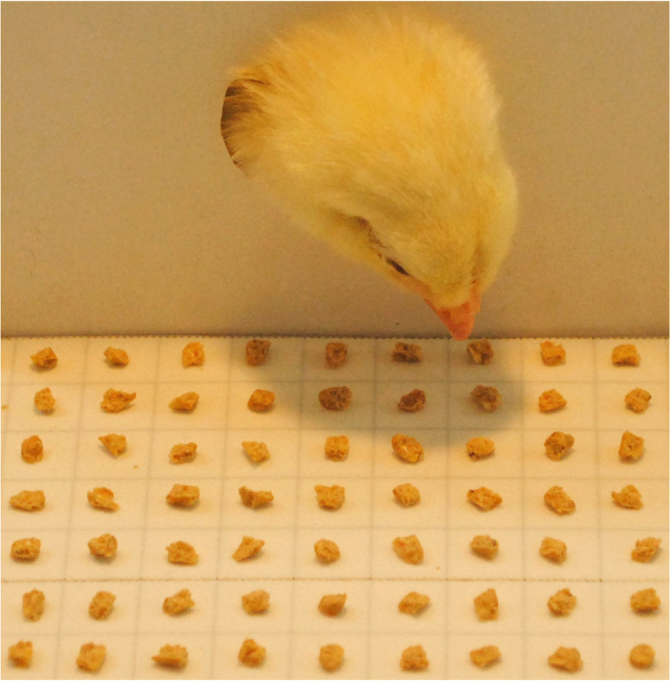
Single grains of food are homogeneously disposed every cm on a double sided sticky tape.
Figure 2. Means (+ s.e.m.) pecks at grains made by Di-chicks (top), EarlyLi-chicks (middle) and LateLi-chicks (bottom) at the cancellation grid toward left (L) and right (R) from the central midline (C) in 3 minutes of activity.
Lighter parts indicate the greater amount of pecks toward left. Single grains of food and the chick's head viewed from above are superimposed in post-editing to the graph for representational purposes only.
In Experiment 2, Di-, EarlyLi- and LateLi-chicks were left free to run from a starting box to a feeder located on the opposite ends of a long runway (Figure 3(a)). The routes covered by the chicks were scored with a video analysis software (Videopoint®) and then analyzed with Matlab® to determine the direction (left vs. right) of each route.
Figure 3. A chick arriving at the target feeder located below a conspicuous landmark (a), three examples of routes (in red) from the starting box to the target as analyzed to establish the route direction (b).
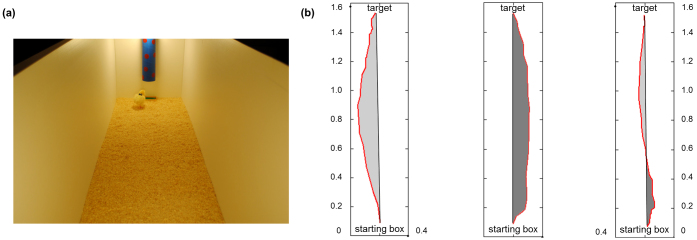
The black line connecting the ends of the route represents the optimal route. Length and width of the apparatus are expressed in metre.
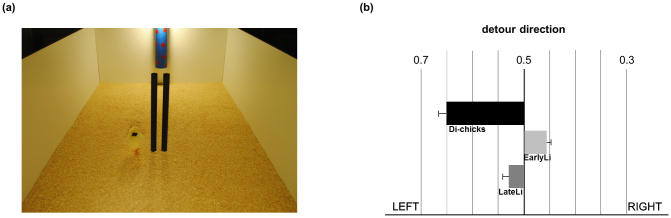
We estimated the area (expressed in m2) between the real route covered by the chick and the optimal route both for leftward and rightward trajectories as visible in Figure 3(b). A repeated measures ANOVA with Hatch (Di-, EarlyLi- and LateLi-chicks) and Route Direction (Left vs. Right) as factors showed no difference across hatching conditions (F(2,58) = 1.115, P = 0.335). By contrast, the main factor Route Direction was significant (F(1,58) = 153.913, P < 0.001) with all animals running more to the left than to the right (respectively, 0.049 m2 ± 0.006 vs. 0.016 m2 ± 0.004; t(60) = 12.514, P < 0.001, Paired Samples t-Test). When an obstacle was inserted in the center of the runway (along the midline of the optimal straight route as shown in Figure 4(a)) and the chick was allowed to reach the target 8 consecutive times, an ANOVA with Hatch as between-subject factor and Detour Direction as dependent variable revealed a significant heterogeneity between groups (F(2,54) = 3.626, P = 0.033). Di-chicks chose to detour the obstacle leftward (t(19) = 3.644, P = 0.002, One-Sample t-Test), whereas EarlyLi- and LateLi-chicks showed no systematic preference for a direction (respectively, t(17) = −0.676, P = 0.508; t(18) = 0.468, P = 0.645) as shown in Figure 4(b).
Figure 4. A chick on the left side of the obstacle while reaching the target (a), Di-chicks choose to detour the obstacle on the left significantly more often than EarlyLi- and LateLi-chicks (b).
Discussion
Chicks hatched from eggs exposed to ambient illumination for three days either at an early or a late stage of embryonic development performed in a comparable way in two different tasks. In the first experiment, both EarlyLi- and LateLi-chicks showed a leftward bias when allowed to peck at crumbles scattered on a grid in front of them. Chicks maintained in dark, in contrast, directed their pecks uniformly toward the left and the right hemi-spaces. The result that LateLi-chicks have a left bias in attending the target position whereas Di-chicks show no systematic asymmetrical preference confirms previous results25. Such a late light action is well-known14 and it is thought to affect brain structures by a retinal route, stimulating photoreceptors of the right eye during a critical period (last three days before hatching) in which retinal ganglion cells start to be active.
The result that an early light stimulation may induce a comparable behavioural lateralization provides the first evidence that illumination also play a role on structures of the nervous system that are not the fully-formed eye.
In the second experiment, we found again a striking similarity of the effects of the early and the late exposure to light. Both EarlyLi- and LateLi-chicks showed no bias when they had to detour an obstacle in order to reach a target, moving around either to the left or the right equally. On the contrary, Di-chicks showed a bias, choosing to detour the obstacle significantly more often to the left. Note that both chicks stimulated by light and chicks incubated in the dark run in a comparable way when they had only to reach for a target, meaning that there were no differences in motor behaviour depending on the different incubation conditions. Rather, a difference in control of attention may explain the results. Both EarlyLi- and LateLi-chicks, having decided on approach to the target, are able to ignore the obstacle altogether, whereas Di-chicks have to actively sustain approach to the target by using the right eye to view the obstacle. Di-chicks seem less able in sustaining attention than the groups of chicks exposed to light much as light chicks use the right eye in initial selection of target. There is evidence that many vertebrates view potential danger with the right eye in order to sustain examination and assessment (reviews5,42).
Here we showed that two different lateralized behaviours are affected by environmental illumination: both the early and the late stimulation seem to affect the lateralized behaviours in the same direction. Despite the observed effects involve visuo-spatial behaviours mediated primarily by visual processing, functions like sustained attention or inhibition of responses are also crucial in performing these tasks. These functions may be differently modulated by asymmetries of other brain regions than the visual pathways on which the late light stimulation operates. The responsible mechanism of the early stimulation needs to be investigated, but we hypothesize that one route to the development of cerebral asymmetries may involve the genetic expression of photosensitive regions.
The early photosensitivity in the epiphyseal area of zebrafish has been proposed as responsible for this early action of light, via gene activation, in cells which are not specialized retinal photoreceptors38,39. It has been demonstrated that these photosensitive cells respond to light very early in fish embryonic development43, well before retinal photoreceptors44,45. To our knowledge it is not known whether the epiphyseal photoreceptors are active during the first 3 days of embryonic development in the chick, though it can be reasonably assumed that a similar developmental pattern may be shared across vertebrates. However, it is not possible to exclude that other photosensitive molecules in the developing telencephalon and diencephalon in early stages of development may be directly involved46.
We know that during the time-window in which we applied the early-light, the chick embryo still lies in a symmetric position within the egg41 but asymmetric processes are likely to be already at play. Kuan and collaborators38 report that lateralized Nodal signaling influences the directional asymmetry of the parapineal which in turn mediates habenular asymmetry in fish. The asymmetry of early action of light may be due to asymmetry of gene expression, presumably in the area where the parapineal develops and where photoreceptors start to be active early. A comparable cascade may be at work in the chick brain, possibly involving different areas outside the habenula. Indeed, in this species the epiphysis is forming as a separate knob at 60 hours after fertilization41. The epiphyseal area could be the target substrate for the early illumination to trigger visuo-spatial asymmetry comparable to the late activity of light on the optic nerve.
Although this explanation has to be taken cautiously, it is clear that structures other than the eye may be modulated by light exposure. Evolution may have provided organisms with more than one sensitive temporal window as a chance for the light to exert its important ecological role in triggering brain asymmetry.
Methods
The study was carried out in compliance with the European Community and the Italian law on animal experiments by the Ministry of Health, under the authorization of the Ethical Committee of the University of Trieste (protocol number 385 pos II/9 dd 16.03.2012).
Subjects
Two hundred and six Hybro (White Leghorn) chicks (Gallus gallus) hatched in our laboratory under controlled conditions were used. The eggs were obtained from a local commercial hatchery immediately after fertilization; thereafter, some eggs (n = 70) were kept in complete darkness until the hatching day (Di-chicks) in an incubator FIEM snc, MG 100 H (45 cm wide × 58 cm high × 43 cm deep), under controlled temperature (36.7°C) and humidity (about 50–60%) conditions; some eggs (n = 66) were exposed to light from day 1 to day 3 (nearly 70 hours) after fertilization (EarlyLi-chicks) and thereafter remained in the dark; some others (n = 70) were maintained in darkness and exposed to light from day 18 of incubation (LateLi-chicks) to day 21 of hatching. A 60 W incandescent light bulb provided light within of the incubator. Immediately after hatching, chicks were reared singly in metal cages (22 cm wide × 30 cm high × 40 cm deep) illuminated by 30 W fluorescent lights (12 L: 12 D cycle) and located in a separate room at 28–30°C. Food and water were available ad libitum. Chicks of each incubation condition were tested on day 4 post-hatch. The experimenter was blind to the hatching condition.
Cancellation task
Apparatus. The apparatus was the same used in previous experiments47,26 and consisted of a white uniform wooden enclosure (50 cm wide × 45 cm high × 50 cm deep) with a brown ground. A white cardboard box (14 cm wide × 14 cm high × 14 cm deep) served as confining box; it was fixed at the middle of the rear wall and presented on its frontal wall a circular window measuring 2 cm in diameter. A Poliplack® array positioned centrally and exactly beyond the window in the cardboard box, 4 cm above the floor, was divided in 170 compartments of 1 × 1 cm (17 columns of 10 compartments each) containing a single grain of chicks crumbles each. It was covered by a double sided sticky tape which provided grains to remain in a fixed position while the chick was pecking at close elements. A lamp of 30 W placed exactly on the top of the cardboard box illuminated the apparatus. The behaviour was videorecorded by a Panasonic® NV-GS27 camera connected to a monitor so that animals' activity could be observed without interference.
Procedure
After 3 h of food deprivation, on day 3 of age, each chick was in turn placed within the confining box and accustomed to protrude head and neck through the circular window in order to feed from a rectangular dish located frontally outside. The chick is motivated to do this because the environment within the box is dark while the external surroundings well lit; the chick spontaneously comes out and goes back inside the confining box. After 15 minutes of activity the chick was brought back in its rearing cage with food and water available until the evening. The next day, after overnight of food deprivation only, the chick was placed in the confining box and observed for a total of 3 minutes during which time it was free to peck at the grains of food regularly scattered in the above described array. The body-restrained condition ensured a continuous alignment with the midline of the searching area used in the test.
Running task
Apparatus. The apparatus consisted of a white uniform rectangular wooden enclosure (40 cm wide × 50 cm high × 160 cm deep) with sawdust (5 cm in depth) on the floor.
At the middle of the opposite smaller ends of the enclosure were fixed a white Poliplack® starting box (12 cm wide × 12 cm high × 12 cm deep) with no frontal wall and a landmark (a blue and red cardboard cylinder) placed 7 cm above the floor. The landmark indicated the presence of a small yellow and green rectangular plastic feeder (target) exactly below it. A uniform illumination of the whole apparatus was provided by two lamps of 25 W placed exactly on the top of the starting box and of the landmark. The behaviour was videorecorded by a Panasonic® NV-GS27 camera and scored offline. In order to keep track of the chick's movements within the apparatus, a black removable piece of paper was temporarily attached on the chick's back.
Procedure
On day 3 of age, after 3 hours of food deprivation, all the chicks were first positioned inside the starting box and left free to move around in order to get acquainted with the novel environment by reaching the target and finding mealworm larvae (Tenebrio molitor). Chicks did this quite spontaneously since the landmark was conspicuous and caught their interest. The habituation phase lasted in average 30 minutes: each chick was placed in the starting box and left free to walk toward the landmark to find the reward. The next day, after overnight of food deprivation, each chick was in turn placed within the apparatus in the starting box and left free to run toward the landmark once. No food was available in the feeder. The trial started as soon as the chick came out of the starting box and ended when the chick rested at the feeder. The positions of the starting box and the target were counterbalanced between subjects.
The same procedure was used for the animals that underwent the running task in presence of the obstacle placed in the centre of the apparatus (this time 80 cm wide). The obstacle consisted of 2 identical black plastic cylinders (2 cm in diameter and 30 cm high), spaced 2 cm from one another and blocked by a plastic bar positioned just underneath the sawdust. The proportion of the left routes over the total 8 routes was calculated to determine detour direction.
Author Contributions
C.C. and G.V. wrote the manuscript text. C.C., G.V. and R.J.A. conceived the experiments. C.C. and J.G. performed the experiments, analyzed the data and prepared the figures. C.C., R.J.A. and G.V. reviewed the manuscript.
Acknowledgments
This work has been realized thanks to the support from the Provincia Autonoma di Trento and the Fondazione Cassa di Risparmio di Trento e Rovereto. We are grateful to Tommaso Mastropasqua and Tommaso Pecchia for technical assistance.
References
- Burdine R. D. & Schier A. F. Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 14, 763–776 (2000). [PubMed] [Google Scholar]
- Capdevila J., Vogan K. J., Tabin C. J. & Belmonte J. C. I. Mechanisms of left-right determination in vertebrates. Cell. 101, 9–21 (2000). [DOI] [PubMed] [Google Scholar]
- Hamada H., Meno C., Watanabe D. & Saijoh Y. Establishment of vertebrate left-right asymmetry. Nat. Rev. Genet. 3, 103–13 (2002). [DOI] [PubMed] [Google Scholar]
- Mercola M. & Levin M. Left-right asymmetry determination in vertebrates. Annu. Rev. Cell Dev. Biol. 17, 779–805 (2001). [DOI] [PubMed] [Google Scholar]
- Rogers L. J., Vallortigara G. & Andrew R. J. Divided brains: The biology and behaviour of brain asymmetries. Cambridge university press, New York. (2013).
- Schier A. F. Nodal signalling in vertebrate development. Annu. Rev. Cell Dev. Biol. 19, 589–621 (2003). [DOI] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 122, 3–25 (2005). [DOI] [PubMed] [Google Scholar]
- Concha M. L., Burdine R. D., Russell C., Schier A. F. & Wilson S. W. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 28, 399–409 (2000). [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Liang J. O. & Gamse J. T. Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci. 26, 308–313 (2003). [DOI] [PubMed] [Google Scholar]
- Ramsdell A. F. & Yost H. J. Molecular mechanisms of vertebrate left-right development. Trends in Genetics. 14, 459–465 (1998). [DOI] [PubMed] [Google Scholar]
- Zhu L., Marvin M. J., Gardiner A., Lassar A. B., Mercola M., Stern C. D. & Levin M. Cerverus regulates left–right asymmetry of the embryonic head and heart. Curr. Biol. 9, 931–938 (1999). [DOI] [PubMed] [Google Scholar]
- Ververs I. A. P., de Vries J. I. P., van Geijn H. P. & Hopkins B. Prenatal head position from 12–38 weeks. I. Developmental aspects. Early Hum. Dev. 39, 83–91 (1994). [DOI] [PubMed] [Google Scholar]
- Previc F. H. A General theory concerning the prenatal origins of cerebral lateralization in humans. Psychol. Rev. 98, 299–334 (1991). [DOI] [PubMed] [Google Scholar]
- Rogers L. J. Light experience and asymmetry of brain function in chickens. Nature. 297, 223–225 (1982). [DOI] [PubMed] [Google Scholar]
- Güntürkün O. Adult persistence of head-turning asymmetry. Nature. 421, 711 (2003). [DOI] [PubMed] [Google Scholar]
- Kuo Z. Y. Ontogeny of embryonic behavior in aves. III. The structural and environmental factors in embryonic behavior. J. Comp. Psychol. 13, 245–271 (1932). [Google Scholar]
- Rogers L. J. & Deng C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 98, 277–287 (1999). [DOI] [PubMed] [Google Scholar]
- Deng C. & Rogers L. J. Social recognition and approach in the chick: Lateralization and effect of visual experience. Anim. Behav. 63, 697–706 (2002). [Google Scholar]
- Skiba M., Diekamp B. & Güntürkün O. Embryonic light stimulation induces different asymmetries in visuoperceptual and visuomotor pathways of pigeons. Behav. Brain Res. 134, 149–156 (2002). [DOI] [PubMed] [Google Scholar]
- Mey J. & Thanos S. Development of the visual system of the chick I. Cell differentiation and histogenesis. Brain Res. Brain Res. Rev. 32, 343–79 (2000). [DOI] [PubMed] [Google Scholar]
- Rogers L. J. Early experiential effects on laterality: Research on chicks has relevance to other species. Laterality. 2, 199–219 (1997). [DOI] [PubMed] [Google Scholar]
- Dharmaretnam M. & Rogers L. J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain Res. 162, 62–70 (2005). [DOI] [PubMed] [Google Scholar]
- Rogers L. J. Light input and the reversal of functional lateralisation in the chicken brain. Behav. Brain Res. 38, 211–221 (1990). [DOI] [PubMed] [Google Scholar]
- Manns M. & Güntürkün O. Development of the retinotectal system in the pigeon: a choleratoxin study. Anat. Embryol. 195, 539–555 (1997). [DOI] [PubMed] [Google Scholar]
- Manns M. & Güntürkün O. Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon's visual system. Behav. Neurosci. 113, 1–10 (1999). [DOI] [PubMed] [Google Scholar]
- Chiandetti C. Pseudoneglect and embryonic light stimulation in the avian brain. Behav. Neurosci. 125, 775–782 (2011). [DOI] [PubMed] [Google Scholar]
- Chiandetti C., Regolin L., Rogers L. J. & Vallortigara G. Effects of light stimulation of embryos on the use of position-specific and object-specific cues in binocular and monocular domestic chicks (Gallus gallus). Behav. Brain Res. 163, 10–17 (2005). [DOI] [PubMed] [Google Scholar]
- Manns M. & Römling J. The impact of asymmetrical light input on cerebral hemispheric specialization and interhemispheric cooperation. Nat. Commun. 3, 1–5 (2012). [DOI] [PubMed] [Google Scholar]
- Mascetti G. G. & Vallortigara G. Why do birds sleep with one eye open? Light exposure of chick embryo as a determinant of monocular sleep. Curr. Biol. 11, 971–974 (2001). [DOI] [PubMed] [Google Scholar]
- Andrew R. J., Johnston A. N. B., Robins A. & Rogers L. J. Light experience and the development of behavioural lateralization in chicks. II. Choice of familiar versus unfamiliar model social partner. Behav. Brain Res. 155, 67–76 (2004). [DOI] [PubMed] [Google Scholar]
- Horn G. & Johnson M. H. Memory systems in the chick: Dissociations and neuronal analysis. Neuropsychol. 27, 1–22 (1989). [DOI] [PubMed] [Google Scholar]
- Johnston A. N. B. & Rogers L. J. Light exposure of chick embryo influences lateralized recall of imprinting memory. Behav. Neurosci. 113, 1267–1273 (1999). [DOI] [PubMed] [Google Scholar]
- Barth K. A., Miklósi A., Watkins J., Bianco I. H., Wilson S. W. & Andrew R. J. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr. Biol. 15, 844–850 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. N., O'Craven K. M., Ticho B. S., Goldstein A. M., Makris N. & Henson J. W. Structural and functional brain asymmetries in human situs inversus. Neurology. 53, 1260–1265 (1999). [DOI] [PubMed] [Google Scholar]
- McManus I. C., Martin N., Stubbings G. F., Chung E. M. K. & Mitchison H. M. Handedness and situs inversus in primary ciliary dyskinesia. Proc. R. Soc. Lond. B. Biol. Sci. 271, 2579–2582 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. J., Osorio D. & Budaev S. Light during embryonic development modulates patterns of lateralisation strongly and similarly in both zebrafish and chick. Phil. Trans. R. Soc. 364, 983–989 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budaev S. V. & Andrew R. J. Shyness and behavioural asymmetries in larval zebrafish (Brachydanio rerio) developed in light and dark. Behaviour. 146, 1037–1052 (2009). [Google Scholar]
- Budaev S. V. & Andrew R. J. Patterns of early embryonic light exposure determine behavioural asymmetries in zebrafish: A habenular hypothesis. Behav. Brain Res. 200, 91–94 (2009). [DOI] [PubMed] [Google Scholar]
- Kuan Y. S., Yu H. H., Moens C. B. & Halpern M. E. Neuropilin asymmetry mediates a left-right difference in habenular connectivity. Development. 134, 857–865 (2007). [DOI] [PubMed] [Google Scholar]
- Andrew R. J. Origins of asymmetry in the CNS. Semin. Cell Dev. Biol. 20, 485–490 (2009). [DOI] [PubMed] [Google Scholar]
- Hamburger V. & Hamilton H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951). [PubMed] [Google Scholar]
- Vallortigara G., Chiandetti C. & Sovrano V. A. Brain asymmetry (animal). WIREs Cogn. Sci. 2, 146–157 (2011). [DOI] [PubMed] [Google Scholar]
- Ekström P., Borg B. & van Veen T. Ontogenetic development of the pineal organ, parapineal organ, and retina of the three-spined stickleback, Gasterosteus aculeatus L. (Teleostei). Development of photoreceptors. Cell Tissue Res. 233, 593–609 (1983). [DOI] [PubMed] [Google Scholar]
- Omura Y. & Oguri M. Early development of the pineal photoreceptors prior to the retinal differentiation in the embryonic rainbow trout, Oncorhynchus mykiss (Teleostei). Arch. Histol. Cytol. 56, 283–91 (1993). [DOI] [PubMed] [Google Scholar]
- Östholm T., Brännäs E. & van Veen T. The pineal organ is the first differentiated light receptor in the embryonic salmon, Salmo salar L. Cell Tissue Res. 249, 641–646 (1987). [DOI] [PubMed] [Google Scholar]
- Tomonari S. Takagi A. Akamatsu S. Noji S. & Ohuchi H. A non-canonical photopigment, melanopsin, is expressed in the differentiating ganglion, horizontal, and bipolar cells of the chicken retina. Dev. Dynamics. 234, 783–790 (2005). [DOI] [PubMed] [Google Scholar]
- Diekamp B., Regolin L., Güntürkün O. & Vallortigara G. A left-sided visuospatial bias in birds. Curr. Biol. 15, R372–373 (2005). [DOI] [PubMed] [Google Scholar]