Abstract
Background:
Rituximab and trastuzumab were the first therapeutic monoclonal antibodies (mAbs) approved in oncology. Both antibodies are delivered by the intravenous (IV) route, but recently subcutaneous (SC) formulations have been developed. Subcutaneous administration of mAbs can offer substantial patient and resource benefits compared with IV, but SC administration of some mAbs can be limited by drug volume. Recombinant human hyaluronidase (rHuPH20) temporarily degrades hyaluronan, allowing SC delivery of drug volumes that might not otherwise be feasible.
Methods:
Clinical trials assessing coformulation of rituximab or trastuzumab with rHuPH20 for SC administration were reviewed.
Results:
Phase I trials of rituximab SC maintenance therapy in patients with follicular lymphoma and trastuzumab SC in healthy volunteers and patients with early breast cancer have demonstrated substantially shorter administration times and comparable tolerability and pharmacokinetics compared with IV formulations. Rituximab SC 1400-mg and trastuzumab SC 600-mg doses were identified for further study. Phase III clinical data for rituximab SC 1400 mg have shown comparable efficacy to rituximab IV, and initial clinical data suggest comparable efficacy of trastuzumab SC 600 mg and the IV formulation.
Conclusion:
Coformulation with rHuPH20 may enable effective, well-tolerated, cost-effective, and convenient SC administration of rituximab and trastuzumab. Additional studies are ongoing.
Keywords: breast cancer, hyaluronidase, lymphoma, subcutaneous administration, rituximab, trastuzumab
Introduction
Rituximab and trastuzumab were the first monoclonal antibodies (mAbs) approved for the treatment of cancer (US FDA, 2011) and have since become the standard of care (Vogel, 2010; National Comprehensive Cancer Network, 2012). Rituximab targets CD20 and is indicated for patients with non-Hodgkin's lymphoma and chronic lymphocytic leukaemia (F. Hoffmann-La Roche, 2008). Trastuzumab is targeted to HER2 (human epidermal growth factor receptor 2) and is indicated for the treatment of HER2-positive early and metastatic breast cancer and metastatic gastric cancer (F. Hoffmann-La Roche, 2011). In oncology, mAbs are typically administered by intravenous (IV) infusion; however, this administration route faces some challenges (Vogel, 2010; Haller, 2007; Sehn et al, 2007; Vescia et al, 2008), including:
The need for trained personnel, dedicated infusion facilities, dose calculation, aseptic preparation of required infusion volumes, and extended post-infusion observation;
Long infusion times and slow workflow for medical staff;
Potential difficulties with IV catheter placements;
Risk of infusion-related reactions and complications (e.g. relevant thromboembolic events, infections), leading to hospitalisation and additional costs;
Costs associated with the placement of permanent IV lines.
Subcutaneous (SC) administration can potentially overcome many of these challenges (Haller, 2007; Bookbinder et al, 2006). Although administration-associated reactions (AARs) may occur with SC administration, they are likely to be less severe than IV-associated infusion-related reactions, given the more gradual absorption of the SC formulation. SC formulations may be more convenient for patients, offering potential improvements in quality of life and treatment adherence (Bookbinder et al, 2006; Haller, 2007). In oncology, SC administration of mAbs has been shown to reduce administration times (Salar et al, 2010; Wynne et al., 2013) and health-care costs relative to IV infusion (Lundin et al, 2002; Stilgenbauer et al, 2009). However, this delivery method is challenged by limitations on drug volume (Bookbinder et al, 2006; Haller, 2007). This article will review how recent technological advances can potentially make SC administration of mAbs more feasible and more beneficial than IV formulations in the oncology setting.
Subcutaneous administration
Subcutaneous administration is commonly used in a number of therapy areas. In diabetes, insulin SC has been available for >50 years and is the cornerstone of treatment (Selam, 2010). Adalimumab SC, a recombinant human immunoglobulin mAb for the treatment of rheumatoid arthritis, was recently approved by the US Food and Drug Administration (FDA) (US FDA, 2011). Currently, no anticancer mAbs are approved for SC administration, although SC formulations of some agents have been developed (Lundin et al, 2002; Stilgenbauer et al, 2009; Salar et al, 2010; Wynne et al, 2013). For example, alemtuzumab is an anti-CD52 mAb approved for the treatment of chronic lymphocytic leukaemia; alemtuzumab SC has been shown to have better tolerability and similar efficacy as alemtuzumab IV (Stilgenbauer et al, 2009). SC formulations of rituximab and trastuzumab could bring clinically meaningful benefits to patients and offer an alternative to the current practice of IV administration.
However, the biggest challenge with SC formulations has been the ability to deliver the required volume of drug to patients. The extracellular matrix, which maintains tissue architecture and controls diffusion/molecule flow (Haller, 2007), limits drug volumes to 1–2 ml, with volumes >2 ml causing tissue distortion and pain (Bookbinder et al, 2006; Haller, 2007). The relatively large volume of the established IV doses of rituximab and trastuzumab has hindered SC administration. These hurdles have been overcome by concentrating IV formulations 12-fold and adding recombinant human hyaluronidase (rHuPH20). By hydrolysing hyaluronan, rHuPH20 reversibly opens the interstitial space in SC tissue and allows the installation of >2–3 ml (Bookbinder et al, 2006; Haller, 2007).
Hyaluronidase
The glycosaminoglycan hyaluronan, together with collagen, creates a volume barrier within the extracellular matrix (Haller, 2007). Hyaluronan occupies a high fluid exclusion volume and has a half-life of 15–20 h (Bookbinder et al, 2006). Because it is broken down and replenished as part of normal bodily functions (Verzijl et al, 2000; Bookbinder et al, 2006), it can be transiently disrupted for drug delivery (Supplementary Figure S1). Hyaluronan can be degraded by hyaluronidase, which has been used for >60 years to facilitate dispersion of coinjected materials (Haller, 2007). Previous formulations of hyaluronidase were animal-derived products whose impurity was associated with immunogenicity (Bookbinder et al, 2006; Haller, 2007). The development of purified soluble human hyaluronidase, rHuPH20, has overcome these limitations (Bookbinder et al, 2006; Haller, 2007). In human microvascular endothelial cultures, rHuPH20 does not elicit inflammatory responses (Bookbinder et al, 2006), and animal studies have shown no adverse local reactions or electrocardiographic, haemodynamic, clinical, or anatomical changes after repeated dosing (Bookbinder et al, 2006). Moreover, the changes within the extracellular matrix induced by rHuPH20 are local, reversible, and transient (Bookbinder et al, 2006).
Subcutaneous administration of mAbs
Clinical development programmes exploring SC formulations of rituximab or trastuzumab coformulated with rHuPH20 are underway. These programmes are underpinned by pharmacokinetic studies: the clinical benefit of rituximab IV and trastuzumab IV is assumed to be dependent on serum concentrations of the respective antibodies (Mager and Jusko, 2001; Yin et al, 2010). As serum trough concentrations (Ctrough) reflect the degree of accessible target site saturation, it is hypothesised that attaining Ctrough levels with SC dosing that are at least as high as those of approved IV doses will provide comparable saturation of target sites and, thus, comparable efficacy. It has been shown that rHuPH20 improves the bioavailability of coadministered SC drugs. With rHuPH20, it may be possible to achieve serum concentrations equivalent to those seen with IV formulations (Bookbinder et al, 2006; Haller, 2007; Thomas et al, 2007; Thomas et al, 2009; Vaughn et al, 2009; Harb et al, 2010).
Rituximab SC coformulated with rHuPH20: preclinical and clinical development
Preclinical efficacy and pharmacodynamics
For rituximab SC to be efficacious, rituximab must be effectively distributed from a single SC injection site to lymphoid tissues throughout the body. In cynomolgus monkeys given two doses of rituximab SC or rituximab IV (10 mg kg−1) 7 days apart (Table 1; Del Nagro et al, 2010), Ctrough levels and the extent of B-cell depletion in distal secondary lymphoid tissues and peripheral blood were similar for both formulations, demonstrating that administration route does not affect non-clinical efficacy (Del Nagro et al, 2010).
Table 1. Key preclinical studies and clinical trials for rituximab SC and trastuzumab SC (Del Nagro et al, 2010; Salar et al, 2010; US NIH, 2011; Ismael et al, 2012; Salar et al, 2012; Wynne et al, 2012).
| Population (n) | Phase | Outcome | Comment |
|---|---|---|---|
|
Rituximab | |||
| Cynomolgus monkeys (n=11) | Preclinical | Pharmacokinetics, B-cell depletion and CD20 target coverage (SC vs IV) | Rituximab SC has similar pharmacokinetics, B-cell depletion and CD20 target coverage to rituximab IV |
| Previously treated or untreated FL (n=124) | Phase Ib (BP22333; NCT00930514) (n=124) | Stage 1: pharmacokinetics (SC vs IV) | Rituximab SC 1400 mg calculated to be non-inferior to an IV dose of 375 mg m−2 |
| Stage 1: safety (SC vs IV) | Rituximab SC has comparable safety profile to rituximab IV | ||
| Phase Ib (BP22333; NCT00930514) (n=155) | Stage 2: pharmacokinetics of rituximab SC (1400 mg) vs IV | Ongoing (due to complete in 2013) | |
| Previously untreated FL (n=530) | Phase III (BO22334; NCT01200758) | Stage 1: pharmacokinetics (SC vs IV) | Ongoing |
| Stage 2: Safety (SC vs IV) | Ongoing | ||
| Previously untreated CLL (n=200) |
Phase I (BO25341; NCT01292603) |
Pharmacokinetics; safety (SC vs IV) |
Ongoing |
|
Trastuzumab | |||
| HMV (n=24) and patients with HER2-positive EBC (n=42) | Phase I/Ib (BP22023; NCT00800436) | Pharmacokinetics | Trastuzumab SC (8 mg kg−1) provides comparable exposure to trastuzumab IV (6 mg kg−1) |
| Safety | There were fewer AEs with trastuzumab SC than IV | ||
| Neoadjuvant HER2-positive EBC (n=595) | Phase III (HannaH; NCT00950300) | Pharmacokinetics; efficacy; safety | Co-primary efficacy and pharmacokinetic end points met |
| HMV (n=119) | Phase I (CP3; NCT01344863) | Pharmacokinetics of the injection system vs manual injection | Study complete, data expected in 2012 |
| HER2-positive EBC patients who have completed (neo)adjuvant chemotherapy (n=400) | PrefHer | Patient preference; HCP satisfaction; HCP-perceived time savings; safety | Ongoing (due to complete in 2013) |
| HER2-positive EBC (n=2500) | SafeHer | Safety of repeated SC injections | Start March 2012 |
Abbreviations: AEs=adverse events; CLL=chronic lymphocytic leukaemia; EBC=early breast cancer; EBCC=European Breast Cancer Conference; FL=follicular lymphoma; HannaH=Enhanced Treatment With Neoadjuvant Herceptin study; HCP=health-care provider; HMV=healthy male volunteers; IV=intravenous; PrefHer=Preferences for Herceptin; SafeHer=Safety and Tolerability Study of Assisted- and Self-administered Therapy in Patients With Early HER2-Positive Breast Cancer; SC=subcutaneous.
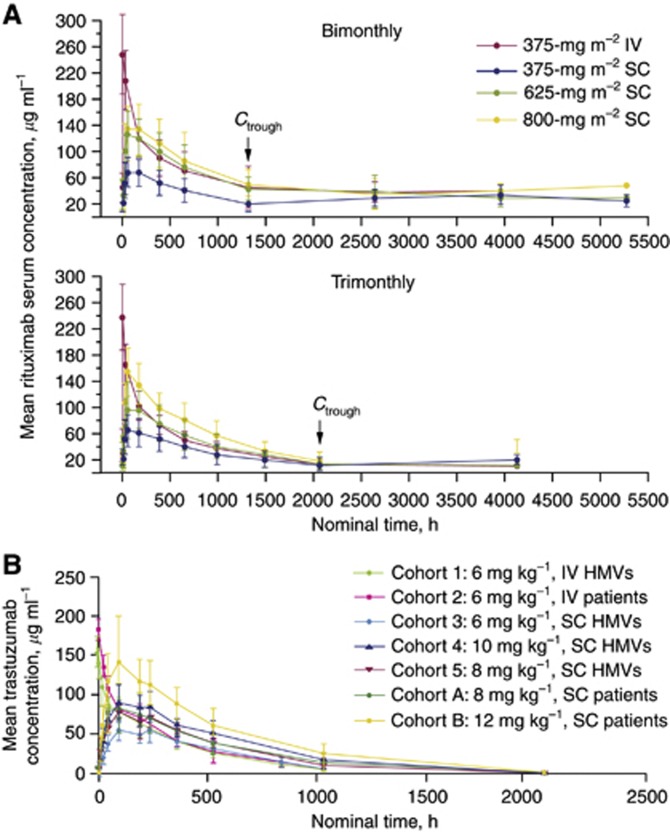
In stage 1 of a randomised, dose-finding, open-label, phase Ib trial (BP22333), 124 patients with previously treated or untreated follicular lymphoma who responded to a rituximab-containing induction regimen and had received ⩾1 dose of rituximab IV as maintenance received a single dose of rituximab 375 mg m−2 IV, 375 mg m−2 SC, 625 mg m−2 SC, or 800 mg m−2 SC, with subsequent IV doses (375 mg m−2) every 2 or 3 months for up to 2 years. Rituximab Ctrough levels and the extent of serum exposure (bimonthly: area under the curve (AUC)0–57; trimonthly: AUC0–85) with SC doses of 625 mg m−2 and 800 mg m−2 were within the range of those in patients receiving rituximab IV 375 mg m−2 (Supplementary Table S1A; Figure 1 A). For mAbs, linear pharmacokinetics are observed once accessible target receptors are saturated (Mager and Jusko, 2001; Del Nagro et al, 2010). Pharmacokinetic parameters after a single rituximab SC dose (375, 625, and 800 mg m−2) were linear for the bimonthly and trimonthly dosing schedules (Supplementary Table S1A; Salar et al, 2010).
Figure 1.
(A) Mean serum concentration of rituximab over time by administration schedule (Salar et al, 2010). (B) Mean (±s.d.) trastuzumab concentration–time profile in (a) all cohorts, (b) HMVs and female patients receiving 6 mg kg−1 IV trastuzumab, and (c) cohorts with comparable SC and IV doses of trastuzumab. Abbreviations: HMVs, healthy male volunteers; IV, intravenous; SC, subcutaneous (Wynne et al, 2013).
Dosing by body surface area has historically been used for cytotoxic agents, which have a narrow therapeutic window. For agents like rituximab, which are well tolerated with a wide therapeutic window, fixed dosing is convenient and minimises error and waste of material (Wang et al, 2009). A flat dose that has undergone rigorous pharmacokinetic and clinical assessment will ensure that target saturation is achieved, even in patients with high tumour burden who may clear antibody more rapidly, and that Ctrough levels will be maintained throughout the treatment course. To achieve comparable target receptor saturation, and thus comparable therapeutic efficacy, simulations of mean Ctrough values predicted that a fixed dose of rituximab SC 1400 mg would be non-inferior to rituximab IV 375 mg m−2. This dose of rituximab SC was selected for formal Ctrough non-inferiority testing vs rituximab IV 375 mg m−2 in stage 2 of the BP22333 trial. Rituximab SC is intended for use in patients who do not experience tolerability issues after infusion with rituximab IV.
In stage 2, 154 patients were randomised (1 : 1) to rituximab SC 1400 mg or rituximab IV 375 mg m−2 on day 1 of each maintenance cycle (administered bimonthly or trimonthly). Rituximab SC 1400 mg was found to be non-inferior to rituximab IV 375 mg m−2, with geometric mean Ctrough,SC : Ctrough,IV ratios of 1.24 and 1.12 for the bimonthly and trimonthly regimens, respectively (Salar et al, 2012).
Safety
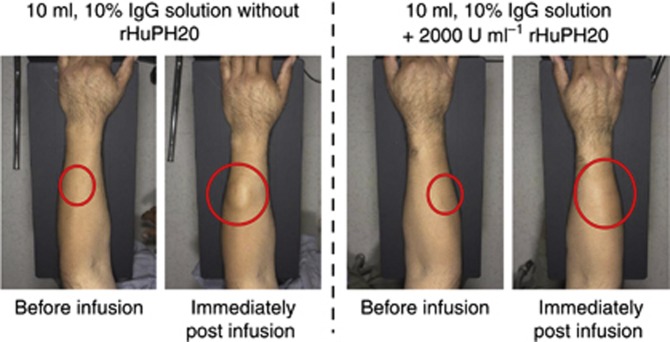
Several clinical studies assessing rituximab SC have been completed or are ongoing (Table 1). In stage 1 of BP22333, 157 adverse events (AEs) were reported by 65 patients (52%) following a single dose of rituximab SC. The most common AEs were AARs (n=30), such as rash, erythema, and mild discomfort; these AARs were reversible, and most were mild in intensity. Mild infections (n=18) and gastrointestinal disorders (n=17) were the most common events after AARs for patients treated with rituximab SC. Skin distortion was not observed at the injection site (Figure 2). No treatment-related serious AEs, grade 4 AEs, or AEs leading to death, withdrawal, or treatment discontinuation were reported. Overall, the incidence and severity of AEs associated with rituximab SC (375, 625, and 800 mg m−2) were comparable with those known for rituximab IV (Salar et al, 2010). As in stage 1, the most frequently reported AEs in stage 2 were AARs, which occurred in 31% of patients receiving rituximab SC and 4% of those administered rituximab IV. The most frequently observed AARs in the rituximab SC arm were erythema (13%), injection site erythema (5%), and myalgia (5%). An overview of AEs reported in stage 2 is presented in Supplementary Table S2A (Salar et al, 2012).
Figure 2.
Administration site before and immediately after infusion of rituximab SC coformulated without (left panel) and with (right panel) rHuPH20. Although the forearm is shown, preclinical evidence suggests that the most appropriate place for rituximab SC administration is the abdomen (Kagan et al, 2012). Abbreviation: IgG, immunoglobulin G; rHuPH20, recombinant human hyaluronidase; SC, subcutaneous.
The BO22334 (NCT01200758) trial is a two-stage, randomised, controlled, open-label, phase III study of patients with previously untreated follicular lymphoma administered induction chemotherapy plus either rituximab SC 1400 mg or rituximab IV 375 mg m−2. Treatment responders (partial response, complete response) were eligible for maintenance treatment with rituximab SC or rituximab IV monotherapy every 8 weeks for 2 years (US NIH, 2011). In stage 1, participants received cyclophosphamide, doxorubicin, vincristine, and prednisone or cyclophosphamide, vincristine, and prednisone per local practice, and were randomized to 8 cycles of either rituximab SC (n=62) or rituximab IV (n=65). Irrespective of randomisation, all patients were given rituximab IV in cycle 1. At a median follow-up of 8.7–8.8 months, the proportions of patients with AEs (SC, 92% IV, 88%), serious AEs (SC, 23% IV, 22%), and grade ⩾3 AEs (SC, 47% IV, 46%) were similar in the two treatment arms. As in the BP22333 study, the incidence of AARs was higher with rituximab SC than rituximab IV (50% vs 32%) (Davies et al, 2012).
The two-stage, randomised, parallel-group, phase Ib BO25341 study (NCT01292603) is comparing the pharmacokinetics and safety of fludarabine and cyclophosphamide combined with either rituximab SC or rituximab IV in patients with previously untreated chronic lymphocytic leukaemia (US NIH, 2011). In stage 1, patients received rituximab IV 500 mg m-2 plus chemotherapy in cycles 1–5. In cycle 6, rituximab IV was replaced by one of three doses of rituximab SC (1400 mg (n=16), 1600 mg (n=17), or 1870 mg (n=22)). Overall, AE incidence was numerically greater in cycle 6 when rituximab SC was administered than in cycle 5 when rituximab IV was administered (63.6% vs 52.7%). A slight increase in AE incidence was seen with increasing rituximab SC doses (Assouline et al, 2012). Similar to patients with follicular lymphoma (Davies et al, 2012; Salar et al, 2012), the rate of ARRs was higher in cycle 6 than in cycle 5 (21.8% vs 3.6%). However, a greater percentage of patients experienced grade ⩾3 AEs during cycle 5 than during cycle 6 (29.1% vs 18.2%) (Assouline et al, 2012).
Efficacy
The only efficacy data currently available for rituximab SC derive from the BO22334 trial. Investigator-assessed overall response rates were numerically greater for rituximab SC plus chemotherapy than rituximab IV plus chemotherapy (90.5% vs 84.4%, respectively). In the rituximab SC plus chemotherapy treatment arm, the percentage of patients with a complete response or complete response unconfirmed was 46.0%. The corresponding value in the rituximab IV plus chemotherapy arm was 29.7% (Davies et al, 2012).
Convenience and added value to patients and health-care providers
Rituximab is currently administered IV with infusion times ranging from 90 min (rapid infusion) to 4–6 h (standard infusion) (Salar et al, 2006; Sehn et al, 2007; F. Hoffmann-La Roche, 2008; Vogel, 2010). Rituximab infusion, particularly at the standard rate, can be labour-intensive and be associated with resource costs, including medical supplies and staff for administration and observation time (Haller, 2007; Chan et al, 2009). SC administration is typically more convenient than IV infusion, with reduced administration times and no requirements for dedicated infusion facilities. Rituximab SC can be delivered in ∼2–8 min; in a phase Ib study, 4.4–15.0 ml of rituximab SC was administered at an average speed of 1.9 ml min−1 (range: 0.4–3.8 ml min−1; Salar et al, 2010). The rituximab SC 1400-mg dose carried forward to phase III will be delivered in a volume of 11.7 ml.
A survey of oncology practitioners showed that most considered SC administration to be more cost-effective than IV infusion in terms of resource utilisation (Gilbert and Cothran, 2005), suggesting that rituximab SC will have a better cost-effectiveness profile than rituximab IV. Furthermore, SC administration was considered to result in higher patient satisfaction than IV administration (Gilbert and Cothran, 2005). Therefore, SC administration has the potential to improve patient quality of life (Haller, 2007). The BO25341 study, which is due to complete in 2014, will also assess (1) patient and nurse preferences regarding SC vs IV administration and (2) physician and nurse opinions on the time savings and convenience of SC administration (US NIH, 2011).
Trastuzumab SC coformulated with rHuPH20: development and clinical experience
Efficacy and pharmacodynamics
To determine the dose of trastuzumab SC that results in an exposure level comparable to the approved dose of trastuzumab IV (6 mg kg−1, given once every 3 weeks following a loading dose of 8 mg kg−1; F. Hoffmann-La Roche, 2011), an open-label, two-part, phase I/Ib dose-finding and dose-confirmation trial comparing the pharmacokinetics of trastuzumab SC vs trastuzumab IV was conducted in healthy male volunteers (HMVs; n=24) and female patients with early breast cancer (n=42; Wynne et al, 2013). HMVs received a single dose of trastuzumab IV (6 mg kg−1) or trastuzumab SC (6, 8, or 10 mg kg−1), whereas female patients received a single dose of trastuzumab IV (6 mg kg−1). The pharmacokinetic profile and mean serum exposure of patients and HMVs receiving trastuzumab IV 6 mg kg−1 were comparable (mean Cmax: 185 μg ml−1 vs 150 μg ml−1, respectively; Figure 2B, Supplementary Table S1B), enabling extrapolation of results in HMVs to patients. In HMVs, mean trastuzumab exposure post dose was similar for trastuzumab SC 8 mg kg−1 and trastuzumab IV 6 mg kg−1 (Figure 1B), suggesting that an SC dose of 8 mg kg−1 would result in comparable serum exposure to that of the approved IV dose. To confirm this, female patients received a single dose of trastuzumab SC 8 mg kg−1; serum exposure was comparable for these patients and those receiving trastuzumab IV 6 mg kg−1 (Figure 1B). As trastuzumab IV is administered at a loading dose of 8 mg kg−1 (F. Hoffmann-La Roche, 2011), female patients were also exposed to a higher dose of trastuzumab SC (12 mg kg−1). Patients who received trastuzumab SC 12 mg kg−1 in this study had higher trastuzumab exposure than patients in the Herceptin Adjuvant study, who received a loading dose of 8 mg kg−1 IV (60.8 μg ml−1 vs 42.9 μg ml−1, respectively, 22 days after the first dose; Wynne et al, 2013). Plasma concentrations of rHuPH20 were below the limit of quantification (∼3 ng ml−1) at all time points, suggesting that rHuPH20 is acting locally and is rapidly eliminated from the body. These data, along with modelling and simulations, were used to calculate a fixed dose of 600 mg for use in phase III trials to further investigate the safety and efficacy of trastuzumab SC (Wynne et al, 2013).
Safety
The safety and tolerability profile of trastuzumab SC vs trastuzumab IV was the secondary objective of the phase I/Ib trial discussed earlier (Wynne et al, 2013). Trastuzumab SC was well tolerated, showing fewer reported AEs vs trastuzumab IV at all dose levels. There was no apparent increase in the incidence or severity of AEs with escalating SC doses (Supplementary Table S2B; Wynne et al, 2013). The most common AEs experienced by patients receiving trastuzumab SC were headache, upper respiratory tract infection, and influenza-like illness, whereas trastuzumab IV was most commonly associated with headache, acne, musculoskeletal pain, diarrhoea, abdominal pain, and IRR. Most of the AEs reported with trastuzumab (IV or SC) were of mild intensity (71–73%). No deaths, serious AEs, AEs leading to withdrawal or dose modification, or clinically relevant changes in any laboratory parameters, vital signs, or electrocardiograms were reported.
Tolerability at the injection site of the SC formulation is important. Among patients who received trastuzumab SC (n=58), 18 local administration site AEs were reported. Of these, 16 were of mild intensity (erythema, n=7; discoloration, n=5; injection site swelling, n=2; injection site discomfort, n=1; and injection site reaction, n=1), and two were of moderate intensity (injection site pain), suggesting that administration site AEs were manageable.
In the same study, analyses were performed to evaluate the immunogenicity of trastuzumab SC and rHuPH20. Anti-trastuzumab antibodies were detected in 8 of the 58 patients who received trastuzumab SC and in none of the 12 patients who received trastuzumab IV (Wynne et al, 2013). The presence of anti-trastuzumab antibodies did not correlate with the AE and pharmacokinetic profiles of these patients. At the time of this study, an anti-trastuzumab antibody-neutralising assay was not available; thus, data for neutralising antibodies will be acquired in future studies to further understand the immunogenicity of trastuzumab SC. No patients tested positive for anti-rHuPH20-neutralising antibodies.
Convenience and added value to patients and health-care providers
Trastuzumab IV is administered over 90 min for the loading dose, with subsequent doses infused over 30 min (if the loading dose is well tolerated). The infusion must be administered by a health-care provider, preferably in an outpatient setting, and patients should be observed for 6 h after the start of the first infusion and 2 h after the start of subsequent infusions for infusion-related reactions (F. Hoffmann-La Roche, 2011). As mentioned, long infusion times are associated with increased resource costs, including those for physical space, medical supplies, and qualified staff (Haller, 2007), with additional costs incurred by the need for long observation times in the hospital.
Trastuzumab SC can be given in ∼5 min, with a median injection rate of 2 ml min−1 and a typical volume of 3.4–11.9 ml (Wynne et al, 2013). Thus, trastuzumab SC has the potential to reduce health-care system burden. The required observation time for trastuzumab SC is under evaluation. The cost-effectiveness of trastuzumab IV is well established in early and metastatic breast cancer (Chan et al, 2009; Perez-Ellis et al, 2009), and it is probable that trastuzumab SC will be associated with additional cost savings over trastuzumab IV.
Ongoing and planned studies with trastuzumab SC
There are a number of studies ongoing with trastuzumab SC (Table 1). The Enhanced Treatment With Neoadjuvant Herceptin study (BO22227; NCT00950300) – a pivotal study comparing trastuzumab SC with trastuzumab IV in the neoadjuvant–adjuvant setting – is evaluating pharmacokinetics, efficacy, and safety. The co-primary end points of efficacy and pharmacokinetics have been met (Ismael et al, 2012). A second study (CP3; NCT01344863) comparing the pharmacokinetics of a trastuzumab SC injection system vs manual injection in HMVs will report results soon (US NIH, 2011). Other studies include Preferences for Herceptin (PrefHer; NCT01401166; US NIH, 2011) and A Safety and Tolerability Study of Assisted- and Self-administered Therapy in Patients With Early HER2-Positive Breast Cancer (SafeHer; NCT01566721; US NIH, 2011). The PrefHer study is a patient preference study on IV vs SC administration and is also assessing health-care provider satisfaction and time savings. The SafeHer study is a global study evaluating the safety of repeated SC injections. Secondary objectives include event-free survival and overall survival; single-use injection device handling; and patient, physician, nurse, and pharmacist perceptions on usage and acceptability. Both PrefHer and SafeHer are assessing the use of an injection system vs manual injection and are due to complete in 2013–2014.
Conclusions
For patients receiving treatment over prolonged periods, SC administration offers significant benefits over IV administration and is considered convenient. For haematologic malignancies, rituximab is typically given for 6 months as induction therapy and for up to 2 years as maintenance therapy. In the adjuvant setting, trastuzumab is recommended for use for 1 year, whereas some patients with metastatic breast cancer receive treatment for 6–8 years or longer. Coformulation of rituximab or trastuzumab with purified rHuPH20 has the potential to overcome the challenges of SC administration by enabling drug dispersion and absorption at the administration site. Unlike previous preparations of hyaluronidase, rHuPH20 is not associated with the immunogenicity observed with animal-derived preparations, allowing well-tolerated delivery of mAbs. The SC formulations of rituximab or trastuzumab coformulated with rHuPH20 are currently under development, with the aim of demonstrating non-inferiority to IV formulations in terms of pharmacokinetics (Ctrough), efficacy, and safety. Given the shorter delivery time, comparable safety profile, and lack of need for dedicated infusion facilities, SC formulations of rituximab or trastuzumab can potentially improve convenience and quality of life for patients and provide cost savings to health-care systems.
Acknowledgments
Support for third-party writing assistance was provided by F Hoffmann-La Roche Ltd.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Assouline S, Buccheri V, Delmer A, Doelken G, Gaidano G, McIntyre C, Brewster M, Hourcade-Potelleret F, Sayyed P, Badoux X.2012Subcutaneous rituximab in combination with fludarabine and cyclophosphamide for patients with CLL: initial results of a phase Ib study (SAWYER [BO25341]) show non-inferior pharmacokinetics and comparable safety to that of intravenous rituximab Blood 120: Abstract1637
- Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114:230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Chan AL, Leung HW, Lu CL, Lin SJ. Cost-effectiveness of trastuzumab as adjuvant therapy for early breast cancer: a systematic review. Ann Pharmacother. 2009;43:296–303. doi: 10.1345/aph.1L504. [DOI] [PubMed] [Google Scholar]
- Davies A, Merli F, Mihaljevik B, Siritanaratkul N, Solal-Céligny P, Boehnke A, Berge C, McIntyre C, Barrett M, Macdonald D.2012Pharmacokinetics (PK), safety and overall response rate (ORR) achieved with subcutaneous (SC) administration of rituximab in combination with chemotherapy were comparable to those achieved with intravenous (IV) administration in patients (pts) with follicular lymphoma (FL) in the first-line setting: stage 1 results of the phase III SABRINA study (BO22334) Blood 120: Abstract1629
- Del Nagro CJ, Mao CP, Brovarney M.2010Comparison of B cell depletion mediated by intravenous (IV) Rituxan and Rituxan given subcutaneous (SC) in cynomolgus monkeys Blood 116Abstract3980 [Google Scholar]
- F. Hoffmann-LaRoche.MabThera SmPC (2008 ) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf (Accessed 3/10/2011).
- F. Hoffmann-LaRoche.Herceptin SmPC (2011 ) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000278/WC500074922.pdf (Accessed 14/10/2011).
- Gilbert D, Cothran D.2005SC versus IV delivery: reducing costs while increasing patient satisfaction Hematol Oncol News Issues. December 25–27Available from URL http://www.towniecentral.com/Hemonctown/Magazine.aspx (Accessed23/05/2013)
- Haller MF.2007. Converting intravenous dosing to subcutaneous dosing with recombinant human hyaluronidase. Pharm Tech http://www.pharmtech.com/pharmtech/article/articleDetail.jsp?id=463578 (Accessed 28/01/2012).
- Harb G, Lebel F, Battikha J, Thackara JW. Safety and pharmacokinetics of subcutaneous ceftriaxone administered with or without recombinant human hyaluronidase (rHuPH20) versus intravenous ceftriaxone administration in adult volunteers. Curr Med Res Opin. 2010;26:279–288. doi: 10.1185/03007990903432900. [DOI] [PubMed] [Google Scholar]
- Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, Pienkowski T, Lichinitser M, Semiglazov V, Melichar B, Jackisch C. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- Kagan L, Turner MR, Balu-Iyer SV, Mager DE. Subcutaneous absorption of monoclonal antibodies: role of dose, site of injection, and injection volume on rituximab pharmacokinetics in rats. Pharm Res. 2012;29:490–499. doi: 10.1007/s11095-011-0578-3. [DOI] [PubMed] [Google Scholar]
- Lundin J, Kimby E, Bjorkholm M, Broliden PA, Celsing F, Hjalmar V, Möllgård L, Rebello P, Hale G, Waldmann H, Mellstedt H, Osterborg A. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1 H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2002;100:768–773. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28:507–532. doi: 10.1023/a:1014414520282. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network 2012Clinical practice guidelines in oncology. Non-Hodgkin's lymphoma . http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf (Accessed 14/03/2012).
- Perez-Ellis C, Gonçalves A, Jacquemier J, Marty M, Girre V, Roché H, Brain E, Moatti JP, Viens P, Le Corroller-Soriano AG. Cost-effectiveness analysis of trastuzumab (Herceptin) in HER2-overexpressed metastatic breast cancer. Am J Clin Oncol. 2009;32:492–498. doi: 10.1097/COC.0b013e3181931277. [DOI] [PubMed] [Google Scholar]
- Salar A, Avivi I, Larouche J-F, Janikova A, Pereira J, Brewster M, Catalani O, McIntyre C, Sayyed P, Haynes A.2012Final results of the BP22333 study demonstrate non-inferior pharmacokinetics (PK) and safety of subcutaneous (SC) administration of rituximab compared with intravenous (IV) administration as maintenance therapy in patients with follicular lymphoma (FL) Blood 120Abstract1641 [Google Scholar]
- Salar A, Bouabdallah R, McIntyre C, Sayyed P, Bittner B.2010A two-stage phase Ib study to investigate the pharmacokinetics, safety and tolerability of subcutaneous rituximab in patients with follicular lymphoma as part of maintenance treatment Blood 116Abstract285820947691 [Google Scholar]
- Salar A, Casao D, Cervera M, Pedro C, Calafell M, Abella E, Alvarez-Larrán A, Besses C. Rapid infusion of rituximab with or without steroid-containing chemotherapy: 1-yr experience in a single institution. Eur J Haematol. 2006;77:338–340. doi: 10.1111/j.1600-0609.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- Sehn LH, Donaldson J, Filewich A, Fitzgerald C, Gill KK, Runzer N, Searle B, Souliere S, Spinelli JJ, Sutherland J, Connors JM. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109:4171–4173. doi: 10.1182/blood-2006-11-059469. [DOI] [PubMed] [Google Scholar]
- Selam JL. Evolution of diabetes insulin delivery devices. J Diabetes Sci Technol. 2010;4:505–513. doi: 10.1177/193229681000400302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilgenbauer S, Zenz T, Winkler D, Bühler A, Schlenk RF, Groner S, Busch R, Hensel M, Dührsen U, Finke J, Dreger P, Jäger U, Lengfelder E, Hohloch K, Söling U, Schlag R, Kneba M, Hallek M, Döhner H, German Chronic Lymphocytic Leukemia Study Group Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2009;27:3994–4001. doi: 10.1200/JCO.2008.21.1128. [DOI] [PubMed] [Google Scholar]
- Thomas JR, Wallace MS, Yocum RC, Vaughn DE, Haller MF, Flament J. The INFUSE-Morphine study: use of recombinant human hyaluronidase (rHuPH20) to enhance the absorption of subcutaneously administered morphine in patients with advanced illness. J Pain Symptom Manage. 2009;38:663–672. doi: 10.1016/j.jpainsymman.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Thomas JR, Yocum RC, Haller MF, von Gunten CF. Assessing the role of human recombinant hyaluronidase in gravity-driven subcutaneous hydration: the INFUSE-LR study. J Palliat Med. 2007;10:1312–1320. doi: 10.1089/jpm.2007.0126. [DOI] [PubMed] [Google Scholar]
- US FDA 2011US Department of Health and Human Services . http://www.fda.gov (Accessed 14/03/2012).
- US NIH 2011. Clinical Trials.gov, http://www.clinicaltrials.gov (Accessed 14/03/2013).
- Vaughn DE, Yocum RC, Muchmore DB, Sugarman BJ, Vick AM, Bilinsky IP, Frost GI. Accelerated pharmacokinetics and glucodynamics of prandial insulins injected with recombinant human hyaluronidase. Diabetes Technol Ther. 2009;11:345–352. doi: 10.1089/dia.2009.0013. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Vescia S, Baumgärtner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, Harbeck N. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008;19:9–15. doi: 10.1093/annonc/mdm272. [DOI] [PubMed] [Google Scholar]
- Vogel WH. Infusion reactions: diagnosis, assessment, and management. Clin J Oncol Nurs. 2010;14:E10–E21. doi: 10.1188/10.CJON.E10-E21. [DOI] [PubMed] [Google Scholar]
- Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body sized based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012–1024. doi: 10.1177/0091270009337512. [DOI] [PubMed] [Google Scholar]
- Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53:192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- Yin A, Li J, Hurst D, Visich J.2010Population pharmacokinetics (PK) and association of PK and clinical outcomes of rituximab in patients with non-Hodgkin's lymphoma J Clin Oncol 28(Suppl 15s):Abstracte13108 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.