Abstract
Purpose
The present meta-analysis aimed at assessing the effectiveness and safety of tranexamic acid (TXA) in reducing blood loss and transfusion in spinal surgery.
Methods
Systematic searches of all studies published through March 2012 were identified from PubMed, EMBase, Cochrane library, Science Direct, and other databases. Only randomized controlled trials (RCTs) were included in the present study. Two independent reviewers searched and assessed the literature. Mean difference (MD) of blood loss and blood transfusions, risk ratios (RR) of transfusion rate and of deep vein thrombosis rate in the TXA-treated group versus placebo group were pooled throughout the study. The meta-analysis was conducted by RevMan 5.1 software.
Results
Six placebo-controlled RCTs encompassing 411 patients met the inclusion criteria for our meta-analysis. The use of TXA significantly reduced both total blood loss [MD = −285.35, 95 % CI (−507.03 to −63.67), P = 0.01] as well as the number of patients requiring blood transfusion [RR = 0.71, 95 % CI (0.54–0.92), P = 0.01]. None of the patients in the treatment group had deep-vein thrombosis (DVT) or pulmonary embolism.
Conclusions
Intravenous use of TXA for patients undergoing spinal surgery is effective and safe. It reduces total blood loss and the need for blood transfusion, particularly in the using of high dosage of TXA (≥15 mg/kg), yet does not increase the risk of postoperative DVT. Due to the limitation of the quality of the evidence currently available, high-quality RCTs are required.
Keywords: Tranexamic acid, Spine, Blood loss, Surgical, Meta-analysis
Introduction
Surgical procedures are inevitably connected with bleeding. The amount of blood loss varies widely between different surgical operations and depends on surgical and non-surgical factors. Spinal operations that entail spinal instrumentation are particularly prone to large blood loss with long operation times and large wound surfaces. Dealing with cancellous bone with its rich blood supply is often associated with significant perioperative blood loss and usually requires multiple blood transfusions [1–3]. Allogeneic blood transfusion carries the significant risk of transmitting infections (viral and bacterial), hemolytic transfusion reactions, transfusion-related lung injury, and increases hospital costs [4–6].
Improved surgical techniques and perioperative anesthetic management may reduce blood loss, but the majority of patients still need blood transfusions [7]. Supplying adequate safe blood remains a challenge always. Therefore, different methods are used to reduce blood loss of spinal operations. Tranexamic acid (TXA) is a synthetic antifibrinolytic drug that competitively blocks the lysine-binding sites of plasminogen, plasmin, and tissue plasminogen activator, thereby delaying fibrinolysis and blood clot degradation [8]. Using TXA perioperatively can reduce intraoperative blood loss and decrease the need for allogeneic blood transfusion. Its efficacy is documented in cardiac surgery as well as hip and knee replacement surgery [9, 10]. Although TXA is used to minimize intraoperative blood loss, each technique has its own benefits and harmful effects. There is still no consensus whether to use antifibrinolytic agents during spinal surgery or not [11–13]. The purpose of the present meta-analysis is to evaluate the evidence from randomized controlled trials (RCTs) that reported the safety and efficacy of TXA in the reduction of blood loss in spinal surgery.
Materials and methods
Search strategy
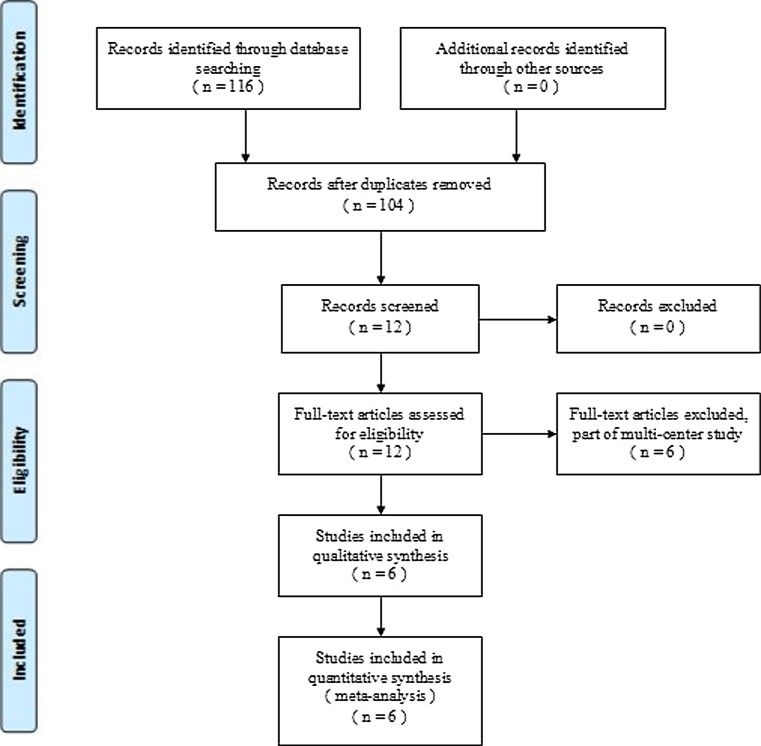
For our meta-analysis we looked at all English and non-English academic articles identified from different electronic databases, including MEDLINE (1966–March 2012), Embase (1980–March 2012), and the Cochrane Central Register of Controlled Trials. The following search terms were used to maximize the search specificity and sensitivity: tranexamic acid, spine surgery, and spinal surgery. Broad MeSH terms and Boolean operators were selected for each database search. The search strategy is presented in Fig. 1, and it only included studies conducted on humans. In addition, using the same search terms we manually searched for further relevant studies such as those of the European Federation of National Associations of Orthopedic and British Orthopedic Association Annual Congress as well as the database of Google. They were also searched for entries up to March 2012 to find further articles which may have been missed in the database search. The reference lists of all the full text papers were examined to identify any initially omitted studies. We made no restrictions on the language of the publication.
Fig. 1.
Flow chart of the study selection and inclusion process
Selection criteria and quality assessment
We included all published RCTs and quasi-randomized controlled trials (qRCTs: method of allocating participants to a treatment which is not strictly random, e.g., by date of birth, hospital record number, alternation) and compared spinal surgery patients using TXA versus placebo. Exclusion criteria comprised the following: trials with retrospective design, those without randomization of patients into two relevant groups as well as studies focusing on a special orthopedic subgroup of patients. We assessed trial quality using the PEDro critical appraisal score that is a reliable and valid scoring system assessing 11 items [14].
Data extraction
For each eligible study, two of the reviewers (Z.J. Li and X. Fu ) extracted all the relevant data independently. Any disagreement was resolved by discussion; when no consensus could be achieved, the third reviewer (Zang J.C.) was the adjudicator and made the final decision. Whenever necessary, we contacted the authors of the studies for the missing data or further information. The following data were extracted: (1) demographic data of participants; (2) indication for spinal surgery; (3) total blood loss, the percentage of postoperative patients who received allogeneic blood transfusion, the amount of transfused allogeneic blood, the drop in hemoglobin or hematocrit, thromboembolic complications, and (4) any other outcomes as mentioned in individual studies were considered for inclusion. In studies in which data were incomplete or unclear, attempts were made to contact the investigators for clarification.
Data analysis and statistical methods
The meta-analysis was conducted with by the Review Manager software 5.1 for Windows (RevMan Version 5.1, The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). We assessed statistical heterogeneity for each study with the use of a standard Chi square test with a significance set at a P value of 0.1, and the quantity of heterogeneity was measured by I2 statistic [15]. An I2 statistic value of 50 % was considered to indicate substantial heterogeneity. If comparing trials showing heterogeneity, pooled data were meta-analyzed using a random effects model [16]. Otherwise, a fixed-effects model was used for the analysis [17]. The clinical heterogeneity prevented a direct quantitative meta-analysis; the data would be pooled by subgroup analysis according to the high or low dosage of Tranexamic acid (dose ≥ 15 mg/kg or dose < 15 mg/kg). For continuous outcomes, such as total blood loss and amount of transfused blood per patient, the means and standard deviations were pooled to a mean difference (MD) and 95 % confidence interval (CI). Risk ratios (RR) and 95 % confidence interval (CI) were calculated for dichotomous outcomes, such as the incidence of the number of patients requiring transfusion and the incidence of deep vein thrombosis.
Results
Search results
We identified a total of 116 citations as potentially relevant studies. By screening the title, reading the abstract and the entire article six placebo-controlled RCT studies were identified that eventually fulfilled the eligibility criteria. The six placebo-controlled RCTs studies include a total of 411 patients with complete follow-up and were thus eligible for data extraction and meta-analysis [18–23]. Fig. 1 shows the flow chart of included and excluded studies.
Quality assessment
Table 1 summarizes the key characteristics of the included studies: of the 411 total patients (182 males and 229 females), the individual sample sizes in the six studies ranged from 40 to 147 patients; a total of 208 patients used TXA for their spinal surgery, and the remaining 203 patients received placebos.
Table 1.
Characteristics of included studies
| Study | Sample size | Mean age | Gender (f/m) | Dose | Operative interventions | Blood transfusion protocol |
|---|---|---|---|---|---|---|
| Elwatidy et al. [18] | ||||||
| TXA | 32 | 51.56 ± 19.08 | 11/21 | 2 g (for adults) or 30 mg/kg (for children) | Multiple level anterior cervical discectomy with or without fixation; spinal decompression or fixation; laminectomy and excision of spinal tumor | Hb < 90 g/L or HCT < 27 % |
| Placebo | 32 | 49.75 ± 21.04 | 14/18 | |||
| Farrokhi et al. [19] | ||||||
| TXA | 38 | 45.5 ± 11.6 | 11/7 | 10 mg/kg | Posterior thoracic or lumbar instrumented spinal fusion of 4–6 vertebrae | Unclear |
| Placebo | 38 | 51.4 ± 11.6 | 27/11 | |||
| Neilipovitz et al. [20] | ||||||
| TXA | 22 | 14.1 ± 2.1 | 13/5 | 10 mg/kg bolus, then 1 mg/kg/h until skin closure | Posterior spinal fusion | Hb < 70 g/L |
| Placebo | 18 | 13.7 ± 2.5 | 10/12 | |||
| Sethna et al. [21] | ||||||
| TXA | 23 | 13.6 ± 1.8 | 6/17 | 100 mg/kg and 10 mg/kg/h until skin closure | Spinal instrumentation | HCT < 25 % |
| Placebo | 21 | 14.0 ± 12.0 | 8/13 | |||
| Tsutsumimoto et al. [22] | ||||||
| TXA | 20 | 68.0 ± 11.0 | 4/16 | 15 mg/kg | Cervical laminoplasty | Unclear |
| Placebo | 20 | 65.8 ± 11.8 | 5/15 | |||
| Wong et al. [23] | ||||||
| TXA | 73 | 56.8 ± 16.2 | 52/21 | 10 mg/kg, then 1 mg/kg/h until skin closure | Posterior thoracic/lumbar Instrumented spinal fusions |
Hb < 70 g/L |
| Placebo | 74 | 50.0 ± 16.2 | 48/26 | |||
Table 2 summarizes the criteria of methodological quality: all of the six placebo-controlled RCT studies have a low risk of bias. Most trials were small, with patient numbers ranging from 40 to 147. However, trials were relatively well designed and the PEDro scores were generally high. The mean score was nine (range 7–11).
Table 2.
PEDro critical appraisal score
| Elwatidy et al. [18] | Farrokhi et al. [19] | Neilipovitz et al. [20] | Sethna et al. [21] | Tsutsumimoto et al. [22] | Wong et al. [23] | |
|---|---|---|---|---|---|---|
| Eligibility criteria | 1 | 1 | 1 | 1 | 1 | 1 |
| Random allocation | 0 | 1 | 1 | 1 | 0 | 1 |
| Concealed allocation | 1 | 1 | 1 | 0 | 0 | 1 |
| Baseline comparability | 1 | 1 | 1 | 1 | 1 | 1 |
| Blind subject | 1 | 1 | 1 | 0 | 0 | 1 |
| Blind clinician | 1 | 1 | 1 | 0 | 0 | 1 |
| Blind assessor | 1 | 1 | 1 | 0 | 1 | 1 |
| Adequate follow-up | 1 | 0 | 1 | 1 | 1 | 1 |
| Intention-to treat analysis | 0 | 1 | 1 | 1 | 1 | 0 |
| Between-group analysis | 1 | 1 | 1 | 1 | 1 | 1 |
| Point estimates and variability | 1 | 1 | 1 | 1 | 1 | 1 |
| Total score | 9 | 10 | 11 | 7 | 7 | 10 |
Different doses and time of delivery of TXA were used. The dosage ranged from approximately 10 to 100 mg/kg. The blood transfusion protocol applied is important for the study designed; however, only four of six trials apart from one clearly specified their transfusion protocol. The other two studies had not clearly specified their transfusion protocol in their trails.
Effects of intervention
Total blood loss
Data on total blood loss were available in all included trials with a total of 411 patients. The pooled results showed a positive, the effect of TXA in all treatment groups, significantly reducing total blood loss by MD = −285.35 ml (95 % CI −507.03 to −63.67, P = 0.01). However, there was significant heterogeneity in the finding (χ2 = 13.04, df = 5, I2 = 62 %, P = 0.02) among the studies included (Fig. 2).
Fig. 2.
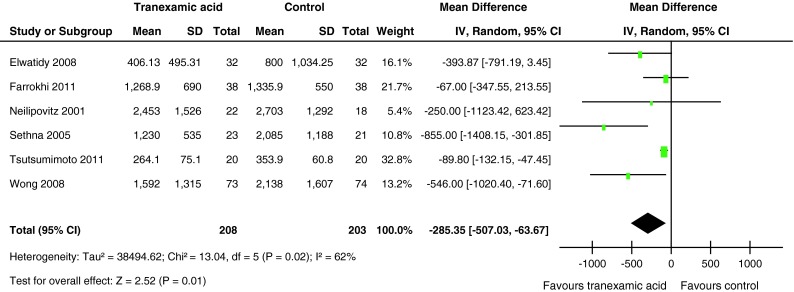
Forest plot diagram showing the effect of tranexamic acid (TXA) on total blood loss
Subgroup analysis was performed to identify possible differences between patients receiving TXA in high or low dosage (dose ≥ 15 mg/kg or dose < 15 mg/kg). The results showed that no significant difference reduced total blood loss compared with the placebo group individually by using of TXA in high or low dosage (Table 3).
Table 3.
Subgroup analysis of transfusion and total blood loss outcome
| Outcome or subgroup | Studies | Effect estimate | ||||
|---|---|---|---|---|---|---|
| χ2 | I2 | MD | 95 % CI | P value | ||
| Total blood loss | ||||||
| Dose ≥ 15 mg/kg | 3 | 9.47 | 79 % | −374.89 | [−791.31,41.53] | 0.08 |
| Dose < 15 mg/kg | 3 | 2.92 | 31 % | −240.23 | [−564.10,83.65] | 0.15 |
| Amount of transfused blood per patient | ||||||
| Dose ≥ 15 mg/kg | 3 | 0.15 | 0 % | −368.00 | [−650.27,−87.16] | 0.01 |
| Dose < 15 mg/kg | 3 | 7.24 | 72 % | −112.42 | [−366.50,141.65] | 0.39 |
Need for transfusion
The number of patients requiring transfusion after surgery was provided in all six included studies. The pooled results indicated that the number of patients requiring transfusion was 27.4 % (57 of 208) patients treated with TXA compared with 38.4 % (78 of 203) patients treated with placebo. There was a significant difference between the groups in the number of patients requiring transfusion (RR = 0.71, 95 % CI 0.54–0.92, P = 0.01). There was no evidence of statistical heterogeneity in the findings of the six studies (χ2 = 3.13, df = 4, I2 = 0 %, P = 0.54) (Fig. 3).
Fig. 3.
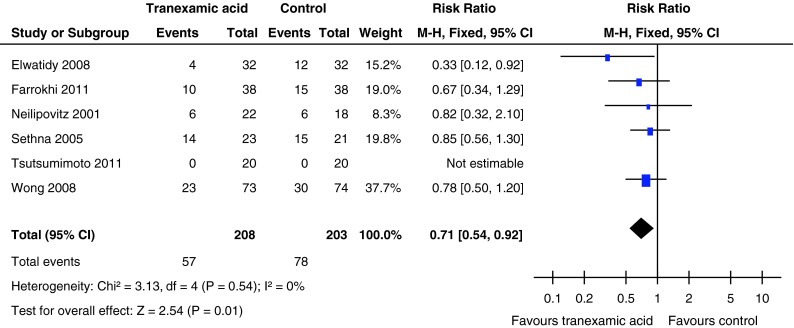
Forest plot diagram showing the effect of tranexamic acid (TXA) on blood transfusion rate
Amount of transfused blood per patient
All six trials, including a total of 411 participants, provided adequate data on the amount of transfused blood per patient. The pooled results indicated that there was no significant difference between the groups in the amount of transfused blood per patient (MD = −198.12 ml, 95 % CI −417.63 to 21.93; P = 0.08). However, there was significant heterogeneity in the finding (χ2 = 12.34, df = 4, I2 = 68 %, P = 0.02) (Fig. 4).
Fig. 4.
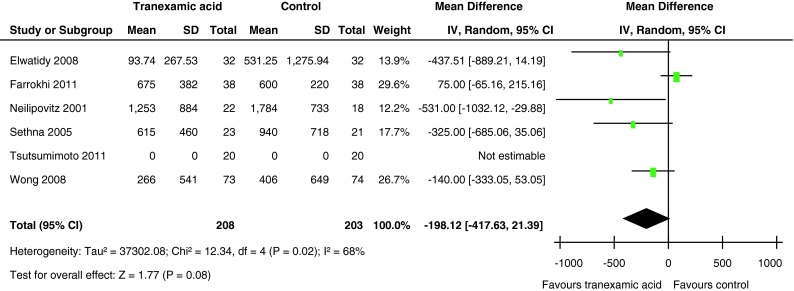
Forest plot diagram showing the effect of tranexamic acid (TXA) on the amount of transfused blood per patient
Subgroup analysis was performed on whether patients received TXA in high or low dosage (≥15 or <15 mg/kg). We found that in the subgroup that used high-dose TXA the amount of transfused blood per patient (P = 0.01) was significantly lower compared with the placebo group. But there was no significant difference between the low-dose TXA and the placebo group (P = 0.39) (Table 3).
Deep-vein thrombosis
The incidence of deep vein thrombosis was documented in all six studies. Only the study by Wong [23] reported one episode of DVT in the placebo-controlled group. The patient had used erythropoietin preoperatively.
Discussion
Many studies have used a variety of methods to reduce blood loss during spinal surgery, including autologous blood transfusion, intraoperative and postoperative blood recovery, drug treatment, controlled hypotension, etc., or a combination of several methods [24]. Antifibrinolytic drugs, especially TXA, have been routinely used in cardiac surgery, orthopedics, and liver surgery to reduce blood loss without increasing related complications or the risk of deep-vein thrombosis [9, 10]. However, few studies have reported safety and efficacy of TXA in spinal surgery. Therefore, the purpose of the present study was to pool the current evidence of TXA in the reduction of blood loss in spinal surgery by meta-analysis and to evaluate the effectiveness and safety of TXA objectively.
The methodological quality assessment identified some limitations of the current evidence bases. Only six randomized controlled trials satisfied the defined eligibility criteria. The size of the comparative groups was small in the two trials. Although all of studies included reported the randomization, two studies did not describe the specific methods of randomization. So, we did not have enough confidence with the information to determine whether to use the exact randomized methods or not. (No blinding of patients and the assessors to their surgical procedure permitted further measurement, which made expectational bias and potential statistical error for type II in these clinical outcomes). All studies included had the consistent baseline; nevertheless no intention to analysis was performed for withdrawals and dropouts. Accordingly, this review of meta-analysis should be considered as appropriate, and the defects in methodological quality should be considered when interpreting the findings.
Although the overall methodological quality of the included studies was high relatively, some degree of clinical heterogeneity was induced by the following factors: first, clinical heterogeneity may be caused by properties of different indication for transfusion, surgical technologies of different centers, and surgical complexity of the procedure. Second, the total blood loss and the amount of transfused blood per person may be affected by the operation time, type of surgery procedure, spinal level of surgery, number of spinal levels operated, surgery involving the sacrum or pelvis, surgery with spinal instrumentation, etc. Finally, the characteristics of patients of individual studies, such as age, pre-existing co-morbidities, and economic condition, may also be confounding factors, which may exert influence on the stability of the pooled results.
This meta-analysis showed that intravenous application of TXA can significantly reduce total blood loss and the needed of transfused blood, and decrease the amount of blood transfusion in spinal surgery compared with the placebo group. None of the patients in the TXA group had DVT. Spinal surgery usually leads to significant blood loss and even coagulation disorder, which often lead to blood transfusion [1–3]. However, the risks such as transmitted infectious diseases, allergic reactions, transfusion reactions, acute lung injury [4–6], etc., caused by allogeneic blood transfusion must be considered (intra and postoperatively). Furthermore, homologous blood transfusion represents a significant cost. Although the indication of blood transfusion varies in the difference studies, the most significant result of this review was that TXA reduced the rate of transfusions (pooled RR = 0.71, 95 % CI 0.54–0.92, P = 0.01). (The results showed that there is significant difference between the treatment versus placebo groups. More patients of the group treated with placebo needed transfusion than patients of the group treated with TXA, which is consistent with the results of the majority of the included studies). However, a retrospective study reported that tranexamic acid reduced blood loss, yet did not reduce transfusion rates in posterior lumbar spine surgery for lower bleeding risk and smaller overall blood loss of a posterior approach [25].
According to the studies included, intravenous TXA did not reduce the total blood loss and amount of transfused blood per patient. However, there were high heterogeneities for total blood loss (62 %) and the amount of transfused blood per patient (68 %). The sub-group analysis showed that use of TXA delivered in high dosage (≥15 mg/kg) had significantly reduced total blood loss and decreased the amount of transfused blood per patient, yet low dosage did not. Yagi et al. [26] did a retrospective, observational study on TXA during posterior spinal fusion for treatment of adolescent idiopathic scoliosis. They reported that in the prophylactic administration of high-dose TXA treatment group (1 g, then 100 mg/h until skin closure), the blood loss was significantly less and its patients received significantly fewer blood transfusions than the control group. There were no significant differences in intra and postoperative complications. (The above-mentioned factors indicate that administration of high dosage of TXA would have an outstanding effect on spinal surgery).
Only one study in this meta-analysis did not support the routine use of TXA in spinal operation. Farrokhi et al. [19] randomized 76 patients to either a single low dose of TXA (10 mg/kg) or a similar volume of saline as a pre-operative bolus. The results showed no significance difference between the two groups. However, other authors and the results of our meta-analysis attribute these findings to the fact that TXA was given in a dose too low to show a significant effect, as most of the other studies administered larger or continuous amounts of TXA, with overall good results.
Deep vein thrombosis is a common complication after spinal surgery, possibly to pulmonary embolism, with its morbidity and mortality. No statistical difference was found in occurrence of deep vein thrombosis, and heterogeneity was not found among them. Only one study reported a patient with DVT in the placebo group [23]. The patients had used erythropoietin preoperatively. Therefore, we have no enough evidence to confirm whether the TXA has the side effect in increasing the risk of DVT.
Henry et al. [27] did a similar meta-analysis on anti-fibrinolytic drugs, and came to the same conclusion. But 88 % of the studies included were about cardiac surgery, and all of them were only from before 2001. This is why their study could not fully assess the value of TXA in spinal surgery. Gill et al. [28] published a meta-analysis of antifibrinolytic agents used in spinal surgery in 2008, which showed that antifibrinolytic agents (including aprotinin, TXA, aminocaproic acid) might reduce blood loss in spinal surgery and the amount of blood transfusions. However, the study did not analyze whether these agents increase the risk of DVT postoperatively. Besides, it involved non-RCT studies, which induces a high risk of bias.
Our meta-analysis has several potential limitations: (1) only six studies were included and their sample size was rather small, which may result in a certain bias of the conclusions. For the safety assessment of the application of TXA in spinal surgery, we cannot make a valid statistical analysis. The statistical efficacy could be improved by including more studies with larger sample sizes in the future; (2) the spinal operations of the included studies are different from each other and different methods were used to calculate blood loss preoperatively. This will induce a bias in the blood loss of surgery as well. (3) Owing to the limited scope of included studies, subgroup analysis can not be performed on any source of heterogeneity. It may exert instability on consistency of outcomes; (4) (Publication bias may from significant conclusions being more easily published and non-English publications not being included in this meta-analysis, which may cause important studies to be overlooked); (5) the potential incompleteness of the reviewed evidence may have limited the validity of the pooled results. However, the present meta-analysis has several strengths, including the inclusion of the highest quality study design-RCTs, quantitative analysis, and comprehensive methodological quality assessment of included studies.
Conclusion
Based on the current evidence, the present meta-analysis showed that TXA reduces blood loss, the amount of transfused blood, and the rate of blood transfusion in spinal surgery without increasing any complications. However, whether the low (<15 mg/kg) dosage TXA is superior to placebo or not is inconclusive. Further high-quality RCTs should be designed to examine the best therapeutic dose and application time of TXA to reduce blood loss in spinal surgery.
Acknowledgments
The authors thank Dr Claudia Juzi for her helping with language and are grateful for the support by the National Natural Science Foundation of China (Grant 21205087) and the Tianjin Health Bureau Science and Technology Foundation (No. 2011kz117).
Conflict of interest
Each author certifies that he has no commercial associations that might pose a conflict of interest related to the submitted article.
Footnotes
Zhi-Jun Li and Xin Fu contributed equally to the study.
References
- 1.Behrman MJ, Keim HA. Perioperative red blood cell salvage in spine surgery. A prospective analysis. Clin Orthop Relat Res. 1992;278:51–57. [PubMed] [Google Scholar]
- 2.Nuttall GA, Horlocker TT, Santrach PJ, Oliver WC, Jr, Dekutoski MB, Bryant S. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976) 2000;25(5):596–601. doi: 10.1097/00007632-200003010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Tate DE, Jr, Friedman RJ. Blood conservation in spinal surgery review of current techniques. Spine (Phila Pa 1976) 1992;17(12):1450–1456. doi: 10.1097/00007632-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112(7):2617–2626. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandler SG, Yu H, Rassai N. Risks of blood transfusion and their prevention. Clin Adv Hematol Oncol. 2003;1(5):307–313. [PubMed] [Google Scholar]
- 6.Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfus Med. 2003;13(4):205–218. doi: 10.1046/j.1365-3148.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 7.Zheng F, Cammisa FP, Jr, Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine (Phila Pa 1976) 2002;27(8):818–824. doi: 10.1097/00007632-200204150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Hardy JF, Desroches J. Natural and synthetic antifibrinolytics in cardiac surgery. Can J Anaesth. 1992;39(4):353–365. doi: 10.1007/BF03009046. [DOI] [PubMed] [Google Scholar]
- 9.Arnold DM, Fergusson DA, Chan AK, Cook RJ, Fraser GA, Lim W, Blajchman MA, Cook DJ. Avoiding transfusions in children undergoing cardiac surgery: a meta-analysis of randomized trials of aprotinin. Anesth Analg. 2006;102(3):731–737. doi: 10.1213/01.ane.0000194954.64293.61. [DOI] [PubMed] [Google Scholar]
- 10.Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, Samama CM, Molliex S. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034–1046. doi: 10.1097/00000542-200611000-00026. [DOI] [PubMed] [Google Scholar]
- 11.Kebaish KM, Awad JN. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004;16(6):e1. doi: 10.3171/foc.2004.16.6.1. [DOI] [PubMed] [Google Scholar]
- 12.Sokolowski MJ, Garvey TA, Perl J, 2nd, Sokolowski MS, Cho W, Mehbod AA, Dykes DC, Transfeldt EE. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine (Phila Pa 1976) 2008;33(1):108–113. doi: 10.1097/BRS.0b013e31815e39af. [DOI] [PubMed] [Google Scholar]
- 13.Yonenobu K, Hosono N, Iwasaki M, Asano M, Ono K. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976) 1991;16(11):1277–1282. doi: 10.1097/00007632-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 17.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976) 2008;33(24):2577–2580. doi: 10.1097/BRS.0b013e318188b9c5. [DOI] [PubMed] [Google Scholar]
- 19.Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol. 2011;23(4):290–296. doi: 10.1097/ANA.0b013e31822914a1. [DOI] [PubMed] [Google Scholar]
- 20.Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93(1):82–87. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102(4):727–732. doi: 10.1097/00000542-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: a prospective randomized study. Spine (Phila Pa 1976) 2011;36(23):1913–1918. doi: 10.1097/BRS.0b013e3181fb3a42. [DOI] [PubMed] [Google Scholar]
- 23.Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, Abrishami A, Baig N, McBroom RJ, Chung F. Tranexamic acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):1479–1486. doi: 10.1213/ane.0b013e3181831e44. [DOI] [PubMed] [Google Scholar]
- 24.Tobias JD. Strategies for minimizing blood loss in orthopedic surgery. Semin Hematol. 2004;41(1 Suppl 1):145–156. doi: 10.1053/j.seminhematol.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Endres S, Heinz M, Wilke A. Efficacy of tranexamic acid in reducing blood loss in posterior lumbar spine surgery for degenerative spinal stenosis with instability: a retrospective case control study. BMC Surg. 2011;11:29. doi: 10.1186/1471-2482-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi M, Hasegawa J, Nagoshi N, Iizuka S, Kaneko S, Fukuda K, Takemitsu M, Shioda M, Machida M. Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine (Phila Pa 1976) 2012;37(21):E1336–E1342. doi: 10.1097/BRS.0b013e318266b6e5. [DOI] [PubMed] [Google Scholar]
- 27.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, Fergusson DA, Ker K (2011) Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev (1):CD001886 doi:10.1002/14651858.CD001886.pub3 [DOI] [PubMed]
- 28.Gill JB, Chin Y, Levin A, Feng D. The use of antifibrinolytic agents in spine surgery. A meta-analysis. J Bone Jt Surg Am. 2008;90(11):2399–2407. doi: 10.2106/JBJS.G.01179. [DOI] [PubMed] [Google Scholar]