Abstract
Purpose
To report three cases of transient perioperative neurological deficit in the absence of direct cord insult following decompression of the severely stenotic thoracic spine.
Methods
The clinical and radiographic electronic medical records of three patients who underwent decompression for severe midthoracic stenosis with transient neurological deficits perioperatively were reviewed. The cases are presented with consideration of possible underlying mechanisms and multimodality intraoperative monitoring (IOM) findings.
Results
Two patients had neurologic changes on IOM and Stagnara wake-up test, the remaining patient had absent motor and sensory potentials at baseline and throughout the case. IOM changes were observed immediately following decompression in the absence of direct cord insult or displacement. Postoperatively all patients experienced neurological motor deficits which presented as complete paralysis of the right lower extremity in two of the patients and the left lower extremity in one patient. The deficit was transient—improvement of motor strength occurred between 1 and 13 months of follow-up in all patients.
Conclusion
Decompression of a severely stenotic region of the thoracic spinal cord may lead to a complete yet transient motor deficit in the perioperative period in the absence of direct mechanical cord insult. Potential etiologies include ischemia-reperfusion injury, microthrombi, and altered perfusion due to internal recoil of spinal cord architecture following decompression. IOM may show conspicuous findings in such events, however, may not be relied upon when baseline potentials are sub-optimal. Recognition of this short-lived neurological deficit following decompression of the severely stenotic thoracic spine will improve preoperative patient counseling and merits further study for determination of the precise pathophysiology.
Keywords: Stenosis, Midthoracic, Decompression, Neurological deficit, Neuromonitoring
Introduction
Neurological deficit in the perioperative period is an under-recognized potential untoward event of spinal decompression surgery. A small subset of patients may develop a neurological deficit in the absence of direct or indirect physical insult to the spinal cord, which at the present time remains a diagnostic conundrum. Careful surgical technique is implemented in spine surgery to avoid any potential trauma to neural tissue during decompression procedures at the cord level. The severely stenotic thoracic spinal cord undergoes changes in its cross-sectional dimensions over time and histological examination of the compressed spinal cord of spondylotic myelopathy patients reported changes such as cord flattening, myelin and axon swelling, or gliosis almost six decades ago [9]. Internal reorganization of the decompressed spinal cord structure is likely accompanied by cellular and biochemical changes to accommodate reperfusion and improved axoplasmic flow, and activation of cellular pathways and release of free radicals contribute to neuronal deterioration associated with cord compression [3]. Recent results from an animal model of spinal cord compression support the hypothesis of alterations in spinal cord blood flow to also play a role in the pathophysiological sequelae of cord compression [7]. Spine surgeons are well aware of the intraoperative appearance of the newly decompressed thecal sac with associated dural pulsations [14], and intraoperative decompression of the cord may be accompanied by recoil in the shape of the spinal cord itself.
There is a paucity of literature on atraumatic thoracic cord neurologic deficit following decompression with prior reports in the English literature limited to those of single cases. It has been previously suggested that decompression of a previously severely compressed region of the spinal cord may lead to sensory or motor deficits during the early postoperative period [4, 8], and reperfusion injury as a mechanism contributing to neural damage has been described in the setting of acute ischemic events [2, 6], however, not for chronic conditions such as thoracic stenosis.
Three patients who underwent decompression for severe midthoracic stenosis with transient neurological deficits perioperatively are presented to raise awareness of the possibility for a transient neurological deterioration, potentially related to reperfusion injury during midthoracic decompression surgery, to discuss the value of intraoperative neuromonitoring (IOM) for avoiding neurological complications in spine surgery, and to contribute to the understanding of a debilitating but transient complication that deserves special consideration in the preoperative informed consent process.
Case presentations
Case 1
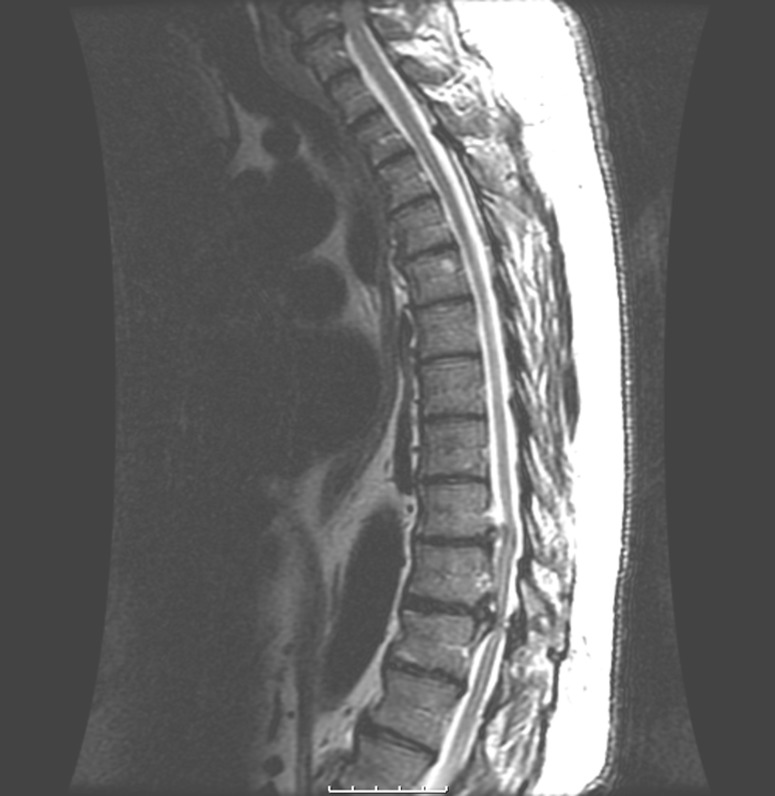
A 69-year-old man presented with a chief complaint of numbness and paresthesia in the toes of his right foot. He reported a 6-week history of thoracic radiculopathy with pain radiating into his right costovertebral angle approximately 3 months prior to presentation. Neurological and radiographic evaluations by attending physicians eventually lead to a diagnosis of severe cord compression secondary to ossification of the posterior longitudinal ligament at the T9–T10, T10–T11 and T11–T12 levels with hyperreflexia. The patient had tried conservative management without significant success and was scheduled for laminectomy with wide decompression and concomitant spinal fusion of the stenotic motion segments. Intraoperatively, immediately following careful decompression with a Kerrison rongeur, motor evoked potentials (MEPs) were lost, initially on the right but soon after that on the left side of the lower extremities. Upper extremity MEPs and somatosensory evoked potentials (SSEPs) remained normal throughout the procedure; however, simultaneously with lower MEP loss a slight increase in latency and subtle degradation of SSEP morphology was witnessed bilaterally via the tibial nerve. Intraoperative fluoroscopy demonstrated satisfactory placement of all pedicle screws. A Stagnara wake-up test showed no movement in the patient’s lower extremities. All rods and screws were removed and screw holes were sounded with a ball tip probe to confirm absence of pedicle breach at all levels. An intravenous steroid protocol was initiated. Magnetic resonance imaging (MRI) was obtained postoperatively and showed a focal area of high signal intensity on T2 weighted image within the spinal cord at the T10–T11 level. The cord edema had not been clearly visible on preoperative MRI (Fig. 1), where severe compression may have obscured such a finding. In the recovery room, examination demonstrated 0/5 motor strength in all muscle groups of the patient’s right lower extremity. On postoperative day 2, the patient had improved to preoperative motor strength, with the exception of a 3/5 motor deficit in his right tibialis anterior and extensor hallucis longus (EHL). Physical therapy was initiated and a postoperative steroid protocol was continued with Decadron. On postoperative day 6, the patient was discharged to a rehabilitation center. At the 13-month follow-up, the patient had regained full motor strength in his right tibialis anterior and EHL.
Fig. 1.
Sagittal magnetic resonance image of the compressed spinal cord (case 1). Large osseous ridges at the posterior margins of the T9–T10 and T10–T11 disc spaces with moderate cord compression at the T9–T10 and severe central canal stenosis at the T10–T11 level
Case 2
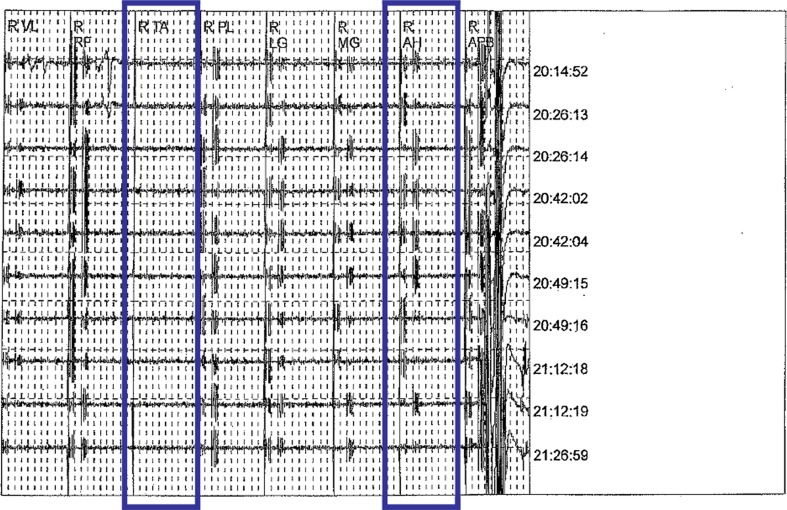
A 64-year-old man presented with a 5-year history of progressive weakness in his bilateral lower extremities and radicular pain in his left lower extremity with numbness and paresthesia extending from his left knee to his left toes. The patient further complained of weakness in both his lower extremities and of feeling “off-balance”. All conservative modalities including steroid injections failed to provide relief. On examination, the patient had a myelopathic gait pattern, Romberg’s sign was positive and muscle strength in his left lower extremity was 4/5 throughout. The patient also showed normal motor strength throughout his right lower extremity, except for a 4/5 weakness on right ankle dorsal flexion, inversion and eversion. Severe spondylosis with thoracic cord compression was evident on MRI and the patient was scheduled for a three-stage procedure, including posterior thoracic decompression, fusion and instrumentation at T9–T10 and T10–T11, posterior lumbar decompression at L3–L4 and L4–L5, and anterior thoracic discectomy at T9–T10 and T10–T11, corpectomy of the T10 vertebral body and anterior thoracic interbody fusion T9–T11. During the final stages of decompression (third stage procedure), bilateral loss of MEPs (Fig. 2) was witnessed initially, followed by bilateral tibial and peroneal SSEP loss shortly thereafter. On Stagnara wake-up test normal movement of the patient’s right lower extremity was elicited, but only minimal movement of his left lower extremity. An intravenous steroid protocol was initiated and on postoperative day 1 the patient had 1/5 motor strength in his left EHL and 0/5 strength of the left gastrocnemius, flexor hallucis longus and tibialis anterior muscles. The patient had full motor strength in his right lower extremity and sensation was intact throughout. MRI ruled out acute compressive processes. By postoperative day 4, the patient displayed 2/5 muscle strength in all major muscle groups of his left lower extremity. At the 2-month follow-up, the patient had improved to 3/5 motor strength on dorsal flexion on the left and showed 4/5 motor strength on plantar flexion and knee extension on the left and hip extension on the right.
Fig. 2.
Loss of motor evoked potentials on intraoperative neuromonitoring (case 2)
Case 3
A 45-year-old female patient with diastrophic dwarfism presented with a 1-year history of bilateral lower extremity weakness and paresthesia resulting in progression to a wheelchair. Physical examination revealed a hypoesthetic right lower extremity and 4/5 motor strength throughout all major muscle groups bilaterally. Imaging studies confirmed thoracic myelopathy due to congenital thoracic stenosis/kyphoscoliosis and lumbar stenosis and the patient was scheduled for lumbar decompression at L3–L5, wide thoracic decompression at T4–T10 and spinal fusion of the T4–T9 levels. Multimodality IOM was employed, but potentials were absent at baseline and throughout the procedure. Following surgery the patient showed no movement in either of her lower extremities. Steroids were administered and the patient was taken to the recovery room for observation. At the 2-week follow-up, motor strength in the right lower extremity was 0/5 throughout all major muscle groups and 2/5 in the left hip flexor, left hip abductor, left hip extensor, left hip adductor and left quadriceps. Motor strength of the left tibialis anterior, EHL and gastrocnemius muscles tested 0/5, but was strongly limited by a patient history of ankle surgery for clubfoot release. At 1-month follow-up, the patient showed considerable improvement and was able to ambulate with a cane. Motor strength was 4/5 throughout all major muscle groups; however, spasticity remained in the lower extremities.
Discussion
Decompression of the severely stenotic thoracic spinal cord in the absence of any direct trauma to the cord resulted in complete but transient neurological deficits in all three of the presented cases. To date, the underlying pathophysiology of such a finding remains unclear. Postoperative neurological deficits are usually detected on physical and neurological examination and may be a substantial disability to the patient. Iatrogenic cord insult is a well-known possible etiology, as it may for instance occur during introduction of the footplate of a larger Kerrison rongeur into a severely stenotic segment. It can be argued that the spinal cord at a midthoracic stenotic level is vulnerable even to minor trauma and that therefore careful decompression is required, potentially using a burr. The timing, nature and underlying circumstances of the neurological deficits observed in the presented cases make alternative underlying mechanisms more likely than direct cord trauma. Transient ischemia as a result of reperfusion injury has previously been suggested as a cause for postoperative paralysis by Hasegawa et al. [4] who reported 49 of 857 (5.7 %) cases of upper extremity palsy after decompression surgery for chronic compressive spinal cord disorders. Reperfusion of neural tissue is well-known to have deleterious clinical sequelae [2, 6] likely related to the role of reactive oxygen radical-mediated neuronal cell death [12]. Animal models have demonstrated that superoxide-mediated injury occurs following reperfusion during acute neuronal ischemic events [6]. However, a potential mechanism for decompression-related reperfusion injury of chronic ischemia (stenosis) has to date not been established. Microthrombi compromising the watershed regions of arterial supply are another possible etiology of transient postoperative paralysis after thoracic decompression surgery [8]. Compromise of the watershed region of the spine deserves special consideration in the presented cases, as surgeries were performed at a watershed zone, supplied by the artery of Adamkiewicz, which usually arises on the left at the T8–L2 level [11] and at T9 or T10 in 50 % of patients [1]. Neurapraxia during recoil in cross-sectional dimensions of the cord, and sudden drop in blood pressure also deserve consideration as possible etiologies.
Multimodality IOM, including MEPs and SSEPs, showed conspicuous changes in two of the three patients. In the remaining patient, baseline potentials could not be established, possibly indicative of a pre-existing abnormality in the sensory pathways, and remained absent throughout the procedure. According to a recent review article, the ability to achieve IOM baseline data in absence of neural axis abnormality varies from 70 to 98 % and 66–100 %, for SSEPs and MEPs, respectively [10]. The IOM changes observed in two of the patients, however, occurred late during surgery (after completion of decompression and for no obvious reason) and did not contribute to preventing neurological complications. A recent report of a patient undergoing posterior spinal fusion for severe kyphoscoliosis associated with spondylo-epiphyseal dysplasia demonstrated the possibility for MEPs to remain entirely normal intraoperatively while a lower extremity paralysis may occur on wake-up examination [5]. False-positive and false-negative readings are a reality of current multimodality neurophysiological intraoperative monitoring technology (0.6–1.38 % and 0–0.79 %, respectively) [10]. A false negative may mislead the surgeon into a false sense of security despite an underlying untoward neurologic event. In 2006, a Japanese study group reported a case of delayed transient paraplegia following laminectomy for thoracic ossification of the posterior longitudinal ligament (OPLL) and ossification of the ligamentum flavum (OLF) in the absence of deleterious changes on intraoperative IOM. [13] In this report, neurologic exam was at baseline immediately postoperatively, however, over the ensuing 18 h the patient deteriorated neurologically to complete paraplegia. A second posterior fusion “rescue procedure” was performed and the patient recovered and had showed 4–5/5 motor strength in both lower limbs at 69 months of follow-up. The authors concluded that laminectomy affected the stability of the thoracic spine thus increasing the compression by OPLL anteriorly [13].
The present cases constitute the largest clinical series to date on atraumatic neurological deterioration following surgical decompression potentially in a region of the thoracic spine with vulnerable vascular supply. IOM—although presenting concern for alarm in two of the reported cases—may not always be able to predict or prevent such events. The cases presented here highlight the possibility of a complete yet transient neurological deficit in the absence of direct insult to the spinal cord following decompression of the severely stenotic thoracic spine and will help to improve the preoperative informed decision making process. These clinical reports merit further investigation into the underlying pathophysiology of this finding.
Conflict of interest
None.
References
- 1.Charles YP, Barbe B, Beaujeux R, Boujan F, Steib JP. Relevance of the anatomical location of the Adamkiewicz artery in spine surgery. Surg Radiol Anat. 2011;33:3–9. doi: 10.1007/s00276-010-0654-0. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976) 1998;23:2730–2737. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa K, Homma T, Chiba Y. Upper extremity palsy following cervical decompression surgery results from a transient spinal cord lesion. Spine (Phila Pa 1976) 2007;32:E197–E202. doi: 10.1097/01.brs.0000257576.84646.49. [DOI] [PubMed] [Google Scholar]
- 5.Hong JY, Suh SW, Modi HN, Hur CY, Song HR, Park JH. False negative and positive motor evoked potentials in one patient: is single motor evoked potential monitoring reliable method?: a case report and literature review. Spine (Phila Pa 1976) 2010;35:E912–E916. doi: 10.1097/BRS.0b013e3181d8fabb. [DOI] [PubMed] [Google Scholar]
- 6.Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurokawa R, Murata H, Ogino M, Ueki K, Kim P. Altered blood flow distribution in the rat spinal cord under chronic compression. Spine (Phila Pa 1976) 2011;36:1006–1009. doi: 10.1097/BRS.0b013e3181eaf33d. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Shim JJ, Doh JW, Yoon SM, Bae HG, Yun IG. Transient paraparesis after laminectomy in a patient with multi-level ossification of the spinal ligament. J Korean Med Sci. 2004;19:624–626. doi: 10.3346/jkms.2004.19.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mair WG, Druckman R. The pathology of spinal cord lesions and their relation to the clinical features in protrusion of cervical intervertebral discs; a report of four cases. Brain. 1953;76:70–91. doi: 10.1093/brain/76.1.70. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra NR, Shaffrey CI. Intraoperative electrophysiological monitoring in spine surgery. Spine. 2010;35:2167–2179. doi: 10.1097/BRS.0b013e3181f6f0d0. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Baeza A, Muset-Lara A, Rodriguez-Pazos M, Domenech-Mateu JM. The arterial supply of the human spinal cord: a new approach to the arteria radicularis magna of Adamkiewicz. Acta Neurochir (Wien) 1991;109:57–62. doi: 10.1007/BF01405699. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki M, Koda M, Okawa A, Aiba A. Transient paraparesis after laminectomy for thoracic ossification of the posterior longitudinal ligament and ossification of the ligamentum flavum. Spinal Cord. 2006;44:130–134. doi: 10.1038/sj.sc.3101807. [DOI] [PubMed] [Google Scholar]
- 14.Yip SL, Woo SB, Kwok TK, Mak KH. Nightmare of lumbar diskectomy: aorta laceration. Spine (Phila Pa 1976) 2011;36:E1758–E1760. doi: 10.1097/BRS.0b013e3182194e1c. [DOI] [PubMed] [Google Scholar]