Abstract
Purpose
Currently degeneration of the intervertebral disc and joint in the degenerative process of the lumbar spine has mainly attracted the attention, however, there are very few literatures focusing on the height of the spinous process. Our objective was to examine in what generation the change in spinous process height occurs and how the change is involved in the degenerative process of the lumbar spine.
Methods
CT or CT myelography of 1,015 patients, 536 males and 579 females were measured in 6 items, including the heights of the L4 and L5 vertebral bodies, the L4 and L5 spinous processes, the L4/5 intervertebral disc, and the L5/S1 intervertebral disc. All data of the 6 items were analyzed and compared between gender in 5 age groups (40s, 50s, 60s, 70s and 80s).
Results
The results indicated a significant increase in the height of the L4 and L5 spinous process (P < 0.01) in the 60- to 70-year-old group for both genders, and also showed that the L4 and L5 vertebral body height was significantly decreased in the 50- to 60-year-old group (P < 0.01 in males, P < 0.001 in females).
Conclusions
Changes in the spinous process morphology followed degenerative changes of the intervertebral disc and vertebral body in the degenerative process of the lumbar spine. This result may suggest that the morphological change of an increase in the height of the spinous process may be a kind of biological defense reaction to stabilize the intervertebral portion.
Keywords: Lumbar spine, Degeneration, Spinous process, Vertebral body, Intervertebral disc
Introduction
Degenerative lumbar spinal disease is reported to start with the degeneration of intervertebral discs, and after a period of progression to different pathologies including herniated intervertebral discs, spinal stenosis, intervertebral instability, etc., a stable stage is reached subsequent to the formation of osteophytes [1]. The degeneration of intervertebral discs and facet joints plays an especially important role in this degenerative change in the lumbar spine [2]. Many reports have been published in this regard [1, 3–6]. Furthermore, to a considerable extent, the heights of the vertebral body and spinous process may indicate in the degenerative changes in the lumbar vertebrae [1, 7–9]. A decrease in vertebral body height is mainly caused by a decrease in bone content and bone density due to aging, whereas an increase in the height of the spinous process is mainly attributable to the formation of enthesopathy spurs [1, 10]. However, very few reports have been published on the actual measurement of the heights of the intervertebral body and spinous process in this regard [8–10]. We determined the heights of the 4th (L4) and 5th (L5) lumbar vertebrae, the spinous processes in L4 and L5, and the vertebral discs between L4/5 and L5/S1 with computed tomography (CT) to investigate how the heights of intervertebral disc, vertebral body, and spinous process are related to aging and gender differences. The purpose of this study was to examine in what generation the change in spinous process height occurs and how the change is involved in the degenerative process of the lumbar spine.
Materials and methods
A total of 1,335 patients underwent CT or myelo-CT of the lumbar vertebrae at D hospital in Tsu City, Japan between January 2007 and December 2011 and had their image data stored in the hospital computer. We excluded those 39 years old or younger, those who had undergone a lumbar operation, those with L4 and L5 fractures, those definitely demonstrating lumbarization and sacralization, those with large osteophytes, and those with severe scoliosis and rotational deformity of the spine. We excluded 320 patients, and the remaining 1,015 patients were investigated. Their mean age was 69.1 years (40–89 years), and there were 536 males and 579 females; 451 (44.4 %) had undergone lumbar vertebral operations. The CT employed was ECROS 8 slices (Hitachi Medico, Tokyo, Japan). The images were taken with the parameters of 1-mm slice thickness, matrix 512 × 512 pixel resolution, 350 mm field of view, and 0.59 × 0.59–0.68 × 0.68 mm in-plane pixel size to prepare the reconstructed sagittal plane. Using the measurement tool of the DICOM Image Work Station (VOX-BASE/VIEW Version 2.69j; J-MAC SYSTEM Inc., Sapporo, Japan), the anterior and posterior wall heights of the L4 and L5 vertebral bodies, front and rear heights of the intervertebral discs between L4/5 and L5/S1, and the heights of the L4 and L5 spinous processes were determined on the computer as shown in Fig. 1. The vertebral body height was obtained by (height of anterior wall + height of posterior wall of vertebral body)/2. The height of the intervertebral disc was obtained by (front height + rear height of intervertebral disc)/2. For the height of the spinous process, a line parallel to the posterior wall of the vertebral body was drawn first, and the maximum length among all the slice lengths was obtained. Two orthopedic surgeons (A and B with 8 and 7 years of experience, respectively, as orthopedic surgeons) who did not know the objective of this research conducted the measurement. Using the reconstructed sagittal plane in the CT of each of the 1,015 cases, a total of six items, including the heights of the L4 and L5 vertebral bodies, the L4 and L5 spinous processes, the L4/5 intervertebral disc, and the L5/S1 intervertebral disc, were determined a total of three times, that is, twice by orthopedic surgeon A and once by B. The mean value of the three measured values was employed as the data point for each item. Furthermore, intra-observer error was calculated from the first and second values determined by orthopedic surgeon A, and the inter-observer error from the values measured by orthopedic surgeons A and B.
Fig. 1.
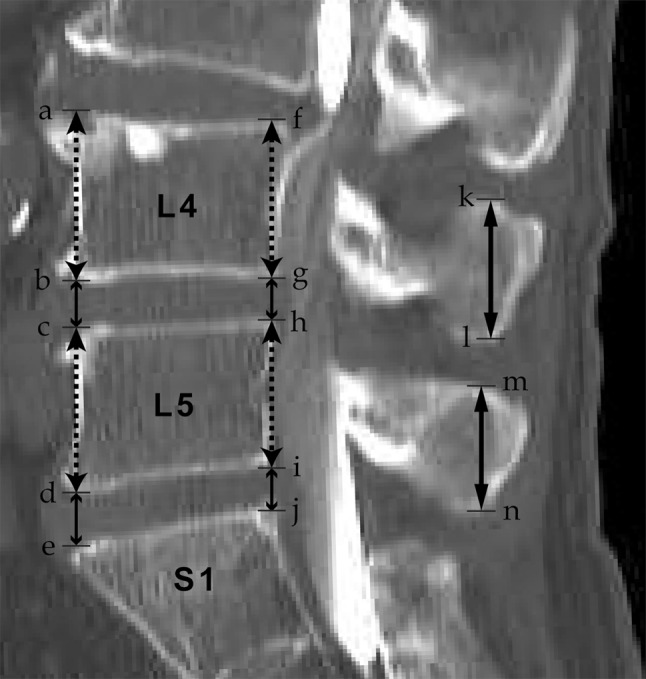
Illustration of measuring method at the L4–L5 and L5–S1 level. Anterior height of L4 vertebral body (a–b), posterior height of L4 vertebral body (f–g), anterior height of L4/5 intervertebral disc (b–c), posterior height of L4/5 intervertebral disc (g–h), anterior height of L5 vertebral body (c–d), posterior height of L5 vertebral body (h–i), anterior height of L5/S1 intervertebral disc (d–e), posterior height of L5/S1 intervertebral disc (i–j), height of L4 spinous process (k–l), height of L5 spinous process (m–n)
The patients were classified into five groups according to age (40–49 years old, 50–59 years old, 60–69 years old, 70–79 years old, and 80–89 years old). The average and standard deviation of the six items (the heights of the L4 and L5 vertebral bodies, the L4 and L5 spinous processes, the L4/5 intervertebral disc, and the L5/S1 intervertebral disc) were calculated for each group, and the data were compared between genders. Among the five age groups of patients, there were no statistically significant differences in the number of patients of each gender in each group as analyzed with the Kruskal–Wallis method (Table 1).
Table 1.
Number of patients of each gender in each group
| Groups | Gender | |
|---|---|---|
| Male | Female | |
| 40s | 57 | 73 |
| 50s | 64 | 88 |
| 60s | 85 | 115 |
| 70s | 120 | 158 |
| 80s | 111 | 144 |
No significant differences in each group
The intraclass correlation coefficient (ICC) and 95 % confidence interval were calculated for inter-observer and intra-observer reproducibility. We used a Welch t test to detect the difference among the five age groups for the six radiographic parameters, and a P value <0.05 was considered significantly different according to the Bonferroni correction.
Results
Table 2 shows the ICC and 95 % confidence interval for the inter-observer and intra-observer reproducibility. ICCs indicated very good intra-observer reproducibility for all radiographic parameters measured, with values of 0.91–0.93 for observer A. The ICC of the inter-observer reproducibility was also very good, with values of 0.86–0.92 for both observers in all parameters.
Table 2.
Inter- and intra-observer reproducibility
| Inter-observer reproducibility (observer A × B) | Intra-observer reproducibility (observer A) | |||
|---|---|---|---|---|
| ICC | 95 % CI | ICC | 95 % CI | |
| Height of L4 vertebral body | 0.86 | 0.79–0.94 | 0.91 | 0.83–0.95 |
| Height of L5 vertebral body | 0.88 | 0.80–0.95 | 0.92 | 0.85–0.96 |
| Height of L4/5 intervertebral disc | 0.91 | 0.85–0.96 | 0.93 | 0.87–0.97 |
| Height of L5/S1 intervertebral disc | 0.92 | 0.86–0.96 | 0.92 | 0.89–0.95 |
| Height of L4 spinous process | 0.89 | 0.83–0.93 | 0.91 | 0.87–0.94 |
| Height of L5 spinous process | 0.91 | 0.85–0.95 | 0.92 | 0.87–0.95 |
The results indicated a decrease in the heights of the L4 and L5 vertebral bodies and the height of the L4/5 intervertebral disc. We also observed an increase in the height of the L4 and L5 spinous processes according to age, but no relationship to age was observed in the height of the L5/S1 intervertebral disc. The results of the six radiographic parameters are shown in Table 3. The height of the L4 spinous process and L5 spinous process had significantly increased in the 60- to 70-year-old group (P < 0.001 in L4, P < 0.01 in L5) for both genders. The L4 vertebral body height was significantly decreased in the 50- to 60-year-old group (P < 0.01 in males, P < 0.001 in females), and then, the L5 vertebral body height was also significantly decreased in the 50- to 60-year-old group (P < 0.01 in males, P < 0.001 in females). The L4–L5 intervertebral disc height had a tendency to decrease with age (beginning in the 40- to 50-year-old group), but the difference was not statistically significant among the age groups in either gender. On the other hand, there was no change in the L5–S1 intervertebral disc height with age.
Table 3.
Morphological data of L4 and L5 (cm; mean ± SD)
| Age | 40s | 50s | 60s | 70s | 80s |
|---|---|---|---|---|---|
| Height of L4 spinous process | 2.07 ± 0.36 | 2.11 ± 0.25 | 2.15 ± 0.31a | 2.27 ± 0.30a | 2.30 ± 0.28 |
| Height of L5 spinous process | 1.76 ± 0.38 | 1.81 ± 0.31 | 1.81 ± 0.30b | 1.90 ± 0.37b | 1.92 ± 0.28 |
| Height of L4 vertebral body | 2.80 ± 0.34 | 2.77 ± 0.32b | 2.64 ± 0.32b | 2.63 ± 0.33 | 2.58 ± 0.31 |
| Height of L5 vertebral body | 2.64 ± 0.33 | 2.63 ± 0.32c | 2.53 ± 0.31c | 2.50 ± 0.31 | 2.47 ± 0.31 |
| Height of L4/5 intervertebral disc | 0.96 ± 0.25 | 0.89 ± 0.28 | 0.83 ± 0.23 | 0.79 ± 0.30 | 0.70 ± 0.29 |
| Height of L5/S1 intervertebral disc | 1.05 ± 0.26 | 1.02 ± 0.35 | 1.03 ± 0.32 | 1.01 ± 0.37 | 0.98 ± 0.36 |
aSignificant difference (P < 0.001) between groups
bSignificant difference (P < 0.01) between groups
cSignificant difference (P < 0.05) between groups
Discussion
Theoretically, intervertebral disc degeneration is thought to be the initial process in the typical degenerative cascade of the spine [1]. After the disc degenerates, the compressive load to the spine shifts from the vertebral body to the neural arch with high stress concentration at the facets [1, 7, 11]. Then, facet degeneration occurs. The vertebral bodies are also involved in the degenerative process, not only in osteophyte formation but also in the loss of vertebral height [1, 11, 12]. Vertebral height loss occurs for two reasons. First, high load bearing by the facet joint effectively shields the anterior region of the vertebral body from stress, so the vertebral body loses bone strength. If the spine is flexed, load bearing is shifted to the anterior body, leading to anterior wedge fracture. Second, vertebral bodies in older persons have high elastic deformability [13] due to the loss of bone mass, which allows increased buckling of the trabeculae and cortex [11]. This can explain the cupping deformation of the vertebral endplates in the spine of older persons [1, 13–15].
Our results showed that the height of the intervertebral disc between L5/S1 scarcely changed with aging. Hangai et al. [16] investigated the height of the intervertebral disc in healthy Japanese people and found that the height of the intervertebral disc between L4/5 and between L3/4 decreased with aging while not decreasing between L5/S1. However, they did not mention the reason. Our results in this study were the same as their results, and we also do not know the reason why the intervertebral disc height between L5/S1 did not decrease with aging.
Regarding the important role of the posterior element in the degenerative process, the neural arch can further oppose vertebral height loss from the compressive deformation so that the vertebral height loss rarely exceeds 3 mm [7]. In the spine of older persons with severely degenerated discs, the neural arch shares 63 % of the compressive load, whereas the anterior and posterior halves of the vertebral body bear only 10 and 26 % of the load, respectively [17, 18]. One biomechanical study has shown that an intact posterior element along with posterior ligamentous tissue increase stiffness and stability in an injury model at the L4–L5 segment [19].
Until now, the role of spinous processes in the degenerative process of the spine was unknown. However, some literature published in English mentions changes in spinous process morphology with age [9, 10]. Aylott et al. [9] measured the height and width of lumbar spinous processes with CT and showed significant increases that were correlated with age. Their study also showed a negative correlation between the height of the spinous processes and the degree of the lumbar lordosis. The group also stated that increases in the height of spinous processes may lead to decreased lumbar lordosis due to these structures abutting each other and blocking lumbar extension or lordosis. Spinous process abutment can be explained by both increases in the height of the spinous processes and decreases in the distance of the interspinous space with age [8, 20]. Although Aylott et al. [9] and Sartoris et al. [20] describe that the spinous process height increases with aging, they did not measure the vertebral body height, and hardly mentioned the degenerative process of the lumbar spine. Our results show that the height of the intervertebral disc becomes low from 40s or 50s, the height of the vertebral body becomes low from 50s or 60s, and the height of the spinous process becomes high from 60s or 70s, which are new findings.
From this study, we found that the heights of the L4–L5 intervertebral disc and L4 and L5 vertebral bodies were decreased, whereas the L4 and L5 spinous process heights were increased with age. The earliest degenerated structure was the L4–L5 intervertebral disc, which began to degenerate in the 40s and 50s and gradually narrowed with age. The second structures that degenerated were the L4 and L5 vertebral bodies. Our study showed that the vertebral bodies at both levels were significantly decreased in height in patients in their 50s and 60s (P < 0.01). The spinous processes were the last structure that changed with age. In patients in their 60s and 70s, the L4 and L5 spinous processes showed significantly increased heights (P < 0.01). Our result showed that males tended to have vertebral bodies and spinous processes that were larger than those in females, and the degenerative process in males tended to be less affected than in females. These results could be explained by the hypothesis that males are slightly protected from bone loss by their bone geometry, hormonal effects, and time-delay of age-related changes in bone density and quality [21–26].
Our hypothesis explaining the result of the increased heights of the L4 and L5 spinous processes with age may be a secondary adaptive response to increase their mechanical properties after sharing a compressive load from anterior structures, according to Wolf’s Law [1, 9]. Because we found that the spinous process height was increased after the intervertebral disc space narrowed and the vertebral body height was decreased, we think that the adaptive response occurred to act as a tethering structure to prevent hyper-extension of the lumbar spine and to increase the stiffness and stability. The advantage of this phenomenon may be a delay in canal stenosis because of blocking of hyper-extension or the lordosis position [27–31]. The disadvantage of this phenomenon is that it can cause sagittal misalignment in elderly persons [9]. As mentioned above, our results confirmed that there was a negative correlation between the vertebral body height and spinous process height with age. This may be a leading cause of flexion misalignment of the lumbar spine in elderly patients.
Regarding the clinical implications, as stated above, changing the spinous process morphology may be a secondary adaptive change to increase the mechanical properties to share the compressive load from the anterior element. Therefore, interspinous process devices and spinous process anchorage devices may play an important role in the treatment of degenerative disc diseases and early spinal stenosis patients [32–34]. These devices can mimic the natural effect of tethered and restricted hyper-extension or lordosis posture of the lumbar spine, spinous processes, and posterior ligamentous structures and also reduce facet loading at the implanted level [32, 33]. The increased size of the spinous processes may also improve anchorage positions for these implants.
Limitations of our study are: (1) this study was cross-sectional, (2) our study did not quantify the condition of the articular facets, (3) we did not perform a dynamic study to measure the distance between the spinous processes during motion, width or bone quality of the spinous process and lumbar lordosis, (4) we did not obtain the patients’ body mass index and bone mineral density data, and (5) we did not perform a biomechanical study to test our hypothesis that a compressive load was shared by the spinous processes and their ligamentous complexes when the disc was degenerated.
In conclusion, our results showed that an increase in height of spinous process was associated with decreased heights of intervertebral disc and vertebral body in the degenerative process of lumbar spine. This result may suggest that the morphological change of an increase in the height of the spinous process as well as the osteophytes are formed in the vertebral body may be a kind of biological defense reaction to stabilize the intervertebral portion. As described above, the spinal surgeon should pay more attention to an increase in the height of the spinous process as a possible characteristic finding of lumbar spinal instability.
Conflict of interest
None.
References
- 1.Adams MA. Degenerative disc and vertebral disease-basic sciences. Surgery (Oxford) 2009;27:297–300. doi: 10.1016/j.mpsur.2009.03.005. [DOI] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.Videman T, Battie MC, Gill K, Manninen H, Gibbons LE, Fisher LD. Magnetic resonance imaging findings and their relationships in the thoracic and lumbar spine. Insights into the etiopathogenesis of spinal degeneration. Spine. 1995;20:928–935. doi: 10.1097/00007632-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lewin T. Osteoarthritis in lumbar synovial joints: a morphologic study. Acta Orthop Scand. 1964;Suppl 73:1–112. doi: 10.3109/ort.1964.35.suppl-73.01. [DOI] [PubMed] [Google Scholar]
- 5.Oegema TR, Jr, Bradford DS. The inter-relationship of facet joint osteoarthritis and degenerative disc disease. Br J Rheumatol. 1991;30:16–20. doi: 10.1093/rheumatology/30.1.16. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop RB, Adams MA, Hutton WC. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg Br. 1984;66:706–710. doi: 10.1302/0301-620X.66B5.6501365. [DOI] [PubMed] [Google Scholar]
- 7.Pollintine P, Przybyla AS, Dolan P, Adams MA. Neural arch load-bearing in old and degenerated spines. J Biomech. 2004;37:197–204. doi: 10.1016/S0021-9290(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 8.Ihm EH, Han IB, Shin DA, Kim TG, Huh R, Chung SS (2011) Spinous process morphometry for interspinous device implantation in Korean patients. World neurosurg [Epub ahead of print] [DOI] [PubMed]
- 9.Aylott CE, Puna R, Robertson PA, Walker C. Spinous process morphology: the effect of ageing through adulthood on spinous process size and relationship to sagittal alignment. Eur Spine J. 2012;21:1007–1012. doi: 10.1007/s00586-011-2029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartoris DJ, Resnick D, Tyson R, Haghighi P. Age-related alterations in the vertebral spinous processes and intervening soft tissues: radiologic-pathologic correlation. AJR Am J Roentgenol. 1985;145:1025–1030. doi: 10.2214/ajr.145.5.1025. [DOI] [PubMed] [Google Scholar]
- 11.Pollintine P, van Tunen MS, Luo J, Brown MD, Dolan P, Adams MA. Time-dependent compressive deformation of the ageing spine: relevance to spinal stenosis. Spine. 2010;35:386–394. doi: 10.1097/BRS.0b013e3181b0ef26. [DOI] [PubMed] [Google Scholar]
- 12.Sevinc O, Barut C, Is M, Eryoruk N, Safak AA. Influence of age and sex on lumbar vertebral morphometry determined using sagittal magnetic resonance imaging. Ann Anat. 2008;190:277–283. doi: 10.1016/j.aanat.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Molloy S, Mathis JM, Belkoff SM. The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine. 2003;28:1549–1554. [PubMed] [Google Scholar]
- 14.Brinckmann P, Frobin W, Hierholzer E, Horst M. Deformation of the vertebral end-plate under axial loading of the spine. Spine. 1983;8:851–856. doi: 10.1097/00007632-198311000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Green TP, Allvey JC, Adams MA. Spondylolysis. Bending of the inferior articular processes of lumbar vertebrae during simulated spinal movements. Spine. 1994;19:2683–2691. [PubMed] [Google Scholar]
- 16.Hangai M, Kaneoka K, Kuno S, Hinotsu S, Sakane M, Namizuka N, Sakai S, Ochiai N. Factors associated with lumbar intervertebral disc degeneration in the elderly. Spine J. 2008;8:732–740. doi: 10.1016/j.spinee.2007.07.392. [DOI] [PubMed] [Google Scholar]
- 17.Adams MA, Pollintine P, Tobias JH, Wakley GK, Dolan P. Intervertebral disc degeneration can predispose to anterior vertebral fractures in the thoracolumbar spine. J Bone Min Res. 2006;21:1409–1416. doi: 10.1359/jbmr.060609. [DOI] [PubMed] [Google Scholar]
- 18.Kazarian LE. Creep characteristics of the human spinal column. Orthop Clin North Am. 1975;6:3–18. [PubMed] [Google Scholar]
- 19.Najarian S, Dargahi J, Heidari B. Biomechanical effect of posterior elements and ligamentous tissues of lumbar spine on load sharing. Biomed Mater Eng. 2005;15:145–158. [PubMed] [Google Scholar]
- 20.Sobottke R, Koy T, Rollinghoff M, Siewe J, Kreitz T, Muller D, Bangard C, Eysel P. Computed tomography measurements of the lumbar spinous processes and interspinous space. Surg Radiol Anat. 2010;32:731–738. doi: 10.1007/s00276-010-0686-5. [DOI] [PubMed] [Google Scholar]
- 21.Patsch JM, Deutschmann J, Pietschmann P. Gender aspects of osteoporosis and bone strength. Wien Med Wochenschr. 2011;161:117–123. doi: 10.1007/s10354-011-0891-9. [DOI] [PubMed] [Google Scholar]
- 22.Peacock M, Koller DL, Lai D, Hui S, Foroud T, Econs MJ. Bone mineral density variation in men is influenced by sex-specific and non sex-specific quantitative trait loci. Bone. 2009;45:443–448. doi: 10.1016/j.bone.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amin S, Khosla S. Sex- and age-related differences in bone microarchitecture in men relative to women assessed by high-resolution peripheral quantitative computed tomography. J Osteoporos. 2012;2012:129760. doi: 10.1155/2012/129760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith JF, Guglielmi G. Vertebral fracture. Radiol Clin North Am. 2010;48:519–529. doi: 10.1016/j.rcl.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 26.Riggs BL, Melton Iii LJ, 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/jbmr.040916. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara A, An HS, Lim TH, Haughton VM. Morphologic changes in the lumbar intervertebral foramen due to flexion-extension, lateral bending, and axial rotation: an in vitro anatomic and biomechanical study. Spine. 2001;2:876–882. doi: 10.1097/00007632-200104150-00010. [DOI] [PubMed] [Google Scholar]
- 28.Inufusa A, An HS, Lim TH, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21:2412–2420. doi: 10.1097/00007632-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Schmid MR, Stucki G, Duewell S, Wildermuth S, Romanowski B, Hodler J. Changes in cross-sectional measurements of the spinal canal and intervertebral foramina as a function of body position: in vivo studies on an open-configuration MR system. AJR Am J Roentgenol. 1999;172:1095–1102. doi: 10.2214/ajr.172.4.10587155. [DOI] [PubMed] [Google Scholar]
- 30.Penning L, Wilmink JT. Posture-dependent bilateral compression of L4 or L5 nerve roots in facet hypertrophy. A dynamic CT-myelographic study. Spine. 1987;12:488–500. doi: 10.1097/00007632-198706000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Willen J, Danielson B, Gaulitz A, Niklason T, Schonstrom N, Hansson T. Dynamic effects on the lumbar spinal canal: axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine. 1997;22:2968–2976. doi: 10.1097/00007632-199712150-00021. [DOI] [PubMed] [Google Scholar]
- 32.Richards JC, Majumdar S, Lindsey DP, Beaupre GS, Yerby SA. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine. 2005;30:744–749. doi: 10.1097/01.brs.0000157483.28505.e3. [DOI] [PubMed] [Google Scholar]
- 33.Wiseman CM, Lindsey DP, Fredrick AD, Yerby SA. The effect of an interspinous process implant on facet loading during extension. Spine. 2005;30:903–907. doi: 10.1097/01.brs.0000158876.51771.f8. [DOI] [PubMed] [Google Scholar]
- 34.Kasai Y, Inaba T, Akeda K, Uchida A. Tadpole system as new lumbar spinal instrumentation. J Orthop Surg Res. 2008;3:41. doi: 10.1186/1749-799X-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]


