Abstract
Purpose
The aim of the present study was to analyze outcome, with respect to functional disability, pain, fusion rate, and complications of patients treated with transforaminal lumbar interbody fusion (TLIF) in compared to instrumented poserolateral fusion (PLF) alone, in low back pain. Spinal fusion has become a major procedure worldwide. However, conflicting results exist. Theoretical circumferential fusion could improve functional outcome. However, the theoretical advantages lack scientific documentation.
Methods
Prospective randomized clinical study with a 2-year follow-up period. From November 2003 to November 2008 100 patients with severe low back pain and radicular pain were randomly selected for either posterolateral lumbar fusion [titanium TSRH (Medtronic)] or transforaminal lumbar interbody fusion [titanium TSRH (Medtronic)] with anterior intervertebral support by tantalum cage (Implex/Zimmer). The primary outcome scores were obtained using Dallas Pain Questionnaire (DPQ), Oswestry disability Index, SF-36, and low back pain Rating Scale. All measures assessed the endpoints at 2-year follow-up after surgery.
Results
The overall follow-up rate was 94 %. Sex ratio was 40/58. 51 patients had TLIF, 47 PLF. Mean age 49(TLIF)/45(PLF). No statistic difference in outcome between groups could be detected concerning daily activity, work leisure, anxiety/depression or social interest. We found no statistic difference concerning back pain or leg pain. In both the TLIF and the PLF groups the patients had significant improvement in functional outcome, back pain, and leg pain compared to preoperatively. Operation time and blood loss in the TLIF group were significantly higher than in the PLF group (p < 0.001). No statistic difference in fusion rates was detected.
Conclusions
Transforaminal interbody fusion did not improve functional outcome in patients compared to posterolateral fusion. Both groups improved significantly in all categories compared to preoperatively. Operation time and blood loss were significantly higher in the TLIF group.
Keywords: Prospective, RCT, Lumbar interbody fusion
Introduction
Spinal fusion has for many years been the treatment of choice to treat low back pain generated from disc degeneration, failed disc surgery, spondylosis, spondylolisthesis, resistant to conservative therapy, and in combination with decompressions performed due to spinal stenosis. Different methods have evolved over the last many years. At the present time, there is no clear cut scientific evidence of which surgical strategy is the best [9]. There have been randomized controlled trials showing no significant improvement in short time outcomes in instrumented fusion compared to uninstrumented fusion [8, 16, 19]. Nevertheless treatment strategies have moved towards global fusion based on the theoretical point of view that restoration of lordosis, sagittal balance, and neuroforaminal decompression due to restoration of the disc height would result in better functional outcomes. However, this theory has been difficult to validate scientifically. During the late 90s and in the beginning of the new century, anterior lumbar interbody fusion (ALIF) was used to minimize pain in patients suffering from chronic low back pain (CLBP). However, only one randomized clinical trial (RCT) with long time follow-up has been able to show benefits of this procedure [20]. In comparison, the Swedish Lumbar Spine Study reported significantly higher complication rates in the interbody fusion group compared to the instrumented and non-instrumented posterolateral fusion groups and no difference in functional outcome between groups [7]. The need of anterior support has also been questioned by Ekman and colleagues [4], comparing posterior lumbar interbody fusion (PLIF) to posterolateral lumbar fusion (PLF) in adult isthmic spondylolisthesis: they did not find any difference in patient reported outcome. Recently, Neumann et al. [17] presented data from an RCT comparing transforaminal lumbar interbody fusion (TLIF) to uninstrumented PLF in degenerative disc disease (DDD), where outcome was significantly in favour of TLIF in visual analogue scale (VAS, pain) and disability Rating Index, but with no significant difference in Oswestry disability Index (ODI). Our group has earlier been able to show benefits of 360° fusion utilizing anterior interbody fusion [3]. Transforaminal lumbar interbody fusion (TLIF) should theoretically offer the same benefits of circumferential fusion, but with less morbidity due to the lesser surgical approach. But as there is conflicting evidence, the aim of the present study was designed to test whether a TLIF procedure resulted in better functional outcome than PLF instrumented posterolateral fusion.
Materials and methods
From November 2003 through November 2008, a total of 100 patients (mean age 49.8 years; TLIF group 50.3 years, PLF group 49.3 years) were included in the present single-centre prospective RCT. The study protocol was approved by the regional ethical committee (No: 20030172). All patients suffered from severe CLBP and/or leg pain, static or dynamic, resulting from localized lumbar or lumbosacral segmental instability, spinal stenosis at levels L2–S1 or caused by isthmic spondylolisthesis (grade 1 and 2). Baseline characteristics concerning demographic and clinical data are presented in Table 1. Of the 14 patients in the TLIF group, who had undergone previous spine surgery, ten had had a prior discectomy and four a decompressive procedure; in the control group there were seven discectomies, three fusions and three decompressive procedures. In the TLIF group 21 patients (41 %) used opioids and six patients (12 %) used anti-depressants before surgery; in the control group it was 21 (43 %) and 8 (16 %), respectively (differences non-significant).
Table 1.
Patient characteristics according to treatment group
| TLIF | Control | p value | |
|---|---|---|---|
| Sex (female/male) | 27/24 | 32/17 | 0.209 |
| Age at surgery (years) | 51 (30–63) | 49 (25–70) | 0.269 |
| Diagnosis | 0.335 | ||
| Spondylolisthesis | 10 | 17 | |
| DDD | 20 | 17 | |
| Spinal stenosis | 9 | 8 | |
| Failed back surgery | 12 | 7 | |
| Pain duration | 0.283 | ||
| <1 year | 8 | 3 | |
| 1–2 years | 14 | 17 | |
| >2 years | 29 | 29 | |
| Work status | 0.740 | ||
| Working | 22 | 16 | |
| Without work | 2 | 2 | |
| Sick-leave | 14 | 15 | |
| Retired | 13 | 16 | |
| Smoking | 24 (44 %) | 21 (46 %) | 0.904 |
| Previous spine surgery | 14 (27 %) | 13 (27 %) | 0.917 |
| Ongoing casea | 16 (31 %) | 7 (14 %) | 0.042 |
| Spondylolisthesis | 1 | 2 | |
| DDD | 6 | 1 | |
| Spinal stenosis | 4 | 0 | |
| Failed back surgery | 5 | 4 | |
| Operated level(s) | 0.479 | ||
| 1 level | 36 | 29 | |
| 2 levels | 14 | 19 | |
| 3 levels | 1 | 1 | |
| Additional neural decompression | <0.001 | ||
| None | 0 | 18 | |
| Laminotomy | 33 | 7 | |
| Spondylolisthesis | 3 | 0 | |
| DDD | 15 | 1 | |
| Spinal stenosis | 4 | 2 | |
| Failed back surgery | 11 | 4 | |
| Laminectomy | 18 | 24 | |
| Spondylolisthesis | 7 | 14 | |
| DDD | 5 | 4 | |
| Spinal stenosis | 5 | 5 | |
| Failed back surgery | 1 | 1 | |
| Operation time (min) | 228 (135–450) | 171 (100–300) | <0.001 |
| Blood loss (ml) | 775 (150–5,000) | 443 (50–1,500) | 0.001 |
| Hospitalisation (days) | 9.8 (5–22) | 9.3 (6–16) | 0.405 |
Values are mean (range) or number (%)
aOngoing case means: insurance, compensatory case, negligence case, etc
Before surgery, the indication for fusion was determined on the basis of anamnesis, several clinical and neurological examinations, and MRI. Exclusion criteria comprised age younger than 25 years, spondylolisthesis grade III and IV, osteoporosis diagnosed via radiography and bone mineral density testing, severe cardiac or vascular disease, brain disorders, kidney problems, former or actual malignancy, use of medicine reducing bone metabolism, dementia or abnormal psychological behaviour, and language problems.
Randomization was done using sealed envelopes with a 20-number-per block randomization. The envelopes were consecutively numbered, thereby assigning a number to each patient in the study. The type of operation remained unknown to both the patient and the surgeon until the patient’s written consent was obtained. By this procedure, 49 patients were allocated to posterolateral fusion with titanium TSRH (Medtronic) pedicle instrumentation (PLF group) (Fig. 1) and 51 patients to transforaminal fusion in the form of a tantalum cage (Implex/Zimmer) placed using an approach lateral to the facet joint (TLIF group) (Fig. 2). The anterior interbody fusion device was supported by a posterolateral fusion using pedicle screws (titanium TSRH, Medtronic).
Fig. 1.
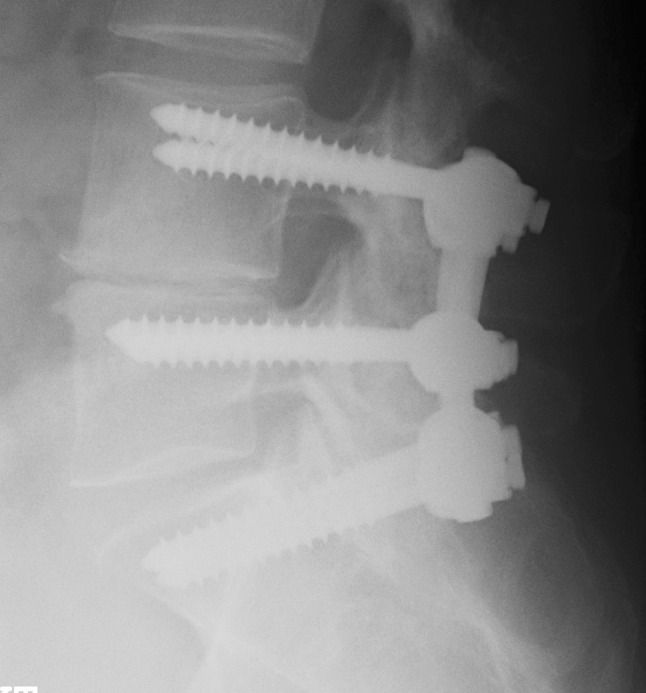
X-ray showing the instrumentation in the control group
Fig. 2.
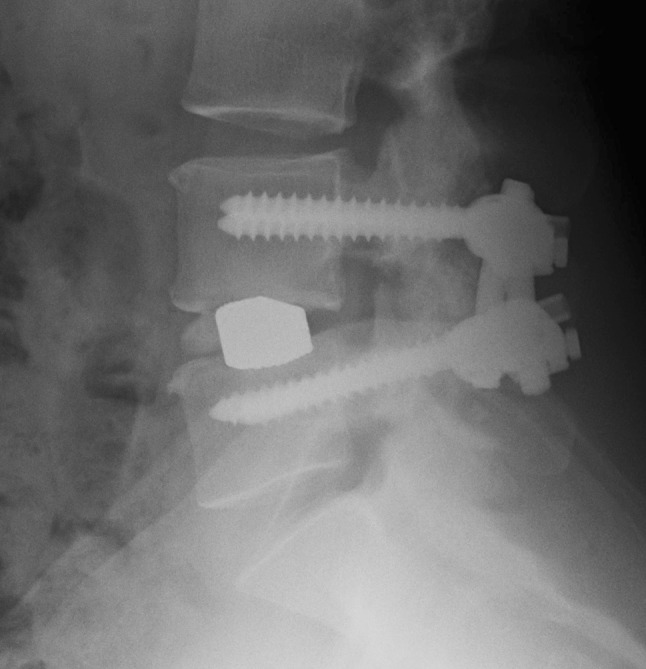
X-ray showing the instrumentation in the TLIF group
During surgery the patients were placed in prone position. We used controlled hypotensive anaesthesia. The patients first underwent insertion of pedicle screws by a midline subperiosteal approach. When indicated, hemilaminectomy or laminectomy for neural decompression was performed. In case the patients were randomized to the transforaminal procedure, the facet joint of the intended levels was indentified and the inferior and superior facets were resected to gain access to the disc space, and by that procedure an indirectly neurolysis or decompression of the nerve was performed. The pedicle screws were used to distract. The upper nerve was indentified and protected. The tantalum cage was placed after cleaning the disc space. Compression over the disc space was done after placement of the cage in order to create lordosis [11]. Cancellous bone from bone bank femoral heads were used as bone graft and placed on the transverse process of the vertebraes fused. A careful preparation of the posterolateral region was performed before positioning of the graft. Before that the decompressed neural structures were covered with a gel-foam (Spongostan) to avoid damage.
Overall, a 2-year follow-up rate of 94 % was achieved, with follow-up evaluation for 47 patients in the PLF group and 47 patients in the TLIF group. One patient from the PLF group had moved to Greenland and did not show up for follow-up. One patient from the TLIF group had died for reasons unrelated to the surgery. One patient from the PLF group and one patient from the TLIF group did not show up to the follow-up due to disappointment with the effect of the procedure. Two patients from the TLIF group did not answer follow-up.
Functional outcome was assessed by means of the Dallas Pain Questionnaire (DPQ), the pain index from the low back pain rating Scale (LBPRS), ODI, and SF-36. The questionnaires were completed by the patients, independently of the surgeon, before the operation and at the 1 and 2 years follow-up assessments. The DPQ is validated and it assesses the functional impact of chronic spinal pain in four categories: daily activity, work-leisure activity, anxiety-depression, and social concerns. A high score indicates great functional impact [13]. Back and leg pain was assessed by the LBPRS pain index. It is a validated index scale that includes measurements of pain intensity ranging from 0 to 10, in which 10 represents the worst possible pain. They are 11 point (0–10) numerical rating scales assessing both back and leg pain in three ways: worst pain within in the last 14 days, average pain within the last 14 days, and actual pain level at the time of completing the questionnaire. The scores for leg pain and back pain are summed giving a pain index ranging from 0 to 60 [14]. The ODI is a condition specific outcome measure for spinal disorders, yielding an index score which ranges from 0 to 100, with a high percentage reflecting a high degree of disability [5]. The SF-36 is a generic health survey measure. It yields a profile of scores in eight scales covering different physical and mental components of health. The score in each scale ranges from 0 (poorest health) to 100 (best health). In addition two summary measures are produced: a physical component summary (PCS) and a mental component summary (MCS). The two summary measures are calculated so that the value of 50 is equal to the US population mean [22]. Before surgery and at the follow-up each patient received a questionnaire concerning work status and employment. At follow-up assessments patients were also asked the question “would you undergo the same treatment again now you know the result?”, the answer to which served as a global outcome parameter.
All analyses comparing the two intervention groups were done using the intention to treat principle. Comparison between groups were done using non-parametric tests (Mann–Whitney rank-sum test for unpaired data, Wilcoxon signed rank test for paired data, Chi-square statistics or the Kruskal–Wallis test for equality at groups, with correction for ties) depending on the nature of the data. Significance level was 5 % using two-tailed testing. Intercooled Stata version 12 for Windows was the software used for statistical analysis. Sample size of the study was calculated using a significance level of 0.05 and a power of 0.80. Based on earlier studies, the standard deviation of the DPQ daily activity score was set at 25 points. A 15-point difference in this category was considered clinically relevant. To fulfill these criteria, the study would need 44 patients in each group. To allow for dropouts, the study was designed with 50 patients in each group.
Results
We could not observe any difference in favour of the TLIF procedure in any of the outcome parameters measured at any time point (Figs. 1, 2). Both groups improved significantly compared to preoperatively, but without any difference between the groups (Fig. 1). There was, however, an insignificant trend towards the TLIF group developing more leg pain during follow-up compared to the controls (Table 2). Subgroup analysis based on diagnosis could not reveal any substantial benefits of the TLIF procedure either (Table 3). Number of patients with ongoing case was the only variable to differ between the two randomization groups (Table 1). After stratifying for ongoing compensatory case still no difference between the two procedures could be observed (data not shown).
Table 2.
Scores at all time points for the subcategories of the LBPRS
| Preoperative | 1-year follow-up | 2-year follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TLIF | Control | p value | TLIF | Control | p value | TLIF | Control | p value | |
| Back pain right now | 6.1 (0.3) | 5.8 (0.3) | 0.535 | 3.6 (0.5) | 3.4 (0.4) | 0.981 | 3.5 (0.4) | 3.6 (0.5) | 1.000 |
| Worst back pain within last 14 days | 7.8 (0.3) | 8.1 (0.3) | 0.707 | 4.9 (0.5) | 4.6 (0.4) | 0.659 | 5.3 (0.5) | 5.1 (0.5) | 0.795 |
| Average back pain within last 14 days | 6.1 (0.4) | 6.1 (0.3) | 0.894 | 3.8 (0.4) | 3.8 (0.4) | 0.883 | 4.1 (0.4) | 4.0 (0.5) | 0.774 |
| Leg pain right now | 5.1 (0.4) | 4.3 (0.4) | 0.215 | 3.0 (0.5) | 2.4 (0.4) | 0.542 | 3.2 (0.4) | 2.5 (0.4) | 0.265 |
| Worst leg pain within last 14 days | 6.2 (0.4) | 6.5 (0.4) | 0.657 | 4.0 (0.6) | 3.2 (0.5) | 0.470 | 4.4 (0.5) | 3.4 (0.5) | 0.137 |
| Average leg pain within last 14 days | 5.1 (0.4) | 5.0 (0.4) | 0.888 | 3.2 (0.5) | 2.7 (0.4) | 0.589 | 3.5 (0.4) | 2.9 (0.5) | 0.214 |
Values are mean (standard error of mean)
Table 3.
Outcome scores at 2-year follow-up in the two treatment groups stratified according to surgical diagnosis
| TLIF | Control | p value | |
|---|---|---|---|
| Spondylolisthesis | |||
| DPQ daily activity | 40.1 (11.1) | 41.8 (9.1) | 0.799 |
| DPQ work/leisure activity | 31.1 (10.4) | 39.6 (11.5) | 0.562 |
| DPQ anxiety/depression | 21.9 (10.7) | 18.2 (4.8) | 0.808 |
| DPQ social interest | 15.6 (5.8) | 21.1 (7.2) | 0.974 |
| LBPRS pain index | 19.6 (2.7) | 17.1 (3.2) | 0.503 |
| ODI | 25.8 (6.9) | 26.7 (4.5) | 0.698 |
| SF-36 BP | 50.8 (7.0) | 57.5 (7.1) | 0.509 |
| SF-36 PF | 60.8 (8.3) | 64.3 (6.6) | 0.976 |
| SF-36 PCS | 40.0 (3.2) | 41.2 (3.5) | 0.659 |
| DDD | |||
| DPQ daily activity | 45.5 (7.4) | 30.4 (7.3) | 0.145 |
| DPQ work/leisure activity | 48.2 (9.2) | 32.0 (7.6) | 0.155 |
| DPQ anxiety/depression | 29.2 (7.2) | 24.3 (6.7) | 0.645 |
| DPQ social interest | 25.8 (7.5) | 20.0 (5.6) | 0.901 |
| LBPRS pain index | 26.3 (4.2) | 25.0 (5.1) | 0.600 |
| ODI | 32.4 (4.5) | 21.8 (4.0) | 0.144 |
| SF-36 BP | 48.7 (6.9) | 51.9 (8.0) | 0.701 |
| SF-36 PF | 63.1 (5.6) | 70.2 (6.2) | 0.431 |
| SF-36 PCS | 37.2 (2.4) | 42.3 (2.8) | 0.184 |
| Spinal stenosis | |||
| DPQ daily activity | 36.4 (9.9) | 38.1 (9.8) | 0.954 |
| DPQ work/leisure activity | 37.5 (12.5) | 35.0 (13.0) | 0.942 |
| DPQ anxiety/depression | 15.6 (7.9) | 28.6 (9.0) | 0.143 |
| DPQ social interest | 18.1 (6.0) | 10.7 (7.1) | 0.231 |
| LBPRS pain index | 23.6 (7.2) | 18.1 (4.5) | 0.728 |
| ODI | 25.0 (6.6) | 27.3 (8.5) | 1.000 |
| SF-36 BP | 52.6 (11.1) | 47.4 (11.2) | 0.728 |
| SF-36 PF | 56.4 (9.7) | 62.1 (9.9) | 0.560 |
| SF-36 PCS | 35.5 (5.4) | 38.5 (4.3) | 0.729 |
| Failed back surgery | |||
| DPQ daily activity | 39.0 (10.3) | 54.4 (12.0) | 0.222 |
| DPQ work/leisure activity | 42.5 (12.6) | 53.8 (16.6) | 0.670 |
| DPQ anxiety/depression | 32.8 (14.1) | 45.8 (13.9) | 0.551 |
| DPQ social interest | 34.5 (13.5) | 28.3 (9.7) | 0.912 |
| LBPRS pain index | 24.0 (4.7) | 25.8 (9.5) | 0.957 |
| ODI | 34.4 (8.0) | 35.7 (5.1) | 0.625 |
| SF-36 BP | 48.5 (8.0) | 53.0 (10.4) | 0.404 |
| SF-36 PF | 61.6 (11.0) | 50.7 (8.9) | 0.463 |
| SF-36 PCS | 39.5 (4.1) | 35.0 (4.4) | 0.540 |
Values are mean (standard error of mean)
Data on work status at 2-year follow-up were available in 96 patients. In the TLIF group 23 patients were working, one was without work, two were on sick-leave and 22 were retired. In the control group the numbers were 23 working, 2 on sick-leave and 27 retired (p = 0.60). In the TLIF group 16 patients used opioids and three patients anti-depressants at the 2-year follow-up compared to 14 and 6 patients, respectively, in the control group (not significant). Fusion rate at 2 years was 94 % (44 of 47 patients with available radiographs) in the TLIF group compared to 88 % (42 of 48 patients with available radiographs) in the control group (p = 0.31). 91 patients, 46 in the TLIF group and 45 in the control group had answered the question “Knowing the result would then undergo the procedure again?” at 2 years. Thirty-three patients in each group answered with a positive response (72 vs. 73 %, p = 0.87) (Figs. 3, 4).
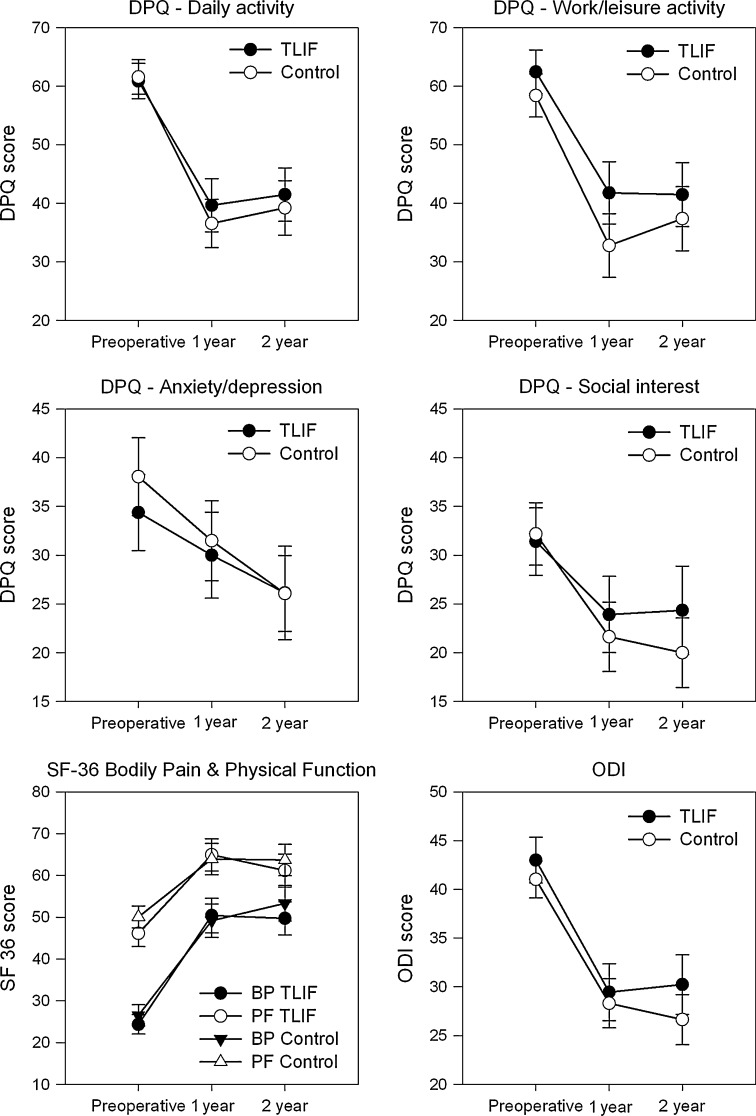
Fig. 3.
Preoperative and 1 and 2 year follow-up scores of the four DPQ categories as well as ODI and the BP (bodily pain) and PF (physical function) subscales from the SF-36. There was no significant difference between the two groups at any time points (p > 0.50, except DPQ work/leisure activity p > 0.29). Both groups improved significantly from preoperatively to last follow-up (p < 0.01, except DPQ anxiety/depression in the TLIF group p = 0.03). Values are mean and errors are standard error of the mean
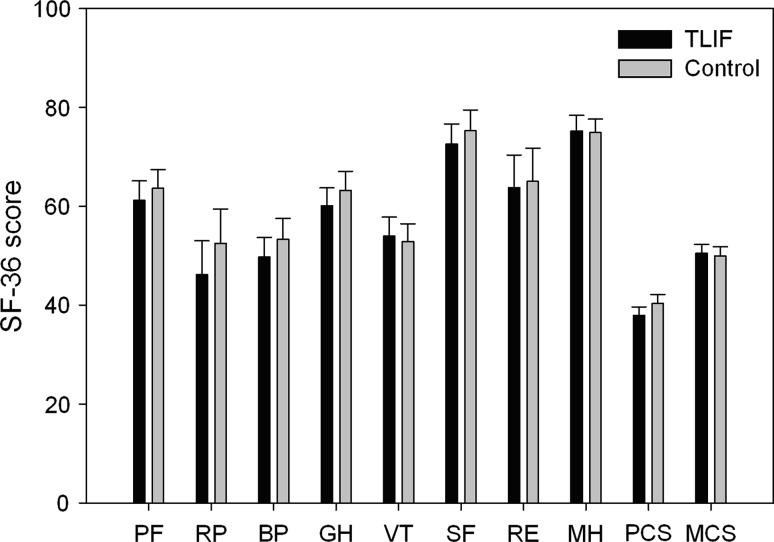
Fig. 4.
Two year follow-up scores, according to randomization group, of the eight subscales and two summary measures of the SF-36. There was no significant difference between the two groups in any of the scores (p > 0.30). Values are mean and errors are standard error of mean
Operation time and blood loss were significantly higher in the TLIF group, but did not result in longer length of hospitalization (Table 1). The complication rate in the TLIF group was 14 % (7 patients) as compared to 6 % (3 patients) in the control group (p = 0.205). In the TLIF group complications were one haematoma, two superficial infections, one nerve root lesion, two dural tears, and one intraoperatively developed pneumothorax, which was treated with drainage. In the control group, there were two haematomas and one dural tear. In two of the TLIF cases the disc space was too small for insertion of the cage and instead the disc space was filled with allograft bone. Also in one case in the TLIF group it was preoperatively decided to convert to an anterior procedure. Among the study patients nine patients (9 %) had undergone a second operation: three patients in PLF group had removal of the hardware due to loosening or failure. Four patients in the TLIF group had removal of the implant: two due to misplaced cages, one due to non-union, (this patient subsequently had an ALIF), and one due to deep infection. In addition, two patients in the TLIF group had secondary decompression at another level performed within 1 year.
Discussion
Transforaminal lumbar interbody fusion is today widely used in lumbar spinal fusion because of less violation to the spinal canal, compared to PLIF, and due to less time consumption and morbidity compared to ALIF, to achieve interbody fusion, which by many authors is considered to be the treatment of choice [2, 3, 15, 20, 23]. To our knowledge, this study is the first randomized prospective study to analyze a standardized instrumented spinal posterolateral fusion procedure with a TLIF procedure.
The present study was a single-centre study, which increases the possibility of a standardized patient selection and uniform surgical technique. This we believe improved the design and power of the present study. We could not demonstrate any superiority of the procedure with respect to function and back pain in a 2 years perspective. Neither could we demonstrate any improvement in leg pain in the TLIF group compared to the PLF group. On the contrary, there was a tendency towards more leg pain at 2-year follow-up in the TLIF group, questioning the concept of indirect foraminal decompression in the interbody fusion method or indicating that the procedure offers a risk of injury to the upper nerve root during access to and cleaning of the intervertebral disc space. Our study consisted of a mixed patient material with and without radicular pain, which could blur the results. Another major difference between the two methods in our study is that the TLIF procedure always is associated with a decompression at one side, irrespective of whether this is needed or not. In case of preoperative sciatica, the procedure was performed on the side with sciatica. The fact that the procedure always included decompression would also put the nerve root at risk of later irritation due to the formation of scar tissue. This could explain why we observed slightly more leg pain in the TLIF group at 2-year follow-up, and it could be an argument in favour of ALIF surgery.
The only variable which differed between the two randomization groups was the number of patients with an ongoing compensatory case. As this has been shown to be a risk factor for poorer outcome it might hide a small effect in favour of the TLIF procedure. But we could not prove any difference between the two procedures in the patients without ongoing compensatory case, suggesting that our results are not skewed significantly by this.
Only one RCT study has shown evidence to support the benefits of the TLIF procedure in DDD [17]. They found significant improvement in the TLIF group in pain index and in disability Rating Index compared to their control group. However, difference in the widely used ODI was not significantly in favour of TLIF. Unfortunately, the data from this study have not been published in full yet. In the abstract they concluded that the results strongly suggested interbody fusion to be considered as the primary choice in the degenerative lumbar spine. This strong conclusion might not be valid considering our results. One explanation to the difference between these two studies could be the choice of control group. Their control group was uninstrumented fusion, a comparison that could be questioned, as it compares a very stable construct to an unstable construct. On the other hand, several studies have failed to show any difference between uninstrumented and instrumented posterolateral fusion, including the Swedish Spine Study, which also failed to show any effect of interbody fusion, performed as a PLIF or an ALIF procedure for patients with CLBP [6, 8, 19].
The positive effects of interbody fusion have been shown in a long-term follow-up study comparing 360° fusion, done as instrumented posterolateral fusion combined with an ALIF in a mixed population. In that study Videbaek et al. [20] showed significantly better functional outcome in the ALIF group, measured using DPQ, ODI, SF-36, leg and back pain NRS, compared to the control group comprising instrumented posterolateral fusion. However, in the 2-year follow-up of that study no functional outcome benefits could be proven, although significant better fusions rates and fewer reoperations were observed in the 360-group. Their long-term results are in contrast to the findings of Swan et al. [18] who were able to show a positive effect of anterior support in 6 months and at 1-year follow-up but not at 2-year follow-up in a mixed population. They concluded that the positive effects of global fusion diminished over time. Comparison of the three fusion techniques uninstrumented posterolateral, instrumented posterolateral and 360° fusion was also done in the SPORT study on degenerative spondylolisthesis [1]. It showed minor differences between the types of fusion, with a slight tendency towards 360° fusion performing better at 1 and 2 year follow-up, but with no difference at the 3 and 4 year follow-ups. This study was, however, performed in a population significantly older than the current study and the comparison between fusion techniques was done in a non-randomized fashion. Kim et al. [12] performed a randomized study comparing instrumented posterolateral fusion with a PLIF procedure, both with and without additional posterolateral bone graft in DDD, and found no significant differences with respect to pain and functional outcome. Neither could Grob et al. [10] demonstrate any advantages of a TLIF procedure compared to posterolateral fusion performed using translaminar facet screw fixation in a mixed population of DDD, degenerative spondylolisthesis and facet syndrome; this was, however, not a randomized study.
In our study, we did not observe a higher complication rate in the TLIF group compared to the PLF group, although it has been shown earlier that an increase in technicality leads to higher complications rates [4, 7, 21]. This could be due to the fact that our study was a single-centre study, possibly reflecting a more standardized patient selection and surgical technique, as compared to the multi-centre Swedish Spine Study, with 19 different orthopedic departments participating in the study, and with different frequencies of performing the procedure [7]. Neither could we observe any significant difference in re-operation as seen in the ALIF study by Christensen et al. [3]. On the contrary, the data from the SPORT study on degenerative spondylolisthesis did not show any difference in complication or repeated surgery rate up to 4 years, between the three fusion groups in that study [1].
These different studies have been performed in patients with varying diagnosis. This could explain some of the differences. The present study was performed in a group of patients with a mixed diagnosis and we could not demonstrate any difference between the two surgical techniques in any of the diagnostic groups, but one has to bear in mind that the study was not powered for subgroup analysis.
In conclusion, this study showed that TLIF demands more extensive resources than posterolateral fusion with pedicle screws, without significant improvement in functional outcome or fusion rate and with a tendency towards more leg pain.
Conflict of interest
None.
References
- 1.Abdu WA, Lurie JD, Spratt KF, et al. Degenerative spondylolisthesis: does fusion method influence outcome? 4-year results of the spine patient outcomes research trial. Spine. 2009;34(21):2351–2360. doi: 10.1097/BRS.0b013e3181b8a829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brantigan JW, Neidre A, Toohey JS. The lumbar I/F Cage for posterior lumbar interbody fusion with the variable screw placement system: 10-year results of a food and drug administration clinical trial. Spine J. 2004;4(6):681–688. doi: 10.1016/j.spinee.2004.05.253. [DOI] [PubMed] [Google Scholar]
- 3.Christensen FB, Hansen ES, Eiskjaer SP, et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion With Titanium Cotrel-Dubousset instrumentation: a prospective, randomized Clinical study of 146 patients. Spine. 2002;27(23):2674–2683. doi: 10.1097/00007632-200212010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ekman P, Moller H, Tullberg T, Neumann P, Hedlund R. Posterior lumbar interbody fusion versus posterolateral fusion in adult isthmic spondylolisthesis. Spine. 2007;32(20):2178–2183. doi: 10.1097/BRS.0b013e31814b1bd8. [DOI] [PubMed] [Google Scholar]
- 5.Fairbank JC, Pynsent PB. The Oswestry disability index. Spine. 2000;25(22):2940–2953. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 6.Fischgrund JS, Mackay M, Herkowitz HN, et al. Volvo award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22(24):2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Fritzell P, Hagg O, Nordwall A. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish lumbar spine study group. Eur Spine J. 2003;12(2):178–189. doi: 10.1007/s00586-002-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritzell P, Hagg O, Wessberg P, Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine. 2002;27(11):1131–1141. doi: 10.1097/00007632-200206010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis: updated cochrane review. Spine. 2005;30(20):2312–2320. doi: 10.1097/01.brs.0000182315.88558.9c. [DOI] [PubMed] [Google Scholar]
- 10.Grob D, Bartanusz V, Jeszenszky D, et al. A prospective, cohort study comparing translaminar screw fixation with transforaminal lumbar interbody fusion and pedicle screw fixation for fusion of the degenerative lumbar spine. J Bone Joint Surg Br. 2009;91(10):1347–1353. doi: 10.1302/0301-620X.91B10.22195. [DOI] [PubMed] [Google Scholar]
- 11.Harms JG, Jeszenszky D. The unilateral transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol. 1998;6(2):88–89. [Google Scholar]
- 12.Kim KT, Lee SH, Lee YH, Bae SC, Suk KS. Clinical outcomes of three fusion methods through the posterior approach in the lumbar spine. Spine. 2006;31(12):1351–1357. doi: 10.1097/01.brs.0000218635.14571.55. [DOI] [PubMed] [Google Scholar]
- 13.Lawlis GF, Cuencas R, Selby D, McCoy CE. The development of the Dallas Pain Questionnaire. An assessment of the impact of spinal pain on behavior. Spine. 1989;14(5):511–516. doi: 10.1097/00007632-198905000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Manniche C, Asmussen K, Lauritsen B, et al. Low back pain rating scale: validation of a tool for assessment of low back pain. Pain. 1994;57(3):317–326. doi: 10.1016/0304-3959(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 15.McAfee PC, Devine JG, Chaput CD, et al. The indications for interbody fusion cages in the treatment of spondylolisthesis: analysis of 120 cases. Spine (Phila Pa 1976) 2005;30(6 Suppl):S60–S65. doi: 10.1097/01.brs.0000155578.62680.dd. [DOI] [PubMed] [Google Scholar]
- 16.Moller H, Hedlund R. Instrumented and noninstrumented posterolateral fusion in adult spondylolisthesis: a prospective randomized study: part 2. Spine. 2000;25(13):1716–1721. doi: 10.1097/00007632-200007010-00017. [DOI] [PubMed] [Google Scholar]
- 17.Neumann P, Jalalpour K, Johansson C, Hedlund R. A RCT between transforaminal lumbar interbody fusion (TLIF) and posterolateral fusion in DDD and postdiscectomy syndrome. Eur Spine J. 2009;18(Supplement 4):S408. [Google Scholar]
- 18.Swan J, Hurwitz E, Malek F, et al. Surgical treatment for unstable low-grade isthmic spondylolisthesis in adults: a prospective controlled study of posterior instrumented fusion compared with combined anterior-posterior fusion. Spine J. 2006;6(6):606–614. doi: 10.1016/j.spinee.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Thomsen K, Christensen FB, Eiskjaer SP, et al. Volvo award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine. 1997;22(24):2813–2822. doi: 10.1097/00007632-199712150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Videbaek TS, Christensen FB, Soegaard R, et al. Circumferential fusion improves outcome in comparison with instrumented posterolateral fusion: long-term results of a randomized clinical trial. Spine. 2006;31(25):2875–2880. doi: 10.1097/01.brs.0000247793.99827.b7. [DOI] [PubMed] [Google Scholar]
- 21.Villavicencio AT, Burneikiene S, Bulsara KR, Thramann JJ. Perioperative complications in transforaminal lumbar interbody fusion versus anterior-posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord Tech. 2006;19(2):92–97. doi: 10.1097/01.bsd.0000185277.14484.4e. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE., Jr SF-36 Health Survey Update. Spine. 2000;25(24):3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 23.Zdeblick TA, Phillips FM. Interbody cage devices. Spine. 2003;28(15):S2–S7. doi: 10.1097/01.BRS.0000076841.93570.78. [DOI] [PubMed] [Google Scholar]