Abstract
Purpose
Percutaneous interspinous stand-alone spacers offer a simple and effective technique to treat lumbar spinal stenosis with neurogenic claudication. Nonetheless, open decompressive surgery remains the standard of care. This study compares the effectiveness of both techniques and the validity of percutaneous interspinous spacer use.
Methods
Forty-five patients were included in this open prospective non-randomized study, and treated either with percutaneous interspinous stand-alone spacers (Aperius®) or bilateral open microsurgical decompression at L3/4 or L4/5. Patient data, operative data, COMI, SF-36, PCS and MCS, ODI, and walking distance were collected 6 weeks, 3, 6, 9, 12, and 24 months post-surgery.
Results
Group 1 (n = 12) underwent spacer implantation, group 2 (n = 33) open decompression. Five patients from group 1 required implant removal and open decompression during follow-up (FU); one patient was lost to FU. From group 2, seven patients were lost to FU. Remaining patients were assessed as above. After 2 years, back pain, leg pain, ODI, and quality of life improved significantly for group 2. Remaining group 1 patients (n = 6) reported worse results. Walking distance improved for both groups.
Conclusion
Decompression proved superior to percutaneous stand-alone spacer implantation in our two observational cohorts. Therapeutic failure was too high for interspinous spacers.
Keywords: Lumbar spinal stenosis, Microsurgical decompression, Percutaneous interspinous spacer, Quality of life, Clinical outcome
Introduction
Increasing life expectancy and the related demand for quality of life among the elderly has contributed to neurogenic claudication becoming one of the most common diagnoses of all degenerative spine diseases [1, 2]. Back and leg pain along with decreasing walking tolerance limit quality of life and can result in social isolation [3, 4]. Non-operative treatment is well recognized for the treatment of early stage disease and mild symptoms, generally yielding satisfactory results within the first 3 months. In cases of severe complaints or a failure of conservative management, surgical treatment is usually offered to the patient [5].
Forward bending tends to relieve patients’ symptoms of neurogenic claudication. Interspinous spacers capitalize on this effect by inducing segmental kyphosis and limiting spine extension. Use of these devices significantly increases canal area and foraminal width radiographically [6, 7]. Implantation of an interspinous spacer is a rapid and uncomplicated procedure. Several studies have reported good short to mid-term results after spacer implantation, with less pain, claudication symptoms, and disability [8–11]. The main indication for interspinous spacer implantation is the presence of discoligamentous lumbar spinal stenosis that results in tightening of the posterior longitudinal ligament and the ligamentum flavum. In cases of bony stenosis or reduced segmental flexibility, expansion of the spinal canal with the placement of an interspinous spacer is unlikely, and thus, this treatment should not be selected. Evidence for the treatment of discogenic back pain with interspinous spacers is minimal [12].
Zucherman and Anderson conducted randomized controlled trials of the X-STOP interspinous spacer device for lumbar spinal stenosis in 2005 and 2006. Both trials assessed outcomes using the Zurich Claudication Questionnaire and provided 2 years of follow-up. In these studies, superior clinical and quality of life outcomes were obtained using interspinous spacers versus conservative management [13, 14]. Zucherman [15] also previously published his 1-year results, with encouraging outcomes for interspinous spacer implantation. However, a systematic review and meta-analysis of eleven RCTs, reviews, and prospective observational studies of spacer implantation found poor quality of evidence, and the cost-effectiveness of this treatment was questioned [16].
Microsurgical open decompression remains the accepted gold standard of treatment. Through a posterior approach, laminotomy as well as partial or total laminectomy is performed. Mid- to long-term results are available and symptom improvement has been confirmed [17, 18]. Superiority to conservative management has already been demonstrated [5, 17, 18]. Thus, new surgical interventions should be compared to open decompression.
Comparative studies of discrete surgical interventions are rare. Postacchini published 2-year results of a comparative study of two cohorts treated with either interspinous spacer or open decompression. Outcome measurement was performed using the Oswestry Disability Index and Zurich Claudication Questionnaire. The authors concluded that indications for spacer use are limited, but did identify patient benefits [19]. In 2010, the study protocol for the Felix trial was published. It offers a randomized controlled trial comparing the Coflex™ interspinous decompression device implanted over a median approach to open decompression. Closing is planned for April 2015 [20].
The current study reports the 2-year follow-up results of clinical outcomes and quality of life after percutaneous stand-alone interspinous spacer implantation versus open decompression in two observational cohorts. The 1-year results from our workgroup were published in 2010 [21].
Methods
Forty-five patients with neurogenic intermittent claudication due to lumbar spinal stenosis were included in this prospective, non-randomized, observational study between February 2007 and March 2010. Inclusion criteria were symptomatic lumbar stenosis between L3 and L5. In addition, all included patients reported relief of symptoms with forward bending. Patients who had undergone previous surgery at the investigated level were excluded from the study.
Patients were divided into two groups. In group 1 (n = 12), stand-alone interspinous spacers (Aperius®, Medtronic, Switzerland) were implanted. Patients in group 2 (n = 33) underwent bilateral microsurgical decompression.
All patients in group 1 received the same implant type. The Aperius® interspinous spacer is made of pure titanium (Ti-6Al-4 V). For implantation, a posterolateral 1.5-cm incision is required. Distraction trocars are used for successive preparation of the interspinous space. Initially, an 8-mm trocar is applied, and preparation continues with trocars of increasing diameters in 2-mm increments up to 14 mm. An interspinous spacer of the corresponding size can then be inserted through the same working channel. Intraoperative fluoroscopy is used to determine the spacer’s final position prior to unfolding of the fins.
For microsurgical decompression in group 2, a midline approach was used. After the skin incision, the paraspinal musculature was dissected and the interlaminar window exposed. Stable bony bilateral decompression was carried out under microscopic visualization.
All data were collected using the international “Spine Tango” register [22]. Patients were asked to complete the core outcome measures index (COMI), the short-form 36 (SF-36) with physical and mental component summaries (PCS and MCS), and the Oswestry disability index (ODI). The operative report form was filled out by the treating physician. It contains disease and surgery-related information as well as peri and postoperative general and surgical complications. Data were collected preoperatively and at 3, 6, 9, 12, and 24 months follow-up. Investigations using patient data from “Spine Tango” received a positive vote from the institutional review board.
Using a treadmill (Proform 615, ICON Health & Fitness Inc., Logan, Utah, USA), walking tolerance was measured in minutes at an individually adjusted velocity. This velocity was maintained during all follow-up tests. Patients were advised to walk until neurogenic claudication forced them to stop. The walking test was completed at 30 min.
Values were all analyzed exploratively. Explorative comparisons between the groups were assessed with corresponding parametric and non-parametric statistical tests. Quantitative variables like age were specified as average, median, standard deviation (SD), minimum (min), and maximum (max). Qualitative variables like gender were given as numbers and percent. Differences were assessed with a Mann–Whitney U Test and considered significant with a probability of 95 % (p < 0.05). Statistical evaluation was carried out using IBM® SPSS® Statistics 20 (SPSS Inc., IBM Company Headquarters, Chicago, IL, USA).
Results
For patient characteristics, see Table 1. From group 1, five of twelve patients required implant removal and underwent open microsurgical decompression during follow-up. All five patients complained of declining effectiveness of therapy. Implant displacement was not observed. Average time between spacer implantation and reoperation was 13.0 months (range: 4.1–20.0 months). One patient from group 1 and seven patients from group 2 were lost to follow-up (lost to FU = 17.8 %). None of the remaining patients in group 2 required reoperation during the course of follow-up. Patient assessed data (COMI, SF-36, ODI and walking tolerance) of these 13 patients (reoperation + lost to FU) were excluded from data analysis.
Table 1.
Patient characteristics
| Group 1 (n = 12) | Group 2 (n = 33) | Total (n = 45) | |
|---|---|---|---|
| Age (mean ± SD) (years) | 64.25 ± 9.6* | 71.12 ± 9.2* | 69.3 ± 9.7 |
| Gender | |||
| Male | 8 | 9 | 17 |
| Female | 4 | 24 | 28 |
| Ratio [m:f] | 2:1 | 1:2.7 | 1:1.6 |
| Number of treated segments | |||
| 1 | 4 | 14 | 18 |
| 2 | 8 | 19 | 27 |
| ASA | |||
| Unknown | 0 | 1 | 1 |
| ASA 1 | 3 | 2 | 5 |
| ASA 2 | 8 | 19 | 27 |
| ASA 3 | 1 | 11 | 12 |
| Complications | |||
| General | 2 (anesthesia-related) | 2 (anesthesia-related) | 4 |
| Surgical | 0 | 4** | 4 |
* Difference between groups not statistically significant at p-level < 0.05
** Four dural leakages, one of them with soft tissue infection
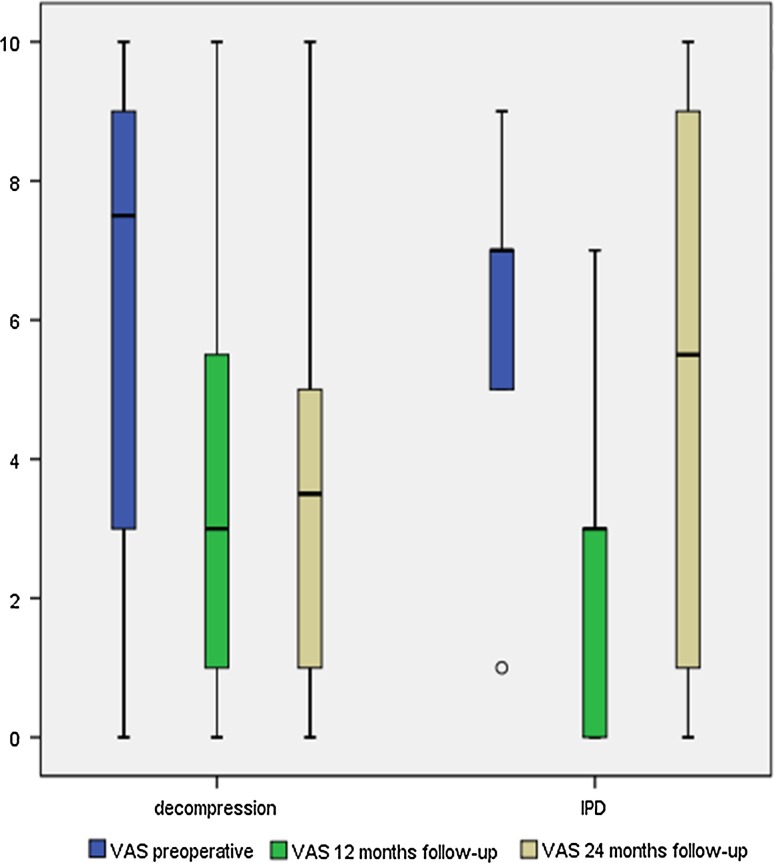
L4/5 was treated in 73.3 % and L3/4 in 26.7 % of all cases. In group 1, two patients received a stand-alone spacer at L3/4, and ten patients at L4/5. Decompression was performed at L3/4 in ten cases, at L4/5 in twenty-three cases. Information regarding intraoperative blood loss was available for six patients in group 1 and 19 patients from group 2. On the Spine Tango Operative Report, categorical values are chosen. In group 1, all six patients had blood loss below 500 ml. For 16 patients in group 2, blood loss was between 500 and 1,000 ml. Walking tolerance on a treadmill was recorded at an average speed of 1.5 km/h (SD ± 0.6 km/h). Results over the 2 years of follow-up are shown in Fig. 1.
Fig. 1.
Back pain levels on visual analog scale over follow-up for both groups
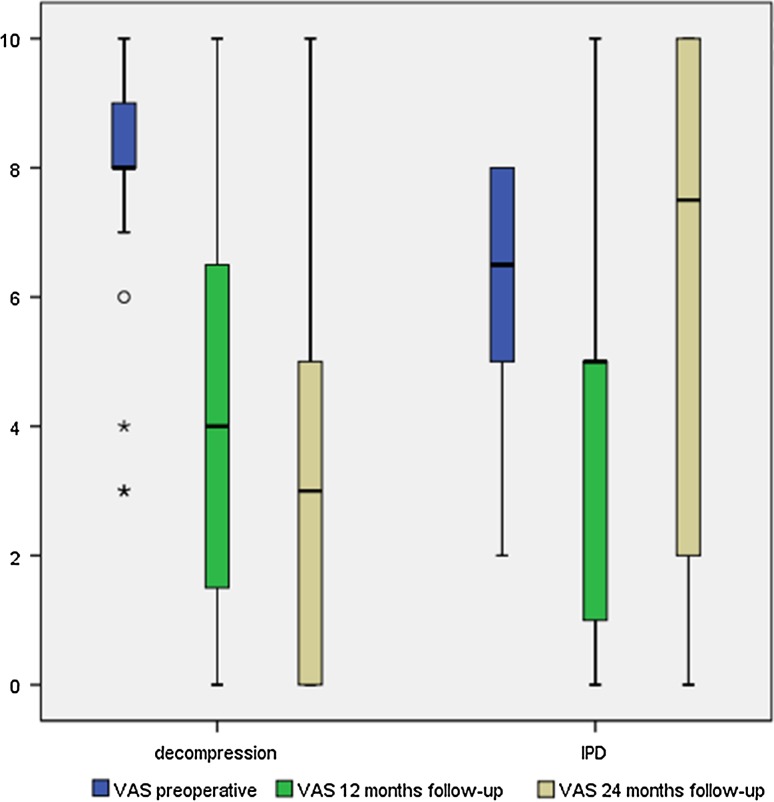
The only significant differences noted on comparison of the two groups were the preoperative leg pain scores, as well as the measured change in leg pain scores at 24 months versus preoperatively. No other measurements for leg pain, back pain, ODI, MCS, and PCS differed significantly between the groups.
The patients remaining (n = 6) in group 1 showed no significant decrease of back or leg pain. There were also no significant improvements in ODI and SF-36 during all follow-ups. These patients in group 1 reported an average decrease of 0.8 points in back pain and a slight increase of 0.6 points in leg pain on the COMI after 24 months. ODI improvement at 12 months was 17.0 points, but later decreased to 0.6 points (SD ± 28.4). Quality of life assessment yielded an average decrease of 5.2 ± 20.6 points in MCS at 12 months. At 24 months, MCS improved by 10.1 points (SD ± 17.0) compared to the preoperative assessment. Physical health summary showed an average increase of 14.5 points and 10.7 points at 12 and 24 months, respectively.
In group 2 (n = 26), there were significant decreases in back and leg pain both at 12 and 24 months. Preoperatively, leg pain was greater than back pain with an average of 7.9 versus 5.9 points. At 24 months post-decompression, back pain decreased to 3.8 (±2.6) and leg pain to 3.0 points (±2.8). Compared to values measured preoperatively, those measured at 24 months were statistically significant. ODI and SF-36 scores, including both subscales, improved significantly in all follow-ups as well. Regarding the entire collective, all assessed parameters improved significantly at 12 and 24 months compared to preoperative values. A detailed overview of all parameters and follow-up scores is given in Table 2. Back and leg pain levels according to a visual analog scale are shown in Figs. 1 and 2.
Table 2.
Results from VAS, ODI and SF-36, preoperatively to 24 months follow-up (FU)
| Group 1 (n = 6) | Group 2 (n = 26) | Total (n = 32) | |
|---|---|---|---|
| VAS back | |||
| Preoperative | 6.0 ± 2.8 | 5.9 ± 3.3 | 5.9 ± 3.1 |
| 12-Month FU | 2.6 ± 2.9 | 3.4 ± 3.2 | 3.3 ± 3.1 |
| 12 Month vs. pre | −4.4 ± 2.7 | −2.7 ± 3.6 | −3.0 ± 3.5* |
| 24-Month FU | 6.2 ± 3.7 | 3.8 ± 2.6 | 3.9 ± 2.9 |
| 24 Month vs. pre | −0.8 ± 2.8 | −2.3 ± 3.4* | −2.0 ± 3.2* |
| VAS leg | |||
| Preoperative | 6.0 ± 2.4 | 7.9 ± 2.0 | 7.6 ± 2.2 |
| 12-Month FU | 4.2 ± 4.0 | 4.3 ± 3.1 | 4.3 ± 3.2 |
| 12 Month vs. pre | −2.6 ± 5.3 | −3.5 ± 3.2* | −3.4 ± 3.6* |
| 24-Month FU | 7.4 ± 3.4 | 3.0 ± 2.8 | 3.5 ± 3.3 |
| 24 Month vs. pre | 0.6 ± 4.0 | −4.9 ± 3.3* | −4.1 ± 3.9* |
| ODI | |||
| Preoperative | 45.7 ± 19.7 | 51.8 ± 15.6 | 50.7 ± 16.3 |
| 12-Month FU | 33.8 ± 21.6 | 30.5 ± 23.6 | 31.1 ± 22.9 |
| 12 Month vs. pre | −17.0 ± 36.0 | −20.0 ± 20.2* | −20.2 ± 22.9* |
| 24-Month FU | 50.2 ± 19.9 | 30.8 ± 21.2 | 32.9 ± 21.7 |
| 24 Month vs. pre | −0.6 ± 28.4 | −20.6 ± 15.2* | −17.8 ± 18.3* |
| SF-36 MCS | |||
| Preoperative | 37.4 ± 15.1 | 36.5 ± 10.7 | 36.6 ± 11.4 |
| 12-Month FU | 37.6 ± 17.8 | 46.6 ± 14.5 | 45.0 ± 15.1 |
| 12 Month vs. pre | −5.2 ± 20.6 | 9.2 ± 13.0* | 8.5 ± 14.2* |
| 24-Month FU | 42.4 ± 15.5 | 47.7 ± 12.5 | 47.3 ± 12.5 |
| 24 Month vs. pre | 10.1 ± 17.0 | 10.4 ± 13.1* | 10.7 ± 13.5* |
| SF-36 PCS | |||
| Preoperative | 31.2 ± 7.2 | 31.0 ± 7.2 | 31.0 ± 7.1 |
| 12-Month FU | 43.2 ± 9.5 | 40.3 ± 11.0 | 40.8 ± 10.7 |
| 12 Month vs. pre | 14.5 ± 10.5 | 9.4 ± 11.3* | 10.3 ± 11.1* |
| 24-Month FU | 39.3 ± 8.7 | 39.8 ± 9.7 | 40.3 ± 9.1 |
| 24 Month vs. pre | 10.7 ± 11.2 | 8.9 ± 9.5* | 9.3 ± 9.4* |
* Statistically significant, p < 0.05
Fig. 2.
Leg pain levels on visual analog scale over follow-up for both groups
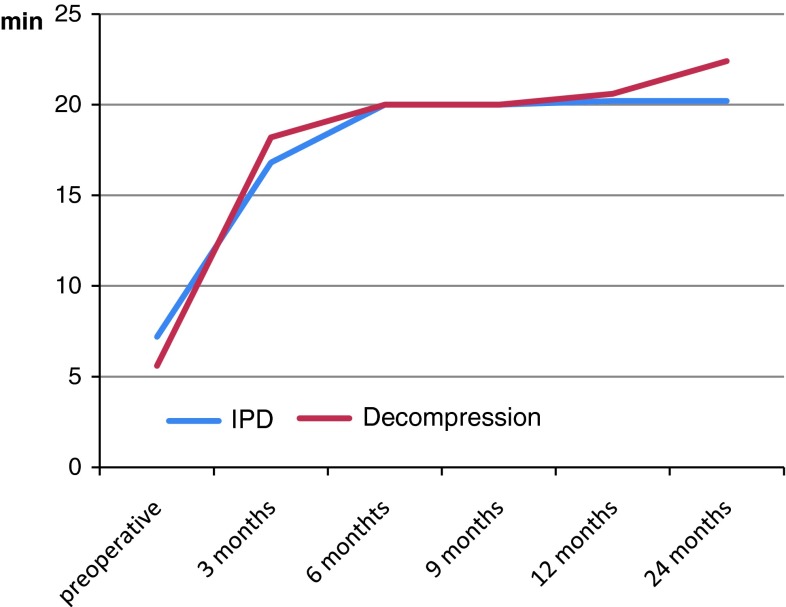
Walking tolerance, as described above, improved in both groups. Group 1 tolerated 7.2 min preoperatively (SD ± 11.3) and 20.2 min at 12 and 24 months (SD ± 13.4 and ±12.0, respectively). Corresponding values for group 2 were 5.6 ± 6.6 min, 20.6 ± 12.3 min and 22.4 ± 10.8 min. Improvements at 12 and 24 months were statistically significant compared to preoperative measurements. Results from walking assessments are presented in Fig. 3.
Fig. 3.
Walking tolerance on treadmill in minutes
Discussion
Over the past few years, interspinous decompression devices or spacers have become widely accepted as a minimally invasive spine surgery. Richter reported 14,000 implantations of the Coflex™ device worldwide prior to 2009 [23]. Insertion is quick and easy to perform, patients require a short hospitalization, and use of local anesthesia is possible [9]. However, because open decompression remains the standard of care, scientific evaluation of surgical and patient-related outcome parameters after interspinous spacer procedures is necessary.
Previously published trials have offered some insight regarding clinical outcomes. In 2005, Zucherman published 1- and 2-year results for the X-STOP® device versus conservative treatment in a randomized controlled trial. Both symptom severity and physical function scores on the Zurich Claudication Questionnaire (ZCQ) improved significantly in the X-STOP® group. Of 91 patients treated non-operatively, 32 received single epidural injections, 22 received two injections, and 21 three injections. There was no significant improvement of symptoms in conservatively treated patients [13, 15]. Anderson conducted a similar trial published in 2006. Patients with degenerative lumbar spinal stenosis with or without additional spondylolisthesis of 5–25 % on lateral radiographs were assessed with the SF-36 and radiologically for a 2-year period. In this study, the authors’ overall success criteria were fulfilled in 63.4 % of all patients undergoing X-STOP® implantation. Control group patients received one epidural steroid injection, and additional injections were administered at the discretion of the investigator [14]. Both above-mentioned trials demonstrated superior outcomes after X-STOP® implantation. However, conservative management was inconsistent and mostly brief. Thus, its effectiveness remains questionable after 2 years of follow-up.
Observational studies focused on safety and efficacy are available for various interspinous devices. In 2006, Kondrashov published a series of eighteen patients after X-STOP® implantation. At an average 51-month follow-up, successful results according to the Oswestry Disability Index were reported for 14 of 18 patients. A 15 point improvement for the ODI was considered as a successful outcome [11]. In a retrospective study of the percutaneous Aperius® device used in 152 patients, treatment success was reported using VAS and ZCQ. Follow-up data were provided for a mean of 9 months [9]. Without a control group, however, and with short follow-up, Nardi’s results are not sufficient to compare the Aperius® device to other treatments. Van Meirhaeghe published his results regarding safety and effectiveness of the Aperius® device in 2012. In 128 patients with a complete 12 months of follow-up, symptom severity according to ZCQ decreased by 26.7 ± 25.8 %, and physical function by 25.3 ± 27.7 %. 58 serious adverse events and twelve serious adverse device-related effects were reported [24]. However, statistical weaknesses of the study as well as conflict of interest should be considered when evaluating his positive conclusion.
In a systematic review and meta-analysis on the effectiveness of interspinous spacers published in 2011, Moojen included three randomized controlled trials and eight prospective cohorts using various interspinous spacers. The Aperius® device was not used in any of these trials. Methodological weakness and selection bias were the primary obstacles to evaluate the outcomes of interspinous spacer use. Thus, there was a slight advantage for interspinous spacers, but the overall level of evidence was poor [16].
Comparative studies for discrete surgical treatments remain rare. In 2011, Postacchini published an observational comparison of two cohorts. As in the current study, patients underwent either open decompression or percutaneous Aperius® implantation. Outcome assessment was carried out using ZCQ and ODI with 2 years of follow-up. After spacer implantation, 60 % of patients with moderate stenosis and 31 % of patients with severe stenosis achieved good outcomes. For the open decompression group, good results were attained in 69 % of cases with moderate stenosis and in 89 % of those with severe stenosis. In the Aperius® group, six out of 36 patients required implant removal and open decompression because of decreasing effectiveness [19].
In our observational cohort study, randomization was also not performed. The small sample size was another important shortcoming. The selective inclusion criteria for our study yielded rather small groups. Dropouts and patients lost to follow-up decreased group sizes even more. On the other hand, strict inclusion criteria for subjects reduce selection bias even without randomization. In addition, use of the Aperius® device as a percutaneous stand-alone spacer is relatively new. Large sample sizes are thus not available. In our opinion, however, beneficial effects of a new device should be established prior to broad clinical application. Taking these restrictions into account, then, a number of conclusions can still be drawn. Group 2 reported significantly better results for all investigated parameters over the entire follow-up. At 2 years, 19 of 26 patients improved by 15 points or greater on the ODI score (73 %). Most remarkable was that almost 50 % of all patients initially included in group 1 required revision surgery with implant removal and open decompression. Revision was performed at an average of 13.0 months (range: 4.1–20.0 months) post-spacer implantation. Thus, only six patients remained for the entire 2-year follow-up. Statistical analysis cannot provide significant results for such a small sample size. This also applies to the subgroup comparison. However, clearly the observed treatment failure within the first 2 years is far too much.
Postacchini concluded that spacers appear to be adequate for patients with moderate stenosis, while decompressive surgery is more appropriate for patients with severe stenosis [19]. Our study did not include MRI measurements; thus, correlations between degree of stenosis and outcome parameters cannot be provided. In any case, diagnostic criteria for imaging and measurement of the lumbar spine in spinal stenosis are inconsistent. Consensus is lacking not only to improve treatment but also to define cut-off values for clinical trials [25]. In addition, MRI measurements, electromyography, and clinical symptoms do not necessarily correlate among each other [26, 27].
The indications for the use of interspinous spacers have yet to be completely defined. “Stand alone” type spacers (e. g. Aperius®) are recommended for cases of discoligamentous lumbar spinal stenosis [12]. However, variations in spacer design, trial methodology, and additional procedures must be considered. Even after thorough review of the pertinent literature, results are difficult to compare and are not always presented objectively.
Conclusions
Randomized controlled trials comparing two discrete surgical options are challenging. In our workgroup, such trials yield low recruitment. Observational cohort studies may not provide a high level of scientific evidence, but they do share a substantial amount of experience. Even considering the methodological weaknesses of our own and other studies, percutaneous interspinous spacers offer a rapid and simple treatment that relieves symptoms of neurogenic claudication. In our experience, thorough medical history and clinical assessment are essential prior to implantation. Nevertheless, treatment will be successful only for a limited number of patients, and most probably for a limited period of time. The results of the current study for the Aperius® device as a percutaneous stand-alone interspinous spacer were disappointing. Failure rate was far too high, and pain levels returned to preoperative values 24 months after spacer implantation. In contrast, patients in the decompression group reported better results and maintained improvements at 2 years. Thus, until better data is forthcoming, we recommend interspinous spacer implantation only within randomized controlled trials for the collection of reliable data and conclusions for the spine surgeon.
Acknowledgments
FB is supported by the German Federal Ministry of Research and Education (BMBF grant 01KN1106).
Conflict of interest
None.
Contributor Information
F. Beyer, Phone: +49-221-4784616, FAX: +49-221-47887296, Email: frank.beyer@uk-koeln.de
A. Yagdiran, Email: ayla.yagdiran@uk-koeln.de
P. Neu, Email: Patrickneu82@web.de
T. Kaulhausen, Email: thomas.kaulhausen@mz-ac.de
P. Eysel, Email: peer.eysel@uk-koeln.de
R. Sobottke, Email: rolf.sobottke@mz-ac.de
References
- 1.Vogt MT, Cawthon PM, Kang JD, Donaldson WF, Cauley JA, Nevitt MC. Prevalence of symptoms of cervical and lumbar stenosis among participants in the osteoporotic fractures in men study. Spine. 2006;31:1445–1451. doi: 10.1097/01.brs.0000219875.19688.a6. [DOI] [PubMed] [Google Scholar]
- 2.Ciol MA, Deyo RA, Howell E, Kreif S. An assessment of surgery for spinal stenosis: time trends, geographic variations, complications, and reoperations. J Am Geriatr Soc. 1996;44:285–290. doi: 10.1111/j.1532-5415.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 3.Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046–2052. doi: 10.1097/00007632-199609010-00024. [DOI] [PubMed] [Google Scholar]
- 4.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F (2000) Lumbar spinal stenosis: conservative or surgical management?: A prospective 10-year study. Spine (Phila Pa 1976) 25:1424–1435; discussion 1435–1426 [DOI] [PubMed]
- 6.Richards JC, Majumdar S, Lindsey DP, Beaupre GS, Yerby SA. The treatment mechanism of an interspinous process implant for lumbar neurogenic intermittent claudication. Spine. 2005;30:744–749. doi: 10.1097/01.brs.0000157483.28505.e3. [DOI] [PubMed] [Google Scholar]
- 7.Sobottke R, Schluter-Brust K, Kaulhausen T, Rollinghoff M, Joswig B, Stutzer H, Eysel P, Simons P, Kuchta J. Interspinous implants (X Stop, Wallis, Diam) for the treatment of LSS: is there a correlation between radiological parameters and clinical outcome? Eur Spine J. 2009;18:1494–1503. doi: 10.1007/s00586-009-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuchta J, Sobottke R, Eysel P, Simons P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18:823–829. doi: 10.1007/s00586-009-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nardi P, Cabezas D, Rea G, Pettorini BL. Aperius PercLID stand alone interspinous system for the treatment of degenerative lumbar stenosis: experience on 152 cases. J Spinal Disord Tech. 2010;23:203–207. doi: 10.1097/BSD.0b013e31819b08da. [DOI] [PubMed] [Google Scholar]
- 10.Fabrizi AP, Maina R, Schiabello L. Interspinous spacers in the treatment of degenerative lumbar spinal disease: our experience with DIAM and Aperius devices. Eur Spine J. 2011;20:20–26. doi: 10.1007/s00586-011-1753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondrashov DG, Hannibal M, Hsu KY, Zucherman JF. Interspinous process decompression with the X-STOP device for lumbar spinal stenosis: a 4-year follow-up study. J Spinal Disord Tech. 2006;19:323–327. doi: 10.1097/01.bsd.0000211294.67508.3b. [DOI] [PubMed] [Google Scholar]
- 12.Sobottke R, Siewe J, Kaulhausen T, Otto C, Eysel P. Interspinous spacers as treatment for lumbar stenosis. Seminars Spine Surg. 2011 [Google Scholar]
- 13.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, 2nd, Skidmore GA, Vessa PP, Dwyer JW, Puccio ST, Cauthen JC, Ozuna RM (2005) A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine (Phila Pa 1976) 30:1351–1358. pii: 00007632-200506150-00003 [DOI] [PubMed]
- 14.Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463–471. doi: 10.3171/spi.2006.4.6.463. [DOI] [PubMed] [Google Scholar]
- 15.Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, Johnson DR, 2nd, Skidmore GA, Vessa PP, Dwyer JW, Puccio S, Cauthen JC, Ozuna RM. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moojen WA, Arts MP, Bartels RH, Jacobs WC, Peul WC. Effectiveness of interspinous implant surgery in patients with intermittent neurogenic claudication: a systematic review and meta-analysis. Eur Spine J. 2011;20:1596–1606. doi: 10.1007/s00586-011-1873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shabat S, Arinzon Z, Folman Y, Leitner J, David R, Pevzner E, Gepstein R, Pekarsky I, Shuval I. Long-term outcome of decompressive surgery for lumbar spinal stenosis in octogenarians. Eur Spine J. 2008;17:193–198. doi: 10.1007/s00586-007-0514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postacchini R, Ferrari E, Cinotti G, Menchetti PPM, Postacchini F. Aperius interspinous implant versus open surgical decompression in lumbar spinal stenosis. Spine J. 2011;11:933–939. doi: 10.1016/j.spinee.2011.08.419. [DOI] [PubMed] [Google Scholar]
- 20.Moojen WA, Arts MP, Brand R, Koes BW, Peul WC (2010) The Felix-trial. Double-blind randomization of interspinous implant or bony decompression for treatment of spinal stenosis related intermittent neurogenic claudication. BMC Musculoskelet Disord 11:100. doi:10.1186/1471-2474-11-100 [DOI] [PMC free article] [PubMed]
- 21.Sobottke R, Rollinghoff M, Siewe J, Schlegel U, Yagdiran A, Spangenberg M, Lesch R, Eysel P, Koy T. Clinical outcomes and quality of life 1 year after open microsurgical decompression or implantation of an interspinous stand-alone spacer. Minim Invasive Neurosurg. 2010;53:179–183. doi: 10.1055/s-0030-1263108. [DOI] [PubMed] [Google Scholar]
- 22.Roder C, Chavanne A, Mannion AF, Grob D, Aebi M. SSE Spine Tango–content, workflow, set-up. www.eurospine.org-Spine Tango. Eur Spine J. 2005;14:920–924. doi: 10.1007/s00586-005-1023-2. [DOI] [PubMed] [Google Scholar]
- 23.Richter A, Schutz C, Hauck M, Halm H. Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow up of a prospective case control study of 60 patients. Eur Spine J. 2010;19:283–289. doi: 10.1007/s00586-009-1229-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Meirhaeghe J, Fransen P, Morelli D, Craig NJ, Godde G, Mihalyi A, Collignon F. Clinical evaluation of the preliminary safety and effectiveness of a minimally invasive interspinous process device APERIUS((R)) in degenerative lumbar spinal stenosis with symptomatic neurogenic intermittent claudication. Eur Spine J. 2012;21:2565–2572. doi: 10.1007/s00586-012-2330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steurer J, Roner S, Gnannt R, Hodler J. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12:175. doi: 10.1186/1471-2474-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiodo A, Haig AJ, Yamakawa KSJ, Quint D, Tong H, Choksi VR. Magnetic resonance imaging vs. electrodiagnostic root compromise in lumbar spinal stenosis: a masked controlled study. Am J Phys Med Rehabil. 2008;87:789–797. doi: 10.1097/PHM.0b013e318186af03. [DOI] [PubMed] [Google Scholar]
- 27.Haig AJ, Geisser ME, Tong HC, Yamakawa KSJ, Quint DJ, Hoff JT, Chiodo A, Miner JA, Phalke VV. Electromyographic and magnetic resonance imaging to predict lumbar stenosis, low-back pain, and no back symptoms. J Bone Joint Surg Am. 2007;89A:358–366. doi: 10.2106/JBJS.E.00704. [DOI] [PubMed] [Google Scholar]