Abstract
Purpose
Major spine surgery with multilevel instrumentation is followed by large amount of opioid consumption, significant pain and difficult mobilization in a population of predominantly chronic pain patients. This case–control study investigated if a standardized comprehensive pain and postoperative nausea and vomiting (PONV) treatment protocol would improve pain treatment in this population.
Methods
A new regimen with acetaminophen, NSAIDs, gabapentin, S-ketamine, dexamethasone, ondansetron and epidural local anesthetic infusion or patient controlled analgesia with morphine, was introduced in a post-intervention group of 41 consecutive patients undergoing multilevel (median 10) instrumented spinal fusions and compared with 44 patients in a pre-intervention group.
Results
Compared to patients in the pre-intervention group, patients treated according to the new protocol consumed less opioid on postoperative day (POD) 1 (P = 0.024) and 2 (P = 0.048), they were mobilized earlier from bed (P = 0.003) and ambulation was earlier both with and without a walking frame (P = 0.027 and P = 0.027, respectively). Finally, patients following the new protocol experienced low intensities of nausea, sedation and dizziness on POD 1–6.
Conclusions
In this study of patients scheduled for multilevel spine surgery, it was demonstrated that compared to a historic group of patients receiving usual care, a comprehensive and standardized multimodal pain and PONV protocol significantly reduced opioid consumption, improved postoperative mobilization and presented concomitant low levels of nausea, sedation and dizziness.
Keywords: Major spine surgery, Multimodal pain treatment, Opioid consumption, Mobilization
Introduction
The frequency of spinal surgery has increased during the last decade, especially in patients aged 65 years and older [1, 2]. This includes multilevel instrumentation which is often associated with severe pain and consumption of large amounts of opioids thereby hindering postoperative mobilization and rehabilitation [3, 4]. Also, patients undergoing major spine surgery often suffer from pre-operative chronic pain, further challenging postoperative pain treatment [5, 6].
Postoperative pain is the result of activation of different pain mechanisms, including nociceptive, neuropathic and inflammatory pains. Likewise, peripheral and central sensitization further contributes to the development of hyperalgesia with increased pain as a result. Therefore, a balanced analgesic treatment addressing the different mechanisms represents a logical therapeutic approach [7]. A reduction in opioid consumption and opioid related side-effects is of special concern in this approach [8, 9].
A number of studies have focused on pain treatment in spine surgery, but most studies have focused on primary degenerative conditions in the lumbar spine treated with discectomy, decompression and lumbosacral fusion [10, 11]. Since an increased number of major spinal procedures, including revision surgery can be anticipated, we found it of relevance to assess a multimodal pain treatment strategy in patients undergoing surgery requiring instrumentation on >3 levels.
Our hypothesis was that a comprehensive pain and postoperative nausea and vomiting (PONV) treatment regimen would reduce opioid consumption and improve postoperative mobilization, while maintaining low levels of side-effects. The aim of this study was to assess the effect of such a standardized regimen and to compare opioid consumption and postoperative mobilization with those of a pre-intervention control group.
Materials and methods
The study was carried out at the Spine Unit, Department of Orthopaedic Surgery, Rigshospitalet, Copenhagen University Hospital, Denmark. Adult patients scheduled for elective posterior instrumented fusion on >3 levels for non-malignant and non-infectious conditions of the spine were included in the study.
Design
A new standardized pain and PONV treatment protocol was introduced from 1 January 2011. Consecutive patients receiving the standardized treatment protocol were monitored prospectively to document treatment effects. No randomization was done. According to The local Regional Ethics Committee guidelines in Denmark, no approval was needed. The study was approved by the Danish data protection agency (2008-41-2128).
Patients
Pre-intervention group
Data from 44 comparable patients operated from October 2009 through November 2010 were collected based on review of the medical records and electronic patient’s medicine (EPM) files. Thus, a total of 44 patients undergoing similar procedures were included.
Post-intervention group
Data from 41 consecutive patients who received the new treatment protocol were registered prospectively from January 2011 to June 2011.
Intervention
All patients had general anesthesia with propofol and remifentanil. Controlled hypotension was employed and 2 g of tranexamic acid was administered intravenously immediately prior to the surgical procedure. All patients underwent posterior instrumented fusion with pedicle screws on >3 levels with a midline incision and subperiosteal exposure of the relevant levels. The primary indications for surgery were adult deformity or primary degenerative conditions.
Postoperatively, patients were extubated and transferred to the postanesthesia care unit (PACU).
No modification of the standard mobilization program was introduced in the study period compared to the period for the pre-intervention data. Patients were trained daily by a physiotherapist except at weekends during which the patients were mobilized with help of the ward staff. The criteria for discharge were mobilization from bed to standing position, independent personal hygiene, and independent or assisted stair climb corresponding to one floor [12].
A detailed description of the treatments in the pre- and post-intervention groups is provided in Table 1. The new standardized protocol covered the handling of the patient from before surgery until discharge and was intended for all patients. However, patients with regular opioid consumption >100 mg morphine (eq.)/day, had the following adjustments: IV morphine dose before the end of surgery was 0.4 mg/kg, S-ketamine 0.03 mg/kg/h was continued for 72 h; prolonged release morphine at the discontinuation of either the epidural or the PCA treatment was substituted by increasing the patient’s usual opioid dosing with 50 %/day.
Table 1.
Pre- and post-intervention treatment
| Pre-intervention protocol | Post-intervention protocol | |
|---|---|---|
| Premedication | Usual opioid medication | Usual opioid medication |
| No standardized treatment | Acetaminophen OR 2 g (sustained release) | |
| Celecoxib OR 400 mg | ||
| Gabapentin OR 900 mg | ||
| General anesthesia | Remifentanil and propofol | Remifentanil and propofol |
| Fentanyl (ADA) | Dexamethasone IV 24 mg | |
| S-ketamine IV 0.5 mg/kg + 0.3 mg/kg/h until 45 min before the end of surgery | ||
| Morphine (ADA) | Morphine IV 0.3 mg/kg supplied 45 min before the end of surgery | |
| Epidural, placed at the discretion of the surgeon, bolus at the discretion of the anesthetist | Epidural catheter (tip at the middle of the surgical field), bolus: bupivacaine 0.5 mg/ml 10 ml | |
| If epidural not possible, local infiltration analgesia 40 ml with bupivacaine 2.5 mg/ml placed subfacially | ||
| PONV prophylaxis: ADA | Ondansetron IV 4 mg | |
| Postoperative analgesia | Usual opioid medication | Usual opioid medication |
| Acetaminophen OR 1 g × 4 | Acetaminophen OR 1 g × 4 | |
| Ibuprofen OR 400 mg × 4 until discharge | ||
| Gabapentin OR 400 + 1,000 mg for 5 days | ||
| Epidural for up to 72 h or PCA morphine for 48 h | Epidural bupivacaine 2.5 mg/ml with 50 μg/ml of morphine 5–8 ml/h for 96 h | |
| Opioid ADA | If epidural infusion was not possible, PCA morphine (bolus 2 mg, lockout 15 min, for 48 h) background infusion 1 mg/h during the first 24 h | |
| Sustained release morphine 20 mg × 2 after end of EPI or PCA | ||
| Morphine as needed | ||
| Additional pain | Epidural bolus of bupivacaine 5 ml 2.5 mg/ml | Epidural bolus of bupivacaine 5 ml 2.5 mg/ml |
| Morphine OR or IV as needed | Morphine OR or IV as needed | |
| PONV treatment | Ondansetron | Ondansetron IV 1 mg |
| Droperidol IV 0.625 mg | ||
| Dexamethasone IV 8 mg |
ADA at the discretion of the anesthetist, OR oral, PCA patient controlled analgesia, PONV postoperative nausea and vomiting, EPI epidural infusion
Endpoints
The primary endpoint was opioid consumption (oral morphine eq.) on postoperative days (POD) 1–6. Secondary endpoints were number of days until the patients could be mobilized from bed and until the patients were able to walk with and without a walking frame, numeric rating scale (NRS) pain score (0 = no pain and 10 = worst pain imaginable) at rest and during mobilization on POD 1–6, levels of side-effects (nausea, sedation and dizziness) on POD 1–6 and postoperative length of stay (LOS) at the PACU and at the surgical department.
Data collection
Data for patients in the pre- and post-intervention treatment protocol were tracked and collected from the anesthesia, the PACU, and the patient and nurse records, as well as the EPM files. All patients answered a pre-operative Short Form (36) Health Survey (SF-36) [13] questionnaire assessing health-related quality of life. Data of mobilization were collected from the physiotherapists’ record. Furthermore, data from patients in the post-intervention protocol were supplied with a scheduled assessment on POD 1–6 at 9 AM. Patients were interviewed by one of the authors about their NRS pain (0–10) at rest and during mobilization (from side to side in bed) and about the intensity (none, mild, moderate, severe) of nausea, dizziness and sedation for the previous 24 h.
For statistical comparisons, the oral morphine equivalent dose was calculated based on the following oral ratios: oxycodone (2:1), ketobemidone (2:1), tramadol (1:10), fentanyl (100:1) and methadone (5:1). For IV opioid consumption a 1:1 ratio was used for morphine, oxycodone and ketobemidone. Comparison of opioid consumption on POD 1 and 2 was only done in patients without a PCA device [group pre-intervention: n = 31 (POD 1) and n = 33 (POD 2); group post-intervention: n = 27 (POD 1 and 2)] because of lack of data regarding infusion.
Patients with a dura lesion requiring bed rest for more than 24 h postoperatively are not included in mobilization data (group pre-intervention: n = 1; group post-intervention: n = 3). Furthermore, patients in whom no data about walking ability were available at discharge or transferral to another hospital were considered able to walk without a walking frame for either the day of discharge or for 1 day after the day of transfer to another hospital.
Statistical methods
Data are presented as medians with interquartile range (IQR) or minimum/maximum. P < 0.05 was considered statistically significant. The Mann–Whitney U test for unpaired data was used to compare differences between groups for morphine consumption on POD 1–6, levels of mobilization and LOS at the PACU and the surgical department. Bonferroni correction for multiple comparisons was performed on data of morphine consumption and mobilization at the surgical department. Numerical values were attributed to verbal scores of pain, nausea, sedation, and dizziness (none = 1; mild = 2; moderate = 3 and severe = 4). Categorical data were compared with Fischer’s exact test. Calculations were performed using SPSS 17 for Windows (SPSS, Chicago, IL). The authors did all statistical analysis.
Results
From January 2011 to June 2011, 41 consecutive patients receiving the post-intervention protocol were included in the study and were compared to a pre-intervention group of 44 patients. The baseline demographics and clinical characteristics were similar between groups (Table 2). Also, no significant differences in SF-36 scores between the two groups could be demonstrated. In all patients, the indication for surgery was spinal deformity or a degenerative condition requiring posterior instrumentation on >3 levels.
Table 2.
Patient characteristics and perioperative data
| Variable | Pre-intervention (N = 44) | Post-intervention (N = 41) | P value |
|---|---|---|---|
| Age (years) | 63 (18–85) | 59 (17–83) | 0.22 |
| Gender (female vs. male) | 30 vs. 14 | 27 vs. 14 | 1.0 |
| Height (cm) | 166 (149–192) | 171 (155–190) | 0.11 |
| Weight (kg) | 70 (45–129) | 69 (54–112) | 0.90 |
| Pre-operative daily opioid consumption (n) | 23 | 26 | 0.38 |
| Duration of surgery (min) | 288 (138–406) | 275 (114–491) | 0.26 |
| Number of instrumented spinal levels | 9.5 (4–15) | 10 (4–16) | 0.61 |
| Surgical approach | |||
| Posterior | 44 | 41 | 1.00 |
| Thoracic | 1 | 0 | 1.00 |
| Smith–Peterson osteotomy | 5 | 1 | 0.20 |
| Pedicle subtraction osteotomy | 9 | 9 | 1.00 |
| Iliac instrumentation | 17 | 28 | 0.009 |
| Postoperative length of stay before discharge | 9 (6.3–10.8) | 7 (5–10) | 0.39 |
Values are median (interquartile range) or number of patients
Opioid consumption and pain on POD 1–6
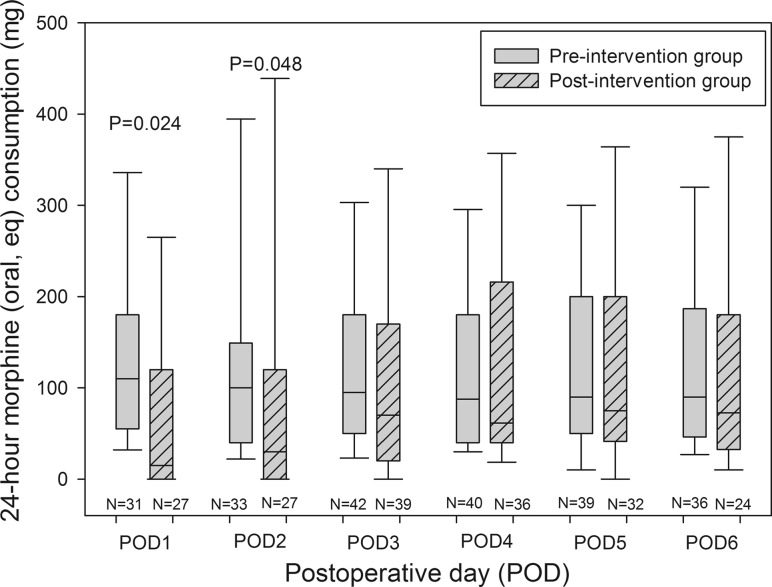
Morphine administration (oral eq.) was significantly reduced with the post-intervention protocol on POD 1 [110 (55–180) vs. 15 (0–120) mg)] (P = 0.024) and on POD 2 [100 (40–149) vs. 30 (0–120) mg)] (P = 0.048), but not on POD 3–6 (Fig. 1).
Fig. 1.
Box plot showing oral morphine (eq.) consumption on postoperative days (POD) 1–6. Data for POD 1 + 2 are without patients treated with patient controlled analgesia (PCA) devices. Plots demonstrate medians and IQR. Whiskers show 5 and 95 % percentiles. The 24-h morphine consumption was significantly reduced on postoperative day 1 (P = 0.024) and 2 (P = 0.048) in the post-intervention group receiving the new multimodal pain treatment protocol
Mobilization
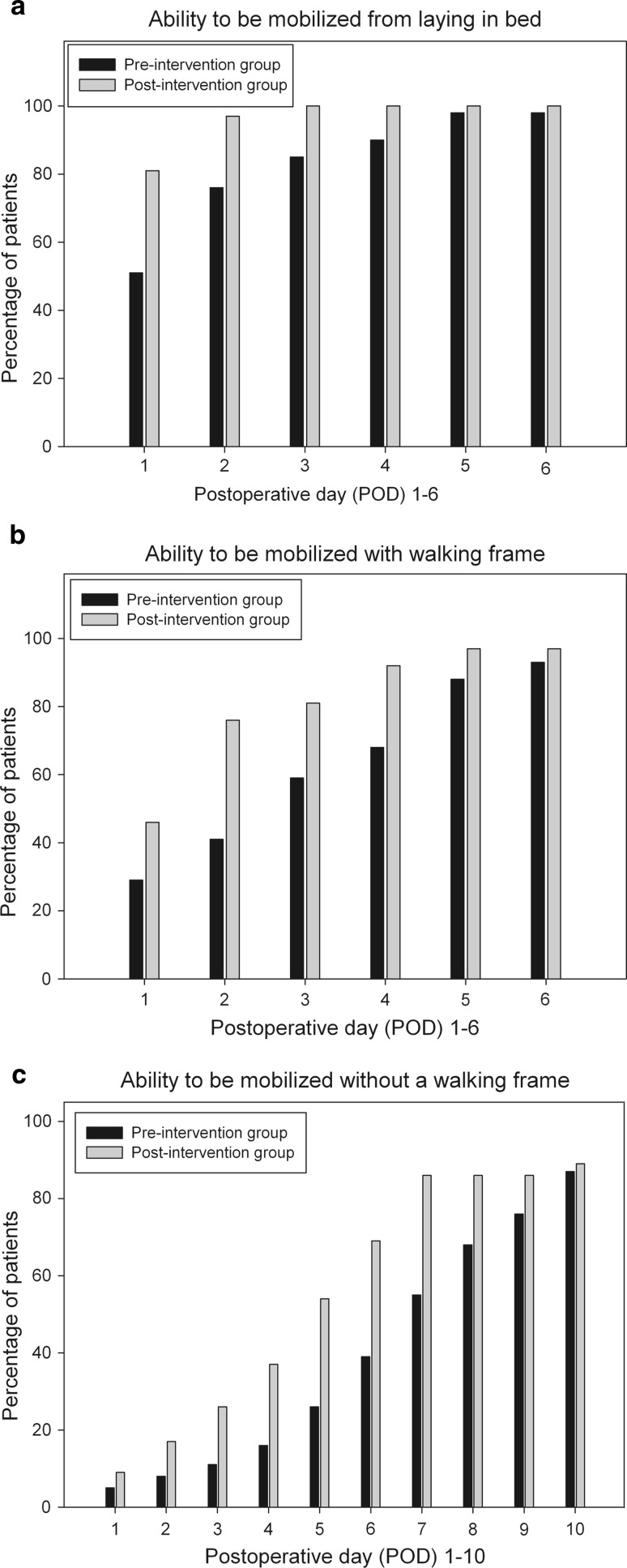
Mobilization was significantly improved for patients in the post- compared to the pre-intervention group. The mobilization ability of patients, i.e., from bed (median and IQR): [1 (1–1) vs. 1 (1–2.5) days (P = 0.003)], walking with [2 (1–2.5) vs. 3 (1–5) days (P = 0.027)] and without a walking frame [5 (3–7) vs. 7 (5–9.3) days (P = 0.027)] is demonstrated in Fig. 2a–c.
Fig. 2.
a–c Mobilization ability as the percentage of patients achieving a fixed endpoint: mobilization from bed, walking with a walking frame and walking without a walking frame. The ability to be mobilized from bed (P = 0.003), as well as walking with (P = 0.027) and without (P = 0.027) the help of a walking frame was significantly improved in the post-intervention group receiving the new multimodal pain treatment protocol
Pain scores and side-effects
Due to inconsistency of pain and side-effect data in the pre-intervention group, comparison between groups was not possible. Instead, data are presented for the post-intervention group only based upon morning assessments on POD 1–6. Median NRS pain at rest was 3–5, and during mobilization 5–7 for the first 6 postoperative days (Table 3).
Table 3.
Postoperative pain scores and levels of side-effects at 9 AM in the post-intervention group
| POD | VAS pain at rest | VAS pain during mobilization | Nausea (0–3) | Sedation (0–3) | Dizziness (0–3) |
|---|---|---|---|---|---|
| 1 | 3 (0.5–6.5) | 7 (5–10) | 0 (0–1) | 1 (0–2) | 0 (0–0) |
| 2 | 3 (0–6) | 5 (2.5–7) | 0 (0–1) | 1 (0–3) | 1 (0–2) |
| 3 | 4 (1–7) | 6 (3.5–8.5) | 0 (0–1) | 1 (0–2) | 1 (0–3) |
| 4 | 5 (2.8–7.3) | 6 (4–8) | 1 (0–1.5) | 1 (0.5–2) | 1 (0–1.5) |
| 5 | 4 (1–7) | 6 (3–8) | 0.5 (0–2) | 1 (0–2) | 1 (0–2) |
| 6 | 5 (2.5–7.5) | 6 (4.5–8) | 0 (0–2) | 0 (0–1.5) | 1 (0–1.5) |
Values are median and interquartile range (IQR), postoperative day (POD), visual analog scale (VAS), range: 0 = no pain, 10 = worst pain imaginable; levels of side-effects: none = 0, slight = 1, moderate = 2, severe = 3
The median intensity of nausea for the first 6 PODs ranges from 0 to 1 (Table 3). PONV treatment was administered in 17–21 % of the patients on POD 1–6. In general, the reported median levels of sedation and dizziness were 1 on POD 1–6, corresponding to a verbal level of mild sedation or dizziness (Table 3).
There was no significant difference in the 1-year revision rate because of pseudarthrosis in the two groups. Two patients in the pre-intervention group were re-operated because of postoperative hematoma; none in the post-intervention group.
There were no postoperative infections in neither of the groups assessed by prolonged perioperative antibiotics and/or revision surgery due to infection.
All patients had a bladder catheter for the duration of the epidural pain treatment, and no patients had urinary retention after removal of the bladder catheter.
Length of stay at the PACU and surgical department
LOS at the PACU was significantly reduced for the post- vs. pre-intervention group: 270 (173–353) vs. 345 (240–480) min (P = 0.007).
In the pre-intervention group, 64 % of the patients were discharged to home vs. 66 % of the patients in the post-intervention group. The remaining patients were transferred to another hospital for convalescence. LOS was not significantly different between groups: 9 (IQR 6.3–10.8) (pre-intervention group) vs. 7 (IQR 5–10) (post-intervention group) days (P = 0.39) (Table 2).
Discussion
In this study, we have reported the effect of a comprehensive multimodal analgesic and anti-emetic treatment protocol in a population of patients undergoing major spine surgery and compared to those of a historic group receiving usual care. Compared to a historic pre-intervention group, postoperative morphine consumption was reduced together with improved mobilization and with concomitant low intensities of nausea, sedation and dizziness for POD 1–6.
To our knowledge, the present study is the first to report a possible effect on pain treatment and mobilization from a multimodal pain treatment strategy involving acetaminophen, NSAIDs, gabapentin, dexamethasone, S-ketamine and epidural pain treatment or PCA morphine in patients undergoing major spine surgery. There is no consensus on the term “major spine surgery” and it is sometimes used synonymously with “complex spine surgery”. Some authors define complex procedures based on the surgical approach or fusion on more than two disk levels [14]. In other studies, multilevel thoracolumbar procedures with instrumentation and fusion qualify as “complex spine surgery” [5]. In the present study, the median number of instrumented levels was 10 and a considerable number of patients underwent pedicle subtraction osteotomy. We find that this reflects the complexity of the procedures and therefore, qualifies as “major spine surgery”.
The terms “mobilization” and “discharge criteria” are poorly defined in the literature on spine surgery. Although length of hospital stay is often used as an outcome variable, the criteria are not always described in detail or standardized [15]. Other studies use mobilization criteria corresponding to the ones used in the present study requiring the patients to be able to transfer and ambulate before discharge [16]. This corresponds to the criteria used in the assessment of fast-track surgery in hip and knee replacement [17]. Since postoperative mobilization and LOS are closely related parameters, we suggest that a detailed description of discharge criteria is required in future studies including these outcome variables to allow comparison of results reported in the literature.
Other studies have focused on pain management related to spinal surgery. This includes the beneficial effect of epidural steroid after lumbar discectomy [18] and from locally applied methylprednisolone in patients undergoing mixed lumbar surgical interventions [19]. The efficacy of glucocorticoids as part of multimodal postoperative pain treatment in different surgical procedures has recently been reviewed [20] demonstrating reductions in both pain scores and morphine consumption. Likewise, Lunn et al. [21] showed that IV methylprednisolone 125 mg after total knee arthroplasty improved analgesia and promoted recovery. Neither of the studies reported on serious complications, especially wound infection, from the use of a single dose of glucocorticoids, but calls for further large-scale studies on safety issues.
The employment of NSAIDs in a surgical population with a high risk of developing pseudoarthrosis can be questioned as it is speculated that NSAIDs may impair bone formation [22]. The debate is ongoing, but it has recently been demonstrated that ketorolac did not predispose to pseudoarthrosis in an adolescent idiopathic scoliosis surgical population [23]. Likewise, Dodwell et al. [24] reviewed NSAID for postoperative pain treatment in seven high-quality retrospective studies in spine surgery and did not find an increased risk of pseudoarthrosis from short-term NSAID use during a 12-month follow-up period. On the other hand, NSAIDs have demonstrated significant analgesic properties with reduced need of opioids followed by reduced levels of PONV [25]. Thus, we included treatment with ibuprofen until discharge as part of the standard treatment protocol. One year follow-up revealed a similar incidence of re-operation for pseudoarthrosis in the two groups, and no increase in postoperative hematoma in the post-intervention group.
This study has several limitations. First, it is not a randomized blinded trial, which may introduce bias, especially with the historic nature of the data in the control group. However, both the pre- and post-intervention data were collected at well-defined time periods, where the surgical treatment principles and discharge criteria in the department did not change. Second, inconsistency of data is of great concern as most data were tracked from patient and nurse records and especially, data on pain and side-effects in the pre-intervention group was scarce. Therefore, we only have pain and side-effect data in the post-intervention group. Third, the calculation of a morphine equivalent parameter from different opiates with different pharmacokinetic and pharmacodynamic profiles may be questioned. Fourth, comparison of opioid consumption on POD 1 and 2 was only done in patients without a PCA device because of lack of data from this device. This reduces our number of patients on these days and may result in wide IQRs. Fifth, we do not have information on pre-operative pain scores and mobility of the two study groups and therefore cannot tell if the groups were comparable on these points. However, based on the pre-operative SF-36 scores, the health-related quality of life was equally affected in the two groups. Sixth, some patients in the pre-intervention group lacked data after discharge or transfer on when they stopped using a walking frame, causing uncertainty on this point.
Overall, this non-randomized and non-blinded study serves as a hypothesis-generating pragmatic description of the results from the implementation of a comprehensive treatment protocol in a population representing a daily clinical challenge and may serve as an inspiration for further investigation in this population. Such studies are warranted since a future increase in complex spine surgery is likely, challenging the request for treatment modalities that can decrease perioperative morbidities like immobilization and pain.
Conclusion
In this study of patients scheduled for multilevel spine surgery, it was demonstrated that compared to a historic group of patients receiving usual care, a comprehensive and standardized multimodal pain and PONV protocol significantly reduced opioid consumption, improved postoperative mobilization and presented concomitant low levels of nausea, sedation and dizziness. The study may serve as inspiration for future randomized studies in this population.
Conflict of interest
Benny Dahl is funded by a grant from the Danish Strategic Research Council (#2142-08-0017). No other funding was received for the study.
References
- 1.Pumberger M, Chiu YL, Ma Y, et al. National in-hospital morbidity and mortality trends after lumbar fusion surgery between 1998 and 2008. J Bone Joint Surg Br. 2012;94:359–364. doi: 10.1302/0301-620X.94B3.27825. [DOI] [PubMed] [Google Scholar]
- 2.Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. doi: 10.1097/BRS.0b013e31820cccfb. [DOI] [PubMed] [Google Scholar]
- 3.Buvanendran A, Thillainathan V. Preoperative and postoperative anesthetic and analgesic techniques for minimally invasive surgery of the spine. Spine (Phila Pa 1976) 2010;35:S274–S280. doi: 10.1097/BRS.0b013e31820240f8. [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk A, Freitag M, Tank S, et al. Quality of postoperative pain using an intraoperatively placed epidural catheter after major lumbar spinal surgery. Anesthesiology. 2004;101:175–180. doi: 10.1097/00000542-200407000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. 2011;112:218–223. doi: 10.1213/ANE.0b013e3181d8a095. [DOI] [PubMed] [Google Scholar]
- 6.Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–646. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 8.White PF, Kehlet H. Improving postoperative pain management: what are the unresolved issues? Anesthesiology. 2010;112:220–225. doi: 10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H. Postoperative opioid sparing to hasten recovery: what are the issues? Anesthesiology. 2005;102:1083–1085. doi: 10.1097/00000542-200506000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kim JC, Choi YS, Kim KN, et al. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine (Phila Pa 1976) 2011;36:428–433. doi: 10.1097/BRS.0b013e3181d26708. [DOI] [PubMed] [Google Scholar]
- 11.Ziegeler S, Fritsch E, Bauer C, et al. Therapeutic effect of intrathecal morphine after posterior lumbar interbody fusion surgery: a prospective, double-blind, randomized study. Spine (Phila Pa 1976) 2008;33:2379–2386. doi: 10.1097/BRS.0b013e3181844ef2. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PR, Jorgensen LD, Dahl B, et al. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clin Rehabil. 2010;24:137–148. doi: 10.1177/0269215509347432. [DOI] [PubMed] [Google Scholar]
- 13.Julious SA, George S, Campbell MJ. Sample sizes for studies using the short form 36 (SF-36) J Epidemiol Community Health. 1995;49:642–644. doi: 10.1136/jech.49.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine (Phila Pa 1976) 2005;30:1565–1575. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 16.Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;32:1155–1162. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 17.Husted H, Holm G, Jacobsen S. Predictors of length of stay and patient satisfaction after hip and knee replacement surgery: fast-track experience in 712 patients. Acta Orthop. 2008;79:168–173. doi: 10.1080/17453670710014941. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen S, Krum-Moller DS, Lauridsen LR, et al. Epidural steroid following discectomy for herniated lumbar disc reduces neurological impairment and enhances recovery: a randomized study with two-year follow-up. Spine (Phila Pa 1976) 2008;33:2028–2033. doi: 10.1097/BRS.0b013e3181833903. [DOI] [PubMed] [Google Scholar]
- 19.Jirarattanaphochai K, Jung S, Thienthong S, et al. Peridural methylprednisolone and wound infiltration with bupivacaine for postoperative pain control after posterior lumbar spine surgery: a randomized double-blinded placebo-controlled trial. Spine (Phila Pa 1976) 2007;32:609–616. doi: 10.1097/01.brs.0000257541.91728.a1. [DOI] [PubMed] [Google Scholar]
- 20.De Oliveira GSJ, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115:575–588. doi: 10.1097/ALN.0b013e31822a24c2. [DOI] [PubMed] [Google Scholar]
- 21.Lunn TH, Kristensen BB, Andersen LO, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth. 2011;106:230–238. doi: 10.1093/bja/aeq333. [DOI] [PubMed] [Google Scholar]
- 22.Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg. 2004;12:139–143. doi: 10.5435/00124635-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Sucato DJ, Lovejoy JF, Agrawal S, et al. Postoperative ketorolac does not predispose to pseudoarthrosis following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2008;33:1119–1124. doi: 10.1097/BRS.0b013e31816f6a2a. [DOI] [PubMed] [Google Scholar]
- 24.Dodwell ER, Latorre JG, Parisini E, et al. NSAID exposure and risk of nonunion: a meta-analysis of case-control and cohort studies. Calcif Tissue Int. 2010;87:193–202. doi: 10.1007/s00223-010-9379-7. [DOI] [PubMed] [Google Scholar]
- 25.Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106:292–297. doi: 10.1093/bja/aeq406. [DOI] [PubMed] [Google Scholar]