Abstract
Purpose
Systematic review comparing biological agents, targeting tumour necrosis factor α, for sciatica with placebo and alternative interventions.
Methods
We searched 21 electronic databases and bibliographies of included studies. We included randomised controlled trials (RCTs), non-RCTs and controlled observational studies of adults who had sciatica treated by biological agents compared with placebo or alternative interventions.
Results
We pooled the results of six studies (five RCTs and one non-RCT) in meta-analyses. Compared with placebo biological agents had: better global effects in the short-term odds ratio (OR) 2.0 (95 % CI 0.7–6.0), medium-term OR 2.7 (95 % CI 1.0–7.1) and long-term OR 2.3 [95 % CI 0.5 to 9.7); improved leg pain intensity in the short-term weighted mean difference (WMD) −13.6 (95 % CI −26.8 to −0.4), medium-term WMD −7.0 (95 % CI −15.4 to 1.5), but not long-term WMD 0.2 (95 % CI −20.3 to 20.8); improved Oswestry Disability Index (ODI) in the short-term WMD −5.2 (95 % CI −14.1 to 3.7), medium-term WMD −8.2 (95 % CI −14.4 to −2.0), and long-term WMD −5.0 (95 % CI −11.8 to 1.8). There was heterogeneity in the leg pain intensity and ODI results and improvements were no longer statistically significant when studies were restricted to RCTs. There was a reduction in the need for discectomy, which was not statistically significant, and no difference in the number of adverse effects.
Conclusions
There was insufficient evidence to recommend these agents when treating sciatica, but sufficient evidence to suggest that larger RCTs are needed.
Electronic supplementary material
The online version of this article (doi:10.1007/s00586-013-2739-z) contains supplementary material, which is available to authorized users.
Keywords: Sciatica, Systematic review, Meta-analysis, Biological agents, Tumour necrosis factor α
Introduction
Sciatica is a symptom defined as unilateral, well-localised leg pain, with a sharp, shooting or burning quality, that approximates to the dermatomal distribution of the sciatic nerve down the posterior lateral aspect of the leg, and normally radiates to the foot or ankle. It is often associated with numbness or paraesthesia in the same distribution [1]. Sciatica caused by lumbar nerve root pain usually arises from a prolapsed intervertebral disc [2], not only from compression of the nerve root [3], but also the release of pro-inflammatory factors from the damaged disc [4]. Sciatica is common [5], disabling [6–8] and costly to society [9]. Typically, sciatica patients initially receive non-surgical treatments such as oral analgesia or physiotherapy. Those with persistent or severe symptoms are referred to more invasive treatments such as epidural injections, and between 5 and 15 % of patients with sciatica are treated with surgery [6, 8], usually involving a lumbar discectomy. In the National Health Service in England 11,765 lumbar discectomies were performed during 2010/2011 [10].
Pro-inflammatory factors released from the prolapsed intervertebral disc include: phospholipase A2, prostaglandin E2, interleukin-1α (IL-1α), IL-1β, IL-6, nitric oxide and tumour necrosis factor α (TNFα). It has been suggested that TNFα is the cytokine of primary importance in the pathophysiology of sciatica [4]. Biological treatments targeting TNFα (etanercept, infliximab, adalimumab) are increasingly used in rheumatological practice to control inflammatory disease, and may be useful in sciatica [11]. A systematic review was conducted to ascertain the effectiveness of biological agents targeting TNFα for the treatment of sciatica, or lumbar nerve root pain, compared with placebo or alternative interventions. Outcomes included global effects, pain intensity, condition-specific outcome measures, adverse effects, work status and disc surgery rates. Non-randomised and randomised controlled trials as well as controlled observational studies were included.
Methods
This review used updated searches from a larger review evaluating the effectiveness and cost-effectiveness of all treatment strategies for sciatica [12], and was prepared in accordance with the PRISMA guidelines [13].
Literature search
The following databases were searched (from inception to February 2012) using strategies designed for each database: MEDLINE, EMBASE, CINAHL, AMED, British Nursing Index, Health Management Information Consortium, PsychINFO, Inspec, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews, Health Technology Assessment database, NHS Economic Evaluation database, System for Information on Grey Literature, Science Citation Index, Social Science Citation Index, Index to Scientific and Technical proceedings, PEDro, BIOSIS, National Research Register, and other trial registries (n = 7) available via the internet. An example of the search strategy for MEDLINE is presented in an “Appendix”. No language restriction was used. The bibliographies of previous systematic reviews and included studies were screened to identify further relevant studies.
Included studies
The following study designs were included: randomised controlled trials (RCTs), non-RCTs and cohort studies with concurrent or historical controls. Studies with adults who had sciatica or lumbar nerve root pain diagnosed clinically or confirmed by imaging were eligible. Any biological agent targeting pro-inflammatory factors such as tumour necrosis factor-α compared with placebo or alternative interventions using any relevant patient based outcome measure were included.
Data extraction
Two reviewers independently screened the titles and abstracts for relevance. Full papers of potentially relevant studies were retrieved and assessed for inclusion, using the criteria reported above, by two independent reviewers. Data were extracted using predefined forms on a Microsoft Access database by one reviewer and checked for accuracy, against the original paper, by a second independent reviewer. Any disagreements were resolved by discussion or by a third reviewer if necessary.
Quality assessment
Quality assessment was undertaken by two independent reviewers with differences being resolved by consensus or by a third reviewer if necessary. Since this was a meta-analysis of the evidence rather than a guideline development review, we adapted a quality checklist [14, 15] to be applicable for both RCTs and controlled observational studies of sciatica containing the criteria: external validity, selection bias and confounding, detection bias, performance bias, and attrition bias (Table 3). The rating per criterion was performed according to the risk of bias for each set of items by two reviewers independently, with the overall rating of study quality depending on the types and extent of bias. The checklist is described in more detail in the larger review evaluating the effectiveness and cost-effectiveness of all treatment strategies for sciatica [12].
Table 3.
Quality of included studies
| Quality checklist | Genevay et al. [29] | Korhonen et al. [27, 28] | Korhonen et al. [23, 24] | Becker et al. [19] | Karppinen et al. [22] | Cohen et al. [20] | Okoro et al. [25] | Genevay et al. [21] | Cohen et al. [26] |
|---|---|---|---|---|---|---|---|---|---|
| External validity | |||||||||
| Are participants representative? | ± | Unclear | Unclear | ± | Unclear | Unclear | Unclear | ± | ± |
| Percentage who agreed to participate? | 80–100 % | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | <60 % | 80–100 % |
| Staff and facilities representative? | ± | ± | ± | ± | + | ± | ± | + | ± |
| Rating | Moderate | Weak | Weak | Weak | Weak | Weak | Weak | Moderate | Moderate |
| Selection bias—confounders | |||||||||
| Study design? | HCS | Non-RCT | RCT | RCT | RCT | RCT | RCT | RCT | RCT |
| Adequate method randomisation? | – | – | + | + | + | Unclear | Unclear | + | + |
| Adequate allocation concealment? | – | – | ± | ± | Unclear | ± | + | + | + |
| Percentage relevant prognostic factors? | 60–79 % | <60 % | 60–79 % | <60 % | <60 % | 60–79 % | <60 % | 80–100 % | 60–79 % |
| Similar baseline prognostic factors? | ± | Unclear | + | Unclear | Unclear | ± | Unclear | ± | + |
| Recruited from same population? | – | – | + | + | + | + | + | + | + |
| Recruited over same time period? | – | – | + | + | + | + | + | + | + |
| Analysis of co-variance or similar? | – | + | + | + | + | – | – | + | + |
| Co-interventions avoided or similar? | Unclear | Unclear | Unclear | + | Unclear | + | Unclear | + | + |
| Rating | Weak | Weak | Moderate | Moderate | Moderate | Moderate | Moderate | Strong | Strong |
| Detection bias | |||||||||
| Valid outcome measurement? | + | + | + | + | + | + | + | + | + |
| Reliable outcome measurement? | + | + | + | + | + | + | + | + | + |
| Similar timing outcome assessment? | + | – | + | + | + | + | + | + | + |
| Outcome assessors blinded? | – | – | Unclear | + | Unclear | + | + | + | + |
| Data analyst blinded? | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Rating | Weak | Weak | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate | Moderate |
| Performance bias | |||||||||
| Participants blinded? | – | – | + | + | Unclear | + | + | + | + |
| Clinicians blinded? | – | – | Unclear | – | Unclear | + | + | + | + |
| Blinding procedure tested? | NA | NA | Unclear | – | NA | Unclear | Unclear | Unclear | Unclear |
| Rating | Weak | Weak | Moderate | Moderate | Weak | Moderate | Moderate | Moderate | Moderate |
| Attrition bias | |||||||||
| Similar characteristics drop-outs? | + | Unclear | + | + | Unclear | + | + | Unclear | Unclear |
| No differential drop-out? | – | Unclear | + | + | – | + | + | – | + |
| Percentage who completed the study? | 80–100 % | Unclear | 80–100 % | 80–100 % | 80–100 % | 80–100 % | 80–100 % | 80–100 % | <60 % |
| Analysis according to treatment allocation? | + | + | + | + | + | + | + | + | + |
| Analysis included all allocated patients? | + | Unclear | + | + | + | + | + | + | – |
| Rating | Strong | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate | Weak |
| Overall rating | Weak | Weak | Moderate | Moderate | Moderate | Moderate | Moderate | Strong | Moderate |
+ yes, − no, ± partial, HCS historical cohort study, RCT randomised controlled trial
Data analysis/synthesis
Pairwise meta-analyses were conducted for dichotomous and continuous outcomes. Continuous data were synthesised using final mean scores as weighted mean differences. RCTs with multiple treatment arms were combined to produce one intervention arm compared with one control arm as recommended by the Cochrane Handbook [16]. Where mean values were unavailable but the medians were reported, these were used instead. Missing standard deviations (SDs) were derived using methods reported in the Cochrane Handbook [16], substituted with baseline values, or imputed using the weighted mean for each intervention category [17]. Studies were pooled using the random effects model [18] in Revman version 5 with between-study heterogeneity examined using I2 and χ2 statistics. Sensitivity analyses assessed the effect of substituting mean values with medians, using imputed SDs and excluding non-randomised studies.
Results
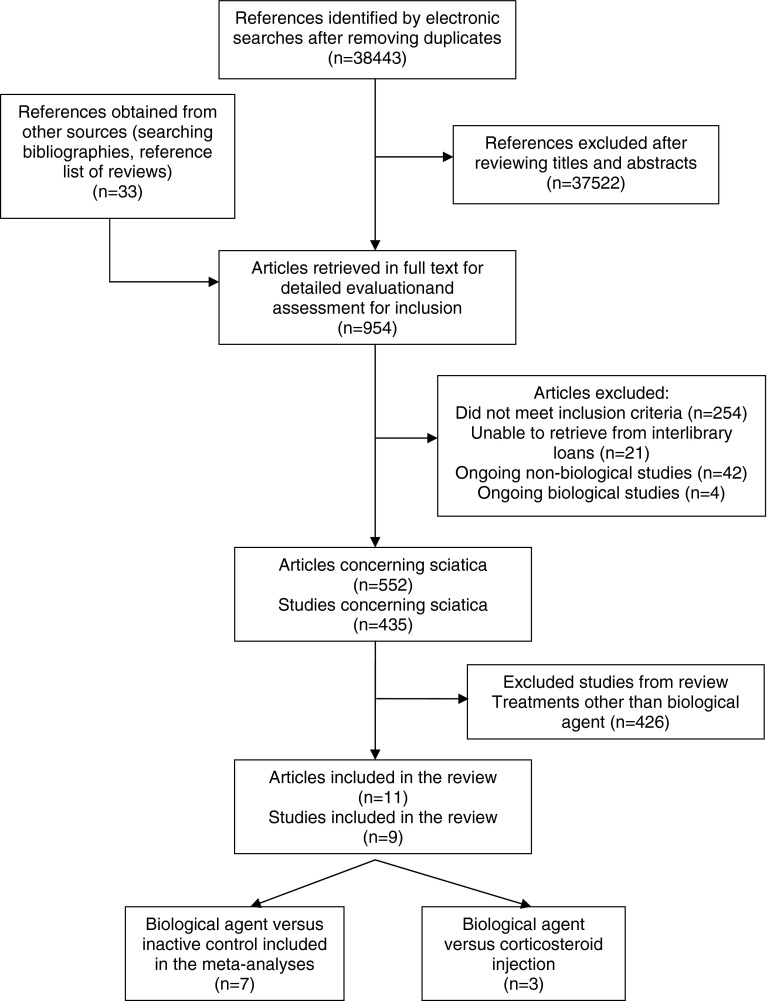
The electronic searches identified 38,443 references and a further 33 references were identified by hand searching, 954 papers were retrieved in full, 435 studies of sciatica were identified, nine of which evaluated biological agents (Fig. 1).
Fig. 1.
Systematic review flow chart
Description of biological agents studies
We identified seven RCTs [19–26] one non-RCT [27, 28] and one historical cohort study [29]. Two studies were reported in two separate publications each [23, 24, 27, 28]. One non-RCT [27, 28] and two RCTs [22–24] compared intravenous infusions of infliximab with placebo injections of saline. One RCT compared subcutaneous injection of etanercept with a placebo injection of saline [25], and another RCT compared three different doses of an epidural injection of etanercept with each other and with an epidural saline injection [20]. One three-armed RCT compared epidural injections of etanercept with epidural injections of corticosteroid and with epidural injection of saline [26]. One RCT compared subcutaneous injections of adalimumab with placebo injections [21] (Table 1). One RCT compared epidural injections of autologous conditioned serum, rich in anti-inflammatory cytokines, compared with epidural injections of corticosteroid and local anaesthetic [19]. One historical cohort study compared sub-cutaneous injections of etanercept with intra-venous injections of corticosteroid [29] (Table 2).
Table 1.
Characteristics of studies comparing biological agents with placebo
| Study | Participants | Intervention | Control treatment | Length of follow-up | Outcomes |
|---|---|---|---|---|---|
| Korhonen et al. [27, 28] Finland, non-RCT | 72 patients with nerve root pain confirmed by imaging. Data for TNF group only (no data for control group); mean duration 7.2 weeks; mean age 39 years; 80 % men | Intra-venous infusion of infliximab 3 mg/kg | Periradicular saline injection | 12 months | Number of painless patients (>75 % decrease from baseline leg pain score); back and leg pain intensity (VAS); Oswestry Disability Index; number of sick leave days; clinical status, adverse effects |
| Korhonen et al. [23, 24] Finland, RCT | 41 patients with first or recurrent episode of nerve root pain confirmed by imaging; median duration 61 days; mean age 41 years; 60 % men | Intravenous infliximab 5 mg/kg | Intravenous saline injection | 12 months | Number of painless patients (>75 % decrease from baseline leg pain score); back and leg pain intensity (VAS)a,b; Oswestry Disability Indexa,b; RAND-36 health questionnaire; number sick leave days; number discectomies; clinical status, adverse effects |
| Karppinen et al. [22] Finland, RCT | 15 patients with nerve root pain confirmed by imaging; disc herniation at L3/4 or L4/5; mean duration 58 days; mean age 53 years; 67 % men | Intravenous infliximab 5 mg/kg | Intravenous saline injection | 6 months | Back and leg pain intensity (VAS)a,b; Oswestry Disability Indexa,b; RAND-36 health questionnaire; number sick leave days; number discectomies; clinical status, adverse effects |
| Cohen et al. [20] USA, RCT | 24 patients with nerve root pain confirmed by imaging; median duration 3–7 months; median age 41–46 years; 71 % men | Transforaminal epidural injection etanercept: 2 mg (Group 1) 4 mg (Group 2) 6 mg (Group 3) |
Transforaminal epidural injection normal saline | 6 months | Number with a positive outcome [>50 % reduction in leg pain + global perceived effect (combination of pain, daily activities improved & satisfaction)]; back and leg pain intensity (numerical rating scale)c; Oswestry Disability Indexc; drug consumption; (Results from groups 1–3 combined for the meta-analysis) |
| Okoro et al. [25] UK, RCT | 15 patients with nerve root pain confirmed by imaging for at least 24 weeks; mean age not stated; 40 % men | Subcutaneous injection of etanercept 25 mg | Subcutaneous injection of saline | 3 months | Leg pain intensity (VAS)d; Oswestry Disability Indexd; modified somatic perception; modified Zung depression index; subjective walking distance; adverse effects |
| Genevay et al. [21] Switzerland, RCT | 61 patients with first or recurrent episode of nerve root pain confirmed by imaging; mean duration 3.6 weeks; mean age 49 years; 57 % men | Subcutaneous injection of adalimumab 40 mg ×2 | Subcutaneous injection of saline ×2 | 6 months | Number of responders (>30 % improvement from baseline leg or back pain score or Oswestry Disability Index); back and leg pain intensity (VAS); Oswestry Disability Index, SF-12v2; drug consumption; number of discectomies; work status; adverse effects |
| Cohen et al. [26] USA, Germany, RCT | 84 patients with nerve root pain confirmed by imaging; mean duration 2.7 months; mean age 42 years; 70 % men | Transforaminal epidural injection etanercept 4 mg + local anaesthetic 0.5 ml × 2 | Transforaminal epidural injection normal saline + local anaesthetic 0.5 ml × 2 | 6 months (large proportion left study after 1 month)e | Positive categorical outcome (>50 % decrease in leg pain + positive global perceived effect obviating the need for further treatment); back and leg pain intensity (NRS); Oswestry Disability Index; reduction in analgesic consumption |
NRS numeric rating scale, RCT randomised controlled trial, SD standard deviation, SF-12 short form 12, TNF tumour necrosis factor, VAS visual analogue scale
aSD not reported, imputed from other studies in meta-analyses
bMedians reported
cResult from only a single patient in control group at 6 month follow-up
dMean leg pain intensity and SDs obtained from authors
eAfter 1 month participants who received no benefit exited the study to pursue other treatments
Table 2.
Characteristics of studies comparing biological agents with alternative interventions
| Study | Participants | Intervention | Control treatment | Length of follow-up | Outcomes |
|---|---|---|---|---|---|
| Biological agents vs. epidural steroid injection | |||||
| Becker et al. [19] Germany, RCT | 84 patients with nerve root pain confirmed by imaging for at least 6 weeks; Mean age 54 years; 62 % men | Epidural injection of autologous conditioned serum (Group 1) | Epidural injection of steroid triamcinolone 5 mg or 10 mg + local anaesthetic 1 ml (Groups 2 and 3) | 22 weeks | Overall pain intensity (VAS)a; Oswestry Disability Index, adverse effects [Results from groups 2 & 3 combined for the forest plot] |
| Cohen et al. [26] USA, Germany, RCT | 84 patients with nerve root pain confirmed by imaging; mean duration 2.7 months; mean age 42 years; 70 % men | Transforaminal epidural injection etanercept 4 mg | Transforaminal epidural injection of steroid methyl prednisolone 60 mg + local anaesthetic 0.5 ml | 6 months (large proportion left study after 1 month)b | Global perceived effect; back and leg pain intensity (NRS); Oswestry Disability Index; reduction in analgesic consumption |
| Biological agents vs. intravenous steroid | |||||
| Genevay et al. [29] Switzerland, HCS | 20 patients with nerve root pain confirmed by imaging; mean duration 3.2 weeks; Mean age 47 years; 50 % men | Subcutaneous injection of etanercept 25 mg (anti-TNF alpha) ×3 | Intravenous injection of methylprednisolone 250 mg ×3 | 6 weeks | Numbers with a good clinical result (leg pain VAS < 30 or Oswestry Disability Index < 20); back and leg pain intensity (VAS); Oswestry Disability Index; Roland-Morris Questionnaire; number of discectomies |
HCS historical cohort study, RCT randomised controlled trial, TNF tumour necrosis factor, VAS visual analogue scale
aResults extracted from graphs
bAfter 1 month participants who received no benefit exited the study to pursue other treatments
The nine studies included 412 participants with mean ages between 39 and 54 years, with 40–80 % men, five with acute [21–24, 27–29], one with chronic [25] and three with acute and chronic symptom duration [19, 20, 26] (Tables 1, 2). Three RCTs included patients with recurrent symptoms [21, 23, 24, 29], but symptom recurrence was not reported in six studies [19, 20, 22, 25, 26, 29]. Sciatica was confirmed by imaging in all studies and previous back surgery was excluded in five trials [22–28]. Participants were selected from consecutive outpatient [19] or inpatient attendances [29], after failure to respond to conservative therapy such as physiotherapy and oral analgesia [20, 25] or were candidates for disc surgery [22–24].
Most of the studies were RCTs (7/9 78 %) and one was of good quality [21]. Five reported an adequate method of random number generation [19, 21–24], but only three documented a secure method of allocation concealment [21, 25, 26]. Three studies had moderately good external validity [21, 26, 29] (Table 3). Two RCTs reported medians rather than means [22–24]. Three RCTs did not report SDs [22–25], but were provided by the authors in one RCT [25]. Imputed SDs were used in the meta-analyses for the remaining missing values [22–24].
Biological agent versus placebo
Global effect
Five studies reported a measure of global improvement (Table 1). One poor quality non-RCT [27, 28], two moderate quality [20, 26] and one good quality RCT [21] were combined in meta-analyses at short-term (4–6 weeks) and medium-term (6 months) follow-up. One poor quality non-RCT [27, 28] and one moderate quality RCT [23, 24] were combined in a meta-analysis at long-term (12 months) follow-up. Combined odds ratios (ORs) were in favour of biological agents at all three time periods, but were only statistically significant at medium-term follow-up. Indeed there was moderate heterogeneity at short- (I2 = 62 %), medium- (I2 = 47 %) and long-term (I2 = 47 %) follow-up. ORs were 1.99 (95 % CI 0.66–5.96) in the short-term, 2.72 (95 % CI 1.04–7.13) in the medium-term and 2.26 (95 % CI 0.53–9.73) in the long-term (Fig. 2). A sensitivity analysis excluding the non-RCT [27, 28] only resulted in minimal changes to the summary OR and measurements of heterogeneity (Online Resource 1).
Fig. 2.
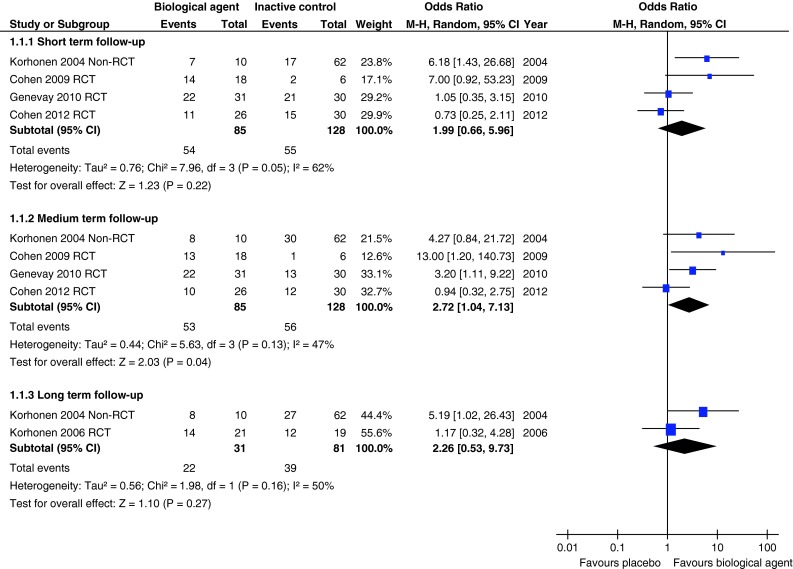
Summary of findings of global effects for studies comparing biological agents with placebo
Leg pain intensity
Seven studies reported leg pain intensity measured with a visual analogue scale. One poor quality non-RCT [27, 28], five moderate quality [20, 22–25] and one good quality RCT [21] were combined in a meta-analysis at short-term (4–6 weeks) follow-up and found a moderate weighted mean difference (WMD) of −13.63 U on a 0–100 visual analogue scale (95 % CI −26.84 to −0.41) in favour of biological agents. Five of these studies were combined in a meta-analysis at medium-term (3–6 months) follow-up [21–25, 27, 28] and found a small WMD of −6.96 (95 % CI −15.42 to 1.51) in favour of biological agents. One poor quality non-RCT [27, 28] and one moderate quality RCT [23, 24] were combined in a meta-analysis at long-term (12 months) follow-up and found no difference with a WMD of 0.18 (95 % CI −20.39 to 20.75) (Fig. 3). There was substantial heterogeneity at short- (I2 = 69 %) and long-term (I2 = 86 %), but not medium-term follow-up. A sensitivity analysis excluding the non-RCT [19, 27] reduced the size of the WMDs and heterogeneity, so that the WMD at short-term follow-up was no longer statistically significant (Online Resource 2). Excluding the two RCTs reporting medians [22–24] or the two RCTs with imputed SDs [22–24] had minimal effect at short- and medium-term follow-up. A funnel plot for publication bias did not appear to show asymmetry, but indicated a lack of large studies (Online Resource 3).
Fig. 3.

Summary of findings of leg pain intensity for studies comparing biological agents with placebo
Oswestry disability index
Seven studies reported the Oswestry Disability Index. One poor quality non-RCT [27, 28], four moderate quality [20, 22–25] and one good quality RCT [21] were combined in a meta-analysis at short-term (4–6 weeks) follow-up and found a WMD of −5.21 U (95 % CI −14.09 to 3.68) on the ODI (range 0–100) in favour of biological agents. Five of these studies were combined in a meta-analysis at medium-term (3–6 months) follow-up [21–25, 27, 28] and found a WMD of −8.16 (95 % CI −14.36 to −1.96) in favour of biological agents. One poor quality non-RCT [27, 28] and one moderate quality RCT [23, 24] were combined in a meta-analysis at long-term (12 months) follow-up and found a WMD of −4.99 (95 % CI −11.78 to 1.80) in favour of biological agents (Fig. 4). There was moderate heterogeneity at short- (I2 = 77 %), medium- (I2 = 45 %) and long-term (I2 = 61 %) follow-up. A sensitivity analysis excluding the non-RCT [27, 28] reduced the size of the WMDs so that the WMD at medium-term follow-up was no longer statistically significant (Online Resource 4). Excluding the two RCTs reporting medians, for which we also imputed SDs [22–24], had minimal effect at short- and medium-term follow-up but increased the WMD at long-term follow-up because only one non-RCT remained with a larger effect size [27, 28]. The funnel plot for publication bias did not appear to show asymmetry, but indicated a lack of large studies (Online Resource 5).
Fig. 4.
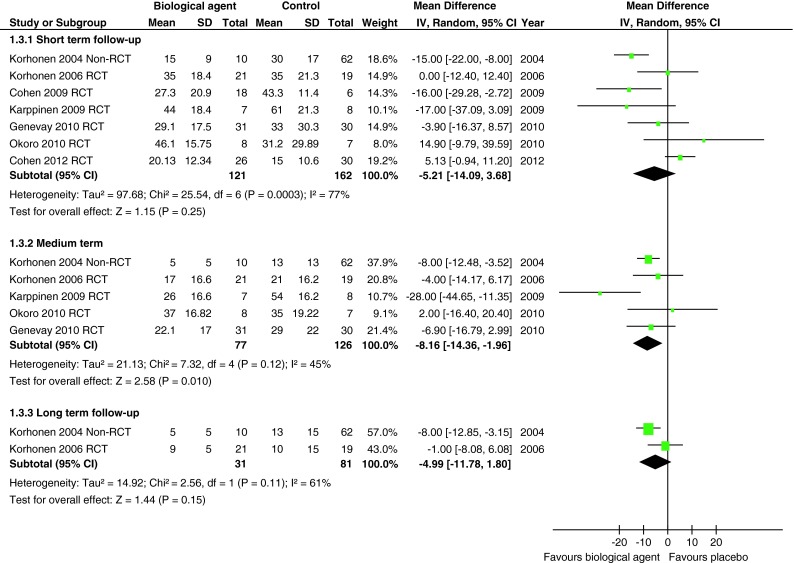
Summary of findings of Oswestry Disability Index for studies comparing biological agents with placebo
Biological agent versus corticosteroid injection
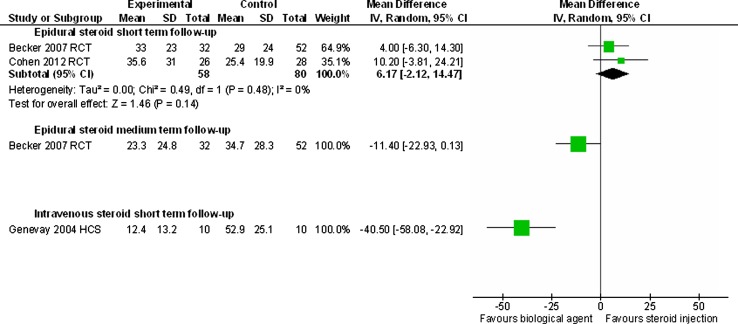
Only three studies compared biological agents with an alternative treatment. Two moderate quality RCTs compared epidural corticosteroid injections with epidural injection of autologous conditioned serum [19] and with epidural injection of etanercept [26] and were combined in meta-analyses at short-term (4–6 weeks) follow-up. For pain intensity the WMD was 6.17 (95 % CI −2.12 to 14.47) in favour of steroid injection with homogeneity amongst the effect sizes (I2 = 0) (Fig. 5). For ODI the WMD was 4.80 (95 % CI −0.88 to 10.48) in favour of steroid injection with moderate heterogeneity (I2 = 62 %) (Fig. 6). At medium-term (22 week) follow-up the RCT comparing etanercept with corticosteroid [26] found a mean difference of 11.4 (95 % CI −22.93 to 0.13) for pain intensity in favour of etanercept (Fig. 5), but a mean difference of only 0.4 in ODI (95 % CI −3.57 to 4.37) (Fig. 6). One poor quality historical cohort study [29] found that sub-cutaneous injections of etanercept were superior to intra-venous injections of corticosteroid in terms of global effects (OR 16.0, 95 % CI 1.8–143) (Online Resource 6), mean leg pain intensity (WMD −40.5, 95 % CI −58.1 to −22.9) (Fig. 5) and ODI (WMD −16.1, 95 % CI −27.5 to −4.7) at short-term (6 weeks) follow-up (Fig. 6).
Fig. 5.
Summary of findings of overall pain intensity for studies comparing biological agents with corticosteroid injection
Fig. 6.
Summary of findings of Oswestry Disability Index for studies comparing biological agents with corticosteroid injection
Need for disc surgery
One poor quality non-RCT [27, 28], three moderate quality [22–25] and one good quality RCT [21] were combined in a meta-analysis of the need for disc surgery for up to 12 months follow-up. The combined odds ratio for needing disc surgery in those receiving biological agents compared with placebo was 0.54 (95 % CI 0.26–1.14) with homogeneity amongst the effect sizes (I2 = 0 %) (Fig. 7). In addition, a poor quality cohort study [29] reported that one patient (10 %) in the etanercept group and one (10 %) in the intravenous corticosteroid group required disc surgery within the first month of the study.
Fig. 7.
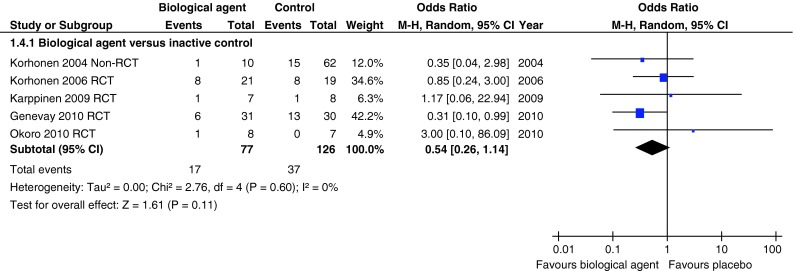
Summary of total numbers of discectomies in studies comparing biological agents with placebo
Employment outcomes
In one moderate quality RCT [23, 24] there was a median of 42 days sick leave in the infliximab group compared with 25 days in the placebo group. In one good quality RCT [21] 16 patients (64 %) in the adalimumab group returned to work by 6 months compared with 13 (42 %) in the placebo group.
Adverse effects
There was no significant difference in the number of adverse events between infliximab, etanercept or adalimumab and placebo in one non-RCT and five RCTs when these were combined in a meta-analysis [21–28], and between epidural injections of etanercept or autologous conditioned serum compared with corticosteroid and local anaesthetic epidural injections in two RCTs [19, 26] (Fig. 8). Only one serious adverse effect of severe gastrointestinal haemorrhage was reported in a patient receiving adalimumab, which was blamed upon concomitant administration of non-steroidal anti-inflammatory medication [21].
Fig. 8.
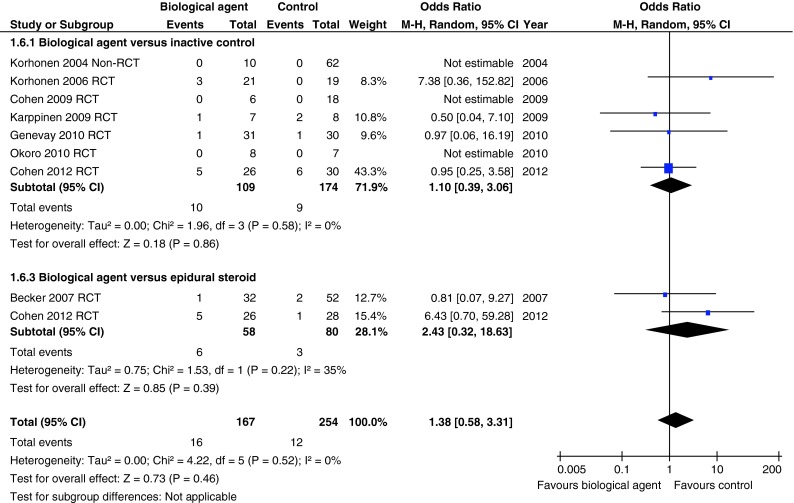
Summary of total numbers of adverse effects for studies comparing biological agents with placebo or corticosteroid injection
Discussion
Summary of main findings
There was insufficient evidence for the efficacy of biological agents targeting TNFα compared with placebo. Meta-analyses found moderate and statistically significant improvements in global effects in the medium-term, leg pain intensity in the short-term and ODI in the medium-term when all study types were included. We did not find any evidence of publication bias, although we only had a limited number of studies in our funnel plots. However, there was moderate to substantial heterogeneity in the leg pain intensity and ODI results and when non-randomised studies, which had a greater risk of bias, were excluded the meta-analysis results were no longer statistically significant. There was a reduction in the need for disc surgery, which was not statistically significant and limited evidence for improved employment outcomes. There was no difference in the number of adverse effects. Only two studies comparing biological agents with an alternative treatment were identified. One was a RCT which, rather than testing a medicinal product, tested serum rich in anti-inflammatory cytokines; the other was a poor quality cohort study. They provided very limited evidence that a biological agent was superior to intra-venous corticosteroids, but not compared with epidural corticosteroid.
Strengths and limitations of the study
One of the strengths of this review was the extensive literature search that was undertaken to identify published, unpublished and grey literature. Observational studies and non-randomised trials were included for completeness as some comparisons may not have been evaluated by RCTs. Observational studies can have better external validity than RCTs [30, 31] and provide more generalisable findings, however, the RCT is widely regarded as the design of choice when assessing the effectiveness of health care interventions [32] and we acknowledge the controversy over the inclusion of non-randomised evidence. In this review, priority was given to RCTs, and the quality of the studies noted. We also conducted a sensitivity analysis excluding non-randomised evidence.
Poor reporting and variation in the way the data were analysed meant that imputation or substitution of missing data was necessary in order for the meta-analyses to be as inclusive as possible. Omitting studies with missing SDs may induce bias in the summary effect estimate [33], and Furukawa et al. [17] have shown that it is safe to borrow SDs from other studies. The use of imputed SDs was tested in a sensitivity analysis.
We identified heterogeneity in many of the meta-analyses performed. It was our intention to explore this heterogeneity with meta-regression, where ten or more studies were included in the meta-analysis, assessing the effect of study level covariates such as: adequacy of randomisation procedure, allocation concealment, attrition rate and blinded outcome assessment. Unfortunately, there were insufficient studies to do this.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system is increasingly being used to rate the quality of evidence and grade the strength of recommendations from systematic reviews [34]. GRADE assesses the quality of a body of evidence in terms of the risk of bias, directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias. Although we did not formally use the GRADE system, we have used the same five principles when considering the overall strength of the evidence. When just considering the RCTs, there was neither serious risk of bias for any of the outcome measures, nor evidence of publication bias; however, there was moderate or serious heterogeneity and high levels of imprecision, which would downgrade the level of evidence. There were also limitations in terms of the directness of the evidence as most of the included studies were placebo controlled trials and only two had a moderate level of external validity; the remainder were weak.
Comparison with existing literature
This is the first systematic review of biological agents targeting TNFα for sciatica. It used updated searches from a larger systematic review examining all management strategies for sciatica, which included indirect comparisons of different management strategies synthesised in a mixed treatment comparison. This mixed treatment comparison (MTC) analysis found a significant improvement in leg pain intensity and condition-specific outcomes compared with inactive control when all studies were included; but when observational studies were excluded these findings were no longer statistically significant [12].
This systematic review focused on biological agents targeting TNFα. Other cytokines have also been implicated in the pathogenesis of sciatica (IL-1α, IL-1β, IL-6, etc.), but we did not identify any other comparator studies of biological agents targeting these alternative cytokines in sciatica. Other non-biological pharmacological agents may also influence cytokines. There has been one small RCT of one such agent; epidural clonidine compared with epidural corticosteroid [35]. The neurophysiology of nerve root pain has been complicated further by the discovery of the anti-inflammatory cytokine IL-10 [36], but agents manipulating this cytokine have yet to be tested in humans.
Implications for future research and clinical practice
There was insufficient evidence to recommend that these agents should be used for treating sciatica. There was heterogeneity in many of the meta-analyses and the improvements in outcome were statistically significant only when non-randomised studies were included. However, these results provide sufficient evidence to suggest that further large RCTs are needed to establish the efficacy of biological agents targeting TNFα compared with placebo. There was a scarcity of RCTs comparing the effectiveness of biological agents with other treatments for sciatica; more are needed. Biological agents are expensive but may lead to cost savings if a reduction in disc surgery is confirmed; economic evaluations alongside RCTs are needed to assess this.
Acknowledgments
We would like to thank Tosan Okoro, Philip Sell and colleagues who provided additional data from their trial. This review used updated searches from a larger review which was funded by the UK NIHR Health Technology Assessment Programme (project number 06/79/01) and has been published in full in Health Technology Assessment; vol. 15 no. 39. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Conflict of interest
There are no conflicts of interest.
Appendix: MEDLINE (OVID)
Search strategy MEDLINE (OVID) 1950 to June week 1 2008 searched on 16-06-2008. Search updated on 04-12-2009 and on 01-02-2011
Sciatica/
(Ischialg$ or sciatic$).ti,ab.
((Lumb$ or sacra$ or spin$) adj5 radicul$).ti,ab.
((Sciatic nerve or lumbar nerve or spinal nerve or sacral nerve) adj5 (irritation or inflammat$ or pain or neuropath$ or dysfunction$ or compressio$ or injur$ or traum$)).ti,ab
Intervertebral disk displacement/
((Intervertebral disk or intervertebral disc or lumbar disc or lumbar disk or lumbosacral disc or lumbosacral disk or lumbo-sacral disc or lumbo-sacral disk) adj5 (hernia$ or slip$ or prolapse or degeneration or fusion or sclerosis or rupture or distortion or fracture or displacement)).ti,ab.
((Lumbosacral nerve root or lumbo-sacral nerve root or lumbar nerve root) adj5 (irritat$ or inflammat$ or pain$ or neuropath$ or dysfunction$ or compressio$ or injur$ or traum$)).ti,ab.
((Refer$ or radiat$) adj5 (back or leg or foot)).ti,ab.
Or/1-8
(Treatment$ or therap$ or manag$ or surg$ or modalit$ or intervention$).ti,ab.
Bed rest/
(Bed rest$ or activ$ or exercise$ or education$ or instruction$ or advice$).ti,ab.
Physical therapy modalities/
((Heat or hot or thermal or infra?red or ultrasound or ultrasonic or short-wave or physio$ or physical or exercise) adj5 (therap$ or treatm$)).ti,ab.
Transcutaneous electric nerve stimulation/
(Transcutaneous electric nerve stimulation or TENS).ti,ab.
Complementary therapies/
Exp musculoskeletal manipulations/
Exp acupuncture therapy/
((Spina$ or chiropract$ or osteopath$ or physi$ or homeopath$ or acupunctur$ or musculo?skeletal or myofunctional) adj5 (massage or manipulat$ or therap$ or treatment$)).ti,ab.
Homeopathy/
Homeopathy.ti,ab.
Herbal Medicine/
Herbal medicine.ti,ab.
Orthotic devices/
(Braces or slings or splints or corset).ti,ab.
Traction/
Traction.ti,ab.
Drug therapy/
Exp analgesics/
Anti-Inflammatory agents, non-steroidal/
((Non-steroidal anti-inflammatory or non-steroidal anti-inflammatory or non?narcotic or narcotic or opioid$ or opiate$) adj5 (drug$ or analges$)).ti,ab.
(Paracetamol or acetaminophen).ti,ab.
(Ibuprofen or aceclofenac or acemetacin or celecoxib or dexketoprofen or diclofenac sodium or etodolac or etoricoxib or fenbufen or fenoprofen or flurbiprofen or indometacin or indomethacin or ketoprofen or mefenamic acid or meloxicam or nabumetone or naproxen or piroxicam or sulindac or tenoxicam or tiaprofenic acid or azapropazone or biarison or acetaminophen or nimesulide or oxyphenbutazone or azapropazone or felbinac or alclofenac or nimesulid or etofenama or loxoprofen or phenylbutazone or valdecoxib or lornoxicam or etoricoxib).ti,ab.
(Buprenorphine or butorphanol or codeine or dextromoramide or dextropropoxyphene or dihydromorphine or diphenoxylate or etorphine or fentanyl or heroin or hydrocodone or hydromorphone or levorphanol or meperidine or meptazinol or methadone or methadyl acetate or morphine or nalbuphine or oxycodone or oxymorphone or pentazocine or phenazocine or phenoperidine or pirinitramide or promedol or sufentanil or tilidine or tramadol).ti,ab.
Epidural analgesia/
Epidural injections/
((Intramuscular or intravenous or peri?neural$ or epidura$ or inject$) adj5 (cortico?steroid$ or steroid$ or ana?lgesic$ or chymopapain)).ti,ab.
(Dexamethasone or hydrocortisone or prednisolone or methylprednisolone or prednisone or methylprednisone or triamcinolone).ti,ab.
Orthopedic procedures/
Intervertebral disk chemolysis/
((Disc or disk) adj5 (chemolysis or chemonucleolysis)).ti,ab.
Vertebroplasty/
Diskectomy/
Neurosurgical procedures/
Laminectomy/
Rhizotomy/
(Discectomy or diskectomy or microdiscectomy or microdiskectomy or rhizotomy or sequestrectomy or vertebroplasty or nucleoplasty or laminectomy).ti,ab.
Surgical decompression/
Surgical decompression. ti,ab.
or/11-50
9 and 51
Limit 52 to human
References
- 1.Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? J Amer Med Assoc. 1992;268:760–765. doi: 10.1001/jama.1992.03490060092030. [DOI] [PubMed] [Google Scholar]
- 2.Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine. 1993;18:1433–1438. [PubMed] [Google Scholar]
- 3.Mixer WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210–215. doi: 10.1056/NEJM193408022110506. [DOI] [Google Scholar]
- 4.Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disc herniation-associated radiculopathy. Semin Arthritis Rheum. 1998;28:60–71. doi: 10.1016/S0049-0172(98)80029-2. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33:2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 6.Bush K, Cowan N, Katz DE, Gishen P. The natural history of sciatica associated with disc pathology; a prospective study with clinical and independent radiological follow-up. Spine. 1992;18:1433–1438. doi: 10.1097/00007632-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Tubach F, Beaute J, Leclerc A. Natural history and prognostic indicators of sciatica. J Clin Epidemiol. 2004;57:174–179. doi: 10.1016/S0895-4356(03)00257-9. [DOI] [PubMed] [Google Scholar]
- 8.Weber H, Holme I, Amlie E. The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine. 1993;18:1433–1438. [PubMed] [Google Scholar]
- 9.van Tulder MW, Koes BW, Bouter LM. A cost of illness study of back pain in the Netherlands. Pain. 1995;62:233–240. doi: 10.1016/0304-3959(94)00272-G. [DOI] [PubMed] [Google Scholar]
- 10.The NHS Information Centre, Hospital Episode Statistics for England, Inpatient statistics, Total procedures and interventions 2010–11. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=210. Accessed 23 August 2012
- 11.Cooper RG, Freemont AJ. TNF-α blockade for herniated intervertebral disc-induced sciatica: a way forward at last? Rheum. 2004;43:119–121. doi: 10.1093/rheumatology/keh013. [DOI] [PubMed] [Google Scholar]
- 12.Lewis R, Williams NH, Matar HE, Din N, Fitzsimmons D, Phillips C, Jones M, Sutton A, Burton K, Nafees S, Hendry M, Rickard I, Chakraverty R, Wilkinson C (2011) The clinical effectiveness and cost effectiveness of management strategies for sciatica: systematic review and economic model. Health Technol Assess 15(39):353–467 [DOI] [PMC free article] [PubMed]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tulder MW, Furlan A, Bombardier C, Bouter L. Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine. 2003;28:1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 15.Effective Public Health Practice Project (2010) Quality assessment tool for quantitative studies. http://www.ephpp.ca/PDF/Quality%20Assessment%20Tool_2010_2.pdf. Accessed 23 August 2012
- 16.Higgins JPT, Deeks JJ. Selecting studies and collecting data. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester (UK): Wiley; 2008. pp. 151–185. [Google Scholar]
- 17.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Becker C, Heidersdorf S, Drewlo S, de Rodriguez SZ, Kramer J, Willburger RE. Efficacy of epidural injections with autologous conditioned serum for lumbar radicular compression. Spine. 2007;32:1803–1808. doi: 10.1097/BRS.0b013e3181076514. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SP, Bogduk N, Dragovich A, Buckemaier CC, III, Griffith S, Kurihara C, Raymond JL, Richter PJ, Williams N, Yaksh TL. Randomized, double-blind, placebo-controlled, dose-response and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology. 2009;110:1116–1126. doi: 10.1097/ALN.0b013e3181a05aa0. [DOI] [PubMed] [Google Scholar]
- 21.Genevay S, Viatte S, Finckh A, Zufferey P, Balague F, Gabay C. Adalimumab in severe and acute sciatica; a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:2339–2346. doi: 10.1002/art.27499. [DOI] [PubMed] [Google Scholar]
- 22.Karppinen J, Korhonen T, Hammond A, Bowman C, Malmivaara A, Veeger N, Seitsalo S, Hurri H. The efficacy of infliximab in sciatica induced by disc herniations located at L3/4 or L4/5: a small-scale randomized controlled trial. Open Spine J. 2009;1:9–13. doi: 10.2174/1876532700901010009. [DOI] [Google Scholar]
- 23.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren K-A, Jarvinen S, Niirimaki J, Veeger N, Seitsalo S, Hurri H. The treatment of disc herniation induced sciatica with infliximab; results of a randomized, controlled 3 month follow-up study. Spine. 2005;30:2724–2728. doi: 10.1097/01.brs.0000190815.13764.64. [DOI] [PubMed] [Google Scholar]
- 24.Korhonen T, Karppinen J, Paimela L, Malmivaara A, Lindgren K-A, Bowman C, Hammond A, Kirkham B, Jarvinen S, Niirimaki J, Veeger N, Haapea M, Torkki M, Tervonen O, Seitsalo S, Hurri H. The treatment of disc herniation induced sciatica with infliximab; one year follow-up results of FIRST II, a randomised controlled trial. Spine. 2006;31:2759–2766. doi: 10.1097/01.brs.0000245873.23876.1e. [DOI] [PubMed] [Google Scholar]
- 25.Okoro T, Tafazal SI, Longworth S, Sell PJ. Tumour necrosis factor α-blocking agent (etanercept); a triple blind randomized controlled trial of its use in treatment of sciatica. J Spinal Disord Tech. 2010;23:74–77. doi: 10.1097/BSD.0b013e31819afdc4. [DOI] [PubMed] [Google Scholar]
- 26.Cohen SP, White RL, Kurihara C, Larkin TM, Chang A, Griffith SR, Gilligan C, Larkin R, Morlando B, Pasquina PF, Yaksh TL, Nguyen C. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter randomized trial. Ann Intern Med. 2012;156:551–559. doi: 10.7326/0003-4819-156-8-201204170-00397. [DOI] [PubMed] [Google Scholar]
- 27.Karppinen J, Korhonen T, Malmivaara A, Paimela L, Kyllonen E, Lindgren K-A, Rantanen P, Tervonen O, Niinimaki J, Seitsalo S, Hurri H. Tumour necrosis factor-α monoclonal antibody, infliximab, used to manage severe sciatica. Spine. 2003;28:750–754. [PubMed] [Google Scholar]
- 28.Korhonen T, Karppinen J, Malmivaara A, Autio R, Niinimaki J, Paimela L, Kyllonen E, Lindgren K-A, Tervonen O, Seitsalo S, Hurri H. Efficacy of infliximab for disc herniation induced sciatica; one year follow-up. Spine. 2004;29:2115–2119. doi: 10.1097/01.brs.0000141179.58778.6c. [DOI] [PubMed] [Google Scholar]
- 29.Genevay S, Stingelin S, Gabay C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann Rheum Dis. 2004;63:1120–1123. doi: 10.1136/ard.2003.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner RM, Spiegelhalter DJ, Smith GCS, Thompson SG. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc. 2009;172:21–47. doi: 10.1111/j.1467-985X.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eddy DM, Hasselblad V, Shachter R. An introduction to Bayesian methods for meta-analysis: the confidence profile method. Med Decis Making. 1990;10:15–23. doi: 10.1177/0272989X9001000104. [DOI] [PubMed] [Google Scholar]
- 32.Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG (2003) Evaluating non-randomised intervention studies. Health Technol Assess 7(27):1–91 [DOI] [PubMed]
- 33.Wiebe N, Vandermeer B, Platt RW, Klassen TP, Moher D, Barrowman NJ. A systematic review identifies a lack of standardization in methods for handling missing variance data. J Clin Epidemiol. 2006;59:342–353. doi: 10.1016/j.jclinepi.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Balshema H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Burgher AM, Hoelzer BC, Schroeder DR, Wilson GA, Huntoon MA. Transforaminal epidural clonidine versus corticosteroid for acute lumbosacral radiculopathy due to intervertebral disc herniation. Spine. 2011;36:E293–E300. doi: 10.1097/BRS.0b013e3181ddd597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milligan ED, Sloane EM, Langer SJ, Hughes TS, Jekich BM, Frank MG, Mahoney JH, Levkoff LH, Maier SF, Cruz PE, Flotte TR, Johnson KW, Mahoney MM, Chavez RA, Leinwand LA, Watkins LR. Repeated intrathecal injections of plasmid DNA encoding interleukin-10 produce prolonged reversal of neuropathic pain. Pain. 2006;126(1–3):294–308. doi: 10.1016/j.pain.2006.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.