Abstract
Purpose
Chronic low back pain (CLBP) is one of the most important pain disorders with increasing social and economic implications. Given that CLBP is a multidimensional process associated with comorbidities such as anxiety and depression, treatment of chronic low back pain is still a challenge. Advancement of in vivo brain imaging technologies has revealed increasing insights into the etiology and pathogenesis of chronic pain; however, the exact mechanisms of chronification of LBP remain still unclear. The purpose of the present study was to analyse the neurostructural alterations in CLBP and to evaluate the role of comorbidities and their neurostructural underpinnings.
Methods
In the present study we investigated a well-characterized group of 14 patients with CLBP and 14 healthy controls applying structural MRI and psychometric measures. Using an improved algorithm for brain normalization (DARTEL) we performed a voxel-based morphometry (VBM) approach. Correlation analyses were performed to evaluate the role of anxiety and depression in neurostructural alterations observed in CLBP.
Results
The psychometric measures revealed significantly higher scores on depression and anxiety in the patient population. VBM analysis showed significant decreases in grey matter density in areas associated with pain processing and modulation, i.e. the dorsolateral prefrontal cortex, the thalamus and the middle cingulate cortex. With respect to anxiety and depression scores, we did not observe any correlations to the structural data.
Conclusions
In the present study we found compelling evidence for alterations of grey matter architecture in CLBP in brain regions playing a major role in pain modulation and control. Our results fit the hypothesis of a “brain signature” in chronic pain conditions. The results of the psychometric assessment underline the importance of an interdisciplinary therapeutic approach including orthopedic, neurological and psychological evaluation and treatment.
Keywords: Chronic low back pain, MRI, Voxel based morphometry, Dorsolateral prefrontal cortex, Thalamus
Introduction
Pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described with terms of such damage” [1]. Low back pain (LBP) is one of the most important pain disorders with increasing social and economic implications. The life-time prevalence of LBP ranges from 60–85 % [2], affecting men and women equally, with onset most often between the age of 30–50 years [3]. Most cases of LBP are idiopathic, i.e. they cannot be assigned to a precise pathoanatomical diagnosis, whereas in about 10–15 % of cases an underlying pathology can be identified (e.g. degenerative processes, regional inflammation, infection or neoplasm) [2, 3]. Most cases of LBP are self-limiting, with 90 % of patients returning to work within several weeks; however, with increasing duration of pain the outcome gets worse [2]. Hashemi [4] reported that in the US population only a minority of LBP cases lasted for >1 year (4.6–8.8 %), but they accounted for a majority of costs of LBP patients. In Germany, back pain accounts for 15 % of all days off work due to sickness and 18 % of all early retirements [5].
Advancement of in vivo brain imaging technologies has revealed increasing insights in the etiology of chronic pain. Structural and functional reorganization of the brain of patients suffering from chronic pain are hypothesized to contribute to the development and maintenance of the disease [6]. As similar brain alterations can be observed in several chronic pain disorders such as chronic back pain, irritable bowel syndrome (IBS), fibromyalgia, chronic headache and chronic regional pain syndrome (CRPS), it may be discussed whether chronic pain might be regarded as a distinct disease [7]. Given that chronic pain is a dynamic, multidimensional process associated with the evolution of comorbidities such as anxiety and depression [8], treatment of chronic pain is still a challenge.
In chronic low back pain (CLBP), studies using in vivo single-voxel proton magnetic resonance spectroscopy (1H-MRS) have revealed evidence for alterations of brain chemistry in chronic pain patients, e.g. within the dorsolateral prefrontal cortex (DLPFC) [9]. Voxel-based morphometry (VBM) studies enabling to compare the volumes of white and gray matter in specific brain areas have shown compelling evidence for grey matter changes of brain regions associated with pain processing (DLPFC, thalamus, dorsolateral pons, somatosensory cortex) [10–12]. However, the exact mechanisms of chronification of LBP remain still unclear.
In the present study, we have examined a well-characterized German population of 14 patients suffering from chronic low back pain (pain duration >1 year) and 14 matched healthy volunteers using MRI-based Voxel-based morphometry to identify neuroanatomical differences between patients and controls that may increase insights in the process of chronification of LBP. Psychometric assessments were performed to evaluate the role of psychological comorbidities in CLBP and their neurostructural underpinnings.
Materials and methods
Description of the patient population
The patient population consisted of 14 patients [8 female and 6 male patients; average age 54 years (range 41–73 years)] suffering from chronic low back pain (CLBP) for at least 1 year (range 1–30 years; mean 10 years) according to IASP criteria [13]. Patients were recruited from the Department of Orthopedic and Trauma Surgery, University of Cologne, Germany. Pain was primarily localized within the lumbar or lumbosacral region, with or without radiation to the buttocks, thighs or legs. 5 out of 14 patients had a history of spinal surgery due to LBP (failed back surgery syndrome, FBSS). Patients suffering from relevant sensimotor deficits (pareses >4/5 and/or sensory deficits within more than 1 dermatom) were excluded from this study. Pain intensity during the 4 weeks before examination was evaluated using a Numerical rating Scale (NRS) with a range from 0 (corresponding to “no pain”) to 10 (corresponding to “worst pain imaginable”); all patients included in this study stated a NRS score ≥4. Experience of chronic LBP was assessed using the Pain Experience Scale (“Schmerzempfindungsskala” SES) [14], a well-proven diagnostic tool allowing quantification of affective and sensory characterization of chronic pain. Pain-related disability was evaluated by the German version of the Oswestry Disability Index version 2.1 (ODI) [15].
Pain-free controls were recruited via advertisements and pairwise matched to the patients with respect to gender and age. All subjects except for one patient and one control were right-handed. Individuals with a history of any disorder with a potential impact on brain structure such as hypertension requiring medical treatment, neurological or psychiatric disorders, traumatic brain injury, diabetes mellitus, rheumatologic disorders, and any chronic pain disease different from CLBP were excluded from this study. In addition, to ensure safety of magnetic resonance imaging (MRI) examination, subjects with known claustrophobia, metal implants in body, tattoos and not removable metallic jewelry were excluded.
For further characterization of patients and controls, all individuals were asked to complete the German version of the NEO-FFI questionnaire [16], based on the big five theory of personality [17] and assessing the personality dimensions “neuroticism”, “extraversion”, “openness for new experiences”, “agreeableness” and “conscientiousness”. In addition, anxiety and depression were measured using the German version of Beck’s Depression Inventory (BDI-II) [18] and Beck’s Anxiety Inventory (BAI) [19].
Imaging
Magnetic Resonance Imaging was performed at the Life&Brain Center in Bonn on a 3 T scanner (Magnetom Trio, Siemens, Erlangen, Germany). A neurovascular eight-channel head coil was used for signal reception. All subjects underwent the same imaging protocol consisting of whole brain T1-weighted, and T2-weighted structural imaging.
T1-weighted images were obtained using an MP-RAGE sequence with 160 slices (TR = 1,300 ms, TI = 650 ms, TE = 3.97 ms, resolution 1.0 × 1.0 × 1.0 mm, flip angle 10°).
Data analysis
All imaging data were transferred to a cluster of Linux workstations for processing. The structural images were visually inspected for any structural abnormalities.
Voxel-based morphometry (VBM8 toolbox, download from http://dbm.neuro.uni-jena.de/vbm8/) was performed via the SPM8 package (Wellcome Department of Imaging Neuroscience, London, UK; available online at http://www.fil.ion.ucl.ac.uk/spm), which was executed on a Matlab 7.9 platform (Mathworks, Sherborn, MA, USA). A number of preprocessing steps were carried out using VBM8 with default parameters (bias regularization 0.0001 and bias cutoff FWHM 30 mm). In brief, each subject’s 3D T1-weighted structural image was de-noised with a spatial adaptive non-local mean (NLM) filter, intensity-corrected with an adaptive Maximum A Posterior (MAP), spatially normalized with Tissue Probability Map (TPM) and segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using a mixed approach of MAP segmentation and Partial Volume Estimation (PVE). Moreover, the data were de-noised with a Markow Random Field model to remove incorrectly classified voxels. As customized DARTEL templates in VBM achieve a closer match between the template and the study participants, a customized DARTEL template for the total sample was created by the affine and DARTEL-warped GM using a 12-parameter affine transformation within the DARTEL toolbox. Each GM segment was morphed into a customized DARTEL template, which was in stereotactic space of the Montreal Neurological Institute (MNI). All segments were non-linearly modulated in order to preserve actual GM values locally in individual brains. Finally, the modulated GM segments were written with an isotropic voxel resolution 1 mm3 and smoothed with a Gaussian kernel set at 8 mm full width at half maximum (FWHM) to reduce possible errors from between-subject variability in local anatomy and render the data more normally distributed. Smoothed GM segments were entered into a voxel-wise multiple regression analysis (based on the general linear model: GLM) to investigate the differences in regional gray matter volume (rGMV). A cluster extent threshold of p < 0.001 with an extend of 100 voxels was applied. Anatomical regions were identified based on Wake Forest University (WFU) Pick Atlas [20]. The neurosynth database was used for discussion of the observed results (http://www.neurosynth.org) [21]. The volumes of global gray matter (gGMV) were exported with the VBM8 option.
Results
Clinical and psychometric data
According to the Numerical Rating Scale (NRS) evaluation, intensity of CLBP during 4 weeks prior to examination ranged from 4 to 10 (mean 7.6; SD 2.07).
Evaluation of chronic LBP experience using the Pain Experience Scale (SES) [14] revealed a mean score of 38 (affective pain) and 22 (sensory pain), both corresponding to a T-score of 55 according to a reference sample of 1,048 pain patients by Geissner [14]. Therefore, 12 individuals displayed average pain, 1 patient displayed below-average pain, and 1 patient showed above-average pain with regard to affective characterization of pain. With regard to sensory pain characterization, 10 patients showed average pain, and 4 patients showed above-average pain.
The Oswestry Disability Index (ODI) scores displayed a range from 20 to 84 (mean 48; SD 17).
Analysis of the NEO-FFI questionnaire [16] revealed a significantly higher score for “neuroticism” (p < 0.001; mean difference−11.79, 95 % CI [−16.6;−7]) and a significantly lower score for “openness for new experiences” (p = 0.029; mean difference 4.86, 95 % CI [0.55;9.12]) in patients than in controls, whereas there was no significant difference with regard to “extraversion”, “agreeableness” and “conscientiousness”.
Evaluation of Beck’s Depression Inventory (BDI-II) [18] showed significantly higher depression scores in CLBP patients (mean 19.86; SD 15.25) than in healthy controls (mean 1.71; SD 2.02) (p < 0,001; mean difference −18.14, 95 % CI [−26.6; −9.7]). According to the cutoff-scores of the German version of BDI-II to assess depression severity as proposed by Hautzinger [22] (0–8: no depression; 9–13: minimal depression; 14–19: mild depression; 20–28: moderate depression, and 29–63: severe depression), 5 CLBP patients showed no depression, 1 minimal depression, 2 mild depression, 3 moderate depression, and 3 severe depression, whereas all 14 controls showed no depression. Analysis of Beck’s Anxiety Inventory (BAI) [19] also showed significantly higher anxiety scores in patients suffering from CLBP (mean 22.71; SD 11.03) than in controls (mean 1.57; SD 1.67). According to cutoff-scores of the German BAI version [23] (0–7: minimal anxiety; 8–15: mild anxiety; 16–25: moderate anxiety; 26–63: severe anxiety), 2 CLBP patients displayed minimal anxiety, 7 moderate anxiety and 5 severe anxiety, whereas 14 controls showed only minimal anxiety. Results of psychometric assessment are given in Table 1.
Table 1.
Psychometric assessment of CLBP patients and controls
| Group | Mean score | SD | p value | |
|---|---|---|---|---|
| BDI-II | Pat. | 19.86 | 15.25 | <0.001 |
| Con. | 1.71 | 2.02 | ||
| BAI | Pat. | 22.71 | 11.03 | <0.001 |
| Con. | 1.57 | 1.67 | ||
| NEO-FFI | ||||
| Neuroticism | Pat. | 24.14 | 7.36 | <0.001 |
| Con. | 12.35 | 4.67 | ||
| Openness to experience | Pat. | 28.07 | 6.04 | 0.029 |
| Con. | 32.93 | 5.00 | ||
| Extraversion | Pat. | 26.21 | 7.40 | 0.51 |
| Con. | 27.79 | 4.71 | ||
| Agreeableness | Pat. | 30.00 | 6.52 | 0.26 |
| Con. | 32.36 | 4.09 | ||
| Conscientiousness | Pat. | 34.79 | 7.39 | 0.61 |
| Con. | 33.57 | 4.55 | ||
Imaging results
The total grey matter volume (GMV) in healthy controls was 600 ± 55 cm3 and significantly reduced in the patient cohort with 553 ± 74 cm3 (T = 1.901; p < 0.05, one-sided). The same effect was observed in the total white matter volume (WMV) with 629 ± 98 vs. 568 ± 74 cm3 (T = 1.847; p < 0.05, one-sided). For both measures we observed an age-dependent decrease in the controls as well as in the patient group [R2 = 0.256 (controls); R2 =0.235 (patients)].
When investigating regional grey matter differences, we observed several significant clusters with reduced grey matter density in CLBP patients compared to healthy controls, including the middle cingulate gyrus, thalamus and dorsolateral prefrontal cortex (DLPFC; for a complete overview of the results see Table 2). No significant increases were observed. Within these regions, we checked for correlations to anxiety and depression (as measured by BAI and BDI) to exclude the possibility that the differences may be explained by differences in these scores. No significant correlations within the patient group (at p < 0.001) to both scores were observed in the regions that showed a significant difference between both groups.
Table 2.
Differences in grey matter density between CLBP patients and controls (controls >patients)
| AAL label | Brodmann area | Peak T-score | Number of voxels | Peak coordinate (MNI) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Lingual Gyrus R | BA 17 | 6.66 | 182 | 16 | −85 | −4 |
| Mid Occipital Gyrus L | BA 39 | 5.80 | 132 | −34 | −69 | 36 |
| Inf Orb Gyrus L | BA 47 | 5.51 | 364 | −37 | 33 | −15 |
| Rectus L | BA 25 | 5.50 | 2859 | −7 | 13 | −25 |
| DLPFC R | BA 9 | 5.43 | 2373 | 18 | 52 | 9 |
| Mid Cingulum R | BA 24 | 5.37 | 775 | 0 | −4 | 28 |
| Supramarginal Gyrus R | BA 40 | 4.91 | 444 | 58 | −27 | 40 |
| Precentral Gyrus L | BA 6 | 4.87 | 542 | −60 | −7 | 13 |
| Inf Temporal Gyrus R | BA 37 | 4.87 | 227 | 46 | −51 | −22 |
| Thalamus R | 4.67 | 259 | 4 | −4 | 12 | |
| Rolandic Operculum R | BA 22 | 4.49 | 154 | 57 | 9 | 1 |
| Postcentral R | BA 3 | 4.16 | 111 | 21 | −34 | 67 |
| Inf Temporal Gyrus R | BA 19 | 4.06 | 120 | 51 | −58 | −6 |
| Medial superior frontal R | BA 8 | 3.97 | 124 | 12 | 31 | 58 |
| Inf Tempral Gyrus R | BA 21 | 3.91 | 120 | 63 | −36 | −15 |
| Rolandic Operculum R | BA 13 | 3.90 | 125 | 46 | −12 | 22 |
In a whole brain analysis, no correlations were observed with the BDI. We did observe significant negative correlations in the patient group to BAI in the anterior cingulate and left lingual gyrus. With regard to the affective Pain Experience Scale we again found a negative correlation in the anterior cingulate gyrus. We did not find any correlations to the sensory Pain Experience Scale and the Numerical Rating Scale with respect to back pain intensity 4 weeks prior to examination.
Discussion
Chronic pain is a stressor that promotes an extended and destructive stress response involving neuroendocrine dysregulation, fatigue and dysphoria, myalgia and impaired mental and physical performance potentially leading to a vicious cycle of stress and disability [24]. Chronic low back pain (CLBP) is a complex multidimensional entity encompassing distinct psychological and behavioral alterations often leading to serious impairment of quality of life. Emerging in vivo brain imaging techniques have revealed increasing insights in functional and morphological changes in chronic pain conditions; however, the exact mechanisms of pain chronification remain unclear.
Maladaptive stress response is playing a substantial role in the pathogenesis of several psychiatric disorders such as depression or anxiety [24]. In the present study, psychometrical analyses revealed compelling evidence for psychological alterations associated with chronic low back pain (Table 1). BAI and BDI-II analyses showed significantly higher anxiety and depression scores within the patient sample than in controls; in addition, the NEO-FFI analysis revealed a significantly increased score for “neuroticism” and a decreased score for “openness for new experiences” in CLBP patients. It is especially noteworthy that we excluded patients with any known history of a psychiatric disorder; therefore, no patient included in the present study had received any psychological or psychiatric treatment yet.
As expected from results of previous chronic pain studies [10, 25, 26], brain morphometric analysis revealed that global grey matter volume was reduced in CLBP patients when compared to controls. Regional VBM analysis revealed significant decreases of grey matter density in CLBP patients in several regions that are discussed to play a potential role in the pathogenesis of chronic pain conditions.
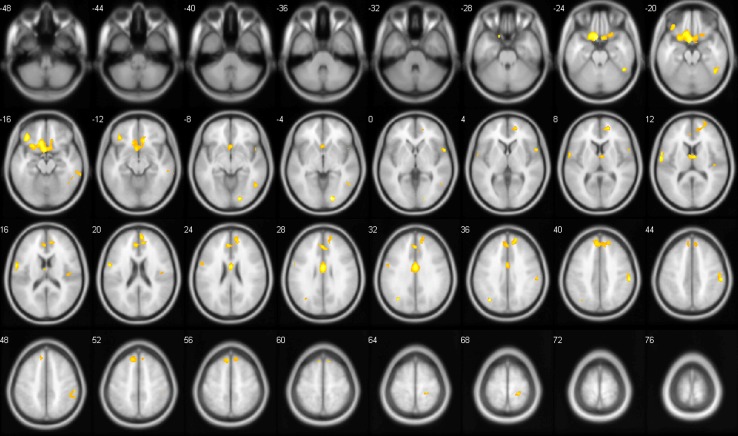
Since the thalamus is an important source of nociceptive input to the cortex, thalamo-cortical processes have been hypothesized to play a major role in the pathophysiology of chronic pain conditions. This hypothesis is underlined by the observation of neurochemical alterations within the thalamus in CLBP patients when compared to healthy volunteers [27]. Based on this hypothesis, the thalamus is now found to be an important target for neurosurgical treatment with deep brain stimulation in pain syndromes [28]. In a previous CLBP study, Apkarian and colleagues [10] showed that the right thalamus was reduced in grey matter density in patients with chronic back pain. Schmidt-Wilcke and colleagues [11], on the other hand, showed an increase in left thalamic grey matter in CLBP patients. Those studies differed with respect to the patient sample under investigation in that Schmidt-Wilcke excluded all patients with radiculopathies, whereas Apkarian included patients suffering from radiating pain. However, the different results are not easily interpreted. In our study population, we were able to reproduce a significant decrease in right thalamus gray matter, underlining the important role of the thalamus in reorganizational processes of chronic pain (Fig. 1).
Fig. 1.
Differences in grey matter density between CLBP patients and healthy controls projected on transversal slices of a standard brain template (p <0.001; extend threshold: 100 voxel). Numbers correspond to MNI coordinates
A region of exceptional interest in brain imaging studies of chronic pain conditions is the dorsolateral prefrontal cortex (DLPFC). In our present study, we found a significant decrease of grey matter density within the DLPFC in CLBP patients. Previous PET studies suggested a major role of the DLPFC in controlling pain perception by modulation of cortico-subcortical and cortico-cortical pathways [29]. In addition, several studies have highlighted the potential role of the DLPFC in placebo analgesia [30–32] and pain catastrophizing [33]. In a brain chemistry study of patients suffering from chronic back pain using in vivo single-voxel proton magnetic resonance spectroscopy (1H-MRS), Grachev and colleagues [9] were able to demonstrate a depletion of N-acetyl aspartate and glucose within the DLPFC. As N-acetyl aspartate may act as a more sensitive marker of neuronal loss in the brain than structural MRI [9], these results indicate a crucial role of the DLPFC in brain reorganization in CLBP. In a subsequent seminal study using voxel-based morphometry, Apkarian et al. [10]. were able to confirm bilaterally reduced grey matter density in chronic back pain patients when compared to healthy controls. Seminowicz and colleagues [34] described a decrease in cortical thickness of the DLPFC in CLBP patients which could even be reversed by treatment, i.e. spinal surgery or facet joint injections. In this study, using an interference task, they additionally showed functional deficits within in the DLPFC of chronic pain patients which were also normalized after effective treatment, hinting at reversibility of chronic pain induced cortical aberrations. In addition, investigating patients suffering from primary osteoarthritis of the hip, Rodriguez-Raecke et al. [35]. demonstrated reversibility of grey matter decrease within the DLPFC after successful total hip replacement. These results are in line with another recent study examining grey matter volumes in patients with different entities of ongoing pain as well as in patients with past-pain, i.e. pain that has stopped more than 12 months ago. In relation to pain-free controls, the DLPFC was significantly decreased only in the group with ongoing pain [26]. These and other studies convincingly show the important involvement of the DLPFC in pain processing and highlight the potential of brain remodeling after successful treatment.
Among the brain regions showing a decreased grey matter density in our CLBP population, the middle cingulate gyrus has also been shown to play a significant role in chronic pain diseases. In a study of older adults suffering from CLBP, Buckalew and colleagues [12] found a non-significant trend to decreased middle cingulate gyrus volumes in the patient group when compared with healthy controls. The results are supported by functional brain imaging studies that have revealed evidence for altered middle CC activity in different pain conditions [36–38] underlining the hypothesis that reduced MCC response is an adaptive cortical mechanism in acute pain that contributes to chronic pain development [12].
Nevertheless, we have to be careful interpreting these results with respect to chronic pain only. As for example the DLPFC has been reported to be involved in major depressive disorders [39], changes in brain grey matter architecture may also be driven by comorbidities such as anxiety and depression. Therefore, using Beck’s Anxiety Inventory (BAI) [19] and Beck’s Depression Inventory (BDI-II) [18], we checked within these regions if the observed structural differences could be explained by anxiety or depression. With respect to the BDI, we did not observe any correlations to the structural data. BAI scores did not correlate with grey matter density within the DLPFC, the thalamus and the middle cingulate gyrus. A significant negative correlation was observed only in the anterior cingulate gyrus. This area is well known to be involved in mood disorders [40], offering also a potential explanation for the negative correlation to the affective Pain Experience Scale.
In the present study of a well-characterized population of CLBP patients we found compelling evidence for alterations of grey matter architecture in brain regions playing a major role in pain modulation and control. Our results fit to the hypothesis of a “brain signature” of chronic pain conditions [41]. As shown above, neuroanatomical brain abnormalities in CLBP patients may at least partly reverse after effective treatment, emphasizing the importance of an optimal, individual therapy of each patient. There is striking evidence for comorbidities such as anxiety and depression in chronic pain conditions; therefore, each CLBP patient should be evaluated individually in an interdisciplinary approach including orthopaedic, neurological as well as psychological examination and specific treatment. However, despite increasing insights into the pathogenesis of pain chronification, the exact causes of brain architecture alterations in CLBP remain unclear yet. Further prospective studies including endogenous factors (e. g. genetics) are necessary to clarify the exact mechanisms of back pain chronification, leading to an optimal individual therapeutic approach for each patient.
References
- 1.Merskey H. Pain terms: a list with definitions and a note on usage. Recommended by the International Association for the study of Pain (IASP) Subcommittee on Taxonomy. Pain. 1979;6:249–252. doi: 10.1016/0304-3959(79)90175-1. [DOI] [PubMed] [Google Scholar]
- 2.Krismer M, van Tulder M. Strategies for prevention and management of musculoskeletal conditions. Low back pain (non-specific) Best Practice & Research Clinical Rheumatology. 2007;21:77–91. doi: 10.1016/j.berh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363–370. doi: 10.1056/NEJM200102013440508. [DOI] [PubMed] [Google Scholar]
- 4.Hashemi L, Webster BS, Clancy EA. Trends in disability duration and cost of worker`s compensation low back pain claims (1988–1996) Journal Occup Environ Med. 1998;40:1110–1119. doi: 10.1097/00043764-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Schneider S, Lipinski S, Schiltenwolf M. Occupation associated with a high risk of self-reported back pain: representative outcomes of a back pain prevalence study in the Federal Republic of Germany. Eur Spine J. 2006;15:821–833. doi: 10.1007/s00586-005-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wand BM, Parkitny L, O´Connell NE, Luomajoki H, McAuley JH, Thacker M, Moseley GL. Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual Therapy. 2011;16:15–20. doi: 10.1016/j.math.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? The Journal of Pain. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Borsook D, Moulton EA, Schmidt KF, Becerra LR. Neuroimaging revolutionizes therapeutic approaches to chronic pain. Molecular Pain. 2007;3:25. doi: 10.1186/1744-8069-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grachev ID, Fredrickson BE, Apkarian VA. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89:7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 10.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic ray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt-Wilcke T, Leinisch E, Gänßbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain Medicine. 2008;9:240–248. doi: 10.1111/j.1526-4637.2008.00412.x. [DOI] [PubMed] [Google Scholar]
- 13.Merskey H, Bogduk N, editors. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Second Edition: IASP Press; 1994. [Google Scholar]
- 14.Geissner E. (1995) The Pain Perception Scale – a differentiated and change-sensitive scale for assessing chronic and acute pain. Rehabilitation (Stuttg.) 1995;34:35–43. [PubMed] [Google Scholar]
- 15.Mannion AF, Junge A, Fairbank JCT, Dvorak J, Grob D. Development of a German version of the Oswestry Disability Index. Part 1: cross-cultural adaptation, reliability, and validity. Eur Spine J. 2006;15:55–65. doi: 10.1007/s00586-004-0815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borkenau P, Ostendorf F. NEO-Fünf-Faktoren-Inventar nach Costa und McCrae. Göttingen: Hogrefe; 2008. [Google Scholar]
- 17.Goldberg LR. An alternative “description of personality”: the big-five factor structure. J Pers Soc Psychol. 1990;59:1216–1229. doi: 10.1037/0022-3514.59.6.1216. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. Beck Depression Inventory - Second Edition. Manual: The Psychological Corporation, San Antonio; 1996. [Google Scholar]
- 19.Beck AT, Steer RA. Beck Anxiety Inventory. Manual: The Psychological Corporation, San Antonio; 1993. [Google Scholar]
- 20.Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 21.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hautzinger M, Keller F, Kuehner C (2009) BDI-II. Beck Depressions-Inventar, Revision. Pearson, Frankfurt
- 23.Margraf J, Ehlers A. Beck Angst-Inventar – BAI. Harcourt, Frankfurt: Manual; 2007. [Google Scholar]
- 24.Chapman CR, Gavrin J. Suffering: the contributions of persistent pain. Lancet. 1999;353:2233–2237. doi: 10.1016/S0140-6736(99)01308-2. [DOI] [PubMed] [Google Scholar]
- 25.Baliki MN, Schnitzler TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS ONE. 2011;6(10):e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusheweyh R, Deppe M, Lohmann H, Stehling C, Flöel A, Ringelstein EB, Knecht S. Pain is assocoiated with regional grey matter reduction in the general population. Pain. 2011;152:904–911. doi: 10.1016/j.pain.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Siddall PJ, Stanwell P, Woodhouse A, Somorjai RL, Dolenko B, Nikulin A, Bourne R, Himmelreich U, Lean C, Cousins MJ, Mountford CE. Magnetic resonance spectroscopy detects biochemical changes in the brain associated with chronic low back pain: a preliminary report. Anesth Analg. 2006;102:1164–1168. doi: 10.1213/01.ane.0000198333.22687.a6. [DOI] [PubMed] [Google Scholar]
- 28.Levy R, Deer TR, Henderson J. Intracranial neurostimulation for pain control: a review. Pain Physician. 2010;13:157–165. [PubMed] [Google Scholar]
- 29.Lorenz J, Minoshima S, Casey KL. Keeping pain out of the mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 30.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 31.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opiod activity in mu-opiod receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derbyshire SWB, Jones AKB, Devani P, et al. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry. 1994;57:1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995;63:225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- 38.Jones AKP. Derbyshire SW (1997) Reduced cortical responses to noxious heat in patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:601–607. doi: 10.1136/ard.56.10.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunelin J, Poulet E, Boeuve C, Zeroug-vial H, d`Amato T, Saoud M. Efficacy of repetitive transcranial magnetic stimulation (rTMS) in major depression: a review. Encephale. 2007;33:126–134. doi: 10.1016/S0013-7006(07)91542-0. [DOI] [PubMed] [Google Scholar]
- 40.Drevets WC, Savitz J, Trimble M. The subgenual anterior cinglate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]