Abstract
Objective
To investigate self-reported illness and household strategies for coping with payments for health care in a city in Bangladesh.
Methods
A cluster-sampled probability survey of 1593 households in the city of Rajshahi, Bangladesh, was conducted in 2011. Multilevel logistic regression – with adjustment for any clustering within households – was used to examine the risk of self-reported illness in the previous 30 days. A multilevel Poisson regression model, with adjustment for clustering within households and individuals, was used to explore factors potentially associated with the risk of health-care-related “distress” financing (e.g. paying for health care by borrowing, selling, reducing food expenditure, removing children from school or performing additional paid work).
Findings
According to the interviewees, about 45% of the surveyed individuals had suffered at least one episode of illness in the previous 30 days. The most frequently reported illnesses among children younger than 5 years and adults were common tropical infections and noncommunicable diseases, respectively. The risks of self-reported illness in the previous 30 days were relatively high for adults older than 44 years, women and members of households in the poorest quintile. Distress financing, which had been implemented to cover health-care payments associated with 13% of the reported episodes, was significantly associated with heart and liver disease, asthma, typhoid, inpatient care, the use of public outpatient facilities, and poverty at the household level.
Conclusion
Despite the subsidization of public health services in Bangladesh, high prevalences of distress financing – and illness – were detected in the surveyed, urban households.
Résumé
Objectif
Étudier les maladies auto-déclarées et les stratégies des ménages pour faire face aux paiements des soins de santé dans une ville du Bangladesh.
Méthodes
Une étude de probabilité menée sur un échantillon de 1593 ménages de la ville de Rajshahi, au Bangladesh, a été réalisée en 2011. Une régression logistique multi-niveaux, avec ajustement pour tous les regroupements au sein des ménages, a été réalisée pour examiner le risque de maladie auto-déclarée dans les 30 jours précédant l'enquête. Un modèle multi-niveaux de régression de Poisson, avec ajustement pour tous les regroupements au sein des ménages et pour les individus, a été utilisé pour examiner les facteurs potentiellement associés au financement «à risque» des soins de santé (par exemple, payer les soins de santé en empruntant, en vendant ses biens, en réduisant ses dépenses de nourriture, en retirant ses enfants de l'école ou en acceptant un travail rémunéré supplémentaire).
Résultats
D'après les personnes interrogées, environ 45% des individus avaient été affectés par une maladie dans les 30 jours qui précédaient. Les maladies les plus fréquemment signalées chez les enfants de moins de 5 ans et les adultes étaient respectivement des infections tropicales courantes et des maladies non transmissibles. Les risques de maladies auto-déclarées dans les 30 jours précédents étaient relativement élevés pour les personnes âgées de plus de 44 ans, les femmes et les membres des ménages du quintile le plus pauvre. Le financement «à risque», mis en place pour couvrir les paiements des soins de santé, et associé à 13% des cas déclarés, était significativement lié aux maladies du cœur et du foie, à l'asthme, à la fièvre typhoïde, aux soins hospitaliers, à l'utilisation des services de soins ambulatoires publics et au niveau de pauvreté des ménages.
Conclusion
Malgré les subventions accordées par les services de santé publique au Bangladesh, les prévalences élevées de financement «à risque» - et la maladie - ont été détectées chez les ménages urbains interrogés.
Resumen
Objetivo
Investigar las enfermedades declaradas por los propios pacientes y las estrategias de los hogares para hacer frente a los pagos sanitarios en una ciudad de Bangladesh.
Métodos
En el año 2011 se llevó a cabo un estudio de probabilidades sobre muestras en grupos de 1593 hogares. Se empleó una regresión logística multinivel con un ajuste para cualquier agrupación dentro de los hogares para evaluar el riesgo de enfermedad declarada por el propio paciente en los 30 días previos. Para examinar los factores que podrían estar asociados con el riesgo de sufrir dificultades económicas relacionadas con la salud (por ejemplo, pagar la atención sanitaria con préstamos, ventas, reducción del gasto en alimentos, retirar a los niños de la escuela o realizar trabajos remunerados adicionales) se utilizó un modelo de regresión de Poisson multinivel con un ajuste para los agrupamientos dentro de los hogares e individuos.
Resultados
De acuerdo con los entrevistados, aproximadamente el 45% de los individuos encuestados había sufrido al menos un episodio de enfermedad en los 30 días previos. Las enfermedades declaradas más frecuentemente entre niños menores de cinco años y adultos fueron, respectivamente, infecciones tropicales comunes y enfermedades no contagiosas. El riesgo de enfermedad declarada por el propio paciente en los 30 días previos fue relativamente elevado en los adultos mayores de 44 años, las mujeres y los miembros de los hogares del quintil más pobre. Las dificultades económicas derivadas de cubrir los pagos sanitarios asociados con el 13% de los episodios declarados estuvieron relacionadas de forma significativa con enfermedades cardíacas y hepáticas, asma, fiebre tifoidea, atención hospitalaria, el uso de los centros ambulatorios públicos y la pobreza del hogar.
Conclusión
A pesar de la subvención de los servicios públicos de salud en Bangladesh, se detectó una prevalencia elevada de dificultades económicas y enfermedades en los hogares urbanos encuestados.
ملخص
الغرض
تحري الاعتلالات المبلغ عنها ذاتياً واستراتيجيات الأسر للتعايش مع مدفوعات الرعاية الصحية في إحدى مدن بنغلاديش.
الطريقة
تم إجراء دراسة استقصائية احتمالية لعينات مجمعة لعدد 1593 أسرة في مدينة راجشاهي في بنغلاديش في عام 2011. وتم استخدام الارتداد اللوجيستي متعدد المستويات – مع التعديل لأي تجميع ضمن الأسر– لدراسة خطورة الاعتلالات المبلغ عنها ذاتياً في الثلاثين يوماً السابقة. وتم استخدام نموذج ارتداد بواسون متعدد المستويات، مع التعديل للتجميع ضمن الأسر والأفراد، لاستكشاف العوامل التي يُحتمل ارتباطها بمخاطر تمويل "الضائقة" ذات الصلة بالرعاية الصحية (مثل دفع مقابل الرعاية الصحية عن طريق الاقتراض أو البيع أو خفض الإنفاق على الطعام أو إخراج الأطفال من المدرسة أو أداء عمل إضافي مدفوع الأجر).
النتائج
وفقاً للأشخاص الذين تم مقابلتهم، عانى حوالي 45 % من الأفراد الذين تم دراستهم استقصائياً من نوبة اعتلال واحدة على الأقل خلال الثلاثين يوماً السابقة. وكانت الاعتلالات المبلغ عنها الأكثر تكراراً بين الأطفال الأقل من 5 سنوات والبالغين هي عدوى المناطق المدارية الشائعة والأمراض غير السارية، على التوالي. وكانت مخاطر الاعتلالات المبلغ عنها ذاتياً خلال الثلاثين يوماً السابقة مرتفعة نسبياً بالنسبة للبالغين الذين تزيد أعمارهم عن 44 سنة والنساء وأفراد الأسر المعيشية في الشرائح الخمسية الأشد فقراً. وكان تمويل الضائقة، الذي تم تنفيذه لتغطية مدفوعات الرعاية الصحية المرتبطة بنسبة 13 % من النوائب المبلغ عنها، مرتبطاً بشكل كبير بأمراض القلب والكبد والربو والتيفويد ورعاية المرضى الداخليين واستخدام مرافق المرضى الخارجيين العمومية والفقر على مستوى الأسرة.
الاستنتاج
على الرغم من الدعم المالي لخدمات الصحة العمومية في بنغلاديش، تم اكتشاف ارتفاع معدلات انتشار تمويل الضائقة – والاعتلالات – في الأسر الحضرية التي تم دراستها استقصائياً.
摘要
目的
探讨孟加拉国城市应对卫生保健支出的自我报告的疾病和家庭战略。
方法
2011 年在孟加拉国拉杰沙希市的1593 户家庭中执行群集抽样概率调查。使用多水平逻辑回归(调整家庭范围内的任何群集)来研究在过去30 天内自我报告疾病的风险。使用多水平的泊松回归模型(调整家庭和个人范围内的群集),探索与卫生保健相关的“砸锅卖铁”筹钱的风险(例如,通过借钱、变卖、减少食品支出、让孩子退学或者参加额外的有偿工作等方式支付卫生保健费用)潜在关联的因素。
结果
在受访者中,约45%的受访个人在过去30 天中至少得过一次病。未满5 岁的儿童和成人最常报告的疾病分别是常见的热带感染和非传染性疾病。年龄超过44 岁的成人、女性和最贫穷地区家庭的成员在过去30 天的自我报告疾病的风险相对较高。报告疾病有13%靠砸锅卖铁筹钱来填补,这种情况与心脏和肝脏疾病、哮喘、伤寒、住院医疗、公共门诊设施的使用以及家庭水平的贫困显著相关。
结论
尽管孟加拉国提供公共卫生服务补助,在受调查的城镇居民家庭中仍发现有很多人得不起病、看不起病。
Резюме
Цель
Исследовать частоту самостоятельных сообщений населением о заболевании и стратегии домохозяйств, помогающие им справиться с расходами на медицинскую помощь в одном из городов Бангладеш.
Методы
В 2011 г. было проведено кластерное вероятностное обследование 1593 домашних хозяйств в г. Раджшахи, Бангладеш. Для изучения уровня сообщений населением о своих заболеваниях, имевших место в течение предыдущих 30 дней, был использован метод многоуровневой логистической регрессии, с поправкой на все кластеризации среди домохозяйств. Для изучения факторов, потенциально связанных с рисковым финансированием медицинских расходов (например, оплата медицинских услуг за счет заемных средств, продажи имущества, сокращения расходов на питание, прекращения посещения детьми школы или выполнения дополнительных платных работ) использовалась многоуровневая регрессионная модель Пуассона с поправкой на кластеризацию среди домохозяйств и отдельных жителей.
Результаты
По словам опрошенных граждан, около 45% из них имели как минимум один случай заболевания за предыдущие 30 дней. Наиболее часто сообщалось о болезни среди детей в возрасте до 5 лет, а среди взрослых были распространены тропические инфекции и неинфекционные заболевания. Риск самообнаружения болезни за предыдущие 30 дней был относительно высок для взрослых старше 44 лет, женщин и членов домохозяйств в беднейшем квинтиле населения. Рисковое финансирование, которое применялось для покрытия медицинских расходов в 13% зарегистрированных эпизодов, было в значительной степени связано с заболеваниями сердца и печени, астмой, тифом, получением помощи в стационаре, использованием государственных амбулаторных услуг и бытовой бедностью.
Вывод
Несмотря на субсидирование здравоохранения, в Бангладеш в обследованных городских домохозяйствах был обнаружен высокий уровень заболеваний и высокая распространенность рискового финансирования.
Introduction
The so-called “double burden” of noncommunicable and infectious diseases is a major challenge for the fragile health systems in many low- and middle-income countries.1–4 In these countries, poverty and illness are closely linked: poverty leads to ill health and ill health perpetuates poverty.1,2,5 Noncommunicable and infectious diseases cause financial hardship both directly, via out-of-pocket spending on treatment, and indirectly, by limiting participation in income-generating activities.6–9 In low- and middle-income countries where public funding for health services is inadequate and mechanisms for “risk-pooling”, such as “demand-side” financing and formal health insurance, are limited or unavailable, out-of-pocket payments and illness-related loss of income can lead to asset depletion, debt and reductions in essential consumption that, together, can result in financial catastrophe.6–10
Although much progress has been made in measuring the impact of out-of-pocket payments for health care on household welfare, knowledge gaps remain. We know relatively little about the strategies that households adopt to cope – or, at least, try to cope – with the financial costs of illness, and we have few data to show how such coping strategies affect the future welfare of the households that implement them.10 In the few relevant studies that have been conducted, the coping strategies that are followed have been found to differ with the type of disease involved,6,7,11–13 with the sector (private or public) providing the outpatient facilities used, if any,9,14 with the need for inpatient care,14–16 and with the economic status of the patients or their households.9,10,14,17
In Bangladesh, a country with high burdens of both noncommunicable and infectious diseases, out-of-pocket payments remain the most important source of funding for health care. Health insurance in Bangladesh is limited to a few small-scale schemes sponsored by nongovernmental organizations.18 The results of only three studies on out-of-pocket payments in Bangladesh have been published. These investigations were focused on household strategies for coping with the health-care expenses associated with pneumonia,11 tuberculosis12 and obstetric care.19 No attempt has been made to investigate the strategies followed by households in Bangladesh to cope with all payments associated with illness. The aims of the present study were to determine the self-reported prevalence of any illness among households in a city in Bangladesh and to identify the associated risk factors for illness and for the “distress” financing of any related health care (e.g. paying for the health care by borrowing, selling, reducing food expenditure, removing children from school or performing additional paid work).
Methods
Study area
Rajshahi city, which lies in Rajshahi district, in north-western Bangladesh, is the third largest city in the country and is considered broadly representative of the country’s urban areas. At the time of the present study, Rajshahi city had a population of about 400 000. About 71% of the males and 62% of the females in Rajshahi district are literate.20 This study was conducted in an urban setting in the absence of risk-pooling mechanisms such as “demand-side” financing (i.e. financing that transfers resources to poor households solely to facilitate the households’ access to health services) or formal health insurance. Although programmes to finance some aspects of health care, including programmes of demand-side financing, exist in rural areas of Bangladesh, these programmes do not currently cover urban areas,21 even though urban households tend to suffer more illness and use more health facilities than rural households.1
Study design and sample size
Between August and November 2011, information was collected from households in Rajshahi city. The households were selected using three-stage cluster-sampling. The primary sampling unit was the mahallah – the lowest administrative unit of a Bangladeshi city. Forty mahallahs were selected, from the 159 forming Rajshahi city, using a method that made the probability of selection proportional to the population of the mahallah. Systematic random sampling was then used to select 40 buildings in each selected mahallah and, subsequently, to select one household from each selected building.
Data collection
Overall, 27 interviewers – all social science, demography or statistics graduates with experience in survey methods – and five supervisors were recruited to administer the pretested, validated, structured questionnaire used to collect data (Appendix A, available at: http://www.ghp.m.u-tokyo.ac.jp/wp-content/uploads/2013/03/Appendix-A-BulletinWHO-MR-20130328.pdf). Before the survey, the interviewers and supervisors each received 10 days’ training and 2 days of practical sessions on the content of the questionnaire, on techniques for eliciting more information and on strategies for obtaining complete and reliable data. Data were collected in face-to-face interviews with an adult member of each selected household (usually a woman or the male head of the household). Only adults who provided informed consent were interviewed. Data on sociodemographic status, household expenditure and illness experienced in the previous 30 days were collected. All illnesses were coded according to a disease list that had been developed in previous studies2,7,10,22,23 and finalized after pilot testing in 100 households (Appendix A). Data were collected on the time of onset and, if possible, duration of illness, diagnosis, treatment response, treatment cost and coping strategies. These data were collected separately for each episode of illness and related care-seeking (n = 4461), for each individual who had been ill (n = 3300) and for each surveyed household (n = 1593). Interviewees were asked about the primary sources of the finances that their households had used to pay for any health care received for each reported episode of illness. These sources were categorized as: routine income; pre-existing savings; loans (from relatives, friends, neighbours, banks or moneylenders); money released by the sale of land or other assets; additional paid work; ex-gratia payments from family members; savings achieved by reducing expenditure on food; and/or savings achieved by removal of children from school. Unless the money used to pay for health care came from the household’s routine income or pre-existing savings, it was considered to have come from “distress” financing.7,9–11,17
Variables
The primary outcome variables that were investigated were the presence of illness in a member of a study household and the distress financing of health care for each reported episode of illness. At episode-of-illness level, the independent variables considered were type of illness and type of health facility used. At the patient level, the independent variables considered were age, sex and educational status; at household level, they were household size (i.e. the number of people in the household) and wealth (i.e. household expenditure quintile).
Statistical analysis
Findings were recorded as frequencies and percentages. Univariate analyses were used to investigate the associations between distress financing and the 20 most commonly reported types of illness, care-seeking behaviour and sociodemographic characteristics, at both the patient and the household levels. A multilevel logistic regression model was used – with a household-level random intercept – to adjust for the clustering effect of households when analysing the presence of illness at the individual level. A three-level Poisson regression model was used – with random intercepts at the individual and the household levels and model selection based on backward stepwise model building – to assess disease-specific strategies for coping with health-care payments. Only predictors that gave P-values of < 0.25 in the univariate analyses were entered into this Poisson regression model. All analyses were adjusted for the probability sampling used for the survey. Data management and statistical analyses were performed using version 12.0 of the Stata/MP software package (StataCorp, LP, College Station, United States of America).
Ethical considerations
The study protocol, questionnaire and disease codes were approved by the Research Ethics Committee of the University of Tokyo and the Bangladesh National Research Ethics Committee.
Results
Background characteristics and prevalence of morbidity
Since the members of seven selected households refused to participate in the study, the data analysis was based on the responses of the members of 1593 households. Table 1 presents the key characteristics of these 1593 households and their members. The households had a mean of 4.6 members (95% confidence interval: 4.5–4.7). The age-specific frequencies of the 20 most frequently reported types of illness, over the 30 days preceding the interview, are presented in Table 2. About 44.9% of the members of the study households had reportedly suffered at least one episode of illness. Most (> 90%) of those who had reportedly suffered typhoid, pneumonia, hypertension, diabetes, heart disease or asthma had had their illness diagnosed by a doctor with a medical degree. The most frequently reported illnesses among children younger than 5 years were infectious diseases such as cold/fever, diarrhoea/gastroenteritis and pneumonia, whereas the elderly members of the study households (i.e. those aged at least 60 years) were more likely to have had noncommunicable diseases such as hypertension, rheumatoid arthritis, heart disease, diabetes, gastritis/peptic ulcer or asthma. Infectious diseases predominated in those younger than 15 years but were less common than noncommunicable diseases among household members aged 30 years or older (Appendix A). Certain illnesses, especially some common tropical infectious diseases, were considerably more frequent among the poorest household quintile than among the richest (Appendix A). In contrast, heart disease and some chronic lifelong conditions, such as hypertension and diabetes, were reported more frequently among members of households in the richest quintile than among those of households in the poorest quintile.
Table 1. Descriptive statistics of surveyed households and household members, Bangladesh, 2011.
| Characteristic | No.a | % (95% CI) |
|---|---|---|
| Household | ||
| Size (no. of members) | ||
| 1–2 | 127 | 7.6 (6.3–9.2) |
| 3–5 | 1132 | 69.7 (67.2–72.2) |
| ≥ 6 | 334 | 22.7 (20.3–27.9) |
| Expenditure | ||
| Quintile 1 (lowest) | 319 | 21.4 (17.5–25.9) |
| Quintile 2 | 319 | 21.5 (18.3–25.1) |
| Quintile 3 | 318 | 20.4 (18.0–23.1) |
| Quintile 4 | 319 | 19.7 (16.9–22.8) |
| Quintile 5 (highest) |
318 |
17.0 (13.2–21.7) |
| Household member (patient) | ||
| Sex | ||
| Male | 3590 | 49.9 (48.7–51.1) |
| Female | 3612 | 50.1 (48.9–51.4) |
| Age (years) | ||
| 0–4 | 449 | 6.2 (5.7–6.9) |
| 5–9 | 565 | 7.8 (7.1–8.6) |
| 10–14 | 740 | 10.6 (9.7–11.5) |
| 15–29 | 2128 | 29.8 (28.3–31.4) |
| 30–44 | 1612 | 22.4 (21.3–23.5) |
| 45–59 | 1119 | 15.3 (14.3–16.3) |
| ≥ 60 | 589 | 7.9 (7.2–8.7) |
| Educational status | ||
| No education | 1265 | 18.0 (16.2–19.9) |
| Primary | 1831 | 26.2 (23.7–28.9) |
| Secondary | 2002 | 28.3 (27.0–29.7) |
| Higher | 2104 | 27.5 (24.1–31.2) |
CI, confidence interval.
Table 2. Self-reported illness among household members, Bangladesh, 2011.
| Illness | No (%) of household members aged (years) |
No. (%) of episodes diagnosed by cliniciana | ||||
|---|---|---|---|---|---|---|
| < 5 (n = 449) | 5–20 (n = 2059) | 20–59 (n = 4 105) | ≥ 60 (n = 589) | Any age (n = 7202) | ||
| Cold/fever | 188 (41.5) | 450 (21.6) | 610 (14.7) | 80 (14.1) | 1328 (18.4) | 342 (24.9) |
| Hypertension | – | – | 393 (9.0) | 156 (26.5) | 549 (7.2) | 509 (92.5) |
| Gastritis/peptic ulcer | 2 (0.4) | 14 (0.7) | 306 (7.4) | 70 (11.9) | 392 (5.4) | 241(61.0) |
| Rheumatic arthritis | 2 (0.6) | 16 (0.8) | 254 (6.1) | 98 (16.8) | 370 (5.1) | 290 (77.3) |
| Diabetes | – | – | 214 (4.9) | 79 (13.0) | 293 (3.8) | 291 (99.5) |
| Heart disease | – | 3 (0.2) | 124 (3.0) | 87 (13.4) | 214 (2.8) | 210 (98.2) |
| Migraine/headache | – | 28 (1.5) | 150 (3.5) | 12 (2.1) | 190 (2.6) | 135 (70.4) |
| Asthma | 4 (0.8) | 26 (1.2) | 87 (2.0) | 37 (6.1) | 154 (2.0) | 139 (90.9) |
| Diarrhoea/gastroenteritis | 25 (5.6) | 27 (1.2) | 78 (2.0) | 10 (1.5) | 140 (2.0) | 66 (47.9) |
| Allergy | 2 (0.4) | 19 (0.9) | 67 (1.6) | 8 (1.4) | 96 (1.3) | 72 (75.5) |
| Injury | – | 11 (0.5)) | 56 (1.5) | 10 (1.7) | 77 (1.1) | 55 (68.2) |
| Skin disease | 3 (0.7) | 20 (1.0) | 45 (1.1) | 6 (1.1) | 74 (1.1) | 54 (74.7) |
| Cataract | 1 (0.1) | 7 (0.3) | 33 (0.8) | 30 (5.0) | 71 (1.0) | 65 (90.6) |
| Dental | 1 (0.1) | 6 (0.2) | 36 (1.0) | 4 (0.6) | 47 (0.6) | 33 (67.1) |
| Nephrolithiasis | – | 3 (0.2) | 25 (0.6) | 5 (1.0) | 33 (0.5) | 33 (100.0) |
| Haemorrhoids | 1 (0.3) | – | 28 (0.7) | 10 (1.6) | 39 (0.5) | 29 (73.5) |
| Urinary tract infection | – | 5 (0.3) | 18 (0.5) | 9 (1.8) | 32 (0.5) | 28 (85.8) |
| Liver disease | 2 (0.6 | 12 (0.4) | 25 (0.6) | 2 (0.4) | 41 (0.5) | 35 (86.5) |
| Otitis media | 3 (0.7) | 3 (0.2) | 16 (0.4) | 2 (0.4) | 24 (0.4) | 19 (82.4) |
| Tumour | 1 (0.3) | 1 (0.1) | 23 (0.6) | – | 25 (0.4) | 19 (79.9) |
| Typhoid | 2 (0.6) | 10 (0.6) | 11 (0.3) | 2 (0.3) | 25 (0.4) | 23 (91.5) |
| Mental disease | – | 6 (0.3) | 18 (0.5) | 2 (0.5) | 26 (0.4) | 23 (88.3) |
| Physical weakness | 1 (0.1) | 2 (0.04) | 18 (0.4) | 4 (0.4) | 25 (0.3) | 17 (67.3) |
| Pneumonia | 9 (2.1) | 1 (0.03) | 2 (0.1) | – | 12 (0.2) | 12 (100.0) |
| Paralysis | – | 1 (0.03) | 5 (0.2) | 10 (1.7) | 16 (0.2) | 14 (88.5) |
| Cancer | – | – | 6 (0.2) | 2 (0.4) | 8 (0.1) | 8 (100.0) |
| Food poisoning | – | 1 (0.1) | 4 (0.1) | – | 5 (0.1) | 3 (60.0) |
| Chicken pox | – | 3 (0.1) | 1 (0.03) | – | 4 (0.1) | 2 (66.7) |
| Insomnia | – | – | 7 (0.2) | 6 (0.8) | 13 (0.1) | 9 (70.1) |
| Uterine prolapse | – | – | 5 (0.1) | – | 5 (0.1) | 5 (100.0) |
| Nasal polyps | – | 4 (0.2) | 3 (0.1) | 1 (0.0) | 8 (0.1) | 7 (94.9) |
| Cholelithiasis/cholecystitis | – | – | 6 (0.2) | 1 (0.2) | 7 (0.1) | 7 (100.0) |
| Tuberculosis | – | – | 4 (0.1) | – | 4 (0.1) | 3 (75.0) |
| Inguinal hernia | – | 1 (0.1) | 5 (0.1) | 3 (0.5) | 9 (0.1) | 9 (100.0) |
| Dengue | – | 1(0.03) | 1 (0.03) | 1 (0.2) | 3 (0.0) | 3 (100.0) |
| Otherb | 6 (1.4) | 19 (0.8) | 57 (1.4) | 18 (3.3) | 102 (1.4) | 84 (82.5) |
| Total | 241 (53.3) | 656 (31.6) | 1958 (46.8) | 436 (73.7) | 3300 (44.9) | 2894 (64.1) |
a Clinicians all had medical degrees.
b Appendicitis, benign prostatic hyperplasia, epilepsy, hypercholesterolemia, anaemia, abdominal, foot or hand pain, swelling/oedema, filariasis, hearing or renal problems, osteoporosis, thyroid goitre, vitamin deficiency and helminth infections.
Determinants of reporting illness
The results of the multilevel analysis of the influence of individual- and household-level characteristics on the reporting of any illness are presented in Table 3. A likelihood-ratio test, in which multilevel modelling was compared with a model without random effects, gave a statistically significant result (χ2 = 1202.54; P < 0.001). This indicates that multilevel modelling was necessary to analyse the frequencies of reported illness. As expected, after early childhood, the age of the individual was found to be significantly associated with reported illness, the higher frequencies of reported illness being observed in the older age groups. The odds of reported illness were, however, broadly similar across the five quintiles of household expenditure and four levels of educational attainment that were considered.
Table 3. Odds of self-reported illness during the 30-day recall period, by household or household member characteristics, Bangladesh, 2011.
| Characteristic | OR (95% CI) (n = 7 202) |
|---|---|
| Household | |
| Size (no. of members) | 0.85 (0.82–0.87) |
| Expenditure | |
| Quintile 1 (lowest) | 0.94 (0.77–1.16) |
| Quintile 2 | 1.00 (0.80–1.26) |
| Quintile 3 | 1.08 (0.87–1.33) |
| Quintile 4 | 1.22 (1.03–1.45) |
| Quintile 5 (highest) |
1.00 |
| Household member (patient) | |
| Age (years) | |
| 0–4 | 1.00 |
| 5–9 | 0.37 (0.26–0.53) |
| 10–14 | 0.33 (0.22–0.48) |
| 15–29 | 0.36 (0.24–0.53) |
| 30–44 | 0.73 (0.50–1.06) |
| 45–59 | 1.78 (1.24–2.57) |
| ≥ 60 | 2.73 (1.77–4.22) |
| Sex | |
| Female | 1.00 |
| Male | 0.73 (0.65–0.82) |
| Educational status | |
| No education | 1.00 |
| Primary | 1.07 (0.85–1.35) |
| Secondary | 0.84 (0.65–1.08) |
| Higher | 0.75 (0.57–0.98) |
CI, confidence interval; OR, odds ratio.
Illness and distress financing
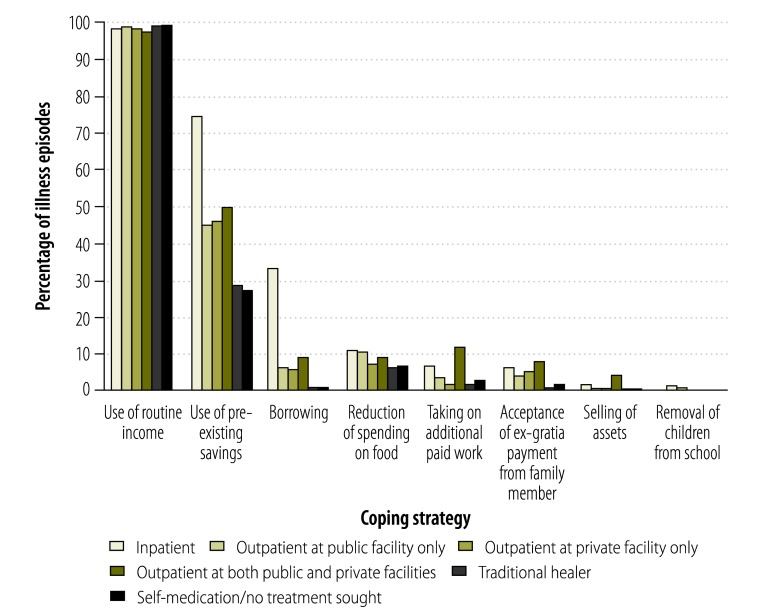
According to the interviewees, most (4127) of the 4461 reported episodes of illness led to increases in household expenditure. As shown in Table 4, heart and liver disease, asthma and tumours were significantly associated with distress financing, as were certain forms of care-seeking behaviour, certain levels of educational attainment, and certain levels of household wealth. Nearly half of all the episodes of illness that had led to inpatient care – but only 8% of those that had been treated by traditional healers – had resulted in distress financing. About 33% of inpatient treatments but only 6% of outpatient treatments and about 0.8% of the treatments by traditional healers had been entirely funded by household loans (Fig. 1).
Table 4. Households implementing distress financing, by household or household member characteristics, Bangladesh, 2011.
| Characteristic | Percentage (95% CI) of households implementing distress financinga |
|---|---|
| Illness | |
| Hypertension | 12.2 (8.0–18.1) |
| Gastritis/peptic ulcer | 11.9 (7.6–18.1) |
| Rheumatoid arthritisb | 16.8 (11.6–23.8) |
| Diabetesb | 12.4 (6.7–21.9) |
| Heart diseaseb | 24.4 (17.4–33.1) |
| Migraine/headache | 14.6 (9.3–22.1) |
| Asthmab | 21.9 (14.3–32.1) |
| Diarrhoea/gastroenteritis | 12.5 (7.4–20.6) |
| Allergyb | 5.8 (1.9–16.4) |
| Injury | 10.1 (4.4–21.7) |
| Skin disease | 17.7 (8.8–32.4) |
| Cataract | 17.1 (8.4–31.6) |
| Dental | 11.9 (5.2–25.0) |
| Haemorrhoids | 12.2 (4.4–29.6) |
| Liver disease (including hepatitis B and C)b | 26.1 (14.3–42.9) |
| Urinary tract infection | 18.1 (8.1–35.7) |
| Nephrolithiasis | 21.0 (8.7–42.4) |
| Mental illness | 15.1 (4.2–42.0) |
| Tumourb | 27.5 (13.7–47.5) |
| Typhoidb | 25.7 (10.7–50.0) |
| Care-seeking behaviourb | |
| Inpatient | 48.4 (35.9–61.0) |
| Outpatient | |
| At public facility only | 17.3 (12.2–24.1) |
| At private facility only | 15.1 (10.1–21.9) |
| At both public and private facilities | 30.5 (17.1–48.2) |
| Traditional healer | 8.1 (4.7–13.7) |
| Self-medication/no treatment sought | 10.0 (7.0–14.1) |
| Educational status of household memberb | |
| No education | 17.3 (12.9–22.9) |
| Primary | 15.5 (11.5–20.6) |
| Secondary | 12.2 (8.1–18.1) |
| Higher | 8.4 (5.5–12.8) |
| Household expenditureb | |
| Quintile 1 (lowest) | 24.0 (18.4–30.8) |
| Quintile 2 | 15.1 (10.7–20.8) |
| Quintile 3 | 9.8 (5.5–16.9) |
| Quintile 4 | 10.9 (6.9–16.6) |
| Quintile 5 (highest) | 6.9 (3.7–12.6) |
CI, confidence interval.
a The analysis was restricted to the 3300 household members who, in the 30 days before the data were collected, reportedly suffered illness that led to household expenditure.
b These characteristics, which each gave a P-value of < 0.25 in the univariate analysis, were included in the multilevel Poisson regression model.
Fig. 1.
Strategies used by households to cope with payments for health care from various sources, Bangladesh, 2011
Note: Multiple strategies were often implemented to finance the care of single episodes of illness.
Determinants of distress financing
Table 5 presents the results of the multiple regression modelling of the relative risks of distress financing among those households that reported expenditure for the treatment of illness. The results of a likelihood ratio test, in which the multilevel modelling was compared with a model without random effects, indicated that the multilevel modelling was appropriate (χ2 = 659.75; P < 0.001). Again, heart and liver diseases, asthma and typhoid were significantly associated with distress financing. The type of health care sought, if any, was also significantly related to the risk of distress financing; those using inpatient care were much more likely to experience distress financing than those who were self-medicated or who had sought no treatment. Outpatient care was also significantly associated with distress financing but treatment by traditional healers was not. Among the households that reported expenditure on health care in the 30 days before the interview, those in the poorest quintile had a sevenfold higher risk of (reported) distress financing than those in the richest quintile.
Table 5. Multilevel Poisson regression model of risk of distress financing, Bangladesh, 2011.
| Characteristic | RR (95% CI)a |
|---|---|
| Illness | |
| Have rheumatoid arthritis? | |
| Yes | 1.19 (0.95–1.49) |
| No | 1.00 |
| Have heart disease? | |
| Yes | 1.22 (1.05–1.42) |
| No | 1.00 |
| Have asthma? | |
| Yes | 1.73 (1.35–2.22) |
| No | 1.00 |
| Have liver disease? | |
| Yes | 1.63 (1.06–2.51) |
| No | 1.00 |
| Have typhoid? | |
| Yes | 1.92 (1.08–3.43) |
| No | 1.00 |
| Have tumour? | |
| Yes | 2.02 (0.92–4.42) |
| No | 1.00 |
| Care-seeking behaviour | |
| Inpatient facility | 8.64 (4.67–15.98) |
| Outpatient facility | |
| Public only | 1.80 (1.42–2.28) |
| Private only | 2.01 (1.40–2.88) |
| Both public and private | 2.14 (1.48–3.09) |
| Traditional healer | 0.89 (0.63–1.25) |
| Self-medication/no treatment sought | 1.00 |
| Age (years) | |
| 0–4 | 1.00 |
| 5–9 | 0.96 (0.58–1.59) |
| 10–14 | 0.83 (0.51–1.36) |
| 15–29 | 1.15 (0.66–1.98) |
| 30–44 | 1.19 (0.78–1.82) |
| 45–59 | 1.45 (0.95–2.23) |
| ≥ 60 | 1.29 (0.84–1.98) |
| Household expenditure | |
| Quintile 1 (lowest) | 7.97 (3.59–17.66) |
| Quintile 2 | 3.94 (1.85–8.39) |
| Quintile 3 | 1.97 (0.81–4.77) |
| Quintile 4 | 1.94 (0.89–4.26) |
| Quintile 5 (highest) | 1.00 |
CI, confidence interval; RR, relative risk.
a The analysis was restricted to the 3300 household members who, in the 30 days before the data were collected, reportedly suffered illness that led to household expenditure.
Discussion
As far as we are aware, this is the first study to analyse illness and strategies for financing the related health care at three different levels (i.e. episode of illness, individual and household). Most previous studies on strategies to cope with health-care costs have focused on the household level;9,10,17 few related investigations have focused on the individual level7,24 and almost none at the episode-of-illness level.14 In the present study, as expected, the frequency of reported illness generally increased with age, but there was a transition from a predominance of infectious illness to one of noncommunicable diseases as age increased. Heart disease, asthma, liver disease, typhoid, inpatient care and pre-existing household poverty were positively associated with “distress” strategies for coping with the costs of health care.
About 94% of the surveyed households reported that they had been affected by illness in the 30 days before the interview. The frequency of illness among all of the members of the households surveyed over the same period (45%) was similar to that reported in Viet Nam25 but higher than the value (35%) observed in a previous study in Bangladesh.22 However, the latter study investigated only illness in adults (older than 20 years) and only illness that had occurred in the 15 days before the interview. In addition, disease profiles and socioeconomic situation often change rapidly in developing countries. This makes the valid interpretation of differences in the results of non-concurrent studies difficult, even if the studies are in the same country.
In the present study, several infectious diseases that are common in tropical settings, such as cold/fever, diarrhoea/gastroenteritis, pneumonia and asthma, were the leading health problems reported among young children living in a city in Bangladesh. Similar observations have been made in other developing countries.2,9 The 10 illnesses that were most frequently reported in adult members of the households surveyed in the present study were mostly noncommunicable diseases such as hypertension, gastritis/peptic ulcer, rheumatoid arthritis, diabetes, heart disease, migraine/headache and asthma. In the present study, hypertension and diabetes, which were reported to have occurred in 11% and 6% of adults in the 30 days preceding interview, appeared to have similar prevalences among adults as previously reported in Bangladesh26–28 and several other developing countries.2,9 It seems clear that Bangladesh, like other low- or middle-income countries,1,2,9 faces heavy burdens of both communicable and noncommunicable disease. In concordance with the results of other studies around the world,1,2,5,22,25 the reported frequency of illness tended to increase with age, at least once early childhood had passed. In the present study, as in studies in Afghanistan9 and Cambodia,2 no significant association was found between the frequency of illness and educational level. The similarity in the conditions recorded in the present study across the five quintiles of household wealth was less expected, since poverty often appears to help to perpetuate illness.1,2,5
Although the members of the households that we surveyed were able to obtain care for most of their episodes of illness, 13% of them were forced to adopt “distress” financing to cope with the costs of the care. In Bangladesh, where an estimated 65% of health-care expenditure is financed from out-of-pocket payments, illness is a major cause of economic hardship and poverty.29 Our results indicate that severe infectious diseases, such as typhoid, are particularly likely to lead to distress financing, presumably because the associated out-of-pocket expenses are relatively high. It appears that some chronic and/or noncommunicable conditions, such as heart or liver disease and asthma, can sometimes lead to distress financing in Bangladesh, as in other low- or middle-income countries.6–8 It has been estimated that, within the Asia Pacific region, Bangladesh faces the greatest challenge from noncommunicable diseases, followed by India, Pakistan and then China.30 It has also been estimated that, in the 10 years between 2006 and 2015, Bangladesh, China and India will lose almost 140 million, 14 billion and 17 billion United States dollars, respectively, in national income as a result of the costs of treating heart disease, stroke and diabetes and of the productivity lost as a result of these three conditions.30 In Bangladesh and many other developing countries, only the implementation of risk-pooling mechanisms, such as demand-side financing and/or formal health-insurance schemes, can protect the poorest households from financial hardship as a result of illness. Out-of-pocket expenses dropped markedly following the introduction of health-insurance schemes in China, Ghana, India, Rwanda and Viet Nam.3,31 However, the introduction of health insurance may not be sufficient to avoid catastrophic health spending and distress financing, especially if the primary health care that is available is not of good quality.31 Even in areas with good primary health care, special programmes that target illnesses with relatively high treatment costs, such as typhoid, pneumonia, liver disease, heart disease and cancer, may also be required.
As predicted – partly from the results of previous studies9,14,17 – the risk that a household will need to implement distress financing to cope with the costs of health care was found to increase as the wealth of the household decreased. The high costs of inpatient care appeared to pose particular difficulties for many of the study households and a loan was often needed. In contrast, care from a traditional healer was relatively inexpensive and rarely required distress financing. One particularly disappointing observation made in the present study was that the costs of care from public health facilities in Bangladesh were high enough to require distress financing by many households, even though such facilities are heavily subsidized by the government.32 This result indicates that health-care subsidization programmes in Bangladesh may not be working properly, especially among disadvantaged groups. One problem may be the inadequacy of the drugs and services available in public health facilities, which may be driving patients or their caregivers to purchase drugs and ancillary health services in the private market.
Study limitations
This study has several limitations. First, we only investigated urban households in a single metropolitan area of Bangladesh. Hence, the findings of the study should not be considered representative of the whole of Bangladesh. However, the results may be applicable to other urban areas of Bangladesh and may therefore reflect reality – in terms of illness, health care and health financing – for a large proportion of the Bangladeshi population. Second, the episodes of illness that we investigated were self-reported and so the types and frequencies of illness that we present here may not be a true picture. However, more than 90% of the episodes of major illness that we considered had been diagnosed by clinicians with medical degrees. Reassuringly, the self-reported frequencies of diabetes and hypertension that we recorded among members of the study households were very similar to those recorded in another recent study in Bangladesh.28 Finally, we made no attempt to estimate the indirect costs of illness, such as income lost because the patient could not work while ill or seeking care. Questions about the indirect costs of health care were dropped from the questionnaire used in the final survey because, in a pilot study, many interviewees were unable to provide any information on such costs or only provided very inaccurate information about them.
Conclusions
In Bangladesh, the costs associated with major infectious and noncommunicable diseases appear to weigh most heavily on those least able to afford them. This puts the families concerned at great risk of financial hardship and impoverishment. The national government and international aid organizations need to give far greater attention to the effects of infectious and chronic noncommunicable diseases on household finances. Progress towards achieving national and international health goals will only be accelerated by three changes:
Increasing government spending on health, and committing to health insurance for the whole population. Such insurance might initially be provided for salaried workers, in both the public and private sectors, while voluntary membership of an insurance scheme might be promoted among the workers’ dependents, farmers and the self-employed, in a strategy similar to those currently followed in Viet Nam and other developing countries.3,33
Improving the quality of primary health care for infectious diseases and the routine management of noncommunicable diseases, to avoid unpredictable medical expenses and also reduce the severity of illness. In its national health policy, the Bangladeshi government should give top priority to major noncommunicable diseases while consistently working on the “unfinished agenda” – of controlling infectious diseases in general and, particularly, diarrhoea, typhoid and pneumonia.
Ensuring standard costs and subsidies across all public health facilities, by tightening the regulation of both official and unofficial payments.
In implementing such systems, policy-makers should consider the disease-specific conditions in Bangladesh and especially the relative roles of major infectious diseases and noncommunicable diseases in driving health-care costs. Incorporation and improvement of our knowledge of the patterns of disease and risks of distress financing will be critical in the development of policies and guidelines to decrease population-level health disparities, excessive expenditures and patient suffering.
Acknowledgements
The authors thank the staff of the Department of Population Science and Human Resource Development, University of Rajshahi and the research project’s trainers, coordinators and supervisors, for their assistance, and the interviewees, for their cooperation and participation.
Funding:
This study was supported in part by a grant for scientific research (24030401) from the Japanese Ministry of Health, Labor and Welfare, an overseas research grant from the University of Tokyo, and grants from the Asian Development Bank. The funders had no role in the study design, data collection, data analysis, interpretation or write up.
Competing interests:
None declared.
References
- 1.Bygbjerg IC. Double burden of noncommunicable and infectious diseases in developing countries. Science. 2012;337:1499–501. doi: 10.1126/science.1223466. [DOI] [PubMed] [Google Scholar]
- 2.Ir P, Men C, Lucas H, Meessen B, Decoster K, Bloom G, et al. Self-reported serious illnesses in rural Cambodia: a cross-sectional survey. PLoS One. 2010;5:e10930. doi: 10.1371/journal.pone.0010930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagomarsino G, Garabrant A, Adyas A, Muga R, Otoo N. Moving towards universal health coverage: health insurance reforms in nine developing countries in Africa and Asia. Lancet. 2012;380:933–43. doi: 10.1016/S0140-6736(12)61147-7. [DOI] [PubMed] [Google Scholar]
- 4.Ruger JP. An alternative framework for analyzing financial protection in health. PLoS Med. 2012;9:e1001294. doi: 10.1371/journal.pmed.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 6.Engelgau MM, Karan A, Mahal A. The economic impact of non-communicable diseases on households in India. Global Health. 2012;8:9. doi: 10.1186/1744-8603-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huffman MD, Rao KD, Pichon-Riviere A, Zhao D, Harikrishnan S, Ramaiya K, et al. A cross-sectional study of the microeconomic impact of cardiovascular disease hospitalization in four low- and middle-income countries. PLoS One. 2011;6:e20821. doi: 10.1371/journal.pone.0020821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith R. Why a macroeconomic perspective is critical to the prevention of noncommunicable disease. Science. 2012;337:1501–3. doi: 10.1126/science.1222569. [DOI] [PubMed] [Google Scholar]
- 9.Steinhardt LC, Waters H, Rao KD, Naeem AJ, Hansen P, Peters DH. The effect of wealth status on care seeking and health expenditures in Afghanistan. Health Policy Plan. 2009;24:1–17. doi: 10.1093/heapol/czn043. [DOI] [PubMed] [Google Scholar]
- 10.Leive A, Xu K. Coping with out-of-pocket health payments: empirical evidence from 15 African countries. Bull World Health Organ. 2008;86:849–56. doi: 10.2471/BLT.07.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alamgir NI, Naheed A, Luby SP. Coping strategies for financial burdens in families with childhood pneumonia in Bangladesh. BMC Public Health. 2010;10:622. doi: 10.1186/1471-2458-10-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrin G, Gray E, Almeida J. Coping with ill health in a rickshaw puller’s household in Chittagong, Bangladesh. Southeast Asian J Trop Med Public Health. 1999;30:136–48. [PubMed] [Google Scholar]
- 13.Ezeoke OP, Onwujekwe OE, Uzochukwu BS. Towards universal coverage: examining costs of illness, payment, and coping strategies to different population groups in southeast Nigeria. Am J Trop Med Hyg. 2012;86:52–7. doi: 10.4269/ajtmh.2012.11-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen KT, Khuat OT, Ma SG, Pham DC, Khuat GT, Ruger JP. Coping with health care expenses among poor households: evidence from a rural commune in Vietnam. Soc Sci Med. 2012;74:724–33. doi: 10.1016/j.socscimed.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson-Wright E, Janssens W, van der Gaag J. The inequitable impact of health shocks on the uninsured in Namibia. Health Policy Plan. 2011;26:142–56. doi: 10.1093/heapol/czq029. [DOI] [PubMed] [Google Scholar]
- 16.Ranson MK, Jayaswal R, Mills AJ. Strategies for coping with the costs of inpatient care: a mixed methods study of urban and rural poor in Vadodara district, Gujarat, India. Health Policy Plan. 2012;27:326–38. doi: 10.1093/heapol/czr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruk ME, Goldmann E, Galea S. Borrowing and selling to pay for health care in low- and middle-income countries. Health Aff (Millwood) 2009;28:1056–66. doi: 10.1377/hlthaff.28.4.1056. [DOI] [PubMed] [Google Scholar]
- 18.Hamid SA, Roberts J, Mosley P. Can micro health insurance reduce poverty? Evidence from Bangladesh. J Risk Insur. 2011;78:57–82. doi: 10.1111/j.1539-6975.2010.01402.x. [DOI] [Google Scholar]
- 19.Powell-Jackson T, Hoque ME. Economic consequences of maternal illness in rural Bangladesh. Health Econ. 2012;21:796–810. doi: 10.1002/hec.1749. [DOI] [PubMed] [Google Scholar]
- 20.Bangladesh Bureau of Statistics. Bangladesh population and housing census 2011. Dhaka: Ministry of Planning; 2012. [Google Scholar]
- 21.Ahmed S, Khan MM. A maternal health voucher scheme: what have we learned from the demand-side financing scheme in Bangladesh? Health Policy Plan. 2011;26:25–32. doi: 10.1093/heapol/czq015. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed SM, Tomson G, Petzold M, Kabir ZN. Socioeconomic status overrides age and gender in determining health-seeking behaviour in rural Bangladesh. Bull World Health Organ. 2005;83:109–17. [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell O, Rannan-Eliya RP, Somanathan A, Adhikari SR, Akkazieva B, Harbianto D, et al. Who pays for health care in Asia? J Health Econ. 2008;27:460–75. doi: 10.1016/j.jhealeco.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Skarbinski J, Walker HK, Baker LC, Kobaladze A, Kirtava Z, Raffin TA. The burden of out-of-pocket payments for health care in Tiblisi, Republic of Georgia. JAMA. 2002;287:1043–9. doi: 10.1001/jama.287.8.1043. [DOI] [PubMed] [Google Scholar]
- 25.Giang KB, Allebeck P. Self-reported illness and use of health services in a rural district of Vietnam: findings from an epidemiological field laboratory. Scand J Public Health Suppl. 2003;62:52–8. doi: 10.1080/14034950310015112. [DOI] [PubMed] [Google Scholar]
- 26.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 27.Zaman MM, Rouf MA. Prevalence of hypertension in a Bangladeshi adult population. J Hum Hypertens. 1999;13:547–9. doi: 10.1038/sj.jhh.1000838. [DOI] [PubMed] [Google Scholar]
- 28.Bangladesh Demographic and Health Survey 2011. Dhaka: Bangladesh National Institute of Population Research and Training; 2013. [Google Scholar]
- 29.WHO Global Health Expenditure Atlas 2011 Geneva: World Health Organization; 2012. [Google Scholar]
- 30.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–38. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 31.Meng Q, Xu L, Zhang YG, Qian J, Cai M, Xin Y, et al. Trends in access to health services and financial protection in China between 2003 and 2011: a cross-sectional study. Lancet. 2012;379:805–14. doi: 10.1016/S0140-6736(12)60278-5. [DOI] [PubMed] [Google Scholar]
- 32.Killingsworth JR, Jr, Hossain N, Hedrick-Wong Y, Thomas SD, Rahman A, Begum T. Unofficial fees in Bangladesh: price, equity and institutional issues. Health Policy Plan. 1999;14:152–63. doi: 10.1093/heapol/14.2.152. [DOI] [PubMed] [Google Scholar]
- 33.Savedoff WD, de Ferranti D, Smith AL, Fan V. Political and economic aspects of the transition to universal health coverage. Lancet. 2012;380:924–32. doi: 10.1016/S0140-6736(12)61083-6. [DOI] [PubMed] [Google Scholar]