Abstract
Objective
To explore what can be learnt about the current composition of the “global landscape” of health research and development (R&D) from data on the World Health Organization’s International Clinical Trials Registry Platform (ICTRP).
Methods
A random 5% sample of the records of clinical trials that were registered as interventional and actively recruiting was taken from the ICTRP database.
Findings
Overall, 2381 records of trials were investigated. Analysis of these records indicated that, for every million disability-adjusted life years (DALYs) caused by communicable, maternal, perinatal and nutritional conditions, by noncommunicable diseases, or by injuries, the ICTRP database contained an estimated 7.4, 52.4 and 6.0 trials in which these causes of burden of disease were being investigated, respectively. For every million DALYs in high-income, upper-middle-income, lower-middle-income and low-income countries, an estimated 292.7, 13.4, 3.0 and 0.8 registered trials, respectively, were recruiting in such countries.
Conclusion
The ICTRP constitutes a valuable resource for assessing the global distribution of clinical trials and for informing policy development for health R&D. Populations in lower-income countries receive much less attention, in terms of clinical trial research, than populations in higher-income countries.
Résumé
Objectif
Étudier et, dans la mesure du possible, connaître la composition actuelle du «paysage mondial» en termes de recherche et de développement (R&D) dans le domaine de la santé à partir de données provenant du système d’enregistrement international des essais cliniques de l’Organisation mondiale de la Santé (ICTRP).
Méthodes
Un échantillon aléatoire de 5% des enregistrements des essais cliniques qui ont été référencés comme étant interventionnels et recrutant activement des patients a été obtenu de la base de données de l’ICTRP.
Résultats
Dans l’ensemble, 2381 enregistrements d’essais ont été étudiés. Leur analyse a montré que pour chaque million d’années de vie corrigées du facteur incapacité (AVCI) causées par des pathologies transmissibles, maternelles, périnatales et des déficiences nutritionnelles, par des maladies non transmissibles ou par des blessures, la base de données de l’ICTRP contenait respectivement environ 7,4, 52,4 et 6,0 essais dont les causes contributives à la charge de morbidité étaient en cours d’étude. Pour chaque million d’AVCI dans les pays à revenu élevé, à revenu intermédiaire de la tranche supérieure, à revenu intermédiaire de la tranche inférieure et à faible revenu, il a été estimé qu’environ 292,7, 13,4, 3,0 et 0,8 essais enregistrés, respectivement, recrutaient des patients dans ces pays.
Conclusion
L’ICTRP constitue une ressource précieuse afin d'évaluer la distribution mondiale des essais cliniques, et une excellente source d’informations sur l’évolution des politiques de recherche et de développement dans le domaine médical. Les populations des pays à revenu faible bénéficient d’une attention bien moindre en matière de recherche axée sur les essais cliniques que les populations des pays à revenu élevé.
Resumen
Objetivo
Analizar qué se puede aprender acerca de la composición actual del «paisaje global» de la investigación y desarrollo sanitarios (I+D) a partir de datos de la plataforma de registros internacionales de ensayos clínicos (ICTRP, por sus siglas en inglés) de la Organización Mundial de la Salud.
Métodos
Por medio de un alistamiento activo se tomó una muestra aleatoria del 5% de los expedientes de los ensayos clínicos registrados como intervencionistas de la base de datos de la ICTRP.
Resultados
En total, se investigaron 2381 expedientes. El análisis de dichos expedientes indicó que, por cada millón de años de vida potencialmente perdidos (AVPP) causados por enfermedades transmisibles, maternas, perinatales y nutricionales, por enfermedades no transmisibles o por lesiones, la base de datos de la ICTRP contenía aproximadamente 7,4, 52,4 y 6,0 ensayos, respectivamente, en los que se investigaban las causas de esas cargas de morbilidad. Por cada millón de AVPP en países con ingresos altos, medios-altos, medios-bajos y bajos, se alistaron aproximadamente 292,7, 13,4, 3,0 y 0,8 ensayos registrados en dichos países.
Conclusión
La ICTRP constituye un recurso valioso para evaluar la distribución global de los ensayos clínicos y para informar sobre el desarrollo de políticas para el I+D sanitarios. Las poblaciones en países con ingresos bajos reciben mucha menos atención, en términos de investigación de ensayos clínicos, que las poblaciones en países con ingresos más altos.
ملخص
الغرض
استعراض الدروس التي يمكن الاستفادة منها بشأن التكوين الراهن "للمشهد العالمي" للبحث والتطوير في مجال الصحة من البيانات المعنية بالبرنامج الدولي لتسجيل التجارب السريرية التابع لمنظمة الصحة العالمية (ICTRP).
الطريقة
تم أخذ عينة عشوائية نسبتها 5 % من سجلات التجارب السريرية التي تم تسجيلها باعتبارها تدخلية وتوظيفية على نحو نشط من قاعدة بيانات البرنامج الدولي لتسجيل التجارب السريرية.
النتائج
بشكل إجمالي، تم فحص 2381 سجلاً من التجارب. وأشار تحليل هذه السجلات إلى أنه بالنسبة لكل مليون سنة من سنوات العمر المصححة باحتساب مدد العجز الناجمة عن الاعتلالات السارية واعتلالات الأمومة واعتلالات الفترة المحيطة بالولادة والاعتلالات التغذوية أو عن الأمراض غير السارية أو عن الإصابات، فقد احتوت قاعدة بيانات البرنامج الدولي لتسجيل التجارب السريرية وفق التقديرات على 7.4 و52.4 و6.0 تجربة تم تحري أسباب عبء المرض هذه فيها، على التوالي. وبالنسبة لكل مليون سنة من سنوات العمر المصححة باحتساب مدد العجز في البلدان المرتفعة الدخل والشريحة العليا من البلدان المتوسطة الدخل والشريحة الدنيا من البلدان المتوسطة الدخل والبلدان المنخفضة الدخل، تم توظيف 292.7 و13.4 و3.0 و0.8 تجربة مسجلة في هذه البلدان على التوالي.
الاستنتاج
يشكل البرنامج الدولي لتسجيل التجارب السريرية مورداً قيماً لتقييم التوزيع العالمي للتجارب السريرية وتزويد عملية وضع السياسات بالمعلومات من أجل البحث والتطوير في مجال الصحة. ويحظى السكان في البلدان المنخفضة الدخل بقدر أقل من الاهتمام، من حيث البحث في التجارب السريرية، عن السكان في البلدان المرتفعة الدخل.
摘要
目的
根据世界卫生组织的国际临床试验注册平台(ICTRP)的数据,探索卫生研发(R&D)“全球景观”的当前组成能够带来哪些讯息。
方法
在ICTRP数据库以介入式和主动招募方式注册的临床试验记录中随机抽取5%的样本。
结果
总计调查了2381 个试验记录。对这些记录的分析表明:对于因传染性、母体遗传、围产期和营养条件、因非传染性疾病或者因受伤造成的每百万残疾调整生命年(DALY),ICTRP数据库估计分别包含有7.4、52.4 和6.0 项正在其中调查这些疾病负担原因的试验。在高收入、中高收入、中低收入和低收入国家中,每百万DALY中分别估计招募有292.7、13.4、3.0 和0.8 项注册试验。
结论
ICTRP是评估全球临床试验分布以及制订翔实卫生研发政策的宝贵资源。就临床试验研究而言,较之高收入国家人口,低收入国家人口得到的关注要少得多。
Резюме
Цель
Изучить доступную информацию о текущем глобальном распределении научных исследований и разработок в области здравоохранения на основе данных Международной платформы для регистрации клинических испытаний (МПРКИ) Всемирной организации здравоохранения.
Методы
Проведена случайная пятипроцентная выборка протоколов клинических испытаний из базы данных МПРКИ, зарегистрированных как интервенционные и с активным набором участников.
Результаты
Всего было изучено 2381 протоколов испытаний. Анализ данных протоколов показал, что на каждый миллион лет жизни, скорректированных на инвалидность (индекс DALYs), обусловленных а) инфекционными заболеваниями, состоянием материнского и перинатального здоровья и условиями питания; б) неинфекционными заболеваниями и в) травмами, в базе данных содержится приблизительно 7,4, 52,4 и 6,0 испытаний, в которых исследовались причины бремени болезней, соответствующие указанным группам. В то же время, на каждый миллион лет жизни, скорректированных на инвалидность, приблизительное число зарегистрированных в базе данных исследований, в которых производился набор участников, в странах с высоким уровнем доходов, выше среднего, ниже среднего и с низким уровнем доходов составило 292,7, 13,4, 3,0 и 0,8 соответственно.
Вывод
Платформа МПРКИ представляет собой ценный ресурс для оценки глобального распределения клинических испытаний и информирования о выработке стратегии для научно-исследовательских и опытно-конструкторских разработок в области здравоохранения. Населению в странах более низкими уровнями доходов уделяется намного меньше внимания при проведении клинических исследований, чем населению в странах с высоким уровнем доходов.
Introduction
More than two decades ago it was shown that only 5% of the world’s resources for health research and development (R&D) were spent on the health problems of developing countries, which then represented 93% of the world’s burden of preventable mortality.1,2 The lack of a rational link between the health R&D that was needed and that which was being conducted resulted in the existence of “neglected populations”.3 This mismatch, which still exists, had and has two main causes. First, the distribution of R&D funding has been – and remains – largely determined by market forces rather than by a more equitable system that is based on health needs.4,5 Second, even when funding for health R&D is distributed by philanthropic or governmental donors, many high-burden diseases and priority areas of R&D can remain badly underfunded.6 This indicates a lack of appropriate mechanisms for the prioritization and coordination of such R&D.7 To start addressing these problems, a sense of agreement on a common R&D agenda will have to grow among funders of health R&D – something that, to date, has proven difficult to achieve.7 As a first step towards such a common agenda, the current composition of the “global landscape” of health R&D needs to be explored so that the gaps in this landscape and neglected populations can be identified. If we are to change how we spend our money on health R&D, we first need to know how we are spending it now.
Unfortunately, we know very little about what health R&D is being conducted, where and how it is being conducted, and who is conducting it.8 Databases of registered clinical trials may offer a new resource for gaining insight into the health R&D “landscape”. In the past decade, trial registration has become broadly accepted as an ethical and scientific responsibility.9–16 Enforcing regulations, policies and legislation has been crucial to the success of trial registration. There has been relevant national legislation,12 the editors of many medical journals have made trial registration a prerequisite for the publication of trial results,9,13–15 such registration may also now be a prerequisite for the ethical approval of a trial’s protocol11,17 and a self-regulating pharmaceutical industry has also promoted trial registration.16 On several continents, many publicly accessible, online registries have been established to allow investigators to register their clinical trials.18 In 2005, the International Clinical Trials Registry Platform (ICTRP) was established by the World Health Organization (WHO) to create a platform for linking these clinical trial registries and provide a single point of access to information on all clinical trials conducted globally.11 Over the last 8 years, the ICTRP has grown into a platform that combines data from 15 different clinical-trial registries, both national and regional, and offers access to more than 200 000 registered records of clinical trials.
This study was conducted to explore what can be learnt from the clinical trial records available on the ICTRP database about the current composition of the “global landscape” of health R&D. We were especially interested in the distribution of trials across different diseases and countries and the identification of any major gaps in the “landscape”.
Methods
Study sample
By using an automated random sampling function that is available as part of the ICTRP’s data management system, we randomly selected from the ICTRP database 5% of all the records for interventional clinical trials that were registered as actively recruiting participants on 10 August 2012. A 5% sample was considered to be sufficient to produce results that could give a general view, but not too large to hamper the manual extraction of relevant data. For trials that were registered in more than one registry, we included only the record with the earliest registration date.19 We excluded trials that, according to the ICTRP’s records, were only observational in nature.
Data extraction
Registry name, date of registration, age and sex inclusion criteria, target sample size, study design, study type, study phase and the countries of recruitment for each record were downloaded from the ICTRP and imported into an Excel (Microsoft, Redmond, United States of America) database on 10 August 2012. We manually reviewed the health condition or problem studied, the intervention and the primary sponsor by examining the registered record, and we then coded the data as described in the next section.
Data coding and classifications
We coded the health conditions or problems studied in each selected trial according to table C3 of the Global burden of disease: 2004 update.20
We categorized the countries in which the subjects of trials were recruited as high-, upper-middle-, lower-middle- or low-income according to the World Bank’s groupings, which are based on gross national incomes per capita.21 We also identified the WHO region to which each country belonged using the current WHO classification of Member States.22 If a trial was recruiting participants in multiple countries that belonged to the same income group or same WHO region, we counted the group or region only once.
We divided primary sponsors (i.e. the individual, organization, group or other legal entity that was responsible for initiating, managing and/or financing a trial) into nine categories: collaborative groups of researchers or doctors; contract research organizations; foundations; government institutions; industries; individuals registered as sponsors; research institutes; universities or hospitals; and “other”. We then classified trials as having an industrial primary sponsor, a non-industrial primary sponsor (including collaborative groups, foundations, governments, research institutes and universities or hospitals) or another type of sponsor (including individuals registered as primary sponsors, contract research organizations and “other” sponsors).
All data were extracted and coded by one author (RFV) and, if ambiguous, discussed with another author (RFT).
Data analysis
For each health condition or problem studied and for each of the categories used for the countries of recruitment, the number of trials detected in the 5% sample was extrapolated to estimate the total number of actively recruiting, interventional trials with the same characteristic that were registered on the ICTRP. The Wilson score interval23 was used to calculate 95% confidence intervals for each estimate.
Whenever possible, for each health condition or problem studied, we mapped the estimated total number of related trials on the ICTRP against the corresponding burden of disease in disability-adjusted life years (DALYs).20,24 Additionally, we divided the estimated total number of related trials by the corresponding burden of disease in DALYs to give an estimate of the total number of trials per million DALYs for each health condition. Burden-of-disease data were not available for all of the health conditions that were being investigated in the selected trials.24 In addition, the subcauses of injuries were ignored in these calculations because the sources of the injuries were not included in the majority of the records pertaining to injuries. Among the health conditions and problems, we also excluded residual (“other”) categories, several overarching categories (i.e. skin disorders, endocrine disorders and “other neoplasms”) and a small number of specific diseases for which uncertainties in the burden-of-disease estimates were large (e.g. chlamydia, gonorrhoea, neonatal infections, polio, all congenital anomalies, all oral diseases and Chagas disease in low-income countries). Trials that recruited participants with malignant neoplasms in general were redistributed proportionally over all of the disease codes for such neoplasms, in a similar approach to that taken by the authors of the Global burden of disease: 2004 update.20
We expressed estimates of the numbers of trials in the ICTRP database that were recruiting in countries in each income group and WHO region as the numbers of trials per capita. For this, we estimated the sizes of the relevant national populations in the year 2012 using the World Bank’s database of health, nutrition and population statistics.25 For each income group and WHO region, we divided the number of trials per capita by the corresponding total burden of disease in DALYs per capita to obtain an estimate of the total number of trials per million DALYs for each category used for the countries of recruitment.
We derived all burden-of-disease data – which were standard DALYs with time discounting and age-weighting – from the most recently published results of WHO’s Global Burden of Disease study.20,24
We used Z-tests23 to compare the proportions of trials whose primary sponsor was industrial with the corresponding proportions of trials with non-industrial primary sponsors.
All of the data analysis was conducted using the Excel software package.
Results
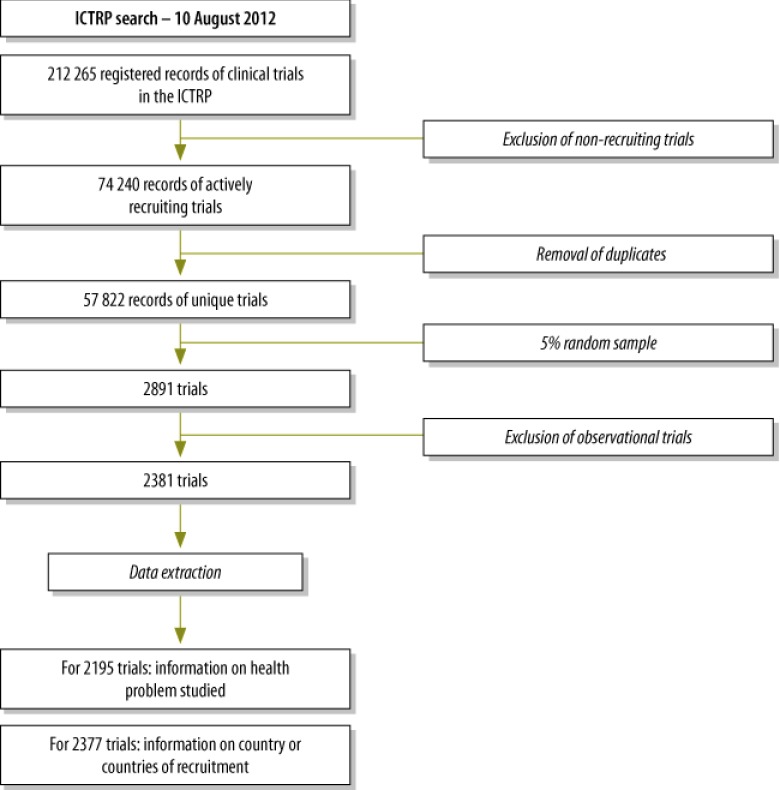
On 10 August 2012, 2381 clinical trials that were registered as interventional and actively recruiting were randomly selected from the ICTRP database (Fig. 1). Baseline information on registry name, intervention type, year of registration, sponsorship, target sample size, study phase and inclusion criteria for sex and age of participants is presented in Table 1.
Fig. 1.
Flowchart of the sampling of the records of interventional and actively recruiting trials in the International Clinical Trials Registry Platform (ICTRP), 2012
Table 1. Baseline information on a 5% sample of trials from the International Clinical Trials Registry Platform, 2012.
| Category | No. (%) of selected trials (n = 2381) |
|---|---|
| Registry name | |
| CT.gov | 1316 (55.3) |
| EU-CTR | 540 (22.7) |
| JPRN | 208 (8.7) |
| ANZCTR | 95 (4.0) |
| ISRCTN | 61 (2.6) |
| ChiCTR | 43 (1.8) |
| CTRI | 36 (1.5) |
| NTR | 31 (1.3) |
| IRCT | 23 (1.0) |
| DRKS | 16 (0.7) |
| CRiS | 9 (0.4) |
| ReBec | 2 (0.1) |
| PACTR | 1 (0.0) |
| RPCEC | 0 (0) |
| SLCTR | 0 (0) |
| Intervention typea | |
| Drugs and biologicals | 1562 (65.6) |
| Surgery and other proceduresb | 281 (11.8) |
| Behaviouralc | 168 (7.1) |
| Device | 167 (7.0) |
| Diagnostic | 119 (5.0) |
| Dietary supplements and diets | 106 (4.5) |
| Physical therapy | 64 (2.7) |
| Radiation | 48 (2.0) |
| Organizational | 42 (1.8) |
| Other | 35 (1.5) |
| Year of registration | |
| Before 2005 | 26 (1.1) |
| 2005 | 127 (5.3) |
| 2006 | 106 (4.5) |
| 2007 | 158 (6.6) |
| 2008 | 245 (10.3) |
| 2009 | 351 (14.7) |
| 2010 | 462 (19.4) |
| 2011 | 544 (22.8) |
| 2012 | 362 (15.2) |
| Primary sponsor | |
| University or hospital | 1459 (61.3) |
| Industry | 495 (20.8) |
| Collaborative group of doctors or researchers | 112 (4.7) |
| Government institution | 99 (4.2) |
| Individual | 97 (4.1) |
| Research institute | 51 (2.1) |
| Foundation | 40 (1.7) |
| Contract research organization | 4 (0.2) |
| Other | 2 (0.1) |
| Not specified or not classifiable | 22 (0.9) |
| Target number of participants | |
| 1–99 | 1184 (49.7) |
| 100–999 | 832 (34.9) |
| ≥ 1000 | 94 (3.9) |
| Not specified | 271 (11.4) |
| Study phase(s) | |
| 0 | 11 (0.5) |
| I | 166 (7.0) |
| I/II | 86 (3.6) |
| II | 432 (18.1) |
| II/III | 44 (1.8) |
| III | 265 (11.1) |
| III/IV | 1 (0.0) |
| IV | 230 (9.7) |
| Not specified | 1146 (48.2) |
| Sex of participants | |
| Both | 2028 (85.2) |
| Female | 257 (10.8) |
| Male | 96 (4.0) |
| Age of participantsa | |
| 0–27 days | 76 (3.2) |
| 28 days–2 years | 111 (4.7) |
| 2–11 years | 200 (8.4) |
| < 12 years | 247 (10.4) |
| 12–17 years | 280 (11.8) |
| < 18 years | 372 (15.6) |
| 18–64 years | 2034 (85.4) |
| ≥ 65 years | 1582 (66.4) |
| Not specified | 127 (5.3) |
ANZCTR, Australian New Zealand Clinical Trials Registry; ChiCTR, Chinese Clinical Trial Register; CRiS, Clinical Research Information Service of the Republic of Korea; CT.gov, ClinicalTrials.gov; CTRI, Clinical Trials Registry – India; DRKS, German Clinical Trials Register; EU-CTR, EU Clinical Trials Register; IRCT, Iranian Registry of Clinical Trials; ISRCTN, International Standard Randomized Controlled Trial Number Register; JPRN, Japan Primary Registries Network; NTR, Netherlands National Trial Register; PACTR, Pan African Clinical Trial Registry; ReBec, Brazilian Clinical Trials Registry; RPCEC, Cuban Public Registry of Clinical Trials; SLCTR, Sri Lanka Clinical Trials Registry.
a As some of the classifications within this category overlap, some trials are included in more than one classification.
b “Other procedures” included acupuncture and cell transplants.
C For example, psychotherapy and lifestyle counselling.
Health conditions or problems studied
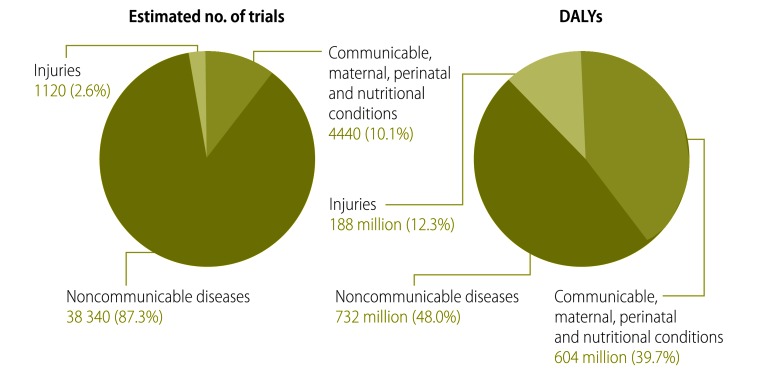
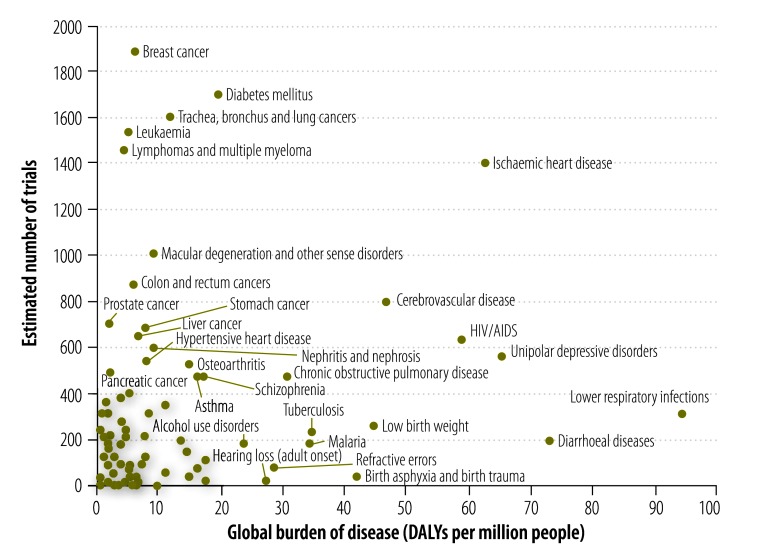
The health condition or problem studied could be classified for 2195 of the 2381 selected trials. The most common focus of investigation – both in terms of the absolute number of trials and the number of trials per million DALYs caused by the condition or problem – was on noncommunicable diseases (52.4), followed first by communicable, maternal, perinatal and nutritional conditions (7.4) and then by injuries (6.0) (Table 2, available at: http://www.who.int/bulletin/volumes/91/6/12-114454, and Fig. 2). The estimated total number of trials registered on the ICTRP for each health condition or problem was mapped against the global burden of the condition or problem (Fig. 3).
Table 2. The health problems being investigated in the actively recruiting, interventional trials registered on the International Clinical Trials Registry Platform (ICTRP), 2012.
| Health condition or problem | No. of trials in samplea | Estimate and (95% CI) |
||
|---|---|---|---|---|
| Percentage of trials in ICTRPb | No. of trials in ICTRP |
|||
| Total | Per 1 000 000 DALYs | |||
| Communicable, maternal, perinatal and nutritional | 222 | 10.1 (8.9–11.4) | 4440 (3916–5025) | 7.4 (6.5–8.3) |
| Infectious and parasitic diseases | 132 | 6.0 (5.1–7.1) | 2640 (2236–3111) | 8.7 (7.4–10.3) |
| Tuberculosis | 11 | 0.5 (0.3–0.9) | 220 (123–393) | 6.4 (3.6–11.5) |
| HIV/AIDS | 32 | 1.5 (1.0–2.1) | 640 (454–900) | 10.9 (7.8–15.4) |
| Diarrhoeal diseases | 10 | 0.5 (0.2–0.8) | 200 (109–367) | 2.7 (1.5–5.0) |
| Childhood cluster diseases | 6 | 0.3 (0.1–0.6) | 120 (55–261) | 4.0 (1.8–8.6) |
| Poliomyelitisc | 1 | 0.0 (0.0–0.3) | 20 (4–113) | – |
| Diphtheria | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 115.2 (20.3–651.6) |
| Measles | 2 | 0.1 (0.0–0.3) | 40 (11–146) | 2.7 (0.7–9.8) |
| Tetanus | 2 | 0.1 (0.0–0.3) | 40 (11–146) | 7.6 (2.1–27.6) |
| Meningitis | 3 | 0.1 (0.0–0.4) | 60 (20–176) | 5.3 (1.8–15.4) |
| Hepatitis B | 8 | 0.4 (0.2–0.7) | 160 (81–315) | 77.4 (39.2–152.4) |
| Hepatitis C | 16 | 0.7 (0.4–1.2) | 320 (197–518) | 335.2 (206.6–543.0) |
| Malaria | 9 | 0.4 (0.2–0.8) | 180 (95–341) | 5.3 (2.8–10.0) |
| Leprosy | 2 | 0.1 (0.0–0.3) | 40 (11–146) | 206.4 (56.6–751.2) |
| Dengue | 2 | 0.1 (0.0–0.3) | 40 (11–146) | 59.7 (16.4–217.4) |
| Intestinal nematode infections | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 5.0 (0.9–28.2) |
| Ascariasis | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 10.8 (1.9–61.1) |
| Other infectious diseasec | 32 | 1.5 (1.0–2.1) | 640 (454–900) | – |
| Respiratory infections | 26 | 1.2 (0.8–1.7) | 520 (355–759) | 5.3 (3.6–7.8) |
| Lower respiratory infections | 16 | 0.7 (0.4–1.2) | 320 (197–518) | 3.4 (2.1–5.5) |
| Upper respiratory infections | 9 | 0.4 (0.2–0.8) | 180 (95–341) | 100.7 (53.0–191.0) |
| Otitis media | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 13.4 (2.4–76.0) |
| Maternal conditions | 36 | 1.6 (1.2–2.3) | 720 (521–993) | 18.5 (13.4–25.5) |
| Maternal haemorrhage | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 4.5 (0.8–25.5) |
| Hypertensive disorders of pregnancy | 5 | 0.2 (0.1–0.5) | 100 (43–234) | 53.0 (22.6–123.7) |
| Obstructed labour | 6 | 0.3 (0.1–0.6) | 120 (55–261) | 41.6 (19.1–90.6) |
| Abortion | 5 | 0.2 (0.1–0.5) | 100 (43–234) | 13.5 (5.8–31.5) |
| Otherc | 19 | 0.9 (0.6–1.3) | 380 (244–592) | – |
| Conditions arising during perinatal period | 20 | 0.9 (0.6–1.4) | 400 (259–616) | 3.2 (2.1–4.9) |
| Low birth weight | 13 | 0.6 (0.3–1.0) | 260 (152–444) | 5.9 (3.4–10.0) |
| Birth asphyxia and birth trauma | 2 | 0.1 (0.0–0.3) | 40 (11–146) | 1.0 (0.3–3.5) |
| Neonatal infections and other conditionsc | 5 | 0.2 (0.1–0.5) | 100 (43–234) | – |
| Nutritional deficiencies | 8 | 0.4 (0.2–0.7) | 160 (81–315) | 4.1 (2.1–8.1) |
| Protein-energy malnutrition | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 1.1 (0.2–6.5) |
| Iron-deficiency anaemia | 4 | 0.2 (0.1–0.5) | 80 (31–205) | 5.0 (1.9–12.7) |
| Otherc |
3 |
0.1 (0.0–0.4) |
60 (20–176) |
– |
| Non-communicable | 1917 | 87.3 (85.9–88.7) | 38 340 (37 700–38 922) | 52.4 (51.5–53.2) |
| Malignant neoplasms | 667 | 30.4 (28.5–32.3) | 13 340 (12 511–14 199) | 171.4 (160.8–182.5) |
| Mouth and oropharynx cancers | 19 | 0.9 (0.6–1.4) | 385 (248–598) | 101.7 (65.4–157.9) |
| Oesophagus cancer | 11 | 0.5 (0.3–0.9) | 214 (119–385) | 44.9 (24.9–80.8) |
| Stomach cancer | 34 | 1.6 (1.1–2.2) | 685 (492–953) | 91.5 (65.7–127.2) |
| Colon and rectum cancers | 44 | 2.0 (1.5–2.7) | 878 (655–1174) | 149.5 (111.6–199.9) |
| Liver cancer | 33 | 1.5 (1.1–2.1) | 664 (474–928) | 98.9 (70.6–138.3) |
| Pancreatic cancer | 25 | 1.1 (0.8–1.7) | 492 (333–727) | 221.9 (150.1–327.5) |
| Trachea, bronchus and lung cancers | 80 | 3.7 (3.0–4.5) | 1606 (1295–1988) | 136.5 (110.1–168.9) |
| Melanoma and other skin cancers | 12 | 0.5 (0.3–0.9) | 236 (134–413) | 333.5 (190.0–584.5) |
| Breast cancer | 94 | 4.3 (3.5–5.2) | 1884 (1546–2293) | 284.3 (233.2–345.9) |
| Cervix uteri cancer | 14 | 0.6 (0.4–1.1) | 278 (166–467) | 74.8 (44.6–125.5) |
| Corpus uteri cancer | 6 | 0.3 (0.1–0.6) | 128 (60–273) | 172.5 (81.1–366.3) |
| Ovary cancer | 16 | 0.7 (0.5–1.2) | 321 (198–520) | 184.1 (113.5–297.9) |
| Prostate cancer | 35 | 1.6 (1.2–2.2) | 707 (510–978) | 383.4 (276.7–530.5) |
| Bladder cancer | 11 | 0.5 (0.3–0.9) | 214 (119–385) | 147.6 (81.8–265.6) |
| Lymphomas and multiple myeloma | 73 | 3.3 (2.6–4.2) | 1456 (1161–1822) | 339.8 (271.1–425.3) |
| Leukaemia | 77 | 3.5 (2.8–4.4) | 1542 (1238–1917) | 311.9 (250.4–387.8) |
| Otherc | 82 | 3.8 (3.0–4.6) | 1649 (1334–2035) | – |
| Other neoplasmsc | 25 | 1.1 (0.8–1.7) | 500 (339–736) | – |
| Diabetes mellitus | 85 | 3.9 (3.1–4.8) | 1700 (1380–2091) | 86.3 (70.0–106.1) |
| Endocrine disordersc | 122 | 5.6 (4.7–6.6) | 2440 (2052–2896) | – |
| Neuropsychiatric conditions | 282 | 12.8 (11.5–14.3) | 5640 (5054–6283) | 28.3 (25.4–31.5) |
| Unipolar depressive disorders | 28 | 1.3 (0.9–1.8) | 560 (388–807) | 8.6 (5.9–12.3) |
| Bipolar affective disorder | 8 | 0.4 (0.2–0.7) | 160 (81–315) | 11.1 (5.6–21.8) |
| Schizophrenia | 26 | 1.2 (0.8–1.7) | 520 (355–759) | 31.0 (21.2–45.3) |
| Epilepsy | 11 | 0.5 (0.3–0.9) | 220 (123–393) | 28.0 (15.7–50.0) |
| Alcohol use disorders | 9 | 0.4 (0.2–0.8) | 180 (95–341) | 7.6 (4.0–14.4) |
| Alzheimer and other dementias | 18 | 0.8 (0.5–1.3) | 360 (228–567) | 32.3 (20.4–50.9) |
| Parkinson disease | 11 | 0.5 (0.3–0.9) | 220 (123–393) | 128.6 (71.9–229.8) |
| Multiple sclerosis | 18 | 0.8 (0.5–1.3) | 360 (228–567) | 235.7 (149.3–371.6) |
| Drug use disorders | 16 | 0.7 (0.4–1.2) | 320 (197–518) | 38.2 (23.6–61.9) |
| Post-traumatic stress disorder | 9 | 0.4 (0.2–0.8) | 180 (95–341) | 51.9 (27.3–98.4) |
| Obsessive–compulsive disorder | 5 | 0.2 (0.1–0.5) | 100 (43–234) | 19.6 (8.4–45.8) |
| Panic disorder | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 2.9 (0.5–16.2) |
| Insomnia (primary) | 5 | 0.2 (0.1–0.5) | 100 (43–234) | 27.6 (11.8–64.5) |
| Migraine | 6 | 0.3 (0.1–0.6) | 120 (55–261) | 15.5 (7.1–33.6) |
| Otherc | 111 | 5.1 (4.2–6.1) | 2220 (1851–2658) | – |
| Sense organ diseases | 73 | 3.3 (2.7–4.2) | 1460 (1165–1827) | 16.8 (13.4–21.0) |
| Glaucoma | 12 | 0.5 (0.3–1.0) | 240 (137–418) | 50.8 (29.1–88.5) |
| Cataracts | 6 | 0.3 (0.1–0.6) | 120 (55–261) | 6.8 (3.1–14.7) |
| Refractive errors | 4 | 0.2 (0.1–0.5) | 80 (31–205) | 2.9 (1.1–7.4) |
| Hearing loss (adult onset) | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 0.7 (0.1–4.1) |
| Macular degeneration and other | 50 | 2.3 (1.7–3.0) | 1000 (760–1313) | 107.6 (81.8–141.2) |
| Cardiovascular diseases | 219 | 10.0 (8.8–11.3) | 4380 (3860–4961) | 28.9 (25.5–32.8) |
| Rheumatic heart disease | 5 | 0.2 (0.1–0.5) | 100 (43–234) | 19.3 (8.2–45.0) |
| Hypertensive heart disease | 28 | 1.3 (0.9–1.8) | 560 (388–807) | 69.8 (48.4–100.6) |
| Ischaemic heart disease | 70 | 3.2 (2.5–4.0) | 1400 (1111–1760) | 22.4 (17.8–28.1) |
| Cerebrovascular disease | 40 | 1.8 (1.3–2.5) | 800 (589–1085) | 17.2 (12.6–23.3) |
| Inflammatory heart disease | 2 | 0.1 (0.0–0.3) | 40 (11–146) | 6.4 (1.8–23.3) |
| Otherc | 74 | 3.4 (2.7–4.2) | 1480 (1183–1849) | – |
| Respiratory diseases | 75 | 3.4 (2.7–4.3) | 1500 (1200–1871) | 25.4 (20.3–31.7) |
| Chronic obstructive pulmonary disease | 24 | 1.1 (0.7–1.6) | 480 (323–712) | 15.9 (10.7–23.6) |
| Asthma | 26 | 1.2 (0.8–1.7) | 520 (355–759) | 31.9 (21.8–46.5) |
| Otherc | 25 | 1.1 (0.8–1.7) | 500 (339–736) | – |
| Digestive diseases | 77 | 3.5 (2.8–4.4) | 1540 (1236–1915) | 36.2 (29.1–45.1) |
| Peptic ulcer disease | 4 | 0.2 (0.1–0.5) | 80 (31–205) | 16.1 (6.3–41.4) |
| Cirrhosis of the liver | 10 | 0.5 (0.2–0.8) | 200 (109–367) | 14.7 (8.0–26.9) |
| Appendicitis | 1 | 0.0 (0.0–0.3) | 20 (4–113) | 47.8 (8.4–270.4) |
| Otherc | 62 | 2.8 (2.2–3.6) | 1240 (970–1582) | – |
| Genitourinary diseases | 84 | 3.8 (3.1–4.7) | 1680 (1362–2069) | 113.9 (92.3–140.2) |
| Nephritis and nephrosis | 30 | 1.4 (1.0–1.9) | 600 (421–854) | 66.2 (46.5–94.2) |
| Benign prostatic hypertrophy | 3 | 0.1 (0.0–0.4) | 60 (20–176) | 22.5 (7.7–66.1) |
| Otherc | 51 | 2.3 (1.8–3.0) | 1020 (778–1335) | – |
| Skin diseasesc | 49 | 2.2 (1.7–2.9) | 980 (743–1290) | – |
| Musculoskeletal disorders | 124 | 5.6 (4.8–6.7) | 2480 (2089–2939) | 80.3 (67.7–95.2) |
| Rheumatoid arthritis | 20 | 0.9 (0.6–1.4) | 400 (259–616) | 79.2 (51.3–122.0) |
| Osteoarthritis | 27 | 1.2 (0.8–1.8) | 540 (372–783) | 34.6 (23.9–50.2) |
| Goutc | 1 | 0.0 (0.0–0.3) | 20 (4–113) | – |
| Low back painc | 9 | 0.4 (0.2–0.8) | 180 (95–341) | – |
| Otherc | 67 | 3.1 (2.4–3.9) | 1340 (1058–1694) | – |
| Congenital anomaliesc | 18 | 0.8 (0.5–1.3) | 360 (228–567) | – |
| Down syndrome | 2 | 0.1 (0.0–0.3) | 40 (11–146) | – |
| Congenital heart anomalies | 2 | 0.1 (0.0–0.3) | 40 (11–146) | – |
| Other | 14 | 0.6 (0.4–1.1) | 280 (167–469) | – |
| Oral conditionsc | 17 | 0.8 (0.5–1.2) | 340 (213–543) | – |
| Dental caries | 2 | 0.1 (0.0–0.3) | 40 (11–146) | – |
| Periodontal disease | 1 | 0.0 (0.0–0.3) | 20 (4–113) | – |
| Edentulism | 3 | 0.1 (0.0–0.4) | 60 (20–176) | – |
| Other |
11 |
0.5 (0.3–0.9) |
220 (123–393) |
– |
| Injuriesc | 56 | 2.6 (2.0–3.3) | 1120 (865–1448) | 6.0 (4.6–7.7) |
CI, confidence interval; DALY, disability-adjusted life year; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome.
a Estimated percentages and numbers for the whole ICTRP were based on the results of the analysis of the records for a 5% sample of the trials registered on the platform. Health conditions or problems for which no trials were found in the sample were excluded from this table.
b The percentages shown are those of the 2195 trials in the sample for which the health condition or problem studied could be classified. The condition or problem investigated in the other 186 trials included in the sample could not be classified because there was insufficient information in the registered records of the trial or because the trials included participants with many different diseases.
c Burden-of-disease data for this condition or problem were either not available or excluded from this table for the reasons given in the methods section.
Fig. 2.
Health problems being investigated by trials registered in the International Clinical Trials Registry Platform (ICTRP), 2012
DALY, disability-adjusted life year.
Note: Only interventional and actively recruiting trials were investigated. The health problems are split according to both the estimated numbers of trials on the ICTRP (lefthand chart) and the burden of disease that they cause globally (righthand chart). Confidence intervals were calculated for the estimates but have been omitted from the figure, for clarity.
Fig. 3.
Estimated number of trials in the International Clinical Trials Registry Platform investigating a specific health problem and the burden of disease posed by that problem, 2012
DALY, disability-adjusted life year; AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
Note: Only interventional and actively recruiting trials were included in the analysis. Data in the grey area is for health problems that have 400 or fewer registered trials and a global burden of disease of less than 20 million. A full list of registered trials by health problem is shown in Table 2 (available at: http://www.who.int/bulletin/volumes/91/6/12-114454). Only trials investigating specific health problems were included in this figure; overarching categories and subcategories of health problems were excluded. Confidence intervals were calculated for the estimates but have been omitted from the figure, for clarity.
Countries of recruitment and sponsorship
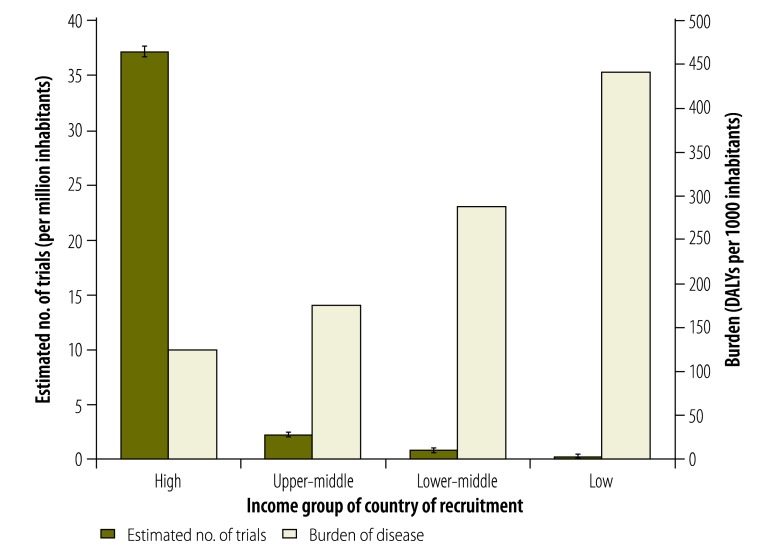
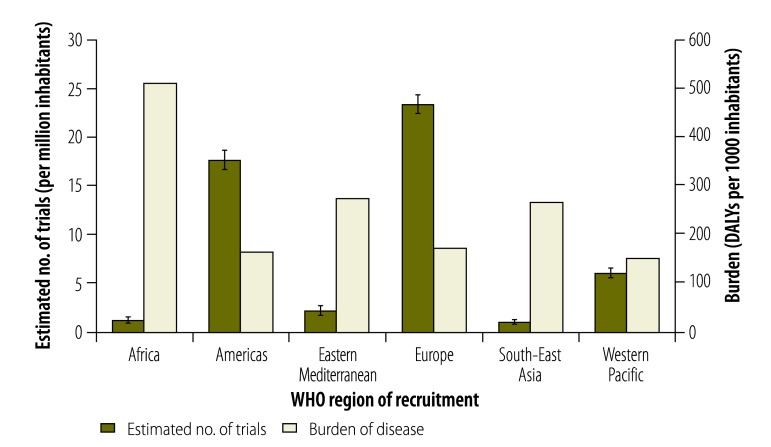
Information on countries of recruitment was available for 2377 of the 2381 selected trials. Trials were found to recruit most often in high-income countries – absolutely, per capita and proportionally to the burden of disease in these countries – followed first by upper-middle-income countries, then by lower-middle-income countries and finally by low-income countries (Table 3 and Fig. 4). Trials recruited most often were in WHO’s European Region and the Region of the Americas (Table 3 and Fig. 5).
Table 3. Areas of recruitment for the actively recruiting, interventional trials registered in the International Clinical Trials Registry Platform (ICTRP), 2012.
| Area of recruitment | No. of trials in samplea | Estimate |
|||
|---|---|---|---|---|---|
| Percentage (95% CI) of trials in ICTRPb | No. (95% CI) of trials in ICTRP |
||||
| Total | Per 1 000 000 inhabitants | Per 1 000 000 DALYs | |||
| World Bank income group21 | |||||
| High-income country | 2115 | 89.0 (87.7–90.2) | 42 300 (41 671–42 869) | 37.2 (36.7–37.7) | 292.7 (288.4–296.7) |
| Upper-middle-income country | 292 | 12.3 (11.0–13.7) | 5840 (5241–6496) | 2.4 (2.1–2.6) | 13.4 (12.0–14.9) |
| Lower-middle-income country | 111 | 4.7 (3.9–5.6) | 2220 (1850–2659) | 0.9 (0.7–1.0) | 3.0 (2.5–3.6) |
| Low-income country | 14 | 0.6 (0.4–1.0) | 280 (167–469) | 0.3 (0.2–0.6) | 0.8 (0.5–1.3) |
| WHO region22 | |||||
| Africa | 50 | 2.1 (1.6–2.8) | 1000 (760–1313) | 1.1 (0.9–1.5) | 2.2 (1.7–2.9) |
| Americas | 840 | 35.3 (33.4–37.3) | 16 800 (15 898–17 724) | 17.7 (16.7–18.7) | 107.9 (102.1–113.8) |
| Eastern Mediterranean | 65 | 2.7 (2.2–3.5) | 1300 (1023–1650) | 2.1 (1.6–2.6) | 7.6 (6.0–9.7) |
| Europe | 1055 | 44.4 (42.4–46.4) | 21 100 (20 156-22 053) | 23.4 (22.3–24.4) | 136.3 (130.2–142.5) |
| South-East Asia | 96 | 4.0 (3.3–4.9) | 1920 (1578–2333) | 1.0 (0.9–1.3) | 3.9 (3.2–4.8) |
| Western Pacific | 548 | 23.1 (21.4–24.8) | 10 960 (10 176-11 785) | 6.0 (5.6–6.5) | 39.7 (36.8–42.6) |
CI, confidence interval; DALY, disability-adjusted life year; WHO, World Health Organization.
a Estimated percentages and numbers for the whole ICTRP were based on the results of the analysis of the records for a 5% sample of the trials registered on the platform.
b The percentages shown are those of the 2377 trials in the sample for which the country or countries of recruitment could be determined from the registered records. When summed, the percentages shown for income groups or regions exceed 100% because some trials were recruiting in multiple countries belonging to more than one income group or region.
Fig. 4.
Estimated numbers of trials in the International Clinical Trials Registry Platform recruiting participants in low-, lower-middle-, upper-middle- and high-income countries, 2012
DALY, disability-adjusted life year.
Note: Only interventional and actively recruiting trials were included in the analysis. For illustration, the burdens of disease in countries in the same income groups are also presented. The error bars on the estimates of trial numbers indicate 95% confidence intervals.
Fig. 5.
Estimated numbers of trials in the International Clinical Trials Registry Platform recruiting in each of WHO’s regions, 2012
DALY, disability-adjusted life year; WHO, World Health Organization.
Note: Only interventional and actively recruiting trials were included in the analysis. For illustration, the burdens of disease in countries in the same regions are also presented. The error bars on the estimates of trial numbers indicate 95% confidence intervals.
We were able to determine country of recruitment and classify the primary sponsor as non-industrial or industrial for 2253 of the 2381 selected trials. Trials with non-industrial primary sponsors recruited more often in low-income countries than trials with industrial primary sponsors (odds ratio, OR: ∞; Z = 2.0; P = 0.0464), whereas trials with industrial primary sponsors recruited more often in lower-middle-income (OR: 4.0; Z = 7.2; P < 0.0001), upper-middle-income (OR: 2.0; Z = 5.0; P < 0.0001) and high-income countries (OR: 2.2; Z = 4.0; P = 0.0001) (Table 4). Trials with industrial primary sponsors were more likely to have multi-country recruitment [222 (44.8%) of 495] than trials with non-industrial primary sponsors [73 (4.1%) of 1758] (OR: 18.8; Z = 23.7; P < 0.0001).
Table 4. Types of primary sponsor for a 5% sample of trials from the International Clinical Trials Registry Platform, 2012.
| Area of recruitmenta | No. (%) of trials with non-industrial sponsor | No. (%) of trials with industrial sponsor |
|---|---|---|
| High-income country | 1550 (88.0) | 467 (94.3) |
| Upper-middle-income country | 183 (10.4) | 93 (18.8) |
| Lower-middle-income country | 49 (2.8) | 51 (10.3) |
| Low-income country | 14 (0.8) | 0 (0.0) |
| All | 1758 (100) | 495 (100) |
a Categorized according to the World Bank income groupings.21 When summed, the percentages shown for income groups or regions exceed 100% because some trials were recruiting in multiple countries belonging to more than one income group.
Discussion
The global monitoring of health R&D requires analyses of the inputs (e.g. investments),2,5,6 processes (e.g. analyses of the R&D “pipeline”)26,27 and outputs (e.g. publications28 or products such as medicines)4 of R&D. Such “triangulation” of different sources of information is essential if we are to obtain a complete picture of what health R&D is being conducted, where and how it is being conducted, and who is conducting it. The increasing public availability of information on clinical trials provides an additional source of information for analysing current processes in health R&D at global, regional or country levels. Evaluations of registered trial data have recently been used to shed light on national clinical trial portfolios29,30 and specific research areas.31–34 This type of evaluation has several strengths: all trials should be registered, even if their final results are never published; registered records contain information that is complementary to that in any published articles on the trials;35 databases of registered trials can provide insight into currently ongoing R&D; and their standardized and searchable format makes databases of registered trials suitable for aggregate analysis.36 For the purpose of obtaining a comprehensive global picture of all ongoing clinical trials, the ICTRP is an unmatched resource of information since it provides access to data from all of the major clinical trial registries around the world that meet the relevant standards of WHO’s registry criteria.37
The results of this study show that, at least on a global scale, there is little correlation between the burden of disease attributable to a particular health condition or problem and the amount of clinical trial research being conducted on that health problem. This finding confirms the mismatch – between health R&D need and relevant health R&D – that has previously been observed using alternative R&D metrics, such as R&D investments and R&D outputs.1–4,6,33,38 A consequence of this mismatch is the existence of several populations that are neglected with respect to health R&D.3 In particular, health R&D currently does not adequately meet the needs of populations in lower-income countries.3,39 In general, communicable, maternal, perinatal and nutritional conditions – which cause a much higher proportion of the burden of disease in lower-income countries than in high-income countries20 – currently receive much less attention, in terms of clinical trial research, than noncommunicable diseases. In addition, clinical trials recruit much less often in lower-income countries than in higher-income countries. For health conditions or problems that cause a large burden in both lower- and higher-income countries, it is important that populations in lower-income countries be included in clinical trial research so that their specific R&D needs can be addressed.3
There are several limitations in using registered trial data for identifying gaps in the health R&D “landscape”. No account is taken of research other than that conducted within the context of a clinical trial. Since a registry for systematic reviews has recently been established40 and the creation of a registry for observational research has been widely advocated,41,42 evaluations of the health R&D “landscape” may soon broaden in scope. Another potential data source could be a registry (or database) of research protocols or even raw datasets43, although the information in such a registry would be much more difficult to analyse than the registered records of clinical trials.
The need for clinical trial research on a given health problem – or the perceived need for such research – is only partly determined by the burden of disease posed by the problem. The severity of the corresponding product shortfall, the state of the relevant science and technology and disease trends can also affect the need for clinical trial research.6,44 In other words, the need for R&D will be relatively high for diseases for which effective product development has been scant and for emerging diseases, diseases posing increasing burdens and diseases on course for eradication, whereas clinical trials may be considered premature if basic science is lacking in new research areas. Caution is therefore warranted in interpreting the correlation – or lack of correlation – between the number of clinical trials conducted on a particular disease and the burden posed by that disease. The main strength of the findings of the present study lies in the general, global trends that the findings reveal. For more specific conclusions about individual diseases, registered trial data will have to be analysed alongside other sources of information.
To date, very little reliable information has been produced on how much clinical trial research is being conducted in lower-income countries.45 Although the present results help to fill this knowledge gap, it is important to note that the registration of trials has not been enforced equally around the world. Many countries still have no legislation to enforce registration12 and not all journals in which clinical-trial data could be published are covered by the journal associations that have committed to enforcing trial registration.9,13 Furthermore, not all clinical trials are conducted with the goal of publication. It is difficult to verify or even estimate how many clinical trials remain unregistered, although it seems likely that at least some trials are never registered, especially in countries where there is no legal requirement for registration.30,46,47 Given that all major medical journals now require evidence of trial registration, as a condition for publication of any data from a trial, and that all studies that assess the effects of new medicines – for which regulatory approval is to be sought internationally – need to be registered, the quality and potential impact of any unregistered trials are questionable. Nonetheless, it is crucial that clinical-trial registration is enforced in every country, by means of national legislation and/or by ethical review boards, to ensure that a complete picture of the global distribution of clinical-trial research can be obtained.11,12,48
Before full use can be made of the ICTRP for exploring the health R&D “landscape”, several other limitations need to be addressed. First, even in those countries that have legislation on the registration of clinical trials, enforced registration is often limited to trials of drugs and – sometimes – devices, phase II–IV trials, and trials that recruit subjects in the country where the legislation is implemented.49 This problem has been recognized in the United States of America, where new legislation to ensure that all clinical trials of interventions are registered has been proposed.50 There also remain concerns about the quality of the data entered into the registered records of clinical trials10,51,52 and about problems with the unique identification of trials, which can lead to duplicate registration.19
Finally, the extraction, aggregation and analysis of the data in the ICTRP database currently require substantial manual labour. The formats of some of the data items differ across the registries covered by the ICTRP, which makes the automated aggregate analysis of data impossible. To remedy this limitation, the staff of the ICTRP are working with individual registries to harmonize the data recording formats across all of the registries that are covered by the platform. An alternative solution would be the development of algorithms to translate the variable information from individual registries into a common format and then classify the information into meaningful categories. ClinicalTrials.gov, one of the registries that provide data to the ICTRP, has already shown that the development of such data classification algorithms is feasible.29,53 Developing similar aggregation algorithms for the ICTRP – and making both the aggregated data and the results of the analysis of those data publicly available – would be an important step forward not only for the ICTRP but also for clinical trial transparency on a global scale.29
In conclusion, this study shows that WHO’s ICTRP constitutes a valuable resource for assessing the global distribution of clinical trials and for informing policy development and priority setting for health R&D. The findings of this study demonstrate that there is little correlation between burden of disease and the global distribution of clinical trial research and that populations in lower-income countries receive much less attention, in terms of clinical trial research, than populations in high-income countries. A more detailed understanding of the global health R&D “landscape” is needed to inform future R&D priorities. The ICTRP is one of several resources of information that will need to be “triangulated” to acquire a complete picture of what health R&D is being conducted, where and how it is being conducted, and who is conducting it. The ICTRP would constitute an essential part of any global observatory on health R&D.39 To increase the usefulness of the ICTRP further, it is important that the enforcement of clinical trial registration be increased, that the quality of the data in registered records be improved and that more possibilities for automated aggregate data analysis on the ICTRP be created.
Acknowledgements
We thank Colin Mathers from the World Health Organization for his help in collecting the burden-of-disease data used for this study. RFV has a dual appointment with the Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, London, England.
Competing interests:
None declared.
References
- 1.Commission on Health Research for Development. Health research: essential link to equity in development New York: Oxford University Press; 1990. [Google Scholar]
- 2.The 10/90 report on health research 2003–2004 Geneva: Global Forum for Health Research; 2004. [Google Scholar]
- 3.Moon S, Bermudez J, ’t Hoen E. Innovation and access to medicines for neglected populations: could a treaty address a broken pharmaceutical R&D system? PLoS Med. 2012;9:e1001218. doi: 10.1371/journal.pmed.1001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirac P, Torreele E. Global framework on essential health R&D. Lancet. 2006;367:1560–1. doi: 10.1016/S0140-6736(06)68672-8. [DOI] [PubMed] [Google Scholar]
- 5.Burke MA, Matlin SA. Monitoring financial flows for health research 2008: prioritizing research for health equity Geneva: Global Forum for Health Research; 2008. [Google Scholar]
- 6.Moran M, Guzman J, Ropars A-L, McDonald A, Sturm T, Jameson N et al. Neglected disease research & development: how much are we really spending? Sydney: George Institute for International Health; 2009. Available from: http://www.policycures.org/downloads/G-FINDER_survey_of_global_R&D_funding_for_Neglected_diseases_2008.pdf [accessed 13 February 2013].
- 7.Viergever RF. Aid alignment for global health research: the role of HIROs. Health Res Policy Syst. 2011;9:12. doi: 10.1186/1478-4505-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry RF, Allen L, Gardner CA, Guzman J, Moran M, Viergever RF. Mapping global health research investments, time for new thinking – a Babel Fish for research data. Health Res Policy Syst. 2012;10:28. doi: 10.1186/1478-4505-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Philadelphia: International Committee of Medical Journal Editors; 2004. Available from: http://www.icmje.org/clin_trial.pdf [accessed 31 August 2012].
- 10.Viergever RF, Ghersi D. The quality of registration of clinical trials. PLoS One. 2011;6:e14701. doi: 10.1371/journal.pone.0014701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghersi D, Pang T. En route to international clinical trial transparency. Lancet. 2008;372:1531–2. doi: 10.1016/S0140-6736(08)61635-9. [DOI] [PubMed] [Google Scholar]
- 12.Bian Z-X, Wu T-X. Legislation for trial registration and data transparency. Trials. 2010;11:64. doi: 10.1186/1745-6215-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The registration of clinical trials. Dallas: World Association of Medical Editors; 2005. Available from: http://www.wame.org/resources/policies#trialreg [accessed 28 March 2013].
- 14.Drazen JM, Zarin DA. Salvation by registration. N Engl J Med. 2007;356:184–5. doi: 10.1056/NEJMe068291. [DOI] [PubMed] [Google Scholar]
- 15.Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, et al. Clinical trial registration: looking back and moving ahead. Lancet. 2007;369:1909–11. doi: 10.1016/S0140-6736(07)60894-0. [DOI] [PubMed] [Google Scholar]
- 16.Joint position on the disclosure of clinical trial information via clinical trial registries and databases Geneva: International Federation of Pharmaceutical Manufacturers and Associations; 2009. Available from: http://clinicaltrials.ifpma.org/clinicaltrials/fileadmin/files/pdfs/EN/November_10_2009_Updated_Joint_Position_on_the_Disclosure_of_Clinical_Trial_Information_via_Clinical_Trial_Registries_and_Databases.pdf [accessed 28 March 2013].
- 17.Resolução da Diretoria Colegiada – RDC no 39, de 05 de Junho de 2008 Brasilia: Agência Nacional de Vigilância Sanitária; 2008. Available from: http://www.fcm.unicamp.br/centros/cpc/index.php?option=com_docman&task=doc_download&gid=11&Itemid=165&ei=FdUaUZb2Oemf0QXr2YGwAQ&usg=AFQjCNE2XZf9UUm4cAO_6NCKryUq7ncyoQ&bvm=bv.42261806,d.d2k&cad=rja [accessed 6 March 2013].
- 18.World Health Organization [Internet]. International Clinical Trials Registry Platform (ICTRP): the WHO Registry Network. Geneva: WHO; 2012. Available from: http://www.who.int/ictrp/network/en/ [accessed 28 March 2013].
- 19.World Health Organization [Internet]. International Clinical Trials Registry Platform (ICTRP): unambiguous identification. Geneva: WHO; 2012. Available from: http://www.who.int/ictrp/unambiguous_identification/en/ [accessed 28 March 2013].
- 20.The global burden of disease: 2004 update Geneva: World Health Organization; 2008. [Google Scholar]
- 21.The World Bank [Internet]. Country and lending groups. Washington: WB; 2012. Available from: http://data.worldbank.org/about/country-classifications/country-and-lending-groups [accessed 28 March 2013].
- 22.World Health Organization [Internet]. Definition of region groupings. Geneva: WHO; 2012. Available from: http://www.who.int/healthinfo/global_burden_disease/definition_regions/en/index.html [accessed 28 March 2013].
- 23.Wallis S. Binomial confidence intervals and contingency tests: mathematical fundamentals and the evaluation of alternative methods London: University College London; 2012. Available from: http://www.ucl.ac.uk/english-usage/staff/sean/resources/binomialpoisson.pdf [accessed 28 March 2013].
- 24.World Health Organization [Internet]. Global burden of disease (GBD). Geneva: WHO; 2012. Available from:http://http://www.who.int/healthinfo/global_burden_disease/en/index.html [accessed 28 March 2013].
- 25.The World Bank [Internet]. The World Bank’s comprehensive database of health, nutrition and population (HNP) statistics: population projections. Washington: WB; 2013. Available from: http://go.worldbank.org/H4UN4D5KI0http://[accessed 28 March 2013].
- 26.Saving lives and creating impact: why investing in global health research works. Sydney: Policy Cures; 2012. Available from: http://www.ghtcoalition.org/files/Savinglivesandcreatingimpact.pdf [accessed 28 March 2013].
- 27.Moran M, Guzman J, Ropars A-L, Jorgensen M, McDonald A, Potter S et al. The malaria product pipeline: planning for the future Sydney: George Institute for International Health; 2008. [Google Scholar]
- 28.Rohra DK. Representation of less-developed countries in pharmacology journals: an online survey of corresponding authors. BMC Med Res Methodol. 2011;11:60. doi: 10.1186/1471-2288-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307:1838–47. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 30.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–23. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 31.Viergever RF, Rademaker CMA, Ghersi D. Pharmacokinetic research in children: an analysis of registered records of clinical trials. BMJ Open. 2011;1:e000221. doi: 10.1136/bmjopen-2011-000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culme-Seymour EJ, Davie NL, Brindley DA, Edwards-Parton S, Mason C. A decade of cell therapy clinical trials (2000–2010). Regen Med. 2012;7:455–62. doi: 10.2217/rme.12.45. [DOI] [PubMed] [Google Scholar]
- 33.Nwaka S, Ilunga TB, Da Silva JS, Rial Verde E, Hackley D, De Vré R, et al. Developing ANDI: a novel approach to health product R&D in Africa. PLoS Med. 2010;7:e1000293. doi: 10.1371/journal.pmed.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourgeois FT, Murthy S, Pinto C, Olson KL, Ioannidis JPA, Mandl KD. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130:285–92. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wieseler B, Kerekes MF, Vervoelgyi V, McGauran N, Kaiser T. Impact of document type on reporting quality of clinical drug trials: a comparison of registry reports, clinical study reports, and journal publications. BMJ. 2012;344(jan03 1):d8141. doi: 10.1136/bmj.d8141. [DOI] [PubMed] [Google Scholar]
- 36.Chan A-W. Out of sight but not out of mind: how to search for unpublished clinical trial evidence. BMJ. 2012;344(jan03 2):d8013. doi: 10.1136/bmj.d8013. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. International Clinical Trials Registry Platform (ICTRP).About registries Geneva: WHO; 2012. Available from: http://www.who.int/ictrp/network/criteria_summary/en/index.html [accessed 28 March 2013].
- 38.Investing in health research and development. Report of the Ad Hoc Committee on Health Research Relating to Future Intervention Options Geneva: World Health Organization; 1996. Available from http://apps.who.int/iris/handle/10665/63024?mode=simple&submit_simple=Show+simple+item+record [accessed 28 March 2013].
- 39.Research and development to meet health needs in developing countries: strengthening global financing and coordination. Report of the Consultative Expert Working Group on Research and Development: Financing and Coordination Geneva: World Health Organization; 2012. Available from: http://www.who.int/phi/cewg_report/en/index.html [accessed 28 March 2013].
- 40.Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M , et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. doi: 10.1186/2046-4053-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loder E, Groves T, Macauley D. Registration of observational studies. BMJ. 2010;340:c950. doi: 10.1136/bmj.c950. [DOI] [PubMed] [Google Scholar]
- 42.Williams RJ, Tse T, Harlan WR, Zarin DA. Registration of observational studies: is it time? CMAJ. 2010;182:1638–42. doi: 10.1136/bmj.c950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehman R, Loder E. Missing clinical trial data. BMJ. 2012;344:d8158. doi: 10.1136/bmj.d8158. [DOI] [PubMed] [Google Scholar]
- 44.Viergever RF, Olifson S, Ghaffar A, Terry RF. A checklist for health research priority setting: nine common themes of good practice. Health Res Policy Syst. 2010;8:36. doi: 10.1186/1478-4505-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A bitter pill: the risks of carrying out clinical drug trials in developing countries Amsterdam: Wemos Foundation; 2007. [Google Scholar]
- 46.Patrone D. Discrepancies between research advertisements and disclosure of study locations in trial registrations for USA-sponsored research in Russia. J Med Ethics. 2010;36:431–4. doi: 10.1136/jme.2010.035378. [DOI] [PubMed] [Google Scholar]
- 47.Reveiz L, Bonfill X, Glujovsky D, Pinzon CE, Asenjo-Lobos C, Cortes M, et al. Trial registration in Latin America and the Caribbean’s: study of randomized trials published in 2010. J Clin Epidemiol. 2012;65:482–7. doi: 10.1371/journal.pone.0033677. [DOI] [PubMed] [Google Scholar]
- 48.Dickersin K, Rennie D. Registering clinical trials. JAMA. 2003;290:516–23. doi: 10.1001/jama.290.4.516. [DOI] [PubMed] [Google Scholar]
- 49.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database – update and key issues. N Engl J Med. 2011;364:852–60. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drazen JM. Transparency for clinical trials – the TEST act. N Engl J Med. 2012;367:863–4. doi: 10.1056/NEJMe1209433. [DOI] [PubMed] [Google Scholar]
- 51.Reveiz L, Chan A-W, Krleza-Jerić K, Granados CE, Pinart M, Etxeandia I, et al. Reporting of methodologic information on trial registries for quality assessment: a study of trial records retrieved from the WHO search portal. PLoS One. 2010;5:e12484. doi: 10.1371/journal.pone.0012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viergever RF, Ghersi D. Information on blinding in registered records of clinical trials. Trials. 2012;13:210. doi: 10.1186/1745-6215-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tasneem A, Aberle L, Ananth H, Chakraborty S, Chiswell K, McCourt BJ, et al. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS One. 2012;7:e33677. doi: 10.1371/journal.pone.0033677. [DOI] [PMC free article] [PubMed] [Google Scholar]