Abstract
Age-related resistance (ARR) is a plant defense response characterized by enhanced resistance to certain pathogens in mature plants relative to young plants. In Arabidopsis thaliana the transition to flowering is associated with ARR competence, suggesting that this developmental event is the switch that initiates ARR competence in mature plants (Rusterucci et al. in Physiol Mol Plant Pathol 66:222–231, 2005). The association of ARR and the floral transition was examined using flowering-time mutants and photoperiod-induced flowering to separate flowering from other developmental events that occur as plants age. Under short-day conditions, late-flowering plant lines ld-1 (luminidependens-1), soc1-2 (suppressor of overexpression of co 1-2), and FRI + (FRIGIDA) displayed ARR before the transition to flowering occurred. Early-flowering svp-31, svp-32 (short vegetative phase), and Ws-2 were ARR-defective, whereas early-flowering tfl1-14 (terminal flower 1-14) displayed ARR at the same time as Col-0. While svp-31, svp-32 and Ws-2 produced few rosette leaves, tfl1-14 produced a rosette leaf number similar to Col-0, suggesting that the development of a minimum number of rosette leaves is necessary to initiate ARR competence under short-day conditions. Photoperiod-induced transient expression of FT (FLOWERING LOCUS T) caused precocious flowering in short-day-grown Col-0 but this was not associated with ARR competence. Under long-day conditions co-9 (constans-9) mutants did not flower but displayed an ARR response at the same time as Col-0. This study suggests that SVP is required for the ARR response and that the floral transition is not the developmental event that regulates ARR competence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11103-013-0083-7) contains supplementary material, which is available to authorized users.
Keywords: Arabidopsis, Pseudomonas syringae, Age-related resistance, Developmental resistance, Flowering, Photoperiod
Introduction
The outcome of a plant-pathogen interaction often depends on the developmental stage of the plant (Agrios 2005). Under short-day conditions (9 h light), young (3- to 4-week-old) Arabidopsis are susceptible to the bacterial pathogen Pseudomonas syringae pv. tomato (Pst), as indicated by the presence of disease symptoms and high in planta bacterial growth. In contrast, mature plants (>5 weeks old) are typically asymptomatic and show a 10- to 100-fold reduction in bacterial growth (Kus et al. 2002). Arabidopsis age-related resistance (ARR) also confers protection against the oomycete Hyaloperonospora arabidopsidis (Rusterucci et al. 2005). ARR has been observed in many plant species and the mechanisms involved appear to differ widely (Reviewed in Develey-Rivière and Galiana 2007; Whalen 2005). For example, tobacco (Nicotiana tabacum) develops enhanced resistance to Phytopthora parasitica during the transition to flowering. Enhanced resistance in reproductive-stage tobacco plants is associated with PATHOGENESIS-RELATED 1 (PR1) accumulation and cytotoxic activity in the apoplast (Hugot et al. 1999). In rice (Oryza sativa), the onset of developmentally regulated resistance to Xanthomonas oryzae pv. oryzae occurs during the vegetative phase (Mazzola et al. 1994). In part this involves the interaction of the rice Xa-21 resistance gene product with the X. oryzae Ax21 effector (Lee et al. 2009; Mazzola et al. 1994).
Arabidopsis salicylic acid (SA) accumulation mutants such as sid2 (salicylic acid induction deficient 2), eds1 (enhanced disease susceptibility 1), eds5/sid1, and pad4 (phytoalexin deficient 4) are defective for the ARR response (Cameron and Zaton 2004; Carviel et al. 2009; Kus et al. 2002) indicating that SA accumulation is important during ARR. In addition, the ARR-defective iap1-1 (important for the ARR pathway 1-1) mutant accumulates little SA in response to Pst (Carviel et al. 2009). The role of SA in defense signaling is well-documented (Reviewed in Vlot et al. 2009), however, the SA-signaling mutant npr1-1 (non-expressor of PR1) shows a wild-type ARR response suggesting that SA may not play a conventional defense-signaling role during ARR (Kus et al. 2002). Moreover, in plants undergoing an ARR response SA accumulates in the intercellular space of leaves (Cameron and Zaton 2004). Based on these data we propose that SA acts as an antimicrobial agent during ARR. Consistent with this hypothesis, it was shown that intercellular washing fluids of mature plants undergoing ARR, as well as purified SA, have an antimicrobial effect on Pst in vitro (Cameron and Zaton 2004).
As a facultative long-day plant, Arabidopsis flowers later in short days than in long days (Gregory and Hussey 1953). We previously observed that in both short- and long-day-grown Col-0, ARR onset is associated with the floral transition at approximately 6 weeks post-germination (wpg) in short days and four wpg in long days (Rusterucci et al. 2005). Several studies indicate that regulatory elements are shared between disease resistance and flowering pathways in Arabidopsis, including SA, which in addition to its role in disease resistance, also plays a role in flowering-time control (Reviewed in Rivas-San Vicente and Plasencia 2011). For example, evidence suggests that the SUMO and ubiquitin E3 ligases SIZ1 and PUB13 modify proteins that affect SA-related defense responses and flowering-time (Jin et al. 2008; Lee et al. 2006; Liu et al. 2012). Moreover, Wang et al. (2011) found that the putative acetylornithine transaminase encoded by WIN3 acts together with other SA regulatory proteins such as NPR1 to control cell death, disease resistance, and flowering time. SA accumulation mutants such as sid2 have been observed to flower later than wild type and also produce greater rosette leaf biomass and seed yield (Abreu and Munné-Bosch 2009; Martínez et al. 2004). However, this has not been observed for the ARR-defective and SA accumulation-deficient mutant iap1-1 (Carviel et al. 2009).
The timing of the floral transition is highly regulated and is controlled by several major pathways that respond to environmental and endogenous stimuli. These pathways converge on a group of integrator genes that regulate the floral meristem-identity genes responsible for floral organ development at the shoot apical meristem (SAM). Figure 1 is a schematic diagram of flowering-time regulation in Arabidopsis that is limited to the flowering-time genes that are relevant to this study. Environmental cues that affect flowering include day length (photoperiod pathway), prolonged periods of cold (vernalization pathway), and ambient temperature. Other pathways respond to endogenous stimuli, for example, the autonomous, gibberellin, and ageing pathways (Reviewed in Amasino 2010; Simpson and Dean 2002).
Fig. 1.
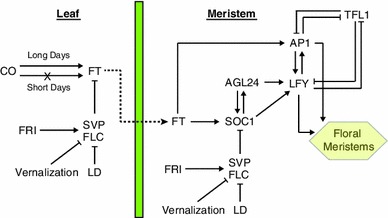
Simplified representation of flowering-time regulation adapted from Amasino (2010) and Fornara et al. (2010). The FLC-SVP complex represses FT in the leaf and SOC1 in the meristem and is regulated by FRI, vernalization, and the components of the autonomous pathway (e.g., LD). In short days FT expression remains low, whereas in long days FT is upregulated by CO and FT protein moves via the phloem (dashed line) from the leaf to the shoot apical meristem where it directly upregulates SOC1 and AP1. SOC1 upregulates AGL24 and LFY. LFY and AP1 are responsible for the production of floral meristems and are repressed by TFL1
FLOWERING LOCUS T (FT) is a floral integrator that links day-length perception in the leaves to floral induction at the SAM. During long days, the CONSTANS (CO) transcription factor accumulates in the phloem companion cells of leaves and upregulates FT transcription (An et al. 2004; Ayre and Turgeon 2004; Suárez-López et al. 2001; Yanovsky and Kay 2002). FT moves from the leaves via the phloem to the SAM (Corbesier et al. 2007; Jaeger and Wigge 2007) where it upregulates another floral integrator SUPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1) (Michaels et al. 2005; Yoo et al. 2005) and the floral meristem-identity gene APETALA 1 (AP1) (Abe et al. 2005; Wigge et al. 2005). SOC1 participates in a positive feedback loop with AGAMOUS-LIKE 24 (AGL24) to up-regulate the LEAFY (LFY) floral meristem identity gene (Lee et al. 2008; Liu et al. 2008).
AP1 and LFY are involved in the production of floral meristems (Weigel et al. 1992, Irish and Sussex 1990) and are repressed by TERMINAL FLOWER 1 (TFL1), which is responsible for maintenance of the indeterminate inflorescence meristem (Alvarez et al. 1992; Liljegren et al. 1999; Schultz and Haughn 1993; Shannon and Meeks-Wagner 1991; Shannon and Meeks-Wagner 1993). Evidently, TFL1 also represses flowering in vegetative plants since tlf1 mutants flower early compared to wild type (Schultz and Haughn 1993; Shannon and Meeks-Wagner 1991). FT and SOC1 also incorporate signals from the vernalization and autonomous flowering pathways. Arabidopsis accessions possessing a dominant FRIGIDA (FRI) allele usually require a vernalization treatment before they become competent to flower (Lee and Amasino 1995; Lee et al. 1993). FRI confers a vernalization requirement by upregulating the floral repressor and MADS-box transcription factor FLOWERING LOCUS C (FLC) (Michaels and Amasino 1999). FLC forms a high-molecular-weight complex with another MADS-box transcription factor SHORT VEGETATIVE PHASE (SVP), and represses flowering by directly binding to regulatory regions of FT and SOC1 in both leaf and SAM tissue (Helliwell et al. 2006; Lee et al. 2007; Li et al. 2008; Searle et al. 2006). Vernalization confers reproductive competence by derepressing SOC1 and FT through epigenetic silencing of FLC (Bastow et al. 2004). Autonomous pathway mutants such as ld-1 (luminidependens-1) are late flowering and exhibit increased expression of FLC and SVP, suggesting that the autonomous pathway is responsible for controlling the levels of FLC and SVP (Li et al. 2008; Michaels and Amasino 1999; Michaels and Amasino 2001; Sheldon et al. 1999).
The aim of this study is to determine whether the floral transition plays a role in regulating ARR competence. To do this we asked whether the association between flowering and ARR is maintained in flowering-time mutants. To examine the role of photoperiod-induced flowering in ARR onset we separated photoperiod-induced flowering from long-day growth conditions. Our results suggest that in both short- and long-day conditions, flowering is not the developmental cue that initiates ARR competence. We present evidence that SVP is required for ARR and propose that in short-day conditions the development of a minimum number of rosette leaves is necessary to initiate ARR competence.
Materials and methods
Plant material and growth conditions
Wild-type Columbia (Col-0) and Wassilewskija (Ws-2) accessions were used. All mutants used in this study were in the Columbia background. Mutants that were previously confirmed to be ARR-defective were sid2-1 (C. Nawrath, University of Fribourg, Fribourg, Switzerland) and iap1-1 (described in Carviel et al. 2009). Flowering-time mutants ld-1 (CS3127), svp-31 (SALK_026551C), and tfl1-14 (CS6238) were obtained from the Arabidopsis Biological Resource Centre, Ohio State University, Columbus OH, USA (Alonso et al. 2003). co-9, ft-10, FRI +, and FRI + flc-3 were supplied by R. Amasino (University of Wisconsin-Madison, WI, USA). soc1-2 was supplied by I. Lee (Seoul National University, Seoul, Korea). 35S:miR156 was obtained from S. Poethig (University of Pennsylvania, PA, USA). svp-32 (SALK_072930) was obtained from J. H. Ahn (Korea University, Seoul, Korea; Lee et al. 2007). Seeds were surface-sterilized and stratified at 4 °C for 2 days before sowing on MS media where they germinated under constant light at 22 °C. Seedlings were transplanted to soil (Sunshine Mix #1) hydrated with 1 g L−1 20–20–20 all-purpose fertilizer approximately 1 week later. Plants were grown in short days unless otherwise specified. Short days consisted of 9 h light, and long days consisted of 16 h light. Light intensity was maintained at approximately 150 μE m−2 s−1 and temperature at 23 °C. Short-day growth chambers had added humidity (75–85 % relative humidity) whereas the long-day chamber did not (50–70 % relative humidity). Rosette leaves that were large enough to be resolved without magnification were scored to determine rosette leaf number.
Bacterial growth, inoculation, and quantification
Virulent P. syringae pv. tomato (Pst) strain DC3000 (pVSP61) was used in all experiments (A. Bent, University of Wisconsin-Madison, WI, USA). Bacteria were cultured in King’s B media with shaking at room temperature to exponential phase (OD600 = 0.2–0.6) and then diluted to 106 colony forming units ml−1 in 10 mM MgCl2. Inoculum was pressure-infiltrated into the abaxial side of leaves using a needle-less syringe. Isolation and quantification of Pst at 3 days post-inoculation was performed as described previously (Kus et al. 2002).
Analysis of gene expression by RT-PCR
Leaf tissue was harvested in the evening (end of photoperiod), flash-frozen in liquid nitrogen, and stored at −80 °C until further use. RNA was isolated using Sigma TRI Reagent according to the manufacturer’s instructions. Residual DNA was degraded using TURBO DNase (Life Technologies) prior to RNA quantification. First-strand cDNA synthesis was carried out using SuperScript III reverse transcriptase (Life Technologies). PCR primers used to amplify FT transcripts were: 5′-TAAGCAGAGTTGTTGGAGACG and 5′-TCTAAAGTCTTCTTCCTCCGCAG (Jang et al. 2009). Primers used to amplify ACTIN1 transcripts were: 5′-GGCGATGAAGCTCAATCCAAACG and 5′-GGTCACGACCAGCAAGATCAAGACG. Twenty-eight PCR cycles were used for both FT and ACTIN1.
Statistical analysis
Statistically significant differences in bacterial densities and average rosette leaf numbers were determined by ANOVA. To account for unequal variance in the means the bacterial density data were transformed prior to analysis (log or square root transformation). Tukey’s HSD post hoc test was used for pair-wise comparisons (p < 0.01). All tests were performed using IBM SPSS Statistics 20.
Results
ARR onset does not coincide with the floral transition in early- and late-flowering plant lines
If the transition to flowering acts as a developmental cue to initiate ARR competence, we should observe delayed ARR onset in late-flowering mutants and early ARR onset in early-flowering mutants. To test this hypothesis, wild-type Col-0, early-flowering svp-31 (Hartmann et al. 2000), late-flowering ld-1 (Rèdei 1962), and the early-flowering Ws-2 accession (Giakountis et al. 2010) were analyzed. In planta bacterial levels were monitored from 3 to 9 weeks post germination (wpg) by inoculating with virulent Pst (106 cfu ml−1) followed by isolation and quantification of in planta bacteria 3 days later. The transition to flowering was approximated by counting the percentage of plants with visible inflorescence stems each week. In this experiment Col-0 flowered earlier than typically observed when grown under short-day conditions, such that 48 % of plants had visible inflorescence stems at four wpg, 71 % at five wpg, and 76 % at six wpg (Fig. 2a), instead of 0 % at 4 wpg, 5 % at 5 wpg and 33 % at 6 wpg (Fig. 2b). A power outage that interrupted the photoperiod regimen in week 3 exposed the plants to a displaced short day. Displaced short days have been shown to cause early flowering in Arabidopsis (Corbesier et al. 1996). While ld-1 did not produce inflorescence stems during the experiment, 95 % of svp-31 produced inflorescence stems by three wpg. Ws-2 also made the transition to flowering earlier than Col-0, with 58 % of plants showing inflorescence stems at three wpg, and 95 % at four wpg.
Fig. 2.
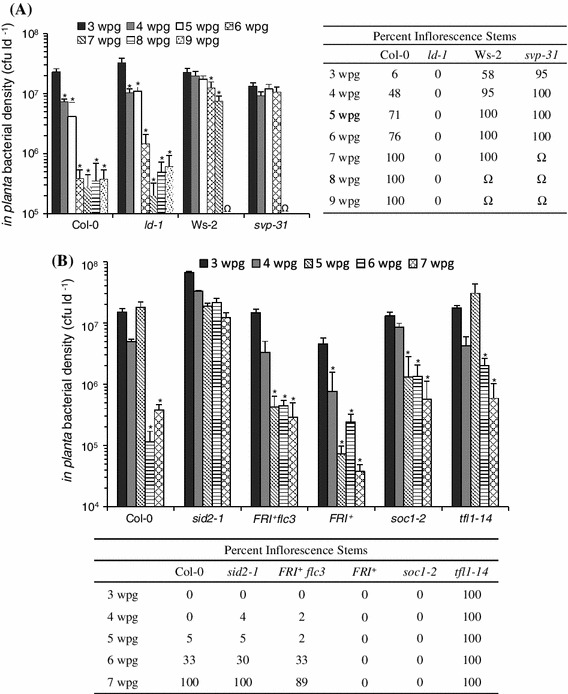
ARR responses of various flowering-time mutants. a Col-0, ld-1, Ws-2 and svp-31 were grown in short days and tested for ARR each week between three and nine wpg. Plants were inoculated with 106 cfu ml−1 virulent Pst (DC3000) and bacterial levels were quantified 3 days later. Data are presented as the mean of three biological replicates. Error bars indicate standard deviation. Asterisk denotes significant differences relative to three wpg plants of the same genotype according to Tukey’s HSD (p < 0.01). Each week at least 12 plants of each genotype were assessed for visible inflorescence stems. Values represent the percentage of plants with visible inflorescence stems. Ω indicates the onset of senescence at which point further testing was not possible. Each genotype was tested at least three times with similar results b Col-0, sid2-1, FRI + flc-3, FRI +, soc1-2, and tfl1-14 were grown in short days and tested for ARR each week between three and seven wpg. Plants were inoculated with 106 cfu ml−1 virulent Pst (DC3000) and bacterial levels were quantified 3 days later. Data are presented as the mean of three biological replicates. Error bars indicate standard deviation. Asterisk denotes significant differences relative to three wpg plants of the same genotype according to Tukey’s HSD (p < 0.01). Each week at least 12 plants of each genotype were assessed for visible inflorescence stems. Values represent the percentage of plants with visible inflorescence stems. This experiment was performed twice with similar results
Col-0 became increasingly resistant to Pst between three and six wpg (Fig. 2a). Young (three wpg) plants supported high levels of Pst (2.3 × 107 cfu ld−1), while 4- and 5-week-old plants supported modestly reduced levels (7.4 × 106 and 4.1 × 106 cfu ld−1) and mature plants (six to nine wpg) supported low levels of Pst (<4.0 × 105 cfu ld−1). There was a 60-fold decrease in Pst between 3- and 6-week-old plants, indicating that Col-0 was fully ARR-competent at six wpg. Late-flowering ld-1 supported Pst levels similar to Col-0 between three and five wpg. At six wpg ld-1 Pst levels dropped to 1.5 × 106 cfu ld−1 (22-fold less than at three wpg) indicative of moderate ARR. At seven, eight, and nine wpg ld-1 displayed a robust ARR response (97-fold reduction in Pst levels between three and seven wpg). Early-flowering svp-31 supported high Pst densities (>1.0 × 107 cfu ld−1) from three to six wpg, remaining ARR-incompetent. At seven wpg, older svp-31 leaves began to senesce as indicated by yellowing and necrosis, therefore these plants were not tested beyond six wpg. A second mutant allele, svp-32, also flowered early and was found to be ARR-defective (Fig. S2). The early-flowering Ws-2 accession also supported high Pst densities (>7.0 × 106 cfu ld −1) at all ages.
Although somewhat delayed compared to Col-0, the ld-1 mutant displayed a robust ARR response even in the absence of flowering, suggesting that the floral transition is not required for ARR competence. In addition, early-flowering does not elicit an early ARR response since svp-31, svp-32 and Ws-2 flowered early but did not display ARR at the time of the floral transition or at any time thereafter. Therefore, the floral transition does not appear to act as a developmental cue for ARR competence.
Photoperiod-induced flowering does not elicit ARR competence
To support the hypothesis that the floral transition does not confer ARR competence we used short day/long day shift experiments to elicit precocious flowering in young, short-day-grown Col-0 followed by an assay for ARR competence. Brief exposure of short-day-grown plants to inductive (long-day) photoperiods activates the photoperiod pathway and initiates the transition to flowering (Corbesier et al. 2007; Imaizumi et al. 2003). Eliciting early flowering in wild-type plants has the advantage of avoiding possible pleiotropic effects of mutations in flowering-time genes. Col-0 was grown under three different photoperiod regimens and tested for ARR competence at four wpg. Photoperiod regimens consisted of either short days, long days, or short days plus three long days followed by return to short days (Fig. 3a). All long-day-grown Col-0 had visible inflorescence stems at four wpg, whereas short-day-grown and photoperiod-induced short-day-grown Col-0 did not. To determine whether photoperiod-induced short-day-grown plants had made the transition to flowering, RT-PCR was used to measure FT expression in leaf tissue taken at time points spanning the induction period (Fig. 3b). FT expression was detected in the leaves of photoperiod-induced short-day-grown Col-0 at the end of the third long day, indicating that the photoperiod pathway had been activated. FT expression was consistently detected in the leaves of long-day-grown Col-0 and was not detected in short-day-grown plants.
Fig. 3.
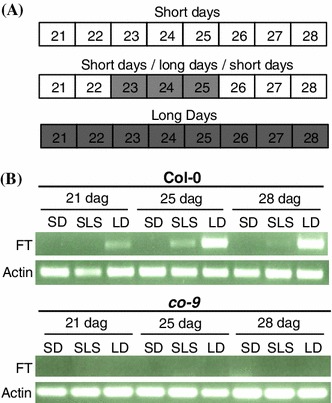
Short-day-grown plants express FT after exposure to three long days. a Schematic representation of the three photoperiod regimens. White bars indicate short days (9 h light), dark bars indicates long days (16 h light). Numbers indicate days after-germination. b FT and ACTIN expression measured by RT-PCR in leaf tissue of Col-0 and co-9 plants grown in short days (SD), short days/long days/short days (SLS), or long days (LD). Tissue was collected in the evening on 21, 25, and 28 days after-germination (dag)
Consistent with previous experiments (Rusterucci et al. 2005), at four wpg long-day-grown Col-0 supported few disease symptoms and low bacterial levels whereas short-day-grown Col-0 was susceptible, supporting 125-fold higher Pst levels than long-day-grown plants (Fig. 4). This indicates that long-day-grown Col-0 was ARR-competent at four wpg whereas short-day-grown plants were not. Photoperiod-induced short-day-grown Col-0 supported high Pst densities at four wpg (1.2 × 107 cfu ld−1), similar to short-day-grown Col-0 (1.4 × 107 cfu ld−1), therefore these plants were not competent for ARR at four wpg. This confirms our previous conclusion that the floral transition does not confer ARR competence.
Fig. 4.
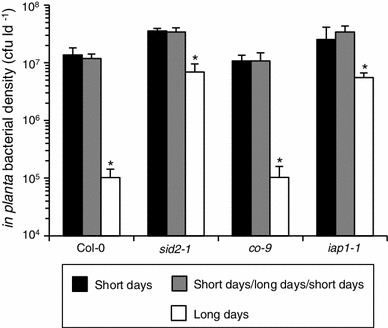
Photoperiod-induced flowering does not elicit ARR. Col-0, sid2-1, co-9, and iap1-1 were grown in short days, short days/long days/short days, or long days and were inoculated with 106 cfu ml−1 virulent Pst (DC3000) at four wpg. Bacterial levels were quantified 3 days later and are presented as the mean of three biological replicates. Error bars indicate standard deviation. Asterisk indicates significant differences as determined by ANOVA (Tukey’s HSD, p < 0.01). This experiment was performed twice with similar results
CONSTANS is not required for ARR in long days
Since ARR onset occurs earlier in long-day conditions we wanted to determine whether the transition to flowering, or a different developmental event accelerated in long days, elicits ARR competence. For example, the vegetative phase change from juvenile to adult vegetative stages occurs earlier in long-day-grown plants (Chien and Sussex 1996; Willmann and Poethig 2005) and could be associated with ARR competence. To separate flowering from other developmental changes that might act as a switch for ARR-competence in long days we tested co mutants which flower late in long days (Koornneef et al. 1991; Putterill et al. 1995) because FT is no longer up-regulated by CO in a photoperiod-dependent manner (Kardailsky et al. 1999; Kobayashi et al. 1999; Samach et al. 2000). If the transition to flowering is the cue for ARR competence in long days, then long-day-grown co mutants should have delayed ARR compared to Col-0 (ARR at four wpg). To test this hypothesis the co-9 mutant was grown in three different photoperiod regimens and tested for ARR competence at four wpg as described previously. In all three photoperiod regimens co-9 lacked inflorescence stems and detectable FT expression throughout the experiment (Fig. 3b), indicating that the photoperiod pathway was not activated. Short-day-grown and photoperiod-induced short-day-grown co-9 supported high Pst levels (1.1 × 107 cfu ld−1), whereas long-day-grown co-9 supported low levels of Pst (1.0 × 105 cfu ld−1; Fig. 4). A 110-fold decrease in Pst levels in long-day-grown compared to short-day-grown co-9 is indicative of a robust ARR response in long-day-grown plants. In long-day-grown co-9 mutants ARR occurs in the absence of flowering, demonstrating that photoperiod-induced flowering is not required for the establishment of ARR competence in long-day conditions.
ARR competence is associated with leaf number in short-day conditions
Our original hypothesis that the floral transition is the developmental cue for ARR competence was not supported, therefore other developmental events that might act as a switch to initiate ARR-competence were considered. The early-flowering plant lines (svp-31, svp-32, and Ws-2) were ARR-defective and produced few rosette leaves (Table 1; Figs. S1 and S2). The SAM switches from production of vegetative to reproductive structures during the transition to flowering, therefore the timing of the floral transition affects vegetative growth (rosette leaf number) such that early-flowering plant lines produce fewer rosette leaves than wild type (Hempel and Feldman 1994; Koornneef et al. 1991). It has been suggested that the timing of some developmental events may be influenced by rosette leaf number (McDaniel et al. 1992; Poethig 1990; Schultz and Haughn 1993) and given that the early-flowering plant lines examined thus far produced few rosette leaves and were ARR-defective, we hypothesized that the development of a minimum number of rosette leaves might initiate ARR competence. To assess whether ARR competence is associated with leaf number we analyzed the early-flowering tfl1-14 mutant (Schultz and Haughn 1993) because it produced more rosette leaves than svp-31, svp-32, and Ws-2 (Table 1; Figs. S1 and S2). In these experiments plants were assessed for ARR competence and average rosette leaf number between three and seven wpg.
Table 1.
ARR onset and leaf number of short-day-grown plants
| Flowering-time phenotypea | ARR onset | Average rosette leavesc at ARR onset | |
|---|---|---|---|
| ARR-competent plant lines | |||
| Col-0 | Wild-type | 6 wpg | 33.9 ± 2.8 |
| FRI + flc-3 | Wild-type | 5 wpg | 26.4 ± 2.8b |
| FRI + | Late | 5 wpg | 27.2 ± 1.3b |
| ld-1 | Late | 6 wpg | 32.7 ± 2.6 |
| soc1-2 | Late | 5 wpg | 27.0 ± 2.1b |
| tfl1-14 | Early | 6 wpg | 28.3 ± 1.7 |
| ARR-defective plant lines | |||
| Ws-2 | Early | na | 22.8 ± 4.3c,d |
| svp-31 | Early | na | 21.7 ± 3.8c,d |
ARR onset and leaf number for experiment presented in Fig. 2a, b. Average rosette leaves for Col-0 from experiment in Fig. 2b
aRelative to wild-type Col-0
bRosette leaf number is presented as the average ± standard deviation (n = 9)
cSignificantly different from Col-0 (ANOVA, Tukey’s HSD, p < 0.01)
dFor ARR-defective plant lines the maximum average rosette leaf number is presented
Col-0 made the transition to flowering between six and seven wpg (33 and 100 % inflorescence stems, respectively) and supported high Pst densities (≥5.0 × 106 cfu ld−1) between three and five wpg (Fig. 2b). At six wpg Pst levels dropped to 1.2 × 105 cfu ld−1, a 131-fold reduction compared to 3-week-old plants, indicative of a robust ARR response. Col-0 had a rosette leaf number of 33.9 ± 2.8 at six wpg (Table 1) for the experiment presented in Fig. 2b. Moreover, in five independent experiments, Col-0 produced an average of 34.7 ± 3.4 rosette leaves at ARR onset (6 wpg), making it possible to compare leaf number across experiments (Tables 1, 2). ARR-defective sid2-1 supported high bacterial densities at all ages (>1.0 × 107 cfu ld−1) and flowered at approximately the same time as Col-0 (30 and 100 % inflorescence stems at six and seven wpg respectively). At all ages sid2-1 had a rosette leaf number similar to Col-0 (Fig. S1). This suggests that sid2-1 is developmentally similar to Col-0 in terms of leaf number and is consistent with previous work suggesting that the sid2-1 ARR defect is due solely to its inability to accumulate SA (Cameron and Zaton 2004). The transition to flowering occurred prior to three wpg in tfl1-14, as 100 % of plants had inflorescence stems by this time (Fig. 2b). tfl1-14 supported high Pst levels at three and five wpg (>1 × 107 cfu ld−1) and a statistically insignificant decline at four wpg (4.2 × 106 cfu ld−1). At six wpg Pst levels in tfl1-14 were reduced to 2.0 × 106 cfu ld−1, characteristic of a modest ARR response (9-fold reduction in Pst levels relative to three wpg). A more robust ARR response was observed at seven wpg (30-fold reduction in Pst levels compared to three wpg). This indicates that ARR occurs in the early-flowering tfl1-14 mutant. At six wpg tfl1-14 had a rosette leaf number of 28.3 ± 1.7, not significantly different from Col-0 (Table 1). The ARR-defective early-flowering plant lines svp-31 and Ws-2 had rosette leaf numbers of 21.7 ± 3.8 and 20.9 ± 6.3 respectively at six wpg; significantly less than tfl1-14 or Col-0. These results suggest that development of a minimum number of rosette leaves is necessary to initiate ARR competence in short-day-grown plants.
Table 2.
ARR in 4-week-old Col-0 and co-9 grown in different photoperiod regimens
| Photoperioda | Floral transitionc | ARR responsec | Average rosette leavesb,c | |
|---|---|---|---|---|
| Col-0 | SD | – | − | 23.1 ± 1.7 |
| SLS | + | − | 24.3 ± 2.3 | |
| LD | + | + | 17.4 ± 2.0 | |
| co-9 | SD | − | − | 24.6 ± 2.0 |
| SLS | − | − | 24.7 ± 1.5 | |
| LD | − | + | 29.1 ± 2.8 |
ARR response and leaf number for experiment presented in Fig. 4
aShort days (SD), long days (LD), or short days/long days/short days (SLS)
bRosette leaf number is presented as the average ± standard deviation (n = 18)
cMeasurements were taken at 4 weeks post-germination
The ld-1 mutant displayed ARR in the absence of flowering, however, the ARR response was somewhat delayed compared to that of Col-0 (Fig. 2a). To test whether ARR is delayed in the absence of flowering, and to determine whether this could be explained in terms of leaf number, we analyzed two additional late-flowering lines; the soc1-2 mutant (Borner et al. 2000) and a FRI + Col-0 line hereafter referred to as FRI + (Lee and Amasino 1995). SOC1 integrates signals from multiple flowering pathways, therefore soc1-2 mutants flower later than wild type (Borner et al. 2000). Wild-type Col-0 has recessive alleles of the FRI gene and as a result, flowers without vernalization (Johanson et al. 2000; Lee and Amasino 1995). A dominant FRI allele introgressed into the Col-0 background severely delays flowering in the absence of vernalization due to upregulation of the floral repressor FLC (Lee and Amasino 1995). FRI + and soc1-2 were chosen primarily for their late-flowering phenotypes. Also, to our knowledge there is no evidence that they exhibit developmental phenotypes aside from late flowering (see discussion on ld-1), however, we also tested a FRI + flc-3 line which flowers at the same time as wild-type Col-0 (Michaels and Amasino 1999) and therefore serves as a control for potential pleiotropic effects of the dominant FRI allele. For example, if FRI + had an ARR defect that was caused by its late-flowering phenotype, this defect should not be observed in FRI + flc-3 which flowers at the same time as wild type. However, if FRI + had an ARR defect for a reason other than late flowering, then FRI + flc-3 should display that same defect. As expected, neither soc1-2 nor FRI + flowered during our experiments while FRI + flc-3 flowered at approximately the same time as Col-0 (Fig. 2b). soc1-2 supported high Pst levels at three and four wpg (>8.0 × 106 cfu ld−1), intermediate levels at five and six wpg (1.4 × 106 cfu ld−1) and lower levels at seven wpg (5.7 × 105 cfu ld−1; Fig. 2b). At five and six wpg there was a 10-fold reduction in Pst levels compared to three wpg, indicative of a moderate ARR response in soc1-2. By seven wpg this difference had increased to 23-fold lower levels of Pst compared to three wpg. soc1-2 had a rosette leaf number of 27.0 ± 2.1 at the time of ARR onset (Table 1). FRI + supported relatively high Pst levels at three wpg (4.5 × 106 cfu ld−1), intermediate levels at four wpg (7.7 × 105 cfu ld−1) and low levels between five and seven wpg (<1.2 × 105 cfu ld−1; Fig. 2b). There was a 63-fold decrease in Pst levels between three and five wpg, indicative of a robust and early ARR response. FRI + had a rosette leaf number of 27.2 ± 1.3 at the time of ARR onset (Table 1). Bacterial levels in FRI + flc-3 were similar to those of FRI + and ARR was also first observed at five wpg (35-fold reduction in Pst relative to three wpg; Fig. 2b). FRI + flc-3 produced a similar rosette leaf number to FRI + at the time of ARR onset (Table 1). Neither FRI + nor soc1-2 flowered during the experiment, but both displayed ARR, further supporting the conclusion that flowering is not necessary for ARR competence. The rosette leaf number of FRI + and soc1-2 at the time of ARR onset was significantly lower than Col-0 but still higher than the maximum reached by the ARR-defective plant lines svp-31, svp-32, and Ws-2 (Table 1, Fig. S2). The observation that one late-flowering mutant (ld-1) had delayed ARR while two other late-flowering plant lines (FRI +, soc1-2) and a wild-type flowering-time plant line (FRI + flc-3) had early ARR indicates that the timing of ARR onset varies between plant lines independently of the timing of the floral transition. Altogether the leaf number data presented in Table 1 is consistent with the hypothesis that development of a minimum number of rosette leaves is required for ARR competence in short-day-grown plants.
To determine whether our hypothesis of a minimum rosette leaf number requirement also applies to long-day-grown plants we analyzed rosette leaf number data collected during the short day/long day shift experiments described above. 4-week-old short-day-grown and photoperiod-induced short-day-grown Col-0 and co-9 had low rosette leaf numbers (between 23 and 25; Table 2) and were ARR-incompetent at this time. This is consistent with our observations that short-day-grown plants remain ARR-incompetent until the production of approximately 30 rosette leaves (Table 1). At four wpg, short-day-grown and photoperiod-induced short-day-grown plants were either vegetative or just beginning the transition to flowering. In contrast, long-day-grown Col-0 made the transition to flowering at approximately three wpg (100 % of plants had inflorescence stems) and therefore had developed fewer rosette leaves (17.4 ± 2.0) than short-day-grown plants at four wpg. The observation that long-day-grown Col-0 was ARR-competent with so few rosette leaves is not consistent with the leaf number-ARR competence relationship observed for short-day-grown plants. This could indicate that the leaf number threshold for ARR competence is lower for plants grown in long days or alternatively, that ARR competence is regulated by a different mechanism in long-day-grown plants.
IAP1 and SID2 are required for ARR in long days
IAP1 and SID2 are important components of the ARR response that occurs in short-day-grown plants (Carviel et al. 2009; Kus et al. 2002). Plants grown in long days display a similar but earlier ARR response (Rusterucci et al. 2005). To obtain clues as to whether the ARR pathway in short-day-grown plants shares components with the ARR pathway in long-day-grown plants, two mutants that are known to be ARR-defective in short-day conditions, iap1-1 and sid2-1, were examined in three different photoperiod regimens as described previously. Short-day-grown and photoperiod-induced short-day-grown iap1-1 and sid2-1 all supported high levels of Pst (>1.0 × 107 cfu ml−1) similar to Col-0 (Fig. 4). Long-day-grown iap1-1 and sid2-1 both supported lower Pst densities compared to their short-day-grown counterparts (5-fold reduction), however, these plants were still susceptible as indicated by high Pst levels (50- to 70-fold higher than long-day-grown Col-0) and characteristic disease symptoms (data not shown), indicative of a defective ARR response. The lower Pst levels in long-day-grown plants were probably due to lower humidity in the long-day chamber (60 %) compared to the short-day chamber (80 %), since high humidity enhances in planta Pst growth (Agrios 2005). Both iap1-1 and sid2-1 had a similar rosette leaf number to Col-0 in all three photoperiod regimens (data not shown). It has been reported that sid2-1 flowers later than Col-0 (greater total leaf number at bolting; Martínez et al. 2004), however this is not observed in our experiments perhaps due to differences in plant growth conditions (day length, light quantity and humidity differences). Taken together, these data suggest that these SA-deficient mutants are developmentally similar to Col-0 in terms of leaf number, and the capacity to accumulate SA is required for ARR in Arabidopsis grown in long-day as well as short-day photoperiods.
Discussion
ARR competence is not associated with flowering in short- or long-day conditions
Previously we demonstrated that ARR competence is associated with the floral transition in Col-0 (Rusterucci et al. 2005). Here we sought to determine if the transition to flowering is responsible for initiating ARR competence by separating the transition to flowering from other developmental events that occur as plants age. To do this, the ARR phenotypes of mutants with three classes of flowering-time phenotype (early, late, and wild-type) were examined under short-day conditions. Overall there was no clear relationship between flowering time and the timing of ARR onset, with all ARR-competent plant lines displaying ARR between five and six wpg irrespective of flowering time. For example, late-flowering plant lines (ld-1, FRI +, and soc1-2) displayed ARR at approximately the same time as Col-0 even though they did not flower during our experiments. This suggests that the floral transition is not required to initiate an ARR-competent state. Of the four plant lines that flowered early, svp-31, svp-32, and Ws-2 were defective for ARR and tfl1-14 displayed a moderate ARR response. Even though tfl1-14 had completed the floral transition by three wpg, ARR was not observed until six wpg, suggesting that early flowering does not initiate early ARR. The observation that svp-31, svp-32, and Ws-2 were ARR-defective further demonstrates that the floral transition is not involved in the initiation of ARR competence and led us to hypothesize that development of a minimum rosette leaf number is required to initiate ARR competence since svp-31, svp-32, and Ws-2 produced significantly fewer rosette leaves than either tfl1-14 or Col-0. The fact that tfl1-14 produced more rosette leaves than svp-31, svp-32, and Ws-2 is counter-intuitive since tfl1-14 appeared to flower slightly earlier than svp-31, svp-32, and Ws-2 and would therefore be expected to have a lower maximum rosette leaf number. This difference could be explained by a higher leaf initiation rate in tfl1-14 or a lower leaf initiation rate in svp-31, svp-32, and Ws-2 although it has previously been shown that tfl1 mutants initiate leaves at a rate similar to Col-0 (Shannon and Meeks-Wagner 1991). Another explanation is that tfl1-14 continued to produce rosette leaves after the transition to flowering, although this is inconsistent with the currently accepted model of organ development in reproductive-stage Arabidopsis which indicates that rosette leaves are not produced after the floral transition (Hempel and Feldman 1994).
To support the conclusion that the floral transition does not initiate ARR competence we looked at the ARR response of short-day-grown Col-0 that were forced to flower early by photoperiod-induced transient expression of FT (exposure to three long days). This treatment initiated the floral transition by four wpg as demonstrated by expression of FT, but did not elicit ARR competence suggesting that photoperiod-induced flowering is not sufficient for the onset of ARR competence in 4-week-old plants. This is consistent with the ARR defects observed in short-day-grown early-flowering plant lines and confirms that an early floral transition does not initiate ARR competence.
In Arabidopsis the floral transition occurs earlier in long days than in short days (Gregory and Hussey 1953). Since ARR onset also occurs earlier in long days and at approximately the same time as the transition to flowering, we suspected that the transition to flowering was the cue for ARR competence (Rusterucci et al. 2005). While this does not appear to be true for short-day-grown plants, we tested whether this might be the case for long-day-grown plants. Long-day-grown co-9 mutants are delayed in photoperiod-induced flowering (Koornneef et al. 1991; Putterill et al. 1995) and remained vegetative at four wpg but still displayed a robust ARR response, similar to long-day-grown Col-0. This suggests that photoperiod-induced flowering is not required for the onset of ARR competence in long-day-grown plants. While it appears that development of a minimum rosette leaf number may initiate ARR competence in short-day-grown plants, the same relationship was not observed for long-day-grown plants since long-day-grown Col-0 displayed ARR at a rosette leaf number similar to short-day-grown, ARR-defective svp-31, svp-32, and Ws-2. This could indicate that the minimum leaf number requirement for ARR competence is lower in long-day-grown plants or that ARR in long-day-grown plants is regulated by a different mechanism altogether.
Vegetative phase change and ARR competence
Another consideration is that the vegetative phase change could be involved in the regulation of ARR competence. The central regulator of the vegetative phase change, miRNA156, targets members of the SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) family, which have been shown to contribute to the onset of adult and reproductive phase characteristics (Schwarz et al. 2008; Usami et al. 2009; Wang et al. 2008; Wu et al. 2009; Wu and Poethig 2006). In wild-type plants miR156 levels decrease over time, leading to a gradual de-repression of SPL genes and transition to the adult vegetative phase. Overexpression of miR156 in 35S:miR156 plants causes the juvenile phase to be dramatically prolonged; with plants producing 90 ± 1.3 juvenile leaves whereas Col-0 produces 7.5 ± 0.7 (Wu et al. 2009). Preliminary results from our lab indicate that short-day-grown 35S:miR156 plants exhibit ARR at six wpg (data not shown), suggesting that the prolonged manifestation of juvenile characteristics does not delay the onset of ARR competence.
Many mutations in flowering-time genes also affect the timing of the vegetative phase change. Alternatively, some mutations alter the timing of either the vegetative phase change or transition to flowering without affecting the other (Telfer et al. 1997; Willmann and Poethig 2005). Interestingly, ARR-defective svp-31, svp-32, and Ws-2 undergo an earlier vegetative phase change relative to Col-0 (Hartmann et al. 2000; Telfer et al. 1997) whereas ARR-positive tfl1-14 undergoes the vegetative phase change normally (Telfer et al. 1997). Although this might suggest that an early vegetative phase change is associated with ARR incompetence, long-day-grown Col-0 also undergoes an early vegetative phase change (Chien and Sussex 1996), and this does not result in an ARR defect. This suggests that the timing of the vegetative phase change does not regulate ARR competence, however, a more detailed analysis is required to fully address this question.
Timing of ARR onset differs between some plant lines
While some plant lines showed early ARR responses, others exhibited delayed ARR, such that robust resistance was not observed until seven wpg (ld-1) or only moderate responses were observed at six or seven wpg (tfl1-14, soc1-2). These differences had no obvious relationship with flowering-time. Instead it may be that some of the mutations that affect flowering time have pleiotropic effects. For example, autonomous pathway genes such as LD are believed to be involved in processes such as chromatin modification and RNA metabolism, and as a result, likely function in aspects of plant development other than flowering-time (Amasino 2010). This proposition is supported by observations of lethality or severe growth and developmental defects in various autonomous pathway mutants (Henderson et al. 2005; Koornneef et al. 1998; Veley and Michaels 2008). Variation in the timing of ARR could also result from differences in the genetic background of various plant lines used in this study (i.e., polymorphisms that are independent of mutations in flowering-time genes). Although all mutants used were in the Columbia background, whole-genome resequencing studies have revealed that different strains of Columbia can harbour thousands of unique polymorphisms (Ossowski et al. 2008). While many of these observed differences could reflect errors in the reference genome, the same group later showed that the rate of spontaneous mutation accumulation is much higher than previously thought (Ossowski et al. 2010). This implies that in some cases mutant lines may possess many genetic differences from wild-type controls (Santuari and Hardtke 2010).
We have demonstrated that the floral transition can be separated from ARR competence in both short- and long-day-grown plants. Therefore, the floral transition is not the developmental cue for ARR competence. Instead, vegetative development of a minimum numbers of leaves appears to be important for ARR competence in short-day grown Arabidopsis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a Natural Science and Engineering Research Council of Canada Discovery Grant to R. Cameron and growth chamber maintenance support from McMaster University. We would like to thank Jen Chin, who performed replicate experiments with svp-31 and Ws-2. D. Wilson, M. Isaacs, and P. Carella were supported by Ontario Graduate Scholarships.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Daniel C. Wilson, Philip Carella and Marisa Isaacs contributed equally to this work.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Abreu ME, Munné-Bosch S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J Exp Bot. 2009;60:1261–1271. doi: 10.1093/jxb/ern363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrios GN. Plant pathology. 5. London: Elsevier Academic Press; 2005. [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Guli CL, Yu X, Smyth DR. terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992;2:103–116. doi: 10.1111/j.1365-313X.1992.00103.x. [DOI] [Google Scholar]
- Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- An H, Roussot C, Suàrez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering in Arabidopsis. Development. 2004;131:3615–3628. doi: 10.1242/dev.01231. [DOI] [PubMed] [Google Scholar]
- Ayre BG, Turgeon R. Graft transmission of a floral stimulant derived from CONSTANS. Plant Physiol. 2004;135:2271–2278. doi: 10.1104/pp.104.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature. 2004;427:164–167. doi: 10.1038/nature02269. [DOI] [PubMed] [Google Scholar]
- Borner R, Kampmann G, Chandler J, Gleiβner R, Wisman E, Apel K, Melzer S. A MADS domain gene involved in the transition to flowering in Arabidopsis. Plant J. 2000;24:591–599. doi: 10.1046/j.1365-313x.2000.00906.x. [DOI] [PubMed] [Google Scholar]
- Cameron RK, Zaton K. Intercellular salicylic acid accumulation is important for age-related resistance in Arabidopsis to Pseudomonas syringae. Physiol Mol Plant P. 2004;65:197–209. doi: 10.1016/j.pmpp.2005.02.002. [DOI] [Google Scholar]
- Carviel JL, Al-Daoud F, Neumann M, Mohammad A, Provart NJ, Moeder W, Yoshioka K, Cameron RK. Forward and reverse genetics to identify genes involved in the age-related resistance response in Arabidopsis thaliana. Mol Plant Pathol. 2009;10:621–634. doi: 10.1111/j.1364-3703.2009.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CJ, Sussex IM. Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 1996;111:1321–1328. doi: 10.1104/pp.111.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Gadisseur I, Silvestre G, Jacqmard A, Bernier G. Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. Plant J. 1996;9:947–952. doi: 10.1046/j.1365-313X.1996.9060947.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Develey-Rivière M, Galiana E. Resistance to pathogens and host developmental stage: a multifaceted relationship within the plant kingdom. New Phytol. 2007;175:405–416. doi: 10.1111/j.1469-8137.2007.02130.x. [DOI] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G. Snapshot: control of flowering in Arabidopsis. Cell. 2010;141(550–550):e2. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Giakountis A, Cremer F, Sim S, Reymond M, Schmitt J, Coupland G. Distinct patterns of genetic variation alter flowering responses of Arabidopsis accessions to different daylengths. Plant Physiol. 2010;152:177–191. doi: 10.1104/pp.109.140772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory FG, Hussey GG. Photoperiodic responses of Arabidopsis thaliana. Proc Linn Soc Lond. 1953;164:137–139. doi: 10.1111/j.1095-8312.1953.tb00676.x. [DOI] [Google Scholar]
- Hartmann U, Höhmann S, Nettesheim K, Wisman E, Saedler H, Huijser P. Molecular cloning of SVP: a negative regulator of the floral transition in Arabidopsis. Plant J. 2000;21:351–360. doi: 10.1046/j.1365-313x.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Wood CC, Robertson M, Peacock WJ, Dennis ES. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006;46:183–192. doi: 10.1111/j.1365-313X.2006.02686.x. [DOI] [PubMed] [Google Scholar]
- Hempel FD, Feldman LJ. Bi-directional inflorescence development in Arabidopsis thaliana: acropetal initiation of flowers and basipetal initiation of paraclades. Planta. 1994;192:276–286. doi: 10.1007/BF01089045. [DOI] [Google Scholar]
- Henderson IR, Liu F, Drea S, Simpson GG, Dean C. An allelic series reveals essential roles for FY in plant development in addition to flowering-time control. Development. 2005;132:3597–3607. doi: 10.1242/dev.01924. [DOI] [PubMed] [Google Scholar]
- Hugot K, Aimé S, Conrod S, Poupet A, Galiana E. Developmental regulated mechanisms affect the ability of a fungal pathogen to infect and colonize tobacco leaves. Plant J. 1999;20:163–170. doi: 10.1046/j.1365-313x.1999.00587.x. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA. FKF1 is essential for photoperiod-specific light signaling in Arabidopsis. Nature. 2003;426:302–306. doi: 10.1038/nature02090. [DOI] [PubMed] [Google Scholar]
- Irish VF, Sussex IM. Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell. 1990;2:741–753. doi: 10.1105/tpc.2.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009;60:614–625. doi: 10.1111/j.1365-313X.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- Jin JB, Jin YH, Lee J, Miura K, Yoo CY, Kim W, Oosten MV, Hyun Y, Somera DE, Lee I, Yun D, Bresan RA, Hasegawa PM. The SUMO E3 ligase, AtSIZ1, regulates flowering by controlling a salicylic acid-mediated floral promotion pathway and through affects on FLC chromatin structure. Plant J. 2008;53:530–540. doi: 10.1111/j.1365-313X.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, West J, Lister C, Michaels S, Amasino R, Dean C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering-time. Science. 2000;290:344–347. doi: 10.1126/science.290.5490.344. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Peeters AJM. Genetic interactions among late-flowering mutants of Arabidopsis. Genetics. 1998;148:885–892. doi: 10.1093/genetics/148.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus JV, Zaton K, Sarkar R, Cameron RK. Age-related resistance in Arabidopsis is a developmentally-regulated defense response to Pseudomonas syringae. Plant Cell. 2002;14:479–490. doi: 10.1105/tpc.010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Amasino RM. Effect of vernalization, photoperiod, and light quality on the flowering phenotype of Arabidopsis plants containing the FRIGIDA gene. Plant Physiol. 1995;108:157–162. doi: 10.1104/pp.108.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Blecker A, Amasino R. Analysis of naturally occurring late flowering in Arabidopsis thaliana. Mol Gen Genet. 1993;237:171–176. doi: 10.1007/BF00282798. [DOI] [PubMed] [Google Scholar]
- Lee J, Nam J, Park HC, Na G, Miura K, Jin JB, Yoo CY, Baek D, Kim DH, Jeong JC, Kim D, Lee SY, Salt DE, Mengiste T, Gong Q, Ma S, Bohnert HJ, Kwak S, Bressan RA, Hasegawa PM, Yun D. Salicylic acid-mediated innate immunity in Arabidopsis is regulated SIZ1 SUMO E3 ligase. Plant J. 2006;49:79–90. doi: 10.1111/j.1365-313X.2006.02947.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering-time by ambient temperature in Arabidopsis. Gene Dev. 2007;21:397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Oh M, Park H, Lee I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 2008;55:832–843. doi: 10.1111/j.1365-313X.2008.03552.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Han S, Sririyanum M, Park C, Seo Y, Ronald P. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science. 2009;326:850–853. doi: 10.1126/science.1173438. [DOI] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell. 2008;15:110–120. doi: 10.1016/j.devcel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell. 1999;11:1007–1018. doi: 10.1105/tpc.11.6.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han J, Liou YC, Yu H. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development. 2008;135:1481–1491. doi: 10.1242/dev.020255. [DOI] [PubMed] [Google Scholar]
- Liu J, Li W, Ning Y, Shirsekar G, Cai Y, Wang X, Dai L, Wang Z, Liu W, Wang GL. The U-box E3 ligase SPL11/PUB13 is a convergence point of defense and flowering signaling in plants. Plant Physiol. 2012;160:28–37. doi: 10.1104/pp.112.199430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C, Pons E, Prats G, León J. Salicylic acid regulates flowering time and links defense responses and reproductive development. Plant J. 2004;37:209–217. doi: 10.1046/j.1365-313X.2003.01954.x. [DOI] [PubMed] [Google Scholar]
- Mazzola M, Leach JE, Nelson R, White FF. Analysis of the interaction between Xanthomonas oryzae pv. oryzae and the rice cultivars IR24 and IRBB21. Phytopathology. 1994;84:392–397. doi: 10.1094/Phyto-84-392. [DOI] [Google Scholar]
- McDaniel CN, Singer SR, Smith SME. Developmental states associated with the floral transition. Dev Biol. 1992;153:59–69. doi: 10.1016/0012-1606(92)90091-T. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell. 2001;13:935–941. doi: 10.1105/tpc.13.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM. Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol. 2005;137:149–156. doi: 10.1104/pp.104.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S, Schneeberger K, Clark RM, Lanz C, Warthmann N, Weigel D. Sequencing of natural strains of Arabidopsis thaliana with short reads. Genome Res. 2008;18:2024–2033. doi: 10.1101/gr.080200.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski S, Schneeberger K, Lucas-Lledó JI, Warthmann N, Clark RM, Shaw RG, Weigel D, Lynch M. The rate and molecular spectrum of spontaneous mutations in Arabidopsis thaliana. Science. 2010;327:92–94. doi: 10.1126/science.1180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig SR. Phase change and the regulation of shoot morphogenesis in plants. Science. 1990;250:923–930. doi: 10.1126/science.250.4983.923. [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell. 1995;80:847–857. doi: 10.1016/0092-8674(95)90288-0. [DOI] [PubMed] [Google Scholar]
- Rèdei GP. Supervital mutants of Arabidopsis. Genetics. 1962;47:443–460. doi: 10.1093/genetics/47.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M, Plasencia J. Salicylic acid beyond defence: its role in plant development. J Exp Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- Rusterucci C, Zhao Z, Haines K, Mellersh D, Neumann M, Cameron RK. Age-related resistance to Pseudomonas syringae pv. tomato is associated with the transition to flowering in Arabidopsis and is effective against Peronospora parasitica. Physiol Mol Plant P. 2005;66:222–231. doi: 10.1016/j.pmpp.2005.08.004. [DOI] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Santuari L, Hardtke CS (2010) The case for resequencing studies of Arabidopsis thaliana accessions: mining the dark matter of natural genetic variation. F1000 Rep 2:85 [DOI] [PMC free article] [PubMed]
- Schultz EA, Haughn GW. Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development. 1993;119:745–765. [Google Scholar]
- Schwarz S, Grande AV, Bujdoso N, Saedler H, Huijser P. The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol Biol. 2008;67:183–195. doi: 10.1007/s11103-008-9310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Gene Dev. 2006;20:898–912. doi: 10.1101/gad.373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell. 1991;3:877–892. doi: 10.1105/tpc.3.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meeks-Wagner DR. Genetic interactions that regulate inflorescence development in Arabidopsis. Plant Cell. 1993;5:639–655. doi: 10.1105/tpc.5.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon CC, Burn JE, Perez PP, Metzger J, Edwards JA, Peacock J, Dennis ES. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell. 1999;11:445–458. doi: 10.1105/tpc.11.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Telfer A, Bollman KM, Poethig SR. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development. 1997;124:645–654. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- Usami T, Horiguchi G, Yano S, Tsukaya H. The more and smaller cells mutants of Arabidopsis identify roles for SQAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development. 2009;136:955–964. doi: 10.1242/dev.028613. [DOI] [PubMed] [Google Scholar]
- Veley KM, Michaels SD. Functional redundancy and new roles for genes of the autonomous floral promotion pathway. Plant Physiol. 2008;147:682–695. doi: 10.1104/pp.108.118927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot CA, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- Wang J, Schwab R, Czech B, Mica E, Weigel D. Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell. 2008;20:1231–1243. doi: 10.1105/tpc.108.058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Seabolt S, Hamdoun S, Ng G, Park J, Lu H. Multiple roles of WIN3 in regulating disease resistance, cell death, and flowering-time in Arabidopsis. Plant Physiol. 2011;156:1508–1519. doi: 10.1104/pp.111.176776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Alvarez J, Smyth DR, Yanofsky MF, Meyerowitz EM. LEAFY controls floral meristem identity in Arabidopsis. Cell. 1992;69:843–859. doi: 10.1016/0092-8674(92)90295-N. [DOI] [PubMed] [Google Scholar]
- Whalen MC. Host defense in a developmental context. Mol Plant Pathol. 2005;6:347–360. doi: 10.1111/j.1364-3703.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Willmann MR, Poethig SR. Time to grow up: the role of smallRNAs in plants. Curr Opin Plant Biol. 2005;8:548–552. doi: 10.1016/j.pbi.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Poethig S. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development. 2006;133:3539–3547. doi: 10.1242/dev.02521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang J, Weigel D, Poethig SR. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


